Abstract
Due to the multisensory input into the balance system, the loss of one input, such as an ear, can generally be compensated for. However, when a mismatch or incomplete loss of inputs occurs, the ability to compensate for the stimulus misrepresentation may be compromised. The inner ear and cerebellum are important input and processing centers for balance but no genetic models have been generated to assess balance or compensation in the abnormal development of both these organs/brain areas. Important to their formation is regulation of proliferation mediated by the proto-oncogene N-Myc. Conditional knockouts (CKOs) of N-Myc using Tg(Pax2-Cre) have a misshapen and smaller ear with a fused utricle, saccule, and cochlea and absent horizontal canal, aberrant cochlear and vestibular innervations, and a size reduction in the cerebellum. CKOs are viable with obvious behavioral deficits, including circling behavior and unstable gait. To test the degree of ataxia and possible compensation of vestibular defects in these mutant mice, we use the Noldus Catwalk System to assess the gait of Tg(Pax2-Cre) N-Myc CKOs over five months. N-Myc CKOs perform worse than control littermates, in particular, in step regularity. We show that disrupting one member of the Myc family during embryonic development coincides with a differential loss of function in the cochlea compared to the vestibular apparatus. In addition, we show that the distortion in the ear morphology combined with a reduction of the cerebellum, rather than a complete loss of the vestibular-cerebellar pathway, leads to partial behavioral compensation that remains unchanged over time.
Keywords: N-Myc, Catwalk, Regularity Index, Stride Length, Stride Speed
Introduction
Balance and coordinated movement orientation in the Earth’s gravity field is necessary for daily life. Unfortunately, ataxia (lack of coordinated movements) is a common symptom of diseases prevalent throughout the world. Ataxia can originate from perturbations to any of three peripheral organ systems: 1) the five vestibular endorgans of the ear, 2) the eye, or 3) proprioception (muscles and joints) (S. M. Jones, Jones, Mills, & Gaines, 2009). From these sensory inputs, afferent neurons project centrally to coordinate balance. Well-coordinated gravistatic responses require balanced afferent inputs from the vestibular ear to the brainstem and cerebellum (Bermingham et al., 2001; S. M. Jones, et al., 2009; Maklad, Kamel, Wong, & Fritzsch, 2010). These sophisticated vestibular inputs, coupled with visual and proprioceptive cues, provide the neuronal substrates for proper oculomotor and postural reflex functioning such as eye movements (Cullen, Minor, Beraneck, & Sadeghi, 2009). Due to these multiple inputs, balance tends to be robust and is often fully compensated for when one input is lost [i.e. balance is maintained when we close our eyes or a vestibular system is surgically removed due to schwannoma (Parietti-Winkler, Gauchard, Simon, & Perrin, 2011) or experimentally removed (Beraneck, McKee, Aleisa, & Cullen, 2008; Cullen, et al., 2009)]. However, when an input is partially lost or sends incorrect information to the brain, marked disorientation occurs, such as in patients suffering from vertigo or vestibular schwannomas (where central processing anatomy is normal but the input is distorted). Cerebellarectomies of cerebellar ataxia mouse models, such as the weaver mouse, show that inaccurate output is more disadvantageous than complete elimination as mice without a cerebellum perform better than those with a disorganized cerebellum (Cutuli et al., 2011; Foti et al., 2011; Grusser-Cornehls & Baurle, 2001; Grusser-Cornehls, Grusser, & Baurle, 1999; Grusser & Grusser-Cornehls, 1998). Debilitation resulting from imbalance due to mismatched inputs can be improved upon when the aberrant signaling ceases. In fact, patients suffering from imbalance due to a unilateral vestibular schwannoma perform better after surgery when all afferent inputs to the vestibular nucleus complex serving the affected ear are removed (Parietti-Winkler, et al., 2011) and even bilateral vestibular removal can be compensated for in rats (Goddard, Zheng, Darlington, & Smith, 2008). This improvement after removal of a mismatched input is due to the central nervous system’s (CNS) plasticity (Dutia, 2010; Helmchen et al., 2011) and is called vestibular compensation (S. M. Jones, et al., 2009).
Many murine models have described isolated defects in either the ear (de Caprona, Beisel, Nichols, & Fritzsch, 2004; Fritzsch, Signore, & Simeone, 2001) or in the cerebellum (Beraneck, et al., 2008; Grusser-Cornehls & Baurle, 2001) yet most of these models result from spontaneous mutations or have otherwise not been fully characterized and may contain abnormalities elsewhere, indicating a need for a well-characterized genetic model. Viable mutants with cerebellar, brainstem, and ear defects exist, but cerebellar defects in these mutants are highly variable minimizing their usefulness (Nichols et al., 2008). At the moment, there are no suitable and viable genetic models that disrupt simultaneously the inner ear, afferents to the cerebellum, and the cerebellum causing a vestibulo-cerebellar syndrome that spares the vestibular nuclei. Combining Cre expressing lines with a relevant floxed gene can result in restricted targeted deletions that narrow down the defected neuronal structures (Cre-LoxP approach). To generate such a mouse model, we took advantage of the genetic similarities between ear and cerebellar development. Many important bHLH transcription factors are known to play roles in both ear and cerebellar development, including Atoh1 (Klisch et al., 2011; Pan et al., 2011), NeuroD1 (Belyantseva et al., 2009; Jahan, Kersigo, Pan, & Fritzsch, 2010; Jahan, Pan, Kersigo, & Fritzsch, 2010), and N-Myc (Dominguez-Frutos et al., 2011; Kopecky, Santi, Johnson, Schmitz, & Fritzsch, 2011; Wey, Martinez Cerdeno, Pleasure, & Knoepfler, 2010). The development of the inner ear and the cerebellum requires a delicate balance between proliferation (using the bHLH transcription factor N-Myc) and directed differentiation of progenitor populations (neurosensory progenitors in the ear and cerebellar granule neural progenitors in the cerebellum). Conditional deletion of N-Myc results in disruption of proliferation (Kopecky, et al., 2011; Pauley, Lai, & Fritzsch, 2006) or differentiation (Jahan, Kersigo, et al., 2010; Pan et al., 2010) in the ear or cerebellum while sparing the hindbrain due to lack of Cre expression of our Tg(Pax2-Cre) line.
The proto-oncogene N-Myc is a member of the highly conserved family of Myc transcription factors (C-Myc, L-Myc, N-Myc) that regulate proliferation throughout the body (Eilers & Eisenman, 2008; Knoepfler & Kenney, 2006; Lasorella et al., 2002; Lasorella, Noseda, Beyna, Yokota, & Iavarone, 2000; Modak & Cheung, 2010; Mott et al., 2010; Ruggero, 2009). In the ear, N-Myc and L-Myc are highly expressed during embryonic development (Kopecky, et al., 2011; Romand, Hirning-Folz, & Ehret, 1994), with, at minimum, N-Myc playing an essential role in ear development and hair cell maintenance (Dominguez-Frutos, et al., 2011; Kopecky, et al., 2011). C-Myc is expressed at low levels in the ear and does not appear to play a major role in ear development (Kopecky, et al., 2011; Romand, et al., 1994). Both C-Myc and N-Myc are necessary for cerebellar development and it is likely (yet unstudied) that L-Myc may also play redundant functional roles in the cerebellum (Wey, et al., 2010). Knockouts of C-Myc and N-Myc combined (Wey, et al., 2010) or N-Myc alone (Kopecky, et al., 2011) result in drastic cerebellar size reduction and abnormal cerebellar development. Based on these studies, knocking out N-Myc will result in abnormal development of the inner ear, afferent neurons, and the cerebellum, generating a vestibulo-cerebellar syndrome mouse. C-Myc or N-Myc null mice are early embryonic lethal (Davis, Wims, Spotts, Hann, & Bradley, 1993; Stanton, Perkins, Tessarollo, Sassoon, & Parada, 1992). Using a Tg(Pax2-Cre) N-Myc CKO, we disrupt ear and cerebellar development. Furthermore, while other models lose vestibular hair cells over time (Pauley, Kopecky, Beisel, Soukup, & Fritzsch, 2008) and mimic disorders such as bilateral vestibulopathy, our mice retain vestibular hair cells comparable to littermates, allowing us to assess compensation in the presence of continued vestibular-cerebellar input. As such, we have generated a mouse model to assess vestibular compensation that can be monitored into late adulthood, if appropriate numbers of viable mice are maintained.
To the best of our knowledge, there has been no consistent or viable genetic murine model that affects both peripheral and central processing of the ear and the cerebellum with sparing of the vestibular nuclei. Additionally, only limited behavioral studies exist to study compensation or gait performance. For the first time, we directly test how the reduction of proliferation in the ear and cerebellum, with resulting dysmorphogenesis in both organs, affects gait regularity, stride length, and stride speed (both static and dynamic parameters of coordination) using the novel Noldus Catwalk System (Hamers, Lankhorst, van Laar, Veldhuis, & Gispen, 2001). Furthermore, we show the differential and progressive loss of cochlear hair cells compared to long-term retention of vestibular hair cells in the inner ear and how this correlates with function. Together, we introduce a viable mouse mutant incapable of full behavioral compensation and show that the Noldus Catwalk System is proficient in detecting ataxic gait of these mice. Our mice represent a novel approach to understanding the residual function of developmentally disrupted ear and cerebellum and the ability to compensate for these perturbations. The future use of conditional deletions that target only the ear or the cerebellum will further elucidate the relative contribution of both organs to gait.
Material and Methods
Mice and Genotyping
We generated a conditional knockout (CKO) line by crossing Tg(Pax2-Cre) mice (Ohyama & Groves, 2004) with N-Myc floxed mice (Jackson Labs B6.129-N-Myctm1Psk/J). Newborn mice are viable; however, they had an apparently increased in utero mortality rate as demonstrated by a skewed Mendelian ratio. CKO mice displayed circling behavior, disorientation, and did not possess a sound elicited startle response. Tail biopsies were used for genomic DNA and polymerase chain reaction for genotyping was performed using the following primers (N-Myc: IMR6727 5′ gtcgcgctagtaagagctgagatc 3′ IMR6729 5′ cacagctctggaaggtgggagaaagttgagcgtctcc 3′ Cre: 1 5′ cctgttttgcacgttcaccg 3′ 2 5′ atgcttctgtccgtttgccg 3′ IMR42 5′ ctaggccacagaattgaaagatct 3′ IMR43 5′ gtaggtggaaattctagcatcatcc 3′). Animal care and usage was in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines for the use of laboratory animals in biological research and approved (ACURF # 0804066 and 1103057).
Gait Analysis
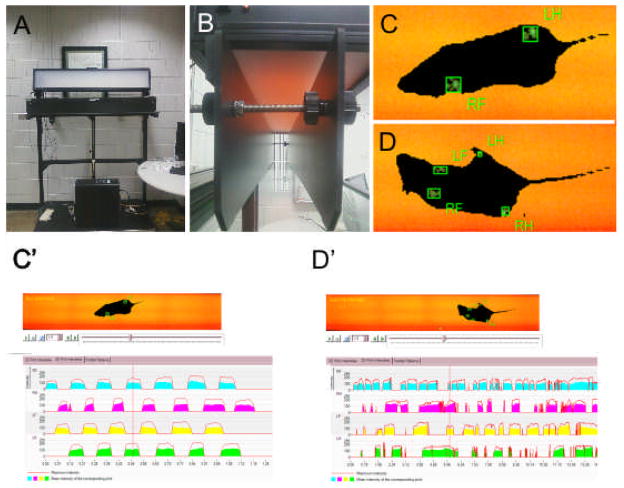
The Noldus Catwalk System consists of a walkway floor containing a sheet of glass encased with a fluorescent light (Figure 1A and 1B). The optical properties of the glass result in internal reflectance of the light unless an external contact is made with the glass. When pressure is applied to the face of the glass, the light changes the dynamic property of the glass and allows the light to escape internal reflectance, illuminating the source of pressure with luminescence proportional to the intensity of pressure (Figure 1C and 1D) (F. P. Hamers, G. C. Koopmans, & E. A. Joosten, 2006; Vrinten & Hamers, 2003). Tg(Pax2-Cre) N-Myc CKOs and control littermates were placed in a corridor 90cm long by 10cm wide and allowed to freely move (movable wire gates prohibited the mice from exiting the walkway and limited the walkway to approximately 60cm in length). A mounted camera captured a 40cm × 10cm field from below the walkway. As the mouse enters the field of view, a run is initiated and stored on a computer. If the mouse exited the field of view within 25 seconds and there was less than a 60% variation in speed, the run was compliant. If the mouse could not exit the field of view or the run was otherwise terminated, the run was considered non-compliant. Furthermore, runs were only considered if the mouse did not stop, change direction, or in any other way did not satisfactorily cross the field of view. Three to five compliant runs were acquired for each mouse at each test date and were termed a trial. Between each trial, the walkway barrier was removed and the glass was cleaned to remove any excrement or smell from the preceding trial using standard glass cleaner. All mice were tested under similar conditions (lights off, “Screenflex” room divider in place to further darken environment, quiet room, and no external impetus for crossing). After each trial, the acquired data was classified. Classification is the semi-automated process of entering which limb (RH=Right Hind, LH= Left Hind, RF= Right Front, LF= Left Front) induced the recorded print (Figure 1C′ and 1D′).
Figure 1.
The Noldus Catwalk System (A) is made up of a transparent glass floor embedded with a light source and two barriers on either side (B), allowing the mouse approximately a 90cm by 10cm walkway. A mounted camera from below captures mouse movement by calculating pressure “prints” as the mouse walks across the field of view for both control (C) and Tg(Pax2-Cre) N-Myc CKO mice (D). The rectangular boxes indicate a pressure mark recorded by the computer. Subsequent classification identifies which paw made the pressure mark. Note that paw prints are regular for control (C and C′) but abnormal for Tg(Pax2-Cre) N-Myc CKO (D and D′). An overhead light allows for easy outline visualization for the researcher. As the mouse walks through the camera’s field of view, pressure intensities for each paw are recorded for both control (C′) and mutant (D′). The catwalk system calculates the mouse regularity, stride speed, and stride length (among other parameters) for each mouse run with paw prints classified. The graph depicts max and average paw pressure as well as sequence (C′ and D′).
Explanation Of Compared Parameters
There are three main independent analytic categories of the catwalk system (with the subcategory we used): coordination parameters (regularity index), dynamic paw parameters (stride speed), and static paw parameters (stride length) (Koopmans et al., 2007). While there are numerous subcategories on the catwalk system, analyses of these three subcategories (in parentheses) were sufficient to conclude that the mutant mice performed worse than control littermates. We therefore limit our results and discussion to regularity index, stride speed, and stride length. The regularity index acts as a measure of generalized coordination (F. P. T. Hamers, G. C. Koopmans, & E. A. J. Joosten, 2006) by computing whether the mouse footfalls fall within one of six regular step patterns. As the animal becomes less coordinated, there becomes an increase in missteps which results in a lower regularity index (Hamers, et al., 2001). Gait regularity tests how consistently the mouse takes “normal strides” compared to “abnormal strides”. Normal strides are considered to occur when either the right front paw is paired with the left hind paw or when the left front paw is paired with the right hind paw. Abnormal strides consist of any other configuration and is broken down into various “support categories” such as “one” where one paw is the only base of support, “three”, “four”, “lateral”, or “none” with the later counting when the mouse is no longer supporting its weight through its limbs. These support subcategories were analyzed and were consistent with the results described through the regularity index (not shown). Stride speed is independent of stride length (Hamers, et al., 2001). Stride speed is measured as the time interval between the same limb’s contacts with the walkway floor. A short stride speed indicates a compensation for unstable gait (F. P. Hamers, et al., 2006). Stride length measures the distance from the previous position of a hindlimb to the current position of the forelimb on the same side. For example, if the right hindlimb does not pass the previous position of the right forelimb, stride length is negative whereas if the left hindlimb exceeds the previous left forelimb location, stride length is positive. The same calculation is performed for forelimb stride length. Stride length is considered a static parameter similar to base of support (width between hindlimbs); both of which indicate unstable gait (Koopmans, et al., 2007). These three parameters are chosen due to their relevance in the three gait assessment categories: coordination, dynamic limb, and static limb parameters.
Statistical Analysis
Regularity and stride length were assessed with a two-way (Line × Time) repeated-measures ANOVA, with time as the within-subjects factor and line as the between-subjects factor. Stride speed was log-transformed and assessed with a two-way (Line × Time) repeated-measures ANOVA, with time as the within-subjects factor and line as the between-subjects factor. After transformation, the assumptions of the statistical model were met. Each mouse successfully completed three, four or five runs at each time point leading to an unbalanced design. Accordingly, the degrees of freedom for tests were computed using the Satterthwaite procedure. Alpha was set at 0.05 for all tests of interest. For brain region measurements, mid-sagittal sections were measured and the surface area was approximated to be equal to width × height of the section. Surface areas of WT and CKOs were compared using student t-Test.
ABR
Mice were anesthetized with 0.025mL/g of the anesthetic Tribromoethanol (Avertin®) and after a surgical level of anesthesia was induced, as assessed by absence of ocular and pedal reflexes, needle electrodes were inserted in the vertex, slightly posterior to the pinna and in the contralateral hindlimb subcutaneously. A loud speaker was placed 10cm from the pinna of the test ear and computer-generated clicks were given in an open field environment in a soundproofed chamber. Clicks were presented and responses were averaged across 512 presentations using Tucker-Davis Technologies System hardware running BioSig® Software. Recorded signals were bandpass filtered (300Hz–5kHz) and 60Hz notch filter. The sound level was decreased in 10-dB steps from a 96-dB sound pressure level until there was no noticeable response. Identical set-up but a different program was used for tone pip where frequencies were given at 4kHz through 60kHz incrementally and with a stepwise decrease in amplitude at each frequency. In total, four Tg(Pax2-Cre) N-Myc CKO and WT mice were tested (P21, P28, P28, and 7 month).
Perfusion
Mice were injected intraperitoneally (IP) with greater than a 0.025mL/g of the anesthetic Tribromoethanol (Avertin®) and after ocular and pedal reflexes ceased, 4% paraformaldehyde (PFA) was pumped continuously with a 30-gauge needle into the left ventricle until blood emptied and peripheral veins appeared clear. The right ventricle was opened to facilitate clearing. After fixation, heads were hemi-dissected and placed in 4% PFA for long-term storage.
Nuclear Staining
Cerebella were hemisected and a near sagittal section was cut and placed in 1:2000 Hoechst nuclear stain for one hour. After several washes in PBS, cerebella were mounted and images were captured with TCS SP5 multiphoton confocal microscope.
Plastic Sectioning
Dissected ears were incubated for 2 hours with 2.5% glutaraldehyde and washed with 0.1 M phosphate buffer three times over a period of two hours. Ears were reacted in 1% osmium tetroxide for approximately one hour or until nerve fibers were dark and rinsed with 0.1 M phosphate buffer. Samples were first dehydrated with an increasing graded ethanol series and then incubated with a 1:1 ethanol and propylene oxide mixture. Samples were mixed with Epon 812 and propylene oxide overnight. Samples were then orientated and embedded with Epon 812 and placed in an incubator for two days at 60°C. Ears were sectioned at 2μm with a Leica Ultratome and sections were retrieved from water bath and placed on heated slides in sequential order. They were allowed to bake onto the glass slide and stained for one minute with Stevenel’s Blue and imaged (Nichols, et al., 2008). Stained slides were rinsed with dH20 and allowed to dry before imaging. Chosen sections were coverslipped using DMX mounting solution and allowed to dry overnight. Samples were imaged with Nikon Eclipse 800 microscope and captured with Image-Pro. Images were processed into plates using CorelDraw software.
Results
Tg(Pax2-Cre) N-Myc CKO Mice Perform Worse Than Control Littermates And Do Not Improve Over Time
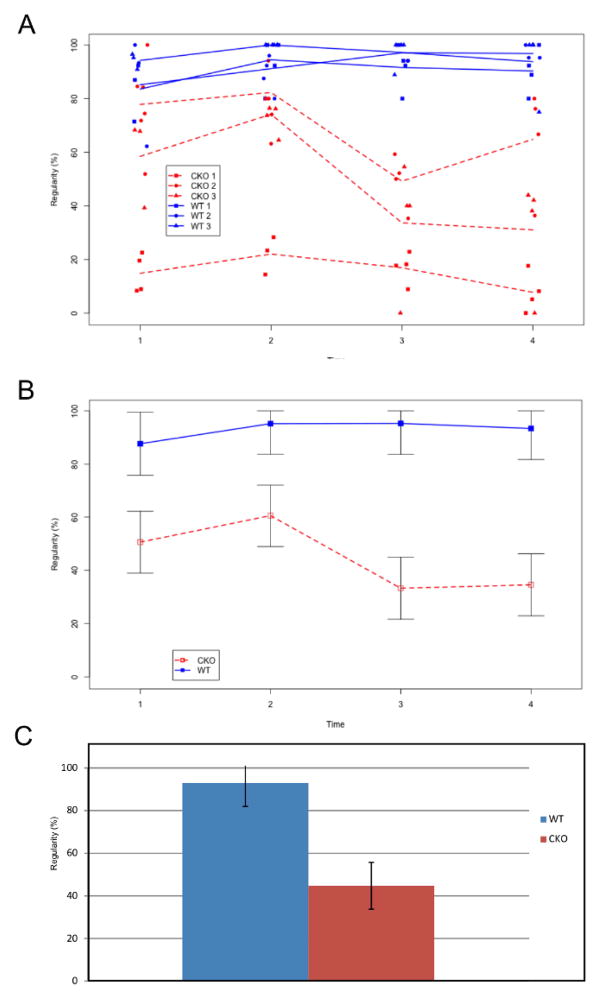
We tested three Tg(Pax2-Cre) N-Myc CKOs and their control littermates on four test dates over a five month period (Date 1: August 9, 2010; Date 2: September 20, 2010; Date 3: November 4, 2010; Date 4: December 2, 2010) to assess their ataxic gait (Supplemental Movie 1 and 2) and to determine if their gait parameters changed over the course of the study. The mice were born March 18, 2010 (WT #1 and CKO #1), April 27, 2010 (WT #2 and CKO #2), and May 3, 2010 (WT #3 and CKO #3) such that their ages at the beginning of the study ranged from 98 days to 144 days. We tested three measures of coordination (for more information please see “Explanation of Compared Parameters” above): gait regularity, stride speed (dynamic paw parameter), and stride length (static paw parameter). For each sub-category, we first investigated the variation between mice of the same type. In general for WT mice, there was minimal variability (Figure 2A), but there was noticeable variability between CKO#1, CKO#2, and CKO#3 (Figure 2A). While there was noted variability between N-Myc CKOs, the overwhelming general trend showed that the N-Myc CKOs performed worse in all measurements of regularity, stride speed, and stride length than WT littermates at each test date as well as over time. Furthermore, our study showed that there did not appear to be any noted improvement in behavior throughout the duration of our study.
Figure 2.
Regularity in WT (Blue) and CKO mice (Red) over four sequential trials (time points) is shown. Time between trials was between one and two months. All three WT mice had high regularity with minimal variability (A-Three blue lines). The worse performing N-Myc CKOs had high variability among the three mice (A-Three red lines). For all time points, when the WT mice average (B-Blue line with SEM) was compared to the CKO mice average (B-Red line with SEM), the WT mice consistently performed higher. This difference was greatest at the third (95.30 +/− 11.62 vs. 33.25 +/− 11.62; p=0.03, Tukey-Kramer) and fourth (93.39+/− 11.67 vs. 34.53+/− 11.62; p=0.05, Tukey-Kramer) test dates. Over the course of the five month study, WT mice showed no change in regularity however, there appeared to be a change in the last test date in our CKO mice but this change was likely due to increased variability among the CKOs (Note: for comparison, there was no change in any other parameter over time). The WT average was considerably highly and significantly different compared to N-Myc CKO mice (92.89 +/− 11.01% vs. 44.703 +/− 11.00%) (C).
The first gait parameter we looked at was gait regularity. Gait regularity provided a generalized measure of coordination. Because the interaction between line and time point was significant [F(3,12)=5.53, p=0.013], we performed a test for line at each respective time point. After applying the Tukey-Kramer adjustment, line was found to be significant at time 3 [p=0.03] and time 4 [p=0.05] with the WT mice consistently having higher regularity than the CKO mice. As Figure 2A and 2B show, time points 1 and 2 also showed a higher regularity for the WT mice, but the difference between lines was not large enough to be significant at the given sample size. A post-hoc test was performed to test for the equality of mean regularity across all four time points within each line (i.e. time effect within line). There was no significant time effect for the WT line [F(3,12)=0.62, p=0.615], but the CKO line did show a significant difference in mean regularity across time points [F(3,12)=9.52, p=0.002]. However, this difference appeared to be due to increased variability between mice rather than a general trend up or down over time. On average the line mean of WT mice was 92.89 (+/− 11.01%) and 44.703 (+/− 11.00%) for N-Myc CKO (Figure 2C).
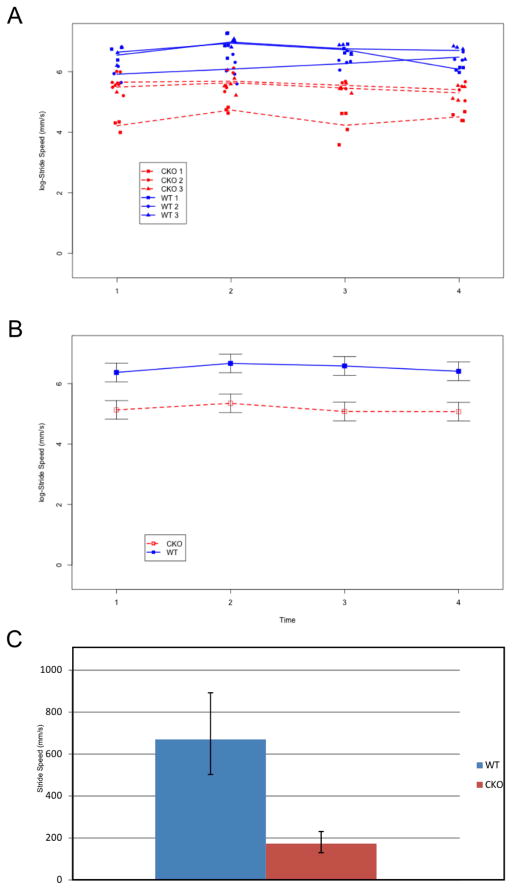
We next looked at the dynamic paw parameter of stride speed. The main effect of line was significant [F(1,4)=11.12, p=0.023] but the main effects of time and the interaction were not significant [F(3,12)=1.84 and F(3,12)=0.36; p=0.195 and p=0.781, respectively; Figure 3A and 3B]. On the log-scale, the mean stride speed for the WT and CKO mice was 6.51 and 5.15, respectively (671.81 mm/s and 172.43 mm/s on the original scale, respectively). A post-hoc test was performed to test for the equality of mean regularity across all four time points within each line (i.e. time effect within line). There was no significant time effect for the WT line, nor the CKO line [F(3,12)=1.18 and F(3,12)=1.00; p=0.357 and p=0.427, respectively], again suggesting there was no learning over time. On average the line mean of WT mice was 669.88 (−166.93, +222.32 mm/s) and 173.49 (−43.22, +57.55 mm/s) for the N-Myc CKO (Figure 3C).
Figure 3.
Stride speed (log-scaled) in WT(Blue) and CKO mice (Red) over four sequential trials (time points) is shown. Time between trials was between one and two months. All three WT mice had rapid strides with minimal variability (A-Three blue lines). The worse performing N-Myc CKOs had higher variability among the three mice (A-Three red lines). When the WT mice average (B-Blue line with SEM) was compared to the CKO mice average (B-Red line with SEM), the WT mice consistently performed higher. There was no significant change over time for either line (p=0.357 (WT); p=0.427 (CKO)). The mean stride speed was higher in the WT mice (669.88 −166.93, +222.32 mm/s vs. 173.49 −43.22, +57.55 mm/s) compared to the N-Myc CKO (C).
The last gait parameter we considered was stride length, which is a sub-category encompassing static paw parameters. Though the general trend in the stride length data was similar to that found in regularity and stride speed, the sample size was not large enough to show statistical significance at the 0.05 for any of the effects [Main effect for Line, F(1,4)=4.49, p=0.102; Main effect for Time, F(3,12)=1.87, p=0.189; Line × Time interaction, F(3,12)=3.11, p=0.067; Supplemental Figure 1A and 1B]. A follow-up investigation showed that the lack of significance was likely due to a wide variation of one CKO mouse at the last test date. Nonetheless, the trend clearly showed that N-Myc CKO mice performed worse than WT mice at each test date and showed no change in performance over the 5 months of our study. On average the line mean of WT mice was 69.63 (+/−10.09mm) and 39.40 (+/−10.08mm) for the N-Myc CKO (Supplemental Figure 4C).
Combined, our data showed that there was minimal variation between runs within any given mouse. There was no notable variation between WT mice. In contrast, the worse performing N-Myc CKO mice had notable variation within their line. N-Myc CKO mice performed worse at every test date than their respective WT littermates. Furthermore, these trends are consistent across time and neither the WT nor the N-Myc CKO appeared to have any change in behavior over time that was detectable within the limitations of our test.
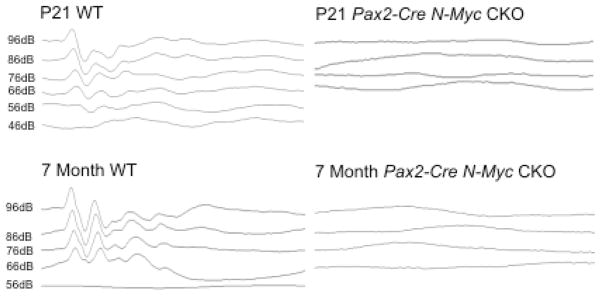
Tg(Pax2-Cre) N-Myc CKOs Show No ABR Response
P21, P28, and 7 month Tg(Pax2-Cre) N-Myc CKO mice and WT littermates were tested for click (Figure 4) and tone pip (not shown) auditory brainstem response (ABR). In both ABR designs, no responses were seen in N-Myc CKOs, regardless of age, frequency, or sound intensity, while normal responses were seen in controls. Attempts to elicit a response in CKOs at higher sound intensities were also unsuccessful (not shown).
Figure 4.
ABR results of P21 and 7 month controls (left) and N-Myc CKOs (right) showed a complete loss of response in mutant mice. P21 WT showed a threshold of approximately 56dB and the 7 month WT had a threshold of 66dB. This increase in threshold in older mice was consistent with the progressive hearing loss in the C57BL6 background.
Tg(Pax2-Cre) N-Myc CKOs Have Abnormally Developed Ear And Cerebellum
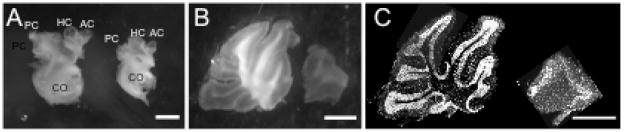
Consistent with previously published data, Tg(Pax2-Cre) N-Myc CKOs had a dramatic reduction in inner ear (Dominguez-Frutos, et al., 2011; Kopecky, et al., 2011) and cerebellar size (Kopecky, et al., 2011; Wey, et al., 2010) (Figure 5 and Supplemental Table 1). Whole mount images showed that the ears we assessed for behavioral abnormalities showed near identical size reductions and morphogenetic defects, regardless of age or litter. Cross sectional area of the CKO inner ears were reduced by 35% (4.19+/−0.14 vs. 6.37+/−0.43 mm2, p= 5.13×10−5; N=6). Likewise the cerebellum was reduced in size. In Tg(Pax2-Cre) N-Myc CKOs, cerebella cross sectional areas were reduced by over 50% (4.16+/−1.03 vs. 8.65+/−0.57mm2, p= 3.30×10−5; N=6).
Figure 5.
Whole mount images of adult dissected inner ears (A) and cerebella (B) showed a size reduction in WT littermates (left) compared to CKOs (right). Both ears and two near mid-sagittal section of the three mutant cerebella (N=6) were quantified (Supplemental Table 1). These data were consistent with previously described and quantified data (Kopecky, et al., 2011). Additionally, Hoechst counterstained adult Tg(Pax2-Cre) N-Myc CKO cerebellum showed cerebella defects in terms of reduced lobule formation (C). Scale bars = 1mm.
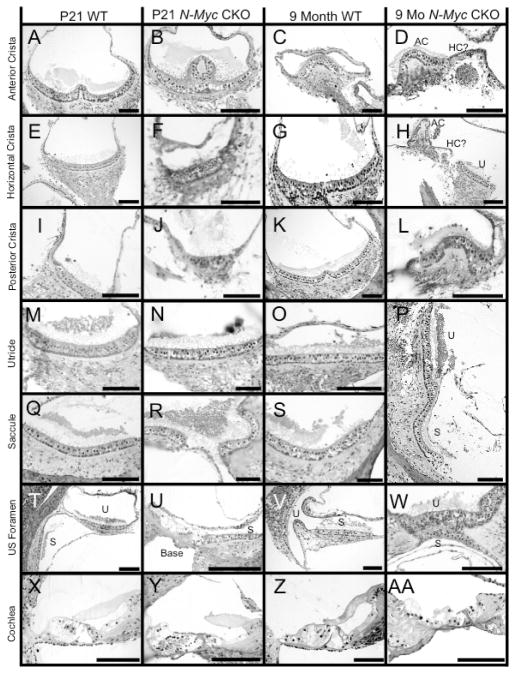
In addition to a reduction in size, we compared a P21 Tg(Pax2-Cre) N-Myc CKO and a WT to a 9 month Tg(Pax2-Cre) N-Myc CKO and a WT to assess changes in sensory epithelia throughout the duration of our study. Sections of a P21 Tg(Pax2-Cre) N-Myc CKO and a WT were consistent with our previously described data (Kopecky, et al., 2011), showing that despite ubiquitous size reductions, all vestibular endorgans retained hair cells. Comparing CKOs to WTs at both time points, the anterior canal appeared to be the least affected vestibular endorgans (Figure 6A–D). Despite the consistent absence of the horizontal canal, the P21 CKO retained horizontal canal epithelia (Figure 6E and 6F). However, by 9 months, it appeared that the horizontal canal epithelium was devoid of hair cells with only a patch of cells remaining (Figure 6G and 6H). The posterior canal was decreased in size, lacked almost all innervations but yet retained hair cells until at least nine months (Figure 6I–L). The utricle and saccule, both gravistatic endorgans, were covered by discontinuous otoconia. At both P21 and 9 months, the utricle and saccule contained hair cells and were innervated in WT and CKOs (Figure 6M–S). However, in P21 WT (Figure 6X) and 9 month WT (Figure 6V) the utricle and saccule were housed each in a unique recess. In contrast, CKOs showed a fused saccular and utricular epithelia (Figure 6P, 6R, and 6W) likely due to a failure of the utriculo-saccular foramen to form (Fritzsch, et al., 2001; Morsli et al., 1999). Furthermore, the saccule was fused with the base of the cochlea in both P21 (Figure 6U) and 9 month CKO (Figure 6W) as the ductus reuniens failed to form. In summary, while the vestibular endorgans were reduced in size and had unique morphogenetic defects (fusion of utricle, saccule, cochlea), and had been previously shown to contain aberrant innervation (Kopecky, et al., 2011), there appeared to be minimal change between CKOs at P21 and 9 months of age. This stability of hair cell and innervation retention in the vestibular system over the course of our study contrasted with the fate of the cochlea. At both P21 and 9 months in WT littermates, three rows of outer hair cells and one row of inner hair cells were surrounded by a variety of supporting resting on the basilar membrane with an overlying tectorial membrane (Figure 6X and 6Z). These cells were densely innervated. In the P21 CKO, at least four rows of outer hair cells and possibly two rows of inner hair cells existed with a disrupted tectorial membrane. Furthermore, the overall organization was abnormal (Figure 6Y). By 9 months, the CKO lacked nearly all recognizable cells (Figure 6AA) and was sparsely innervated. Combined, these data suggest a progressive loss of cochlear hair cells whereas little to no change occurs in the vestibular ear.
Figure 6.
Histologic sections at P21 and 9 months of age of both WT and N-Myc CKO mice showed the dramatic loss of the organ of Corti and the relative sparing of the vestibular system. At all ages, the anterior canal crista was the least affected as innervation and hair cells remained in both P21 WT (A) and mutant (B) and up until at least 9 months in both WT (C) and mutant (D). Despite the absence of a horizontal canal in the N-Myc CKO, the horizontal canal crista had remaining hair cells and was innervated at P21 in both WT (E) and mutant (F). An obvious reduction of nerve fibers to the horizontal canal crista was found in 9 months old mice suggesting that neurons innervating the crista underwent degeneration as only a remaining aggregation of cells and no identifiable hair cells remained in the mutant (D and H). The posterior canal contained hair cells until nine months but was reduced in size at both P21 (I,J) and 9 months (K,L). At all ages, the utricle (M–P) and saccule (P,Q–S) contained hair cells, innervation, and overlying otoconia. In the N-Myc CKOs, the utricle and saccule were fused, as shown in both P21 CKO (R) and 9 month CKO (P, W). This fusion of epithelia was an obvious contrast to WT at P21 (T) and 9 month (V) where two distinct recesses with sensory epithelia exist. Furthermore, the saccule was fused with the base of the cochlea as the ductus reuniens failed to form (U). The P21 WT cochlea had three rows of outer hair cells and one row of inner hair cells (X) with an overlying tectorial membrane. The P21 CKO had at least four rows of outer hair cells and one to two rows of inner hair cells (Y), with an overlying tectorial membrane that was abnormal. The 9 month WT cochlea (Z) was unchanged from the P21 WT cochlea whereas the 9 month CKO cochlea (AA) had lost nearly all identifiable cells in the organ of Corti. Scale bars = 100μm.
Summary Of Results
We comprehensively tested WTs and Tg(Pax2-Cre) N-Myc CKO mice longitudinally at four different test dates, approximately one month apart for three gait performance parameters: regularity index, stride speed, and stride length using the Noldus Catwalk System. Despite small mouse numbers due to disproportionate lethality rates, our data showed that there was little variation among WTs but noticeable variation among mutants. Tg(Pax2-Cre) N-Myc CKOs performed substantially worse than their respective littermates and our longitudinal study showed that in general vestibular function neither improved nor declined in either WTs or CKOs. We tested N-Myc CKOs with broadband (click) and frequency specific stimuli (tone pips) ABR and in both cases, CKOs showed no response. Both the inner ear and cerebellum had numerous defects, including a reduction in size and abnormal development, but inner ear vestibular hair cells, afferent innervations, and cerebella remained until at least 9 months of age.
Discussion
Ataxia is the disruption of coordination where a mismatch occurs between sensory input and motor control and output. Numerous forms of ataxia exist and can result from damage to proprioception (dorsal columns), vestibular system of the ear, or the cerebellum. Various mouse models exist providing insights into the cause of ataxia and the mechanisms of compensation, yet these models focus on either solely peripheral or central lesions. Mice with ataxia due to peripheral defects include stargazer (Khan et al., 2004), Jackson waltzer (Fritzsch, et al., 2001), and tilt (de Caprona, et al., 2004) while mice with ataxia due to cerebellar defects include Purkinje cell degeneration (pcd) (Mullen, Eicher, & Sidman, 1976), leaner (Sidman, Appel, & Fullier, 1965), weaver (Grusser-Cornehls & Baurle, 2001), staggerer (Sidman, Lane, & Dickie, 1962), stumbler (Caddy, Sidman, & Eicher, 1981), nervous (Grusser-Cornehls & Baurle, 2001), meander tail (Hollander & Waggie, 1977), vibrator (Weimar, Lane, & Sidman, 1982), tottering (Green & Sidman, 1962), and lurcher (Grusser-Cornehls & Baurle, 2001). Behaviorally, these mice range from normal (full compensation) to mild or severe ataxia with variation between mutants due to partial or varied compensatory mechanisms (Grusser-Cornehls & Baurle, 2001). Many of the cerebellar defective mutants have additional (yet widely uncharacterized) defects outside of the cerebellum, making interpretation of behavioral defects and compensation complicated. For example, Lmx1a mutants have variable cerebellar defects, massive defects in the ear, the hindbrain, and the spinal cord (Chizhikov & Millen, 2004; Millonig, Millen, & Hatten, 2000; Nichols, et al., 2008). On the other hand, mice with vestibular peripheral defects are rarely analyzed for behavioral defects or compensation. Recent work by Jones and colleagues have shown potential for electrophysiological analyses of vestibular function of various mouse mutants lacking otoconia (including head tilt and tilted) using vestibular sensory evoked potentials (T. A. Jones, Jones, & Hoffman, 2008; T. A. Jones et al., 2011). Yet, these testing methods have yet to combine electrophysiological data with quantifiable behavioral characterization. Future studies must incorporate electrophysiological analyses of peripheral endorgans with quantifiable behavioral and morphological/neuroanatomical deficits using appropriate mouse models.
The Catwalk System Is An Appropriate Tool To Measure Ataxic Behavior And Can Be Used To Assess Compensation
The Noldus Catwalk System allows mice to freely and spontaneously move across a walkway and quantifies not only static gait parameters such as stride length and base of support but also dynamic gait parameters such as stride speed and walking rate. Previous assays to investigate behavioral deficits (Cendelin, Voller, & Vozeh, 2010; Kaemmerer & Low, 1999; Kale, Amende, Meyer, Crabbe, & Hampton, 2004; Simon et al., 2004) include swimming, over-ground locomotion and treadmill locomotion (Cendelin, et al., 2010) but due to differences in methodology these methods yielded (at times) incompatible results (Herbin, Hackert, Gasc, & Renous, 2007; Wooley, Xing, Burgess, Cox, & Seburn, 2009). A consistent, sensitive, and comprehensive assessment system is needed. Given the profound contrasts shown in our results quantifying the behavioral differences in our N-Myc CKO mice compared to our control littermates, the Noldus Catwalk appears to provide such a tool to determine gait differences between animals. In assessing static and dynamic paw parameters, along with a generalized coordination calculation, the Noldus Catwalk yielded consistent data across our experiments, despite the embryonic lethality of the N-Myc CKO mice and resulting small sample size. Furthermore, it showed little to no variation between controls, as expected, and while there was significant variation between individual mutants, this variation is consistent with previously published results on ataxic mice where variable performance is common between mice of the same genetic background, presumably due to varied degrees of compensation. The catwalk system has been shown to be both reliable and sensitive in detecting motor disorders unrelated to those tested here (Vandeputte et al., 2010). Therefore, we conclude that the catwalk system is sensitive to ataxic behavior and can be utilized as at least one method to determine behavioral compensation. Furthermore, the catwalk does not generate negative feedback upon repeated use due to falling, as seen with the common Rota rod tests, and can thus assess long-term effects without introducing a behavioral bias.
Tg(Pax2-Cre) N-Myc CKO Mice Provide A Model For Partially Compensated Ataxia
Current mouse models do not adequately mimic the full spectrum of ataxic diseases, for example, patients suffering from vertigo have abnormal inner ear function but normal afferents to the cerebellum and a normal cerebellum. Patients suffering from vestibular schwannomas have normal inner ears and normal cerebella, yet suffer from imbalance due to acquired conductance changes. This suggests that in the presence of abnormal signaling and continued mismatch between central processing and peripheral inputs, compensation is impaired. However, there are no models that have investigated defects in both peripheral and central mechanisms and how this affects the dynamics of compensation (Beraneck, et al., 2008; de Caprona, et al., 2004).
In Tg(Pax2-Cre) N-Myc CKOs at 9 months of age, vestibular hair cells and innervation remain whereas cochlear hair cells are absent. We have previously shown that L-Myc is more strongly expressed in the vestibular epithelia than N-Myc (Kopecky, et al., 2011). It is possible that the loss of cochlear hair cells seen in Tg(Pax2-Cre) N-Myc CKO mice is due to N-Myc’s stronger role in the cochlea while L-Myc is sufficient for the vestibular development and the long-term retention of vestibular hair cells. Nonetheless, it is noteworthy that in both P21 and 7 month CKO mice, hearing is completely absent. However, despite a nonfunctional cochlea, it is likely that the vestibular ear functions but sends incorrect information centrally, or at minimum, correct information is sent abnormally. This is based on three lines of evidence. First, the presence of normal appearing vestibular hair cells and innervation in 9 month old mutant mice [compared to grossly abnormal and nonfunctional (No ABR response) cochlear neurosensory cells], suggests that vestibular neurosensory cells are functional. Secondly, there are no substantial changes in the vestibular inner ear or in the cerebellum between P21 and 9 months indicating no gross cellular atrophy due to disuse. Lastly, the WT controls have very little variation between mice of different age or litter while the mutants have widely variable performance. This is consistent with partial compensation. In general, when vestibular input is completely lost, full (or nearly full) compensation is possible, yet when vestibular input is incorrect (either due to peripheral or innervation defects), only partial and variable compensation occurs. Based on these observations, the Tg(Pax2-Cre) N-Myc CKOs provide a new model for ataxic behavior unable to be fully compensated for over time due to abnormal vestibular information acquisition and processing.
Conclusion
N-Myc is one of two essential Myc proto-oncogenes responsible for the proliferation of inner ear precursors and is necessary for proper sensory and non-sensory development of the ear. In its absence, the inner ear develops abnormally, including an overall reduction in size, progressive loss of cochlear hair cells, and aberrant development of the vestibular system. N-Myc is also necessary for proper neuronal pathfinding as shown by aberrant projections to the saccule/basal turn of the cochlea (Kopecky, et al., 2011). Lastly, N-Myc is necessary for cerebellar development and, in its absence, the cerebellum is reduced in size. ABR testing suggests that after the onset of hearing around P14, progressive loss of cochlear hair cells leads to a complete loss of hearing. Despite the loss of hair cells in the cochlea, hair cells and innervations remain in the canal cristae and in the fused utricle and saccule. Our results suggest that there remains residual function of the vestibular system until at least nine months after birth, but this functionality is significantly worse in N-Myc CKOs than in control littermates and does not improve over time. This residual function of the vestibular ear results in mismatch between sensory inputs in the CKOs and likely leads to the inability of the mutant to fully compensate and develop an ataxic gait with lack of improvement. Finally, we argue that the Noldus Catwalk System combines multiple behavioral parameters to faithfully quantify ataxic behavior in the conditional mutant of the present study as well as other mutants to be tested with this system in the future.
Supplementary Material
Acknowledgments
We would like to thank Drs. T. Ohyama and A. Groves for providing the Tg(Pax2-Cre) line. We thank Jackson Lab for providing the N-Mycf/f mice and Dr. Knoepfler for creating them. The Leica TCS SP5 confocal microscope was purchased in part with a grant from the Roy. J. Carver foundation. Grant funding for Bernd Fritzsch was provided through the NIH and NIDCD RO1-DC055095590. We thank the NIH P30 grant (DC010362) to make the ABR equipment and the Noldus Catwalk System available as part of the supported core facilities. We thank the University of Iowa Carver College of Medicine, Medical Scientist Training Program, and Office of the Vice President for Research for support.
References
- Belyantseva IA, Perrin BJ, Sonnemann KJ, Zhu M, Stepanyan R, McGee J, et al. Gamma-actin is required for cytoskeletal maintenance but not development. Proc Natl Acad Sci U S A. 2009;106(24):9703–9708. doi: 10.1073/pnas.0900221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100(2):945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30(2):411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Caddy KW, Sidman RL, Eicher EM. Stumbler, a new mutant mouse with cerebellar disease. Brain Res. 1981;208(1):251–255. doi: 10.1016/0006-8993(81)90643-0. [DOI] [PubMed] [Google Scholar]
- Cendelin J, Voller J, Vozeh F. Ataxic gait analysis in a mouse model of the olivocerebellar degeneration. Behav Brain Res. 2010;210(1):8–15. doi: 10.1016/j.bbr.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Control of roof plate formation by Lmx1a in the developing spinal cord. Development. 2004;131(11):2693–2705. doi: 10.1242/dev.01139. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB, Beraneck M, Sadeghi SG. Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. J Vestib Res. 2009;19(5–6):171–182. doi: 10.3233/VES-2009-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutuli D, Rossi S, Burello L, Laricchiuta D, De Chiara V, Foti F, et al. Before or after does it matter? Different protocols of environmental enrichment differently influence motor, synaptic and structural deficits of cerebellar origin. Neurobiol Dis. 2011;42(1):9–20. doi: 10.1016/j.nbd.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7(4):671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- de Caprona MD, Beisel KW, Nichols DH, Fritzsch B. Partial behavioral compensation is revealed in balance tasked mutant mice lacking otoconia. Brain Res Bull. 2004;64(4):289–301. doi: 10.1016/j.brainresbull.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Dominguez-Frutos E, Lopez-Hernandez I, Vendrell V, Neves J, Gallozzi M, Gutsche K, et al. N-myc controls proliferation, morphogenesis and patterning of the inner ear. Journal of Neuroscience. 2011 doi: 10.1523/JNEUROSCI.0785-11.2011. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutia MB. Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):420–424. doi: 10.1097/MOO.0b013e32833de71f. [DOI] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22(20):2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti F, Laricchiuta D, Cutuli D, De Bartolo P, Gelfo F, Angelucci F, et al. Exposure to an enriched environment accelerates recovery from cerebellar lesion. Cerebellum. 2011;10(1):104–119. doi: 10.1007/s12311-010-0236-z. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211(8–9):388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Goddard M, Zheng Y, Darlington CL, Smith PF. Locomotor and exploratory behavior in the rat following bilateral vestibular deafferentation. Behav Neurosci. 2008;122(2):448–459. doi: 10.1037/0735-7044.122.2.448. [DOI] [PubMed] [Google Scholar]
- Green MC, Sidman RL. Tottering--a neuromusclar mutation in the mouse. And its linkage with oligosyndacylism. J Hered. 1962;53:233–237. doi: 10.1093/oxfordjournals.jhered.a107180. [DOI] [PubMed] [Google Scholar]
- Grusser-Cornehls U, Baurle J. Mutant mice as a model for cerebellar ataxia. Prog Neurobiol. 2001;63(5):489–540. doi: 10.1016/s0301-0082(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Grusser-Cornehls U, Grusser C, Baurle J. Vermectomy enhances parvalbumin expression and improves motor performance in weaver mutant mice: an animal model for cerebellar ataxia. Neuroscience. 1999;91(1):315–326. doi: 10.1016/s0306-4522(98)00618-6. [DOI] [PubMed] [Google Scholar]
- Grusser C, Grusser-Cornehls U. Improvement in motor performance of Weaver mutant mice following lesions of the cerebellum. Behav Brain Res. 1998;97(1–2):189–194. doi: 10.1016/s0166-4328(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23(3–4):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18(2):187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- Hamers FPT, Koopmans GC, Joosten EAJ. CatWalk-assisted gait analysis in the assessment of spinal cord injury. Journal of Neurotrauma. 2006;23(3–4):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Klinkenstein JC, Kruger A, Gliemroth J, Mohr C, Sander T. Structural brain changes following peripheral vestibulo-cochlear lesion may indicate multisensory compensation. J Neurol Neurosurg Psychiatry. 2011;82(3):309–316. doi: 10.1136/jnnp.2010.204925. [DOI] [PubMed] [Google Scholar]
- Herbin M, Hackert R, Gasc JP, Renous S. Gait parameters of treadmill versus overground locomotion in mouse. Behav Brain Res. 2007;181(2):173–179. doi: 10.1016/j.bbr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hollander WF, Waggie KS. Meander tail: a recessive mutant located in chromosome 4 of the mouse. J Hered. 1977;68(6):403–406. doi: 10.1093/oxfordjournals.jhered.a108869. [DOI] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010;341(1):95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010;5(7):e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Jones TA, Mills KN, Gaines GC. Anatomical and Physiological Considerations in Vestibular Dysfunction and Compensation. Semin Hear. 2009;30(4):231–241. doi: 10.1055/s-0029-1241124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Hoffman LF. Resting discharge patterns of macular primary afferents in otoconia-deficient mice. J Assoc Res Otolaryngol. 2008;9(4):490–505. doi: 10.1007/s10162-008-0132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Vijayakumar S, Brugeaud A, Bothwell M, Chabbert C. The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs) Hear Res. 2011 doi: 10.1016/j.heares.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaemmerer WF, Low WC. Cerebellar allografts survive and transiently alleviate ataxia in a transgenic model of spinocerebellar ataxia type-1. Exp Neurol. 1999;158(2):301–311. doi: 10.1006/exnr.1999.7099. [DOI] [PubMed] [Google Scholar]
- Kale A, Amende I, Meyer GP, Crabbe JC, Hampton TG. Ethanol’s effects on gait dynamics in mice investigated by ventral plane videography. Alcohol Clin Exp Res. 2004;28(12):1839–1848. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- Khan Z, Carey J, Park HJ, Lehar M, Lasker D, Jinnah HA. Abnormal motor behavior and vestibular dysfunction in the stargazer mouse mutant. Neuroscience. 2004;127(3):785–796. doi: 10.1016/j.neuroscience.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci U S A. 2011;108(8):3288–3293. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle. 2006;5(1):47–52. doi: 10.4161/cc.5.1.2292. [DOI] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Brook G, Gerver J, Honig WM, Hamers FP, et al. Strain and locomotor speed affect over-ground locomotion in intact rats. Physiol Behav. 2007;92(5):993–1001. doi: 10.1016/j.physbeh.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn. 2011;240(6):1373–1390. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorella A, Boldrini R, Dominici C, Donfrancesco A, Yokota Y, Inserra A, et al. Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 2002;62(1):301–306. [PubMed] [Google Scholar]
- Lasorella A, Noseda M, Beyna M, Yokota Y, Iavarone A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature. 2000;407(6804):592–598. doi: 10.1038/35036504. [DOI] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340(2):303–321. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403(6771):764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Modak S, Cheung NK. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat Rev. 2010 doi: 10.1016/j.ctrv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126(11):2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem. 2010;110(5):1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334(3):339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38(4):195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, et al. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2010 doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, et al. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275(1–2):66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parietti-Winkler C, Gauchard GC, Simon C, Perrin PP. Pre-operative vestibular pattern and balance compensation after vestibular schwannoma surgery. Neuroscience. 2011;172:285–292. doi: 10.1016/j.neuroscience.2010.10.059. [DOI] [PubMed] [Google Scholar]
- Pauley S, Kopecky B, Beisel K, Soukup G, Fritzsch B. Stem cells and molecular strategies to restore hearing. Panminerva Med. 2008;50(1):41–53. [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235(9):2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R, Hirning-Folz U, Ehret G. N-myc expression in the embryonic cochlea of the mouse. Hear Res. 1994;72(1–2):53–58. doi: 10.1016/0378-5955(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Ruggero D. The role of Myc-induced protein synthesis in cancer. Cancer Res. 2009;69(23):8839–8843. doi: 10.1158/0008-5472.CAN-09-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Appel SH, Fullier JF. Neurological Mutants of the Mouse. Science. 1965;150(3695):513–516. doi: 10.1126/science.150.3695.513. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Lane PW, Dickie MM. Staggerer, a new mutation in the mouse affecting the cerebellum. Science. 1962;137:610–612. doi: 10.1126/science.137.3530.610. [DOI] [PubMed] [Google Scholar]
- Simon D, Seznec H, Gansmuller A, Carelle N, Weber P, Metzger D, et al. Friedreich ataxia mouse models with progressive cerebellar and sensory ataxia reveal autophagic neurodegeneration in dorsal root ganglia. J Neurosci. 2004;24(8):1987–1995. doi: 10.1523/JNEUROSCI.4549-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton BR, Perkins AS, Tessarollo L, Sassoon DA, Parada LF. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992;6(12A):2235–2247. doi: 10.1101/gad.6.12a.2235. [DOI] [PubMed] [Google Scholar]
- Vandeputte C, Taymans JM, Casteels C, Coun F, Ni Y, Van Laere K, et al. Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci. 2010;11:92. doi: 10.1186/1471-2202-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrinten DH, Hamers FFT. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102(1–2):203–209. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- Weimar WR, Lane PW, Sidman RL. Vibrator (vb): a spinocerebellar system degeneration with autosomal recessive inheritance in mice. Brain Res. 1982;251(2):357–364. doi: 10.1016/0006-8993(82)90754-5. [DOI] [PubMed] [Google Scholar]
- Wey A, Martinez Cerdeno V, Pleasure D, Knoepfler PS. c- and N-myc regulate neural precursor cell fate, cell cycle, and metabolism to direct cerebellar development. Cerebellum. 2010;9(4):537–547. doi: 10.1007/s12311-010-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley CM, Xing S, Burgess RW, Cox GA, Seburn KL. Age, experience and genetic background influence treadmill walking in mice. Physiol Behav. 2009;96(2):350–361. doi: 10.1016/j.physbeh.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.