Abstract
Introduction
Low striatal dopamine 2/3 receptor (D2/3) availability and low ventrostriatal (VST) dopamine (DA) release have been observed in alcoholism, cocaine and heroin dependence. Less is known about the dopaminergic system in cannabis dependence. We assessed D2/3 availability and DA release in abstinent cannabis users compared to controls and explored relationships to parameters of cannabis use history, using [11C]raclopride Positron Emission Tomography (PET) and an amphetamine challenge paradigm.
Methods
16 recently abstinent, medically and psychiatrically healthy cannabis-using participants (CD, 27.3 ± 6.1 years, 1 female, 15 males) and 16 matched controls (HC, 28.1 ± 6.7 years, 2 females, 14 males) completed two PET scans, before and after injection of i.v. d-amphetamine (0.3 mg/kg). Percent change in [11C]raclopride binding after amphetamine (ΔBPND) in subregions of the striatum was compared between groups. Correlations with clinical parameters were examined.
Results
Cannabis dependent participants had an average consumption of 517± 465 estimated puffs per month, indicating overall mild to moderate cannabis dependence. Neither baseline BPND nor ΔBPND differed from controls in any ROI, including VST. In CD, earlier age of onset of use correlated with lower [ΔBPND] in the associative striatum (AST) when controlling for current age.
Conclusions
Unlike other addictions, cannabis dependence of mild to moderate severity is not associated with striatal DA alterations. However, earlier use, or longer duration of use, is related to lower DA release in the AST. These observations suggest a more harmful effect of use during adolescence; more research is needed to distinguish effects of chronicity versus onset.
Keywords: cannabis, dopamine, PET imaging, addiction, amphetamine challenge, adolescent onset
Introduction
Cannabis is the most frequently used illicit drug, with a lifetime prevalence in North America of up to 54 % [1]. It is perceived by many users as harmless compared to other drugs of abuse. Yet, cannabis use has been shown to induce dependence [2], anxiety and depression in some [3, 4] but not all studies [5]. It has been linked to acute psychosis and chronic schizophrenia, possibly as a result of gene-environment interactions [6]. Cannabis use beginning in adolescence may have long-lasting effects on the brain by interacting with normal maturational processes [7]. It has been associated with abnormal brain structure, such as reduced hippocampal and amygdala volumes [8] as well as cognitive disturbances in verbal learning and working memory, particularly in adolescents who are more adversely affected by heavy use than adults [9]. In light of these negative effects, and the addictive potential, it is important to understand how cannabis and its main active component, Δ9tetrahydrocannabinol (THC), may affect normal brain functions.
One neurotransmitter system that is clearly involved in the addictive properties of drugs of abuse in general, and may be involved in mediating THC’s effects, is dopamine (DA). Cannabinoids, including THC [10], stimulate neuronal firing of mesolimbic DA neurons [11, 12] and may elevate striatal DA levels, as measured with microdialysis [13–15]. The direct psychopharmacological effects of THC are mediated by endocannabinoid (CB1) receptors, which are predominantly localized in the basal ganglia, and at lower density in the ventral midbrain, among other regions [16]. CB1 receptors localize to glutamate and gamma-aminobutyric acid (GABA) terminals where their function is inhibitory, but also co-localize with dopamine-2/3 (D2/3) receptors in the midbrain, prefrontal cortex and basal ganglia and may have synergistic effects with D2/3 activation through augmentation of cAMP [17]. Thus, CB1 receptor activation through cannabinoid stimulation affects multiple neurotransmitter systems, including DA.
Four studies have examined the effect of THC on DA release in vivo, including an anecdotal case report by Voruganti et al. [18]. A pilot study in 7 healthy participants found that THC inhalation reduced [11C]raclopride binding in the VST and the precommissural dorsal putamen (preDPU), compared to placebo, consistent with an increase in DA levels in these regions [19].
A third study using the synthetic cannabinoid dronabinol compared to placebo reported no significant difference in [11C]-raclopride binding between conditions despite an increase in psychosis-like symptoms [20]. A closer examination of the results shows a radiotracer displacement of 0.6 to 3% in some striatal subregions, within a similar range to that reported by Bossong et al [19]. Finally, Barkus and colleagues compared intravenous administration of THC compared to placebo in 11 healthy men using Single Photon Emission Computed Tomography (SPECT) with [123I] IBZM, and found no significant change in the caudate or putamen [21]. Unfortunately, this study was not conducted at radiotracer equilibrium conditions, thus not allowing quantifiable information regarding the effects of the challenge.
Overall, these studies suggest a small effect of acute THC on DA release in the human striatum. Since dependence on drugs is associated with long term blunting of DA release [22], we examined the long-term effects of chronic use of cannabis on DA parameters. We hypothesized that similarly to users of other DA enhancing drugs of abuse [23–25], cannabis abusing and dependent participants will have a reduction in D2/3 binding and a blunted DA release with largest magnitude in the VST. We also hypothesized that the severity of these changes would be associated with earlier use during adolescence.
Methods
Study population
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute. All participants provided written informed consent. Inclusion criteria for cannabis users (CD) were: age 21–55 years; regular cannabis use of at least 5 times per week or meeting DSM-IV criteria for cannabis dependence, but no other current or past Axis I disorder (including current nicotine dependence), no or minimal exposure to other drugs of abuse, willingness to detoxify from cannabis and documentation of a negative urine drug screen on study day. For inclusion in the study, current cannabis users were required to abstain until their urine toxicology screen became negative, confirmed by bi-weekly observed urine toxicology and negative urine toxicology on the day of the scan. As urine toxicology in regular users can remain positive for cannabis metabolites (cannabinoids, main metabolite 11-nor-D9-carboxy-THC) for over 1 month [26], abstinence was confirmed by a decrease in urine cannabinoid levels between two individual samples (negative result: < 50 ng/ml). Cannabis users with a negative drug screen at the time of screening were included if the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV (PRISM-IV) [27] confirmed current cannabis dependence. Participants were recruited through advertisements, word of mouth, and referrals from other researchers.
Assessments
Psychiatric diagnoses were ascertained with the PRISM, individual and parental socioeconomic status (SES) was scored for all participants using the Hollingshead scale [28]. Because of the effects of catechol-O-methyl-transferase (COMT) on dopamine transmission, blood samples for COMT genotyping were obtained and groups were matched for COMT genotype.
CD participants were further evaluated with a series of instruments including the Alcohol Time Line Follow-Back (TLFB) [29], modified for cannabis.
Participants reported their cannabis use in number of their preferred units of smoking (pipe, joint, blunt, bong or bowl). The number of "puffs" was estimated as in Gray et al. [30], where 1 pipe equals 5 puffs, 1 "joint" equals 10 puffs, 1 "bong" or "bowl" equals 12 puffs, and 1 "blunt" (very large joint, cannabis “cigar”) equals 20 puffs. Use during the last 30 days was based on the TLFB. Use during the last 12 months was extrapolated from the TLFB and the pattern of use/periods of abstinence reported in the PRISM.
PET Data Acquisition
The study consisted of two [11C]raclopride PET scans on the same day. [11C]raclopride was administered as a bolus plus constant infusion (Kbol of 105 min). Twenty min into the constant infusion, participants were positioned in the scanner (ECAT EXACT HR+, Siemens Medical Systems, Knoxville, TN, USA) and a 10 min transmission scan was acquired. Emission data were collected as eight frames of 5 min each obtained over 40 min, beginning 40 min after the bolus injection. The second [11C]raclopride injection was initiated 2 min following the amphetamine injection (0.3 mg/kg iv), and participants were monitored for cardiovascular changes. During each scan, five venous samples (collected at 40, 50, 60, 70 and 80 min) were obtained to measure the plasma concentration and non-protein bound fraction (fp) of [11C]raclopride. During the second scan, three additional blood samples were obtained at 10, 20 and 40 min for measuring amphetamine levels.
Both in CD and HC, the behavioral effects of amphetamine were rated with PANSS [31] at baseline (immediately prior to amphetamine injection), 10 min post-injection, and after completion of the scan. Self report of participants’ experience of happiness, restlessness, energy, and anxiety was rated with the Amphetamine Interview Rating Scale (AIRS) [32], at baseline and 5, 10, 20, 30 and 45 min after amphetamine injection.
A T1-weighted anatomical MRI was acquired for each participant on a GE 1.5-T Signa Advantage system on a separate day (GE Healthcare, Waukesha, Wisconsin).
PET Data Analysis
Regions of interest (ROIs) were drawn on each individual’s MRI and applied to the co-registered PET images. Image analysis was performed in MEDx (Sensor Systems, Sterling, Virginia) as described previously [33]. The striatum as a whole (STR) was divided into five anatomical ROIs [34] including pre-commissural caudate and putamen (preDCA, preDPU), post-commissural caudate and putamen (postCA, postPU) and ventral striatum (VST). Based on their cortical and subcortical connections, the ROIs were classified as belonging to the associative striatum (AST = preDCA, preDPU, postCA), the VST, or the sensorimotor striatum (SMST = postPU). Activities from left and right were averaged [33, 34]. Cerebellum (CER) was used as a reference region to measure free and non-specifically bound [11C]raclopride activity. Equilibrium analysis was used to derive the specific to non-displaceable equilibrium partition coefficient BPND (unitless) as ROI activity/CER activity − 1 during steady-state. [23]. Cerebellar activity was assumed to be equal to free plus nonspecifically bound activity because the concentration of D2 receptors in the cerebellum is negligible.
The primary outcome measure was the percent change in BPND between conditions (ΔBPND), calculated as:
This expresses the relative reduction in DA D2/3 receptor availability for [11C]raclopride binding after amphetamine-induced DA release.
Statistical Analysis
Comparisons between scan conditions were performed with paired t tests; comparisons between groups were performed with two-group t tests at the ROI level. A linear mixed model across all striatal subregions with regional ΔBPND as repeated measure was performed to test for a global effect of diagnosis (CD vs. HC) on ΔBPND. Relationships between the PET data and the clinical characteristics and response to amphetamine were analyzed with Pearson-Product-Moment correlation coefficients or Spearman’s rho in the case of non-normality or potential outliers. A two-tailed probability value of p < 0.05 was chosen as statistically significant. We also performed an omnibus linear mixed model for overall effect of age of onset and a post-hoc analysis for individual ROI.
We performed a multiple regression analysis of ΔBPND in all ROIs with amphetamine blood levels as a covariate.
Baseline assessments were made 0 min (AIRS) and −5 and 0 min (cardiovascular parameters; baseline calculated as the average) prior to amphetamine. Total changes in cardiovascular parameters according to Martinez et al [24] and AIRS scores were calculated as the area under the curve (AUC), adjusted to baseline. AUC (score*minutes) relative to baseline was computed as the trapezoidal sum of the difference between assessments and baseline, starting from the time of amphetamine administration.
Results
Participant
Thirty-one participants with cannabis dependence and one with cannabis abuse, but similar amount and chronicity of use to the dependent participants, were initially enrolled in the study. Seven participants were lost to follow-up or withdrew consent, one participant was disqualified for cerebral vascular malformation, and 7 participants were disqualified due to their inability to abstain from cannabis use (no decrease in urine cannabinoid levels over a period of at least 3 weeks). 17 participants with cannabis dependence (n = 16) or abuse (n = 1) were scanned with [11C]raclopride. One participant was found to have a systolic blood pressure above 140 mm Hg on the day of the scan and received only the baseline scan. One participant’s PET data was excluded due to motion. Thus, we included n=16 cannabis users (n=15 dependent, n=1 abusing) in the analysis of baseline D2/3 binding (BPND), and n=15 (n=14 dependent, n=1 abusing) in the analysis of [11C]raclopride displacement (ΔBPND) after amphetamine administration. Cannabis users (n=16) were matched to 16 healthy controls for age, sex, socioeconomic status, ethnicity and COMT genotype. One CD and HC participants received their respective baseline and challenge scans on separate days due to technical problems on the initial scan day. See Table 1 for demographics and history of cannabis use. Two CD participants reported stopping cigarette smoking during the enrollment period (10 cigarettes/day, stopped within one month of scan). While they were not considered nicotine dependent at the time of the scan, one HC with a similar pattern of smoking was included for better matching. These participants are listed as “smokers” in table 1. There was minimal exposure to other drugs of abuse (only in 4 participants and no more than 10 times during lifetime for any of the participants).
Table 1.
Demographics.
| Demographics | HC | CD |
|---|---|---|
| Characteristic | (n=16) | (n=16) |
| Age (years)* | 28.1 ± 6.9 | 27.3 ± 6.1 |
| Sex | 2F, 14M | 1F, 15M |
| Ethnicity | 7C, 5AA, 4H | 7C, 5AA, 4H |
| Smoking status | 1 smoker | 2 smokers |
| SES (individual)* | 31.9 ± 16.0 | 29.0 ± 14.4 |
| SES (parental)* | 39.7 ± 14.9 | 42.4 ± 12.0 |
| COMT (Val/val, val/met, met/met) | 3,7,3 | 3,8,2 |
| Age of onset of cannabis use (years) | --- | 17.9 ± 2.0 |
| Duration of use (years) | --- | 9.1 ± 6.7 |
| Days used (past 30 days) | --- | 19.8 ± 9 |
| Severity (estimated no. of puffs, 30 days) | --- | 517.1 ± 465 |
| Severity (estimated no. of puffs, past 12 mnths) | --- | 7816.4 ± 6953 |
| Abstinence before scan (weeks) | --- | 4.1 ± 2.1 |
| Relapse during detoxification period | --- | 6 yes/10 no |
Demographic parameters for healthy controls and cannabis users. There was no significant difference in age or individual or parental socioeconomic status (SES, [28]), (*). Groups were well-matched for sex, ethnicity, smoking status and COMT genotype. Val = valine, met = methionine. Also shown are clinical parameters of cannabis use. “Relapse during detoxification” refers to participants with initial decrease in cannabinoid levels who presented with one or more intermittent increases in cannabinoid levels after enrollment (based on serial urine screening), but were able to eventually to fully abstain (< 3 months).
Amphetamine blood levels
Amphetamine blood levels (AL) did not differ between groups at 10 and 20 min post injection (CD n=15; HC n=13; AL10 min: CD = 31.8 ± 7.8 ng/ml, HC = 35.8 ± 7.2 ng/ml; p = 0.15; AL20 min min: CD = 34.7 ± 9 ng/ml, HC = 38.7 ± 7.5 ng/ml; p = 0.24). There was a significant difference at AL40 min (CD n=15, HC n=15; CD: 34.6 ± 8 ng/ml, HC: 44.0 ± 6.8 ng/ml, p=0.004). This difference, however, was not associated with the main outcome measure ΔBPND; Pearson-Product-Moment correlations between ΔBPND and any AL parameter (10, 20, 40 min concentrations or AUC) failed to reach statistical significance in any ROI.
Behavioral and physiological effects of amphetamine
Both groups showed an increase in systolic (area under the curve - HC: 50.8 ± 13.9; CD: 25.2 ± 9.3; p=0.44), and diastolic blood pressure (HC: 13.3 ± 10.6; CD: 10.7 ± 7.3; p=0.46) and heart rate (HC: 27.5 ± 12.4; CD: 5.6 ± 7.1; p = 0.46) in response to amphetamine, but there were no significant differences between groups.
Cannabis users (n=15) and HC (n=15, one participant without AIRS rating) did not differ significantly in ratings for any of the categories of subjective experience measured with AIRS. Both HC and CD reported subjective experience of amphetamine as described previously in healthy controls [35]. Ratings for increase in “energy” were significantly correlated with increased DA release in AST (r = 0.380, p = 0.04) and STR (r = 0.375, p = 0.04) for the group as a whole.
PANSS scores were compared between baseline and the score representing the largest change from baseline for each individual item, whether this occurred at 10 min post amphetamine or at the end of the second scan (90 min post amphetamine).
PANSS scores at baseline differed significantly between groups in the “negative symptoms” subscore, but not other subscores or total score, with lower baseline ratings in CD.
Post-amphetamine, HC had significantly higher scores in all symptom domains, and change in symptoms was significant only for HC, but not CD (Table 2). Group differences were largely caused by higher scores in HC for “excitement” (P4), “difficulty in abstract thinking” (N5), “anxiety” (G2), and “tension” (G4).
Table 2.
PANSS scores in response to amphetamine.
| HC (n=15) | CD (n=15) | CD vs. HC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| sx scores | baseline | p-amph | ΔPANSS | p | baseline | p-amph | ΔPANSS | p | ΔPANSS, p |
| p-tot | 7.5 ± 1.1 | 8.7 ± 1.9* | 1.30 ± 1.9 | 0.01 | 7.6 ± 1.2 | 7.5 ± 0.8* | −0.27 ± 1.4 | 0.65 | 0.02 |
| n-tot | 8.3 ± 1.4* | 7.7 ± 0.8* | −0.80 ± 1.5 | 0.12 | 7.3 ± 0.5* | 7.1 ± 0.4* | −0.13 ± 0.5 | 0.19 | 0.12 |
| g-tot | 17.4 ± 1.8 | 19.9 ± 2.7* | 2.53 ± 2.4 | 0.001 | 17.3 ± 3.0 | 17.5 ± 3.3* | 0.13 ± 1.9 | 0.79 | 0.005 |
| PANSS tot | 33.0 ± 3.0 | 36.3 ± 3.8* | 3.0 ± 3.4 | 0.002 | 32.3 ± 4.1 | 31.9 ± 3.3* | −0.27 ± 2.6 | 0.65 | 0.006 |
p-tot: positive symptom subscore (maximum possible score: 49), n-tot: negative symptom subscore (max. possible score: 49), g-tot: general symptom subscore (max. possible score 112).
indicates significant difference between groups for this parameter, p < 0.05.
Change in symptom scores was significant only for healthy controls. The difference in change between groups was significant for all subscores except for negative symptoms.
Evaluation of symptoms according to the 5-factor model of the positive and negative syndrome scale [36] which removes P4 from the positive and N5 from the negative symptom subscores, showed that only the factor “excitement” remained significantly larger in HC, compared to CD.
There was no correlation between peak change in positive or negative symptom subscores and ΔBPND in any ROI (standard PANSS and 5-factor analysis; Spearman’s rho) for either group.
PET analysis
The average ROI volumes, injected activities, specific activities, injected mass and plasma free fraction (fp) of [11C]raclopride did not differ between conditions or groups (Table 3). Non-specific distribution volume (VND) differed between conditions but not between groups (Table 3).
Table 3.
Scan parameters.
| HC (n=16) | CD (n=15, BL; n = 16, PA) | |||
|---|---|---|---|---|
| PET parameters | Baseline | p-amph | Baseline | p-amph |
| ID (mCi) | 8.5 ± 1.4 | 8.1 ± 1.6 | 7.8 ± 1.8 | 8.1 ± 2.3 |
| IM (μg) | 4.1 ± 1.8 | 3.6 ± 1.7 | 3.8 ± 1.8 | 3.5 ± 2.0 |
| SA (Ci/mmol) | 1395 ± 1055 | 1554 ± 1054 | 1328 ± 727 | 1448 ± 919 |
| VND* | 0.42 ± 0.07 | 0.37 ± 0.07 | 0.40 ± 0.12 | 0.33 ± 0.12 |
| fp | 0.041 ± 0.01 | 0.039 ± 0.01 | 0.038 ± 0.01 | 0.036 ± 0.01 |
Injected dose (ID), injected mass (IM), specific activity (SA), distribution volume of the reference region (VND) and plasma free faction (fp) are shown. ID refers to the effective injected activity associated with the bolus plus constant infusion paradigm, accounting for decay occurring during the course of the infusion. Parameters did not differ between conditions or groups except for VND, where the difference between baseline and amphetamine scan reached significance at p <0.05 for both HC and CD, but was not different between groups (*). BL = baseline, PA = post-amphetamine.
Main outcome measures
There were no group differences in baseline D2/3 binding (BPND) or in ΔBPND in any ROI (Table 4). The results for the main outcome measures were unchanged when excluding the participant with a diagnosis of cannabis abuse, so he was included in the final analysis. Linear mixed modeling across all striatal subregions with regional ΔBPND as repeated measure found no significant effect of diagnosis.
Table 4.
[11C]raclopride binding.
| BPND | BPND controls (n=16) | BPND cannabis users (BL: n=16; p-amph: n=15) | CD vs. HC | |||||
|---|---|---|---|---|---|---|---|---|
| ROI | baseline | post-amphetamine | ΔBPND | baseline | post-amphetamine | ΔBPND | p BPND | p ΔBPND |
| VST | 2.31 ± 0.3 | 2.05 ± 0.3 | −11 ± 9% | 2.35 ± 0.3 | 2.16 ± 0.3 | −9 ± 8% | 0.49 | 0.98 |
| AST | 2.66 ± 0.4 | 2.44 ± 0.3 | −8 ± 9% | 2.73 ± 0.4 | 2.57 ± 0.3 | −7 ± 6% | 0.58 | 0.74 |
| Pre-DCA | 2.53 ± 0.4 | 2.36 ± 0.3 | −6 ± 8% | 2.65 ± 0.4 | 2.56 ± 0.3 | −5 ± 6% | 0.38 | 0.54 |
| Pre-DPU | 2.95 ± 0.4 | 2.67 ± 0.3 | −9 ± 10% | 2.97 ± 0.4 | 2.73 ± 0.3 | −9 ± 7% | 0.89 | 0.98 |
| Post-CA | 2.03 ± 0.3 | 1.82 ± 0.2 | −9 ± 10% | 2.15 ± 0.3 | 2.00 ± 0.3 | −8 ± 7% | 0.31 | 0.75 |
| SMST | 3.09 ± 0.4 | 2.57 ± 0.3 | −16 ± 12% | 3.18 ± 0.3 | 2.62 ± 0.3 | −18 ± 6% | 0.48 | 0.48 |
| STR | 2.74 ± 0.4 | 2.43 ± 0.3 | −11 ± 10% | 2.83 ± 0.3 | 2.55 ± 0.3 | −11 ± 6% | 0.49 | 0.89 |
There was no statistically significant difference in BPND or ΔBPND between groups in any ROI. BL= baseline; p-amph = post-amphetamine, CD = cannabis users, HC = controls.
Severity of use and abstinence
Neither BPND nor ΔBPND correlated significantly with severity of use. There was no correlation between either BPND nor ΔBPND and duration of abstinence (time since last use, on average 4.1 ± 2.1 weeks).
Age of onset
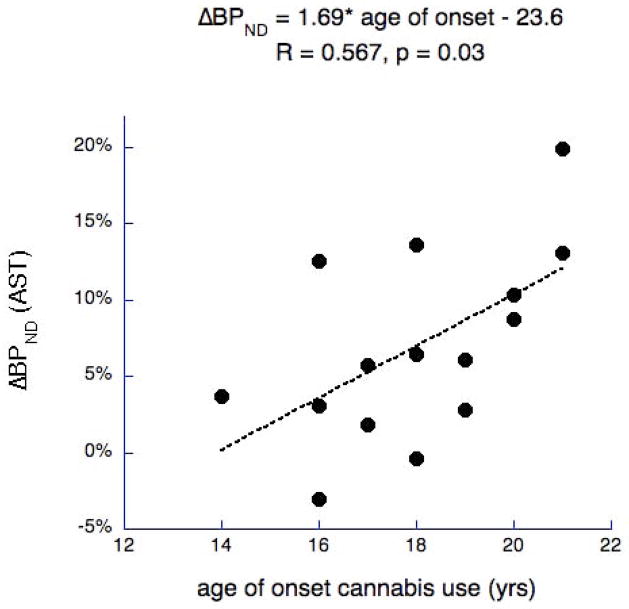
Omnibus linear mixed model for overall effect of age of onset on ΔBPND was significant for “age of onset” alone with all participants (F(1,13) = 6.68, p = 0.023) and when including current age as covariate (F(1,12) = 4.926, p = 0.046). It remained significant when excluding one participant with diagnosis of cannabis abuse. Post hoc analysis by region revealed a significant correlation between age of onset of cannabis use and the absolute value of ΔBPND (n=15) in preDPU (r=0.528, p=0.04), preDCA (r=0.556, p = 0.03), and AST as a whole (r =0.567, p=0.03, Fig. 1), and at trend level for STR (p=0.07). When controlling for age, the correlation between AOO and ΔBPND remained significant in the AST (r=0.549, p=0.042), and preDCA (r=0.537, p=0.048) and survived at trend level in preDPU (r=0.508, p=0.063), and STR (r=0.469, p=0.091).
Figure 1.
Association between age of onset of cannabis use and DA release in the associative striatum (AST). The younger the age of onset, the smaller the participants’ DA release (absolute value of ΔBPND).
Age of onset was significantly correlated with duration of use (r=0.526, p=0.04), but not current age. When partial correlation analysis for the relationship of age of onset to BPND or ΔBPND was performed with duration of use as a co-variate, no relationship between age of onset and BPND or ΔBPND survived as significant in any ROI. The correlation of age of onset with baseline BPND was not significant for any ROI.
Discussion
This is the first report to evaluate both D2/3 receptor availability and striatal DA release capacity in chronic cannabis dependent participants compared to matched controls. Unlike other addictions, such as to alcohol, heroin, cocaine, and methamphetamine [22], chronic cannabis abuse or dependence is not associated with alterations in either of these indices. We therefore confirm the absence of alterations in D2/3 receptors previously reported [37] in a larger cohort and show for the first time that amphetamine-induced DA release is not affected either. Our study used similar methodology to the above-cited studies in other addictions. Participants were imaged within a similar time frame of abstinence, within a few weeks of last use, as confirmed by urine toxicology.
Using the results of Bossong et al. [19] of 3.4% ΔBPND in VST after THC, we can estimate the level of the fractional increase in VST DA induced by THC administration using the simplifying assumptions that the interaction between DA and [11C]raclopride at the D2/3 receptor is purely competitive, that the dissociation constant for DA to D2/3 receptors does not change between conditions, and that receptor-bound DA during the placebo condition is comparable with baseline values. Using the baseline occupancy of D2/3 receptors by DA in healthy volunteers as calculated by Laruelle et al. [38] (10%) and in vivo estimates of the fraction of D2/3 receptors in a high-affinity state for agonists (80%) [39], we estimate that the THC challenge increased extracellular DA levels by 45%. This is lower than the effect of alcohol, estimated to induce an increase in DA release of 138 % (in men) [40], comparable to the effect of low dose amphetamine [35].
The absence of a detectable effect on striatal DA transmission in this sample and overall low effect of acute THC on DA release suggests that striatal DA transmission may not be altered in cannabis dependence, or may not play a major role in mediating the behavioral effects, which would be primarily mediated by the endocannabinoid system. Alternatively, this lack of alteration may be related to the low severity of dependence in our group, with an estimated average of 517 puffs per month (1–2 puffs daily, 5 times per week, for most participants; up to 4 blunts daily in one participant only). We should note here that we did not collect information on the strength of cannabis used by individual participants, but only frequency and amount of use. It is well known that cannabis preparations may vary in potency depending on plant strains, -parts, and preparation [41]. Most of the participants who underwent PET scanning had no difficulty in remaining abstinent on an outpatient basis and without any further therapeutic interventions. The majority of those able to detoxify within less than three months did not relapse into marijuana use after enrollment (11 of 17 participants originally scanned), and only one participant relapsed several times, requiring more than twice as long as the group average of 4.1 weeks to detoxify from cannabis. This time frame corresponds to the average time cannabis metabolites remain detectable after cessation in regular users [26]. However, 21 % of the initially enrolled participants were disqualified from the study due to their inability to abstain from cannabis use at all. These cases presented with more severe use (2628 estimated puffs per month on average, between 4 and 10 blunts per day and generally daily use) and likely more severe dependence than the participants we scanned. Severe cannabis dependence has a similarly high relapse rate and significant withdrawal symptoms comparable to other drugs of abuse [2, 42]. It is possible that more severe use could be associated with alterations in the DA system similar to what is seen in other addictions. Stimulant dependent participants with lower dopamine D2/3 receptor availability and lower dopamine transmission are more likely to relapse into drug use than those with higher dopamine activity [43, 44], and in our study we may have excluded the former, possibly accounting for the absence of a group difference. A study of heavier users is warranted to confirm absence of dopaminergic alterations in the more severe stages of cannabis dependence.
Another possible explanation for our negative results is that differences in striatal DA transmission may have resolved by the time we performed the study in the recent abstinence phase. A reversal or normalization within a few weeks of abstinence was recently reported for the CB1 receptor in cannabis users, using the novel CB1 receptor-selective radioligand [18F]FMPEP-d2. Chronic heavy users showed reduction of CB1 receptor binding in most brain regions that began to reverse after 4 weeks of abstinence [45]. Also cognitive impairment found in users immediately after cannabis cessation resolved and showed no difference compared to healthy controls after 28 days of monitored abstinence [46], suggesting that the impact of THC on DA might have attenuated as well. However, the absence of a relationship between release and duration of abstinence is not in support of this explanation.
Association with age of onset of cannabis use
Despite the absence of measurable overall differences in parameters of DA transmission, we found a moderate positive association between DA release following amphetamine administration and age of onset of drug use: the earlier participants started smoking marijuana, the lower their DA release. This was most noticeable in subregions of the associative striatum (AST).
Since age is associated with a decrease in DA transmission [35], we controlled for age, and found that the relationship weakened, but survived at the level of the AST. However, it was not possible to separate age of onset from duration of cannabis use: earlier use, or longer duration of use, may both be associated with similar effects as in other addictions, i.e, blunting of DA release. While it has been demonstrated that for example decreased frontal glucose metabolism in chronic cocaine users correlates with the duration of drug use [47], further studies are needed to disentangle these two factors. The effect of cannabis use during adolescence is of great interest to public health as experimentation with marijuana generally starts during adolescence. By 12th grade, more than 50% of youths have tried marijuana and more than 6% report daily use [48]. Studies in adolescent users regularly find more pronounced detrimental effects on neurocognitive functioning than in adults [9]. Recent functional imaging research suggests that heavy long-term use, particularly with adolescent onset, may also lead to abnormalities in brain structure [8].
The association with age of onset in AST rather than VST is unexpected. The AST serves to integrate inputs from orbito-frontal and dorsolateral prefrontal cortex [49]. We could speculate that alterations of DA in this area, presumably involving THC-induced DA release acutely and chronic blunting, may partly explain the psychopathological effects reported with cannabis use, encompassing emotional and cognitive domains.
Conclusion
Chronic cannabis abuse or dependence, of mild severity, is not associated with the same magnitude of DA alterations observed in other addictions. Adolescent onset of cannabis use and duration of use, however, may have an impact on the dopaminergic system especially in the AST, an area that has emerged as the main area of dopaminergic dysregulation in psychosis, and a younger age of onset of cannabis use is associated with a higher risk for developing schizophrenia. Studies focusing on use in adolescence are needed to further clarify the effects of cannabis use during adolescence on brain structure, function and behavior.
Acknowledgments
The authors acknowledge the excellent technical assistance of Elizabeth Hackett, Sung-A Bae, Felipe Castillo and John Castrillon.
Funded by the National Institute on Drug Abuse (R01: DA022455).
Footnotes
Financial disclosures: M. Slifstein: Amgen, Glaxo-Smith-Kline (consultant); R. Girgis: Lilly Pharmaceuticals (research support); M. Haney: Celtic Pharmaceuticals, Teva Pharmaceuticals, GW Pharmaceuticals (consultant), Bristol-Meyers Squibb (research support); A. Abi-Dargham: Bristol-Myers Squibb-Otsuka (consultant and speaker), Bohringer-Engelheim (consultant), GlaxoSmithKline (research support). All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMHSA; O.o.A. Studies. Summary of National Findings. I. NSDUH; Rockville, MD: 2010. Results from the 2009 National Survey on Drug Use and Health. [Google Scholar]
- 2.Budney AJ, et al. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 3.Troisi A, et al. Psychiatric symptoms in male cannabis users not using other illicit drugs. Addiction. 1998;93(4):487–92. doi: 10.1046/j.1360-0443.1998.9344874.x. [DOI] [PubMed] [Google Scholar]
- 4.Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–14. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- 5.Moore TH, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–28. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 6.Henquet C, et al. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008;34(6):1111–21. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pistis M, et al. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56(2):86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Yucel M, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 9.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaoni YMR. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 11.French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226(3):159–62. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- 12.Gessa GL, et al. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 13.Ng Cheong Ton JM, et al. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451(1–2):59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen JP, et al. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102(2):156–62. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 15.Fadda P, et al. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17(15):1629–32. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- 16.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83(3):1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 17.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17(14):5327–33. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voruganti LN, et al. Cannabis induced dopamine release: an in-vivo SPECT study. Psychiatry Res. 2001;107(3):173–7. doi: 10.1016/s0925-4927(01)00104-4. [DOI] [PubMed] [Google Scholar]
- 19.Bossong MG, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–66. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 20.Stokes PR, et al. Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage. 2009;48(1):186–90. doi: 10.1016/j.neuroimage.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Barkus E, et al. Does intravenous {triangleup}9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol. 2010 doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- 22.Martinez D, Narendran R. Imaging neurotransmitter release by drugs of abuse. Curr Top Behav Neurosci. 2010;3:219–45. doi: 10.1007/7854_2009_34. [DOI] [PubMed] [Google Scholar]
- 23.Martinez D, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–86. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Martinez D, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–9. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 26.Ellis GM, Jr, et al. Excretion patterns of cannabinoid metabolites after last use in a group of chronic users. Clin Pharmacol Ther. 1985;38(5):572–8. doi: 10.1038/clpt.1985.226. [DOI] [PubMed] [Google Scholar]
- 27.Hasin DS, et al. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153(9):1195–201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 28.Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. [Google Scholar]
- 29.Maisto SA, et al. Comparison of two techniques to obtain retrospective reports of drinking behavior from alcohol abusers. Addict Behav. 1982;7(1):33–8. doi: 10.1016/0306-4603(82)90022-3. [DOI] [PubMed] [Google Scholar]
- 30.Gray KM, Watson NL, Christie DK. Challenges in quantifying marijuana use. Am J Addict. 2009;18(2):178–9. doi: 10.1080/10550490902772579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44(3):215–24. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- 33.Mawlawi O, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 35.Abi-Dargham A, et al. Dopamine mediation of positive reinforcing effects of amphetamine in stimulant naive healthy volunteers: results from a large cohort. Eur Neuropsychopharmacol. 2003;13(6):459–68. doi: 10.1016/j.euroneuro.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 36.van der Gaag M, et al. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 2006;85(1–3):280–7. doi: 10.1016/j.schres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Sevy S, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197(4):549–56. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laruelle M, et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17(3):162–74. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 39.Narendran R, et al. Measurement of in vivo affinity of [11C]NPA and the proportion of D2 receptors configured in agonist high affinity state (%Rhigh) in baboons using PET. Neuroimage. 2004;22:T19–T20. [Google Scholar]
- 40.Urban NB, et al. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry. 2010;68(8):689–96. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NIDA; U.S.D.O.H.A.H. SERVICES. Marijuana: Facts parents need to know. 2011. rev. [Google Scholar]
- 42.Kadden RM, et al. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32(6):1220–36. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang GJ, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez D, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168(6):634–41. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirvonen J, et al. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pope HG, Jr, et al. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42(11 Suppl):41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 48.Johnston LD, et al. N.I.o.D. Abuse. The Monitoring the Future national survey results on adolescent drug use: Overview of key findings, 2009. Bethesda, MD: 2010. [Google Scholar]
- 49.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20(6):2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]