Abstract
Vocal production is crucial for successful social interactions in multiple species. Reward can strongly influence behavior; however, the extent to which reward systems influence vocal behavior is unknown. In songbirds, singing occurs in different contexts. It can be spontaneous and undirected (e.g., song produced alone or as part of a large flock) or directed towards a conspecific (e.g., song used to attract a mate or influence a competitor). In this study, we developed a conditioned place preference paradigm to measure reward associated with different types of singing behavior in two songbird species. Both male zebra finches and European starlings developed a preference for a chamber associated with production of undirected song, suggesting that the production of undirected song is tightly coupled to intrinsic reward. In contrast, neither starlings nor zebra finches developed a place preference in association with directed song; however, male starlings singing directed song that failed to attract a female developed a place aversion. Unsuccessful contact calling behavior was also associated with a place aversion. These findings suggest that directed vocal behavior is not tightly linked to intrinsic reward but may be externally reinforced by social interactions. Data across two species thus support the hypothesis that the production of undirected but not directed song is tightly coupled to intrinsic reward. This study is the first to identify song-associated reward and suggests that reward associated with vocal production differs depending upon the context in which communication occurs. The findings have implications for understanding what motivates animals to engage in social behaviors and ways in which distinct reward mechanisms function to direct socially appropriate behaviors.
Keywords: reward, reinforcement, motivation, social context, communication, birdsong
1 INTRODUCTION
Vocal behavior is critical for successful social interactions in a wide range of animal species, including songbirds. Reward associated with production of particular behaviors is a powerful behavioral incentive, influencing feeding, social, and sexual behaviors (e.g., [1–5]). Songbirds produce song at high rates within multiple social contexts, suggesting that they are highly motivated to sing and that singing behavior may be linked to reward. Progress has been made in understanding the neural basis of song learning and sensorimotor processing (see multiple reviews in [6]), but little is known about reward associated with vocal production.
One roadblock for testing the role of reward in vocal production is the lack of a metric for evaluating reward associated with the act of singing. Here we modified a conditioned place preference (CPP) paradigm, one of the most commonly used methods for measuring reward [7–10], to evaluate song-associated reward. The methods we used are based on the idea that the neural state associated with singing behavior acts as an unconditioned stimulus. When this neural state is paired with a distinct place (in our study a distinct compartment in a CPP apparatus), this place becomes associated with the state associated with the singing behavior. Thus, if song is associated with or induces a pleasurable or rewarding neural state, then males should develop a CPP.
As a first step in the evaluation of CPP as a measure of song-associated reward, we examined two commonly studied songbird species known to produce high rates of song within distinct social contexts in a laboratory setting. Specifically, we examined song-associated reward using CPP in male zebra finches (Taeniopygia guttata) and European starlings (Sturnus vulgaris) singing within functionally distinct social contexts. Directed song is used to attract mates or repel competitors and can evoke an immediate, obvious behavioral response in conspecifics (e.g., cause a female to approach or mate with a male [11–14]). Male songbirds also sing spontaneous song that does not result in immediate, obvious behavioral responses in conspecifics. This type of song is referred to as undirected [11–14] and in adults is considered important for song practice and the maintenance of large flocks [15,16]. Our predication was that if reward plays an important role in the initiation or maintenance of singing behavior, then males will develop a place preference (indicative of reward state) for the side of a CPP apparatus previously paired with song. Our results support this prediction but additionally suggest that the relationship between reward and singing behavior differs depending upon whether song is undirected or directed. To our knowledge, this research is the first to evaluate reward and communication in an animal model system using CPP, and it sets the stage for future studies examining the role of reward in different types of social communication.
2 MATERIALS AND METHODS
All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guidelines for the Use of Animals in Research.
2.1 Rationale for conditioned place preference methods
CPP involves associating the primary rewarding properties of a specific unconditioned reward stimulus (e.g., food or drugs, or in the present study singing behavior) with a previously neutral stimulus (e.g., a distinct chamber in a place preference apparatus). After pairing the unconditioned stimulus (e.g., the affective state induced by singing behavior) with a neutral stimulus (e.g., a distinct chamber), the neutral stimulus then becomes a conditioned stimulus and can elicit an approach response (if the properties associated with the unconditioned stimulus [e.g., the act of singing] are rewarding) or avoidance (if the properties associated with the unconditioned stimulus are aversive) [7–10]. The CPP paradigm that we use in the present study was developed based on studies of copulation-associated reward in male rats [2,8,17]. Copulation is similar to the act of singing in that it cannot be administered in the same way as commonly studied food or drug rewards (e.g., one cannot “inject” the act of singing or sexual behavior; either an animal produces the behavior or it does not). In past rat studies, males were presented with a female prior to being restricted to one side of a CPP apparatus (rather than being presented with the female while being restricted to one side of the apparatus), and the males that ejaculated developed a CPP [2,17]. This is considered evidence that this behavior induced a rewarding neural state, and using this method the physiological consequences of the behavior (and not the factors triggering the behavior, such as female presence) are measured [8,18]. We employ similar methods here 1) because few male songbirds sing in the CPP apparatus (likely due to the stress of being placed alone into a chamber), and 2) to induce directed song we must present males with a social partner, which we do not wish to do in the CPP apparatus as this would conflate the physiological causes or consequences of song production with reinforcement associated with the presence of a conspecific in the CPP apparatus.
2.2 Conditioned place preference in zebra finches
Thirty-two adult male zebra finches (Taeniopygia guttata) from our breeding colony were housed for the duration of the experiment in groups of 4 in cages (approximately 59 cm × 59cm × 59 cm) containing food, water, perches, and enrichment materials. Males were exposed to a 16h light: 8h dark photoperiod.
2.2.1 Habituation days
Male zebra finches with distinct combinations of colored leg bands were observed by an individual positioned in front of the cage. To habituate males to the presence of the experimenter, males were observed for at least 30 min on 5 days during a one-week period prior to conditioning. All observations and testing occurred at the same time each day between 11:00 and 13:00 hours.
2.2.2 Conditioning day
On a single conditioning day, males were observed for 30 min in home cages at least 2 days following habituation. We selected 30 minutes as a starting point based on studies of ejaculation-induced reward in rats [2] and because 30 min provides an adequate amount of time to quantify individual differences in singing behavior. During this period the numbers of directed and undirected songs were counted. Directed song was defined as song produced by a male oriented towards another individual while displaying distinct postural changes, including fluffing of cheek and nuchal feathers. Undirected song was defined as song produced by a male facing away from other individuals or facing towards others but without any obvious postural changes or conspecific-directed orienting or approach behaviors. Long contact calls were also quantified. Long contact calls can be considered another form of directed communication used to establish contact with conspecifics by a bird that has been separated from its flockmates [13,19,20]. Immediately after the 30 min observation period each male was rapidly captured, removed from the home cage, and placed into one side of a CPP apparatus for 30 min. The CPP apparatus consisted of a standard cage (approximately 118 cm x 59 cm x 59cm) divided into two distinct compartments by an opaque divider (the same color as the compartment in which the bird was confined). Each compartment was decorated with colored construction paper covering the walls, floor, and perches. One side was decorated with green materials and the other side was decorated with yellow materials. The colors of the left and right sides were counterbalanced across cages.
During conditioning, half of the birds were placed on the side of the CPP apparatus decorated with one color and the other half were placed on the side decorated with the other color to control for any initial color preference bias. Vocal behavior was also quantified during this period. After the 30 min period, males were placed back into the home cages.
2.2.3 Test day
The following day, each male was placed into the center of the same CPP apparatus in which he was conditioned; however, on this day the partition separating the 2 sides of the CPP apparatus was removed, allowing males free access to both of the distinctly colored sides of the apparatus. Each male was selected arbitrarily from the home cage. Leg bands were checked to determine in which cage the bird had been placed during conditioning, but to prevent observer bias the experimenter had no information about the color/side of the apparatus in which a male had been confined during conditioning. The total amount of time each male spent on each side of the apparatus during a 30 min observation period was recorded to determine side preference. The number of times males moved from one side of the apparatus to the other was also quantified to ensure that males explored both sides of the CPP apparatus.
2.3 Conditioned place preference in European starlings
The results of the first study in male zebra finches singing to males indicated that our measure of song associated CPP was effective. We planned to follow up with tests of male zebra finches singing to females; however contagious bacteria were identified in a few birds in our colony and we have subsequently stopped all research with zebra finches. Therefore we ran additional studies in starlings, which expand the scope of this study to include relationships between song and reward in another species singing in additional social contexts. Twenty-six males with distinct combinations of colored leg bands were housed in groups of 3 to 4 in indoor aviaries (approximately 2m x 2m x 2.5m). Each aviary contained 4 nest boxes, food, water, and perches. The endocrine state of male starlings is sensitive to the photoperiod. Prior to the study, all males were exposed to photoperiods of 18 h light: 6 h dark for 12 weeks followed by 6 h light: 18 h dark for 8 weeks. This schedule of long followed by short photoperiods causes starlings to enter a state of photosensitivity that is characteristic of the fall non-breeding season [21]. In this state males have regressed gonads, form large flocks, and sing high rates of undirected song [22]. Photosensitive males do not display sexual responses or song production in response to females [22]. Fourteen males were tested singing undirected song in the fall-like photosensitive state (fall condition males).
Twelve photosensitive male starlings received subcutaneous testosterone (T) implants and were placed on a photoperiod of 11L:13D to induce a spring-like breeding season endocrine state (spring condition males). Treating photosensitive male starlings with T implants stimulates male sexual responses to females, including high rates of sexually-motivated, directed song production (e.g., [23,24]). Each male received 2, 14-mm long silastic implants (i.d., 1.47; o.d., 1.96 mm; Dow Corning, Midland, MI) packed for 10 mm with crystalline testosterone proprionate (Sigma-Aldrich, St. Louis, MO). Males were anesthetized using isoflurane gas anesthesia and implants were inserted through a small incision over the breast muscle. Males were tested 8 days after receiving implants.
2.3.1 Conditioning day
On a single day of conditioning, males were observed for 30 min in home aviaries. Fall condition males sing high rates of undirected song and do not orient directly towards single conspecifics while singing or sing in response to the introduction of another individual [22,25]. Thus all of the songs produced by fall condition males were considered undirected. For spring condition males a female conspecific was released into the aviary immediately prior to the 30 min observation period. Male starlings do not face females during courtship song but typically fly to a nest site (a nest box in this case) and sing from the perch or from inside the box (a location from which they cannot see the female). Although it is possible that some of the songs produced by males in a spring condition were undirected, our assumption based on past studies of males with elevated T (e.g.,[22]) is that a larger proportion of the songs produced by spring condition males tested in the presence of a female are directed than undirected; and certainly spring condition males produce more directed songs than fall condition birds (who based on past literature are unlikely to sing any female-directed songs [22]).
For observations of fall and spring condition male starlings we recorded several behavioral measures in addition to vocal behavior based on questions generated by the results of the zebra finch study. Specifically, in addition to the number of times each male sang we quantified bouts of preening, eating, and drinking (separate bouts were separated by 2 seconds). We additionally, counted the number of “prurrp” calls [26] produced by each male. These calls are proposed to call together flocks of starlings [26] and may represent another form of directed communication in the sense that they can immediately attract conspecifics. Finally, we recorded the number of times females landed on nest boxes of males singing in the spring condition.
At the end of the 30 min observation period, each male was rapidly captured, removed from the home aviary, and placed into one side of a CPP apparatus for 30 min. The CPP apparatus consisted of a standard cage. One side was decorated with green materials and the other side was decorated with yellow materials. During conditioning, half of the birds were placed on the side of the CPP apparatus decorated with one color and the other half were placed on the side decorated with the other color to control for any initial color preference bias. Vocal behavior was also quantified during this period. After the 30 min period males were placed back into the home aviaries.
2.3.2 Test day
The following day CPP was assessed in starlings following the identical protocol described for male zebra finches.
2.4 Statistical Analysis
For both the zebra finch and starling studies, on test day the proportion of time each male spent on the side of the apparatus in which he was previously confined during conditioning was calculated as a measure of CPP. For each analysis the CPP measure was arcsine transformed to improve normality [27].
Ten male zebra finches spent the entire test period on one side of the apparatus, indicating that they were not exploring the apparatus prior to making a side choice. These males were excluded from the analysis. (During conditioning they did not differ from the remaining males in comparisons of undirected song, calls or number of movements from the floor of the apparatus to the perch [results of independent Student’s t-tests revealed p > 0.31 in all cases]). The remainder of the males on test day moved from one side of the CPP apparatus to the other a mean of 7.36 times (sd = 4.5). For zebra finches each of the 3 vocal behaviors (undirected singing, directed singing, and calling) were collected during the same time period for each individual, thus the data could be normalized against each other by calculating the proportion of the total vocal behavior produced the 30 min prior to and the 30 min during conditioning that consisted of undirected songs, directed songs, and calls. The proportion data were analyzed using simple linear, logarithmic, and polynomial regression analyses to identify the model that best explained correlations between CPP and the proportion of undirected song, directed song, and calling behavior. (Normalized data were inter-related, rendering multiple regression analysis inappropriate.) The best model was selected based on examination of the adjusted R2, normal and residual plots, and standard error (and in each case proved to be linear).
Male starlings were not discarded from analyses for not exploring the apparatus (all but one bird moved back and forth during testing). Fall condition males moved from one side of the CPP apparatus to the other a mean of 54.21 times (sd = 46.67) and spring condition males moved from one side of the CPP apparatus to the other a mean of 34.42 times (sd = 21.38). For starlings, song (undirected for fall condition birds and directed for spring condition birds) and prurrp calls were recorded during the same time period for each individual, thus these data could be normalized against each other by calculating the proportion of the total vocal behavior (songs plus calls) produced for 30 min during and the 30 min prior to conditioning that were undirected songs (for fall condition males) or directed songs (for spring condition males) and calls. Stepwise multiple regression analyses were run to determine the extent to which the proportion of song/calls, preening, and bouts of feeding and drinking (entered as independent variables) explained variation in CPP (entered as the dependent variable).
For each species and for each vocal behavior males were split into two groups based on median vocal production during and 30 min prior to conditioning (those below the median were considered to vocalize at low rates [low group], those above or equal to the median were considered to vocalize at high rates [high group]). Comparisons of males in these groups were made using Student’s t-tests or Mann-Whitney U tests when assumptions of normality or homogeneity of variance were violated.
Additional analyses were run on the spring condition starlings to examine whether social responses of females contributed to male CPP. Male starlings sing to attract females to a nesting location. We considered the number of times females landed on an individual male’s nest box an indicator of his social success. A correlation was used to examine CPP and the number of times a female landed on a male’s nest box. Males were also divided based on whether or not a female ever landed on his nest box. Data violated assumptions of parametric statistics, thus a Mann-Whitney U test was used to compare CPP in males that did compared to those that did not attract a female to his nest box.
In regression analyses outliers were identified using deleted residuals and Cook’s distances. No outliers were identified in the zebra finch data set. For fall condition starlings, a single outlier was identified (proportion undirected song = 1; proportion time spent on conditioned side of CPP apparatus = 0.24; Fig. 2E). For spring condition males, an outlier was identified for a single male (number of directed songs = 15; proportion directed song = 0.34; proportion time spent on conditioned side of CPP apparatus = 0.17; Fig. 2B, 2F). These outliers were not included in the analyses but are shown in the figures to illustrate that inclusion would not alter correlations identified between CPP and measures of vocal behavior.
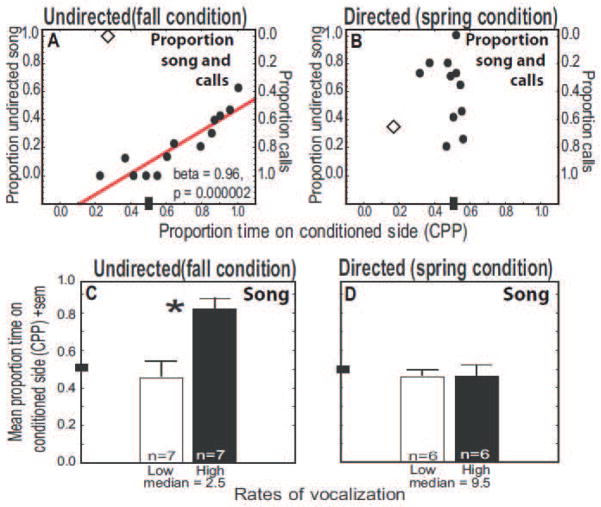
Figure 2.
Evidence linking undirected song and reward in male starlings. A–B) Scatterplots showing correlations between CPP and the proportions of song (y axis on left) and calls (y axis on right) that were A) undirected and B) directed. C–D) Bar graphs showing CPP in males producing low and high rates of C) undirected and D) directed songs. See Figure 1 for additional description of axes. Diamonds in scatterplots indicate statistical outliers not included in the analyses.
3 RESULTS
3.1 Zebra finches
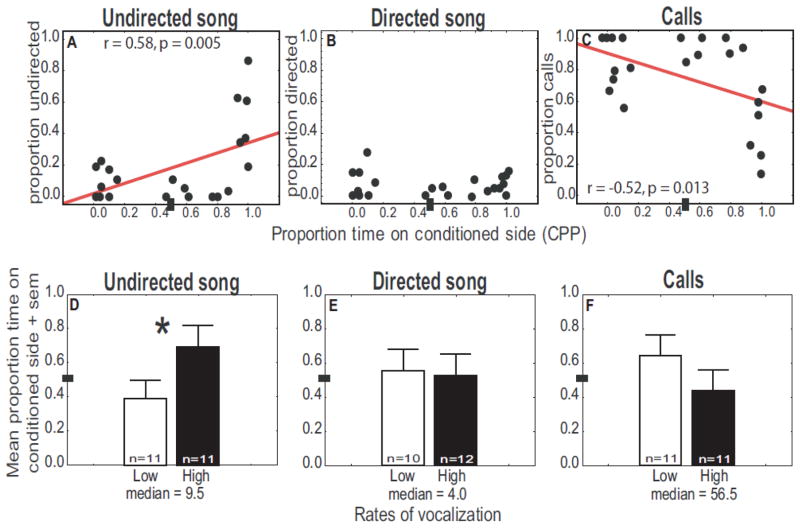
Zebra finches produced multiple undirected and directed songs as well as calls (Table 1). CPP correlated positively with the proportion of undirected song (linear regression: r = 0.58, N = 22, p = 0.005; Fig. 1A). CPP did not correlate with the proportion of directed song (linear regression: r = −.05, N = 22, p = 0.82, Fig. 1B); however, a significant negative correlation was identified between CPP and the proportion of calling behavior (linear regression: r = −0.52, N = 22, p = 0.013; Fig. 1C).
Table 1.
Mean (sd) vocal production for birds in each condition
| Prior to conditioning | During conditioning | ||
|---|---|---|---|
| Zebra finch | undirected song | 6.77 (8.4) | 6.59 (13.30) |
| directed song | 5.5 (7.04) | n/a | |
| calls | 9.14 (8.42) | 46.27 (39.87) | |
|
| |||
| Starling | |||
| fall condition | undirected song | 3 (2.66) | 0 |
| calls | 5.36 (4.93) | 5.78 (9.40) | |
|
| |||
| spring condition | directed song | 2.58 (4.76) | 8.75 (4.07) |
| calls | 6.33 (8.77) | 7.00 (11.15) | |
Figure 1.
Evidence linking undirected song and reward in male zebra finches. A–C) Scatterplots illustrating correlations between CPP and proportions of total vocal behavior consisting of A) undirected song, B) directed song, and C) calls. Y-axis represents the proportion of each vocal behavior produced for 30 min during and 30 min just prior to being placed on one side of the CPP apparatus (conditioned-side). The X- axis represents the proportion of time males spent on the conditioned side of the apparatus the following day. Each point represents data from a single male. Regression lines indicate the slope of significant correlations. D–F) Bar graphs showing the mean proportion time spent on the conditioned side of the CPP apparatus for males that produced low (open bars) or high (filled bars) rates of D) undirected song, E) directed song, and F) calls. * = p < 0.05. For all legends: Analyses were performed on arcsine transformed proportion data. Untransformed data are shown to illustrate actual vocal responses. A proportion of 0.50 is marked by small dark rectangle.
CPP was significantly higher in males singing at the highest compared to the lowest rates (based on a median split of the raw singing values) of undirected song (Mann-Whitney U test: U =25.50, Nbelow median =11, Nabove median = 11, p = 0.022; Fig. 1D). In contrast, CPP did not differ in males producing low or high rates of directed song (Independent t test: t20 = 0.001, p = 0.999; Fig. 1E) or calling behavior (Independent t test: t20 = 1.05, p = 0.308; Fig. 1F).
To provide data related to the function of calls, we compared calling behavior in isolated birds compared to birds in social groups. Consistent with past data showing zebra finch contact calls to be produced primarily by birds separated from flocks [13,19,20], the majority of contact calls were produced when males were isolated during conditioning as compared to during the behavioral observation period 30 min prior to conditioning (Repeated measures t test: t21 = 4.33, p = 0.0003; Table 1).
3.2 European starlings
3.2.1 Undirected song (fall condition)
Starlings in fall condition produced multiple undirected and directed songs as well as calls (Table 1). Results of both forward and backward stepwise multiple regression analyses were identical and significant (adjusted R2 = 0.88, N = 13, p = 0.000008). Undirected song (Beta = 0.96, SE = 0.10, t10 = 1.29, p = 0.000002) contributed significantly CPP (Fig. 2A). (Because for starlings only two vocal behaviors were recorded during the same time period for each male, in contrast to the zebra finch study, the correlation between CPP and calls is the inverse; Fig 2A.) No other variable contributed to CPP.
Fall condition males did not sing while in the CPP apparatus, thus singing was produced in the 30 min prior to conditioning. An analysis comparing males categorized as low or high singers (based on a median split of the raw singing values) indicated that CPP was significantly higher in males that sang high compared to low rates of undirected song (Independent t test: t12 = 3.36, p = 0.006; Fig. 2C). CPP did not differ in males calling at low compared to high rates in fall condition (Independent t test: t12 = 0.98, p = 0.347).
To provide data related to the function of calls, we compared calling behavior in isolated birds compared to birds in social groups. No significant differences were observed in prurpp call production when males were isolated during the 30 min conditioning compared to during the behavioral observation period 30 min prior to conditioning (p > 0.05; Table 1).
3.2.2 Directed song (spring condition)
Starlings in spring condition also produced multiple undirected and directed songs as well as calls (Table 1). Results of forward and backward stepwise regression analyses were identical (adjusted R2 = 0.38, N = 11, p = 0.06). Although the overall regression was not significant, preening behavior did significantly contribute to the prediction of the dependent variable (preening Beta = −0.77, SE = 0.28, t8 = 2.77, p = 0.02). (Males that preened at high rates spent more time away from the conditioned side of the apparatus). No other behavior contributed to CPP, including the proportion of directed song or inversely, calling behavior (Fig. 2B).
No significant differences were identified in CPP for spring condition males producing low or high rates of directed song (Mann-Whitney U test: U = 15.00, Nbelow median =6, Nabove median = 6, p = 0.631; Fig. 2D) or producing prurpp calls at low compared to high rates (Independent t test: t10 = 0.18, p = 0.859).
To provide data related to the function of calls, we compared calling behavior in isolated birds compared to birds in social groups. No significant differences were observed in prurpp call production when males were isolated during the 30 min conditioning compared to during the behavioral observation period 30 min prior to conditioning (p > 0.05; Table 1).
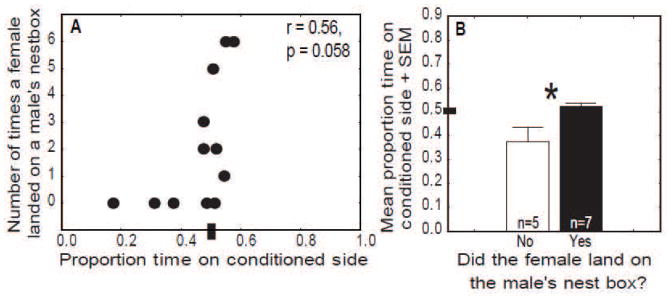
Additional analyses were run to examine the possibility that directed song is primarily rewarded by external social responses of conspecifics rather than intrinsically. A trend was observed for CPP to correlate with the number of times a female landed on a male’s nest box (an indicator of the extent to which a male was successful at attracting a female) during the 30 min prior to conditioning (r = 0.56, p = 0.0585; Fig. 3A). This trend reflected a place aversion in males that failed to attract a female to a nest box.
Figure 3.
Evidence that failing to attract a female has negative valence in spring condition males. A) Scatterplot showing a correlation between the number of times a female landed on an individual male’s nest box and CPP in male starlings. Y-axis represents the number of times a female landed on a male’s nest box during the 30 min prior to conditioning. The X- axis represents the proportion of time males spent on the conditioned side of the apparatus the following day. B) Bar graph showing the mean proportion of time spent on the conditioned side of the CPP apparatus for spring condition males that failed to attract (open bar) or successfully attracted (filled bars) a female to a nest box. Each point represents data from a single male. * = p < 0.05.
Males were then divided based on whether (n = 7) or not (n = 5) a female ever landed on his nest box. Although males in neither group showed an overall CPP (Fig. 3), males that never attracted a female to a nest box spent significantly more time away from the conditioned side of the apparatus compared to males that attracted females to the nest box (Mann-Whitney U test: U = 4, z = 2.19, p = 0.028; Fig. 3B).
4 DISCUSSION
The present study demonstrates a tight link between individual singing behavior and reward state and highlights the utility of the CPP paradigm for examination of reward related to song production. Interestingly, in both zebra finches and starlings only undirected singing behavior was tightly coupled to reward state (as reflected in the development of a song-associated place preference), indicating that the role of reward in vocal production differs depending on the function and social context in which an individual is communicating. Data across species thus suggest that undirected and directed song rely on partially distinct reward mechanisms.
4.1 Undirected song and intrinsic reward
Undirected song is not directed towards other individuals, appears to be ignored by other birds, and occurs without any obvious immediate external reinforcement (such as the attraction of a mate [11–14]). The link identified in the present study between undirected song and individual reward state suggests that in the absence of any obvious, immediate external reinforcement, undirected communication may be intrinsically rewarded. It is possible that a reward state induced by environmental stimuli (e.g., the presence of adequate food or the absence of predators) or individual factors (e.g., low anxiety levels) facilitates undirected singing behavior. Alternatively the act of producing undirected song itself may activate reward neural systems to facilitate further song production (perhaps in part by reducing stress and leading to a positive affective state). We expect these effects are bi-directional, much like singing behavior in humans which can induce positive affect [28–31] and is widely thought to be facilitated by a positive affective state.
4.2 Directed song may be externally reinforced by the behavioral responses of conspecifics
The absence of a link between directed song and individual reward state in the present study indicates that directed song may not be as dependent on intrinsic reward as undirected song. Given that directed singing behavior functions to attract conspecifics (such as mates) or repel competitors, directed song may primarily be externally reinforced, for example through reward resulting from mate attraction and copulation. We formulated this hypothesis after completing the zebra finch study and therefore do not have data on the behavioral responses of the recipients of directed song for zebra finches. In starlings however, we did collect this information. Directed song in the starling study was elicited by the presence of a female and was highly sexually-motivated. Interestingly, males that failed to attract a female spent the majority of time on the side of the apparatus not paired with song, suggesting that the failure to attract a female induced a negative affective state resulting in a place aversion. Somewhat unexpectedly, attracting a female to a nest box did not induce a CPP in this study; however, interactions with a female lasted only 30 minutes and males did not interact physically or copulate with a female. Had behavioral interactions resulted in copulation, we predict that males which successfully copulated with females would develop a mating-induced CPP as in prior studies in rats [2,17]. Although future work is required, the present results are consistent with the idea that directed song may be externally reinforced by the behavioral responses of conspecifics.
4.3 Calling behavior is associated with a place aversion
In the zebra finch and starling studies, contact calls related negatively to CPP. In zebra finches loss of social contact (i.e., separation from a flock or sexual partner) rapidly triggers production of distance contact calls, which are infrequent in birds in flocks or in contact with sexual partners [13,19,20]. Consistent with these past studies, the majority of calling behavior by zebra finches in the present study was observed in male isolated in the CPP chamber during conditioning rather than during the period of behavioral observations in flocks. Thus, similar to male starlings singing to attract females, vocal behavior in isolated male zebra finches can result in immediate social contact (i.e., reunion with flockmates). Likewise, the starling calls that we recorded are thought to call together conspecifics to form flocks [26]. Thus in starlings and zebra finches high rates of calling are likely elicited by males seeking social contact; however, because birds were isolated, calling behavior did not result in conspecific attraction. Therefore, it is possible that for both calling behavior and female-directed song in starlings the failure to attract a conspecific induces in a negative affective state resulting in a place aversion.
4.4 Potential mechanisms underlying song-associated reward
The differences identified here between reward state and undirected compared to directed singing behavior suggest different roles for rewarding neurochemicals in communication in these two contexts. Dopamine plays a primary role in incentive motivation and reward-directed behaviors, including sexually-motivated song directed towards a female [5,23,32–42]; however, it has been argued that dopamine does not underlie reward per se [5,33,43]. Instead, other neurochemicals such as opioid neuropeptides are proposed to play a primary role in pleasure or reward associated with multiple behaviors, including social, sexual, and feeding behaviors [44–50]. Recent data in zebra finches and starlings indicate that opioids in brain regions involved in reward, including the ventral tegmental area and medial preoptic nucleus, are more tightly linked to undirected than directed song [51–53]. Peripheral opioid pharmacological manipulations also demonstrate that opioids differentially regulate directed and undirected song [37,51,54]. Furthermore, in female ring doves (Streptopelia risoria), both auditory and proprioceptive aspects of vocal production feed back onto hypothalamic neural systems to influence opioid rich neural circuits [55,56]. Based on these data, it is possible that opioid reward initiates undirected song or that self-stimulation of opioid release in limbic systems by the act of vocal production contributes to reward associated with undirected song (reviewed in [56–58]). With the use of the CPP paradigm, a next step will be to directly examine whether the reward state associated with the act of singing undirected song is mediated by opioid release in reward neural circuitry.
4.5 Methodological considerations
The CPP methodology employed in the present study differs somewhat from CPP tests often used in studies of other rewards (such as food or drug rewards) [7,9,10]. For example, in studies of food and drug reward it is common during conditioning to repeatedly place an animal in one side of a CPP apparatus with a reward and one side of the apparatus without a reward. Unlike food or drug rewards birds cannot be administered the “act of singing” (either a bird sings or it does not), which means that an experimenter cannot control how much, when or if a bird will sing. Thus pairing “the act of singing” (e.g. 20 song bouts) with one side of the apparatus and “a lack of singing” (0 song bouts) with the other side repeatedly is not possible. This means that the novelty of the song-paired and unpaired CPP compartments in the present study was not controlled as it can be in rats placed an equal number of times in the two sides of a CPP apparatus. Thus novelty may contribute to the present results. Although this cannot be ruled out, in the absence of a compelling argument for why preferences for novelty would correlate linearly with the rate of undirected but not directed song production, we believe the results of the present study more likely reflect links between song and individual reward state than differential responses to novelty.
It is also common in studies of food and drug reward for preexisting individual side preferences to be determined during habituation and then subtracted from preferences observed on test day, to control for preexisting preferences [7,9,10]. In the present study, although we did not test individual baseline CPP side preferences prior to conditioning, across individuals we counterbalanced conditioning across the side and color of the CPP apparatus to control for any general side or color preferences. We plan to examine this issue in our future work but here believe that the consistent associations identified between CPP and undirected but not directed song in two songbird species are not likely artifacts related to baseline preferences.
Finally, it is likely that stress is inherent to any study involving CPP as animals are moved from home cages and restricted to relatively novel chambers. We expect that the contact calls measured in both zebra finches and starlings reflect individual stress responses to being placed alone in a CPP chamber. Thus individual stress levels may interact with the positive affective state associated with song to determine individual CPP. It is important to note however that all birds (whether singing directed or undirected song) were tested under identical conditions. Despite the fact that stress levels would be expected to be similar across birds singing directed and undirected song, only birds singing undirected song developed a strong place preference.
4.6 Conclusions
The present study provides a novel method for assessing song-associated reward and suggests that reward associated with vocal production differs depending upon the social context in which communication occurs. Specifically, the data indicate that undirected but not directed song may be primarily facilitated and maintained by intrinsic release of rewarding neurochemicals. The results also suggest that reward is differentially associated with directed and undirected communication across species and contexts irrespective of whether vocal behavior is complex and learned (as is typically the case for song) or relatively simple and less dependent on learning (as is typically the case for calls) [59]. The data have potentially broad implications, providing insight into what motivates animals to practice social behaviors during development and ways in which distinct reinforcement mechanisms function to direct socially appropriate adult behaviors.
Highlights.
A place preference paradigm was used to measure reward associated with singing behavior
Male songbirds directed song to conspecifics or sang spontaneously (undirected song)
Males singing undirected but not directed song developed a place preference
Results suggest that undirected but not directed song is linked to intrinsic reward
Factors rewarding vocal production may differ depending on the social context
Acknowledgments
The data presented in this paper are based upon work supported by a grant from NIH to LVR (R01 MH080225). The authors thank Dr. Deborah L. Duffy and Dr. Stephen C. Gammie for thoughtful comments on drafts of this manuscript and Dr. Thomas Werner for assistance with translation of an article from German.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren V. Riters, Email: LVRiters@wisc.edu.
Sharon A. Stevenson, Email: sstevenson@wisc.edu.
References
- 1.Watson KK, Shepherd SV, Platt ML. Neuroethology of pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; Oxford: 2008. pp. 85–95. [Google Scholar]
- 2.Agmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav Neurosci. 1990;104:177–82. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- 3.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–80. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson A, Balleine B. Hedonics: the cognitive-motivational interface. In: KML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; Oxford: 2008. pp. 74–84. [Google Scholar]
- 5.Panksepp J, Moskal J. Dopamine and seeking: Subcortical reward systems and appetitive urges. In: Elliot AJ, editor. Handbook of Approach and Avoidance Motivation. Taylor and Francis; New York: 2008. pp. 67–88. [Google Scholar]
- 6.Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. Vol. 1016. New York Academy of Sciences; New York: 2004. [DOI] [PubMed] [Google Scholar]
- 7.Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. The neuropharmacological basis of reward. In: Liebman JM, Cooper SJ, editors. The neuropharmacological basis of reward, Topics in experimental psychopharmacology. Clarendon Press/Oxford University Press; New York: 1989. pp. 264–319. [Google Scholar]
- 8.Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- 9.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 10.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 11.Dunn AM, Zann RA. Undirected song in wild zebra finch flocks: context and effects of mate removal. Ethology. 1996;102:529–39. [Google Scholar]
- 12.Dunn AM, Zann RA. Effects of pair bond and presence of conspecifics on singing in captive zebra finches. Behaviour. 1997;134:127–42. [Google Scholar]
- 13.Zann RA. The zebra finch: A synthesis of field and laboratory studies. Oxford University Press; 1996. [Google Scholar]
- 14.Sossinka R, Bohner J. Song types in the zebra finch Poephila guttata castanotis. Z. Tierpsychol. 1980;53:123–32. [Google Scholar]
- 15.Hausberger M. Social influences on song acquisition and sharing in the European starling (Sturnus vulgaris) In: Snowdon CT, Hausberger M, editors. Social influences on vocal development. 1997. pp. 128–56. [Google Scholar]
- 16.Hausberger M, Richard-Yris M-A, Henry L, Lepage L, Schmidt I. Song sharing reflects the social organization in a captive group of European starlings (Sturnus vulgaris) Journal of Comparative Psychology. 1995;109:222–41. [Google Scholar]
- 17.Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–7. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paredes RG. Evaluating the neurobiology of sexual reward. ILAR J. 2009;50:15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- 19.Guttinger VHR, Nicolai J. Struktur und funktion der rufe bei practfinken (Estrildidae) Z Tierpsychol. 1973;33:319–34. [PubMed] [Google Scholar]
- 20.Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J Neurosci. 1990;10:1541–56. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 22.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 23.Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009:962–73. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heimovics SA, Riters LV. Breeding-context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) Horm Behav. 2006;50:726–35. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eens M. Understanding the complex song of the European starling: An integrated approach. Adv Study Beh. 1997;26:355–434. [Google Scholar]
- 26.Feare CJ. The Starling. Oxford: Oxford Press; 1984. [Google Scholar]
- 27.Lehner PN. Handbook of Ethological Methods. 2. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- 28.Kreutz G, Bongard S, Rohrmann S, Hodapp V, Grebe D. Effects of choir singing or listening on secretory immunoglobulin A, cortisol, and emotional state. J Behav Med. 2004;27:623–35. doi: 10.1007/s10865-004-0006-9. [DOI] [PubMed] [Google Scholar]
- 29.Clift SM, Hancox G. The perceived benefits of singing: findings from preliminary surveys of a university college choral society. J R Soc Promot Health. 2001;121:248–56. doi: 10.1177/146642400112100409. [DOI] [PubMed] [Google Scholar]
- 30.Skingley A, Vella-Burrows T. Therapeutic effects of music and singing for older people. Nurs Stand. 2010;24:35–41. doi: 10.7748/ns2010.01.24.19.35.c7446. [DOI] [PubMed] [Google Scholar]
- 31.Skingley A, Bungay H. The Silver Song Club Project: singing to promote the health of older people. Br J Community Nurs. 2010;15:135–40. doi: 10.12968/bjcn.2010.15.3.46902. [DOI] [PubMed] [Google Scholar]
- 32.Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–16. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–21. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fibiger HC, Nomikos GG, Pfaus JG, Damsma G. Sexual behavior, eating and mesolimbic dopamine. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):566A–67A. doi: 10.1097/00002826-199201001-00294. [DOI] [PubMed] [Google Scholar]
- 36.Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–66. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song production in male European starlings. Physiology and Behavior. 2006;88:575–84. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubikova L, Wada K, Jarvis ED. Dopamine receptors in a songbird brain. J Comp Neurol. 2010;518:741–69. doi: 10.1002/cne.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–4. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–71. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behavioral Neuroscience. 2010;124:300–04. doi: 10.1037/a0018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 44.Agmo A, Paredes R. Opioids and sexual behavior in the male rat. Pharmacol Biochem Behav. 1988;30:1021–34. doi: 10.1016/0091-3057(88)90135-9. [DOI] [PubMed] [Google Scholar]
- 45.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res Brain Res Rev. 1995;21:162–84. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- 47.Van Ree JM, Niesink RJ, Van Wolfswinkel L, Ramsey NF, Kornet MM, Van Furth WR, Vanderschuren LJ, Gerrits MA, Van den Berg CL. Endogenous opioids and reward. Eur J Pharmacol. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- 48.Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–28. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 49.Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–20. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 50.Carden SE, Hernandez N, Hofer MA. The isolation and companion comfort responses of 7- and 3-day-old rat pups are modulated by drugs active at the opioid receptor. Behav Neurosci. 1996;110:324–30. doi: 10.1037//0735-7044.110.2.324. [DOI] [PubMed] [Google Scholar]
- 51.Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings. Behavioral Neuroscience. 2005;119:245–55. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- 52.Kelm CA, Forbes-Lorman RM, Auger CJ, Riters LV. Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings (Sturnus vulgaris) defending nest sites and courting females. Behav Brain Res. 2011;219:15–22. doi: 10.1016/j.bbr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khurshid N, Jayaprakash N, Shahul Hameed L, Mohanasundaram S, Iyengar S. Opioid modulation of song in male zebra finches (Taenopygia guttata) Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Khurshid N, Jayaprakash N, Shahul Hameed L, Mohanasundaram S, Iyengar S. Opioid modulation of song in male zebra finches (Taenopygia guttata) Behavioural Brain Research. 2010;208:359–70. doi: 10.1016/j.bbr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Cheng MF, Zuo M. Proposed pathways for vocal self-stimulation: met-enkephalinergic projections linking the midbrain vocal nucleus, auditory-responsive thalamic regions and neurosecretory hypothalamus. J Neurobiol. 1994;25:361–79. doi: 10.1002/neu.480250403. [DOI] [PubMed] [Google Scholar]
- 56.Cheng MF, Durand SE. Song and the limbic brain: a new function for the bird’s own song. Ann N Y Acad Sci. 2004;1016:611–27. doi: 10.1196/annals.1298.019. [DOI] [PubMed] [Google Scholar]
- 57.Riters LV. Evidence for opioid involvement in the motivation to sing. J Chem Neuroanat. 2010;39:141–50. doi: 10.1016/j.jchemneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riters LV. Pleasure seeking and birdsong. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marler PR, Slabbekoorn H. The Science of Birdsong. Academic Press; 2004. Nature’s Music; p. 504. [Google Scholar]