Abstract
A rigorously controlled, cell culture paradigm was used to assess the role of HIV-1 gp120 ± morphine in mediating opioid-HIV interactive toxicity in striatal neurons. Computerized time-lapse microscopy tracked the fate of individual neurons co-cultured with mixed-glia from mouse striata during opioid and gp120 exposure. Subpopulations of neurons and astroglia displayed μ-opioid receptor, CXCR4, and CCR5 immunoreactivity. While gp120 alone was or tended to be neurotoxic irrespective of whether X4-tropic gp120IIIB, R5-tropic gp120ADA, or dual-tropic gp120MN was administered, interactive toxicity with morphine differed depending on HIV-1 strain. For example, morphine only transiently exacerbated gp120IIIB-induced neuronal death; however, in combination with gp120MN, morphine caused sustained increases in the rate of neuronal death compared to gp120MN alone that were prevented by naloxone. Alternatively, gp120ADA significantly increased the rate of neuron death, which was unaffected by morphine. The transient neurotoxic interactions between morphine and gp120IIIB were abrogated in the absence of glia suggesting that glia contribute significantly to the interactive pathology with chronic opiate abuse and neuroAIDS. To assess how mixed-glia might contribute to the neurotoxicity, the effects of morphine and/or gp120 on the production of reactive oxygen species (ROS) and on glutamate buffering were examined. All gp120 variants, and to a lesser extent morphine, increased ROS and/or decreased glutamate buffering, but together failed to show any interaction with morphine. Our findings indicate that HIV-1 strain-specific differences in gp120 are critical determinants in shaping both the timing and pattern of neurotoxic interactions with opioid drugs.
Keywords: neuro-acquired immunodeficiency syndrome (neuroAIDS), opiate drug abuse, oxidative stress, regulated cell death, CXCR4, CCR5, astroglia, microglia, basal ganglia
Introduction
Aberrant glia-neuron signaling is revealed in the pathogenesis of neuro-acquired immunodeficiency syndrome (neuroAIDS) in human immunodeficiency virus type-1 (HIV-1) infected individuals, which is underscored by neuronal injury or death secondary to an infection that is largely restricted to glia. Infected microglia, and to a lesser extent reactive astroglia (Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005), release products that can be broadly defined as “virotoxins” consisting of toxic viral proteins and inflammatory “cellular toxins” (Nath, 2002). Glial-derived cellular toxins include cytokines such as TNF-α, IL-1β and IL-6, excess glutamate (Wang et al., 2003), platelet activating factor (Bellizzi et al., 2005), and reactive oxygen (ROS) or nitrogen species (RNS). Within the confined extracellular space of the central nervous system (CNS) (Sykova, 2005), glial-derived viral proteins and cellular toxins establish deleterious autocrine/paracrine neurochemical feedback loops, spiraling inflammation, and reactive gliosis, the combination of which inevitably leads to neuronal injury and death (Navia et al., 1986; Gonzalez-Scarano and Martin-Garcia, 2005; Ellis et al., 2007; McArthur et al., 2010). While astroglia contribute less than microglia to the production of new virus, astroglia are significant sources of cellular toxins (Benos et al., 1994a; Conant et al., 1998; Nath et al., 1999; Kramer-Hammerle et al., 2005), and are key sites where opiates exacerbate the CNS effects of HIV-1 (El-Hage et al., 2005; Hauser et al., 2007; El-Hage et al., 2008b; Zou et al., 2011b).
Not only does opioid drug abuse spread the virus through needle sharing or exchange of sex for drugs, but opioid drug use appears to modify HIV-1 pathogenesis through direct interactions with the immune system (Adler et al., 1993; Peterson et al., 1998; Donahoe and Vlahov, 1998). More recently, we and others have proposed that opioids can selectively exacerbate the CNS consequences of HIV-1 infection (Bell et al., 1998; Nath et al., 2000; Gurwell et al., 2001; Nath et al., 2002; Kumar et al., 2004; Hauser et al., 2005; Bell et al., 2006; Turchan-Cholewo et al., 2006; Anthony et al., 2008; Banerjee et al., 2011). Despite in vitro evidence that opioid abuse per se worsens the neurotoxicity, identifying the cellular and molecular sites where opioids first trigger the damage is challenging. Neurons, astroglia, and microglia can all possess μ opioid receptors (MOR), and activating MOR in each cell type appears to modify the disease process uniquely (Peterson et al., 1990; Chao et al., 1994; Peterson et al., 1998; El-Hage et al., 2005; Hu et al., 2005; Turchan-Cholewo et al., 2006; Zou et al., 2011b). To add to the complexity, opioids appear to interact with each viral protein differently (Hauser et al., 2005; Hauser et al., 2007). In the case of gp120, opioids potentiate gp120-induced cytokine release and/or receptor expression from an astroglial-derived cell line (Mahajan et al., 2002; Mahajan et al., 2005) and exacerbate gp120 toxicity in human neurons (Hu et al., 2005). Morphine withdrawal disrupts normal homeostatic synaptic repair mechanisms in gp120-expressing transgenic mice (Bandaru et al., 2011). Lastly, the viral proteins themselves appear to intrinsically modulate opioid receptor expression by astroglia and microglia (Turchan-Cholewo et al., 2008), and throughout the CNS (Fitting et al., 2010), while morphine alone can alter the expression of HIV-1 co-receptors (Steele et al., 2003), suggesting a complex interplay of drug and viral actions in glia.
Gp120 can be directly or indirectly neurotoxic through a variety of mechanisms. Soluble gp120 can interact with CXCR4 or CCR5 chemokine receptors in human and murine neural cells (Meucci et al., 1998; Kaul et al., 2007), via CD4-dependent or -independent mechanisms to induce CNS toxicity and inflammation (Jordan et al., 1991; Moore, 1997; Kaul and Lipton, 1999; Khan et al., 2004; Misse et al., 2005; Kaul et al., 2007). Although intrinsically neurotoxic, gp120 activates/overactivates macrophages/microglia imparting a generalized state of excitotoxicity (Dreyer et al., 1990; Lipton et al., 1991; Kaul et al., 2007). In addition, gp120 can disrupt ion homeostasis in astroglia, which likely compromises neuron function (Pulliam et al., 1993; Benos et al., 1994a; Benos et al., 1994b; Holden et al., 1999). Furthermore, gp120 can inhibit glutamate uptake by astrocytes through reductions in excitatory amino acid transporter-2 (EAAT-2) mRNA and protein levels (Wang et al., 2003). Less is known about how opioids might exacerbate neuroAIDS through interactions with gp120.
Recently, we found basic differences in morphine interactions with X4 versus R5-tropic strains of HIV-1 in an infectious model of hepatitis C virus (El-Hage et al., 2011). Prompted by these findings, we questioned whether morphine would interact with HIV-1 gp120 to cause neurotoxicity in a strain-dependent manner and posed this hypothesis in the present study. Moreover, influenced by new findings that glia largely mediate morphine and HIV-1 Tat interactive neurotoxicity (Zou et al., 2011b), the studies herein begin to consider whether glia might play a similar role in mediating morphine and HIV-1 gp120 neurotoxic interactions in the CNS.
Methods
Mixed-glial cultures
Mixed glial cultures (astroglia and microglia) were prepared from P0-P1 ICR (CD-1) mouse striata (Charles River Co., Wilmington, MA). Striata were dissected, minced, and incubated with 10 ml trypsin (2.5 mg/ml) and DNase (0.015 mg/ml) in serum free medium (30 min, 37 °C). Tissue was triturated, resuspended in culture medium with 10% fetal bovine serum (FBS), filtered through 135 µm and then 45-µm pore nylon mesh, prior to plating at moderate density (~7.5 × 105 cells/cm2) on poly-l-lysine coated cell culture plates. The cultures contained 8.8 ± 0.6% microglia, 90.2 ± 0.4% astroglia, and less than 0.2% each of neurons, oligodendroglia, and glial precursors as previously described for our striatal cultures (Zou et al., 2011b).
Neuron cultures: neuron-enriched or co-cultured with mixed glia
Striatal neurons were prepared from E15-E16 ICR (CD-1) mouse embryos. Striata were dissected, minced, and incubated with trypsin (2.5 mg/ml) and DNase (0.015 mg/ml) in Neurobasal Medium with 25 µM glutamate (30 min, 37 °C). Tissue was triturated, resuspended, and cells filtered through 70 µm pore nylon mesh. Cultures were established in which (i) isolated neurons were plated alone on poly-l-lysine coated coverslips, or (ii) neurons were co-cultured directly on a bedlayer of mixed-glia. Neuron cultures and neuron-glia co-cultures were similarly maintained in neurobasal media supplemented with B27 (Invitrogen, Carlsbad, CA), 0.5 mM L-glutamine, 0.025 mM glutamate, and allowed to mature for about 1 wk prior to the start of experiments.
Opioid and HIV-1 gp120 treatments
Cultures were treated with X4-tropic gp120 HIV-1IIIB, R5-tropic HIV-1ADA, or dual-tropic gp120MN (500 pM; ImmunoDiagnostics, Woburn, MA); morphine sulfate (500 nM; Sigma, St. Louis, MO); and/or (−)naloxone (1.5 µM, Sigma). The concentration of gp120 was chosen for its significant induction of caspase-3 induced neuronal death (Singh et al., 2004), while the morphine concentration used is able to fully activate MOR and synergistically enhance HIV-1 Tat mediated neurotoxicity (Gurwell et al., 2001; Zou et al., 2011b). Multiple treatments without naloxone were applied simultaneously. Naloxone was added 1 h before morphine when treatments contained both drugs.
MOR, CXCR4, and CCR5 immunofluorescence
Striatal neurons or astrocytes were fixed for 15 min in 4% paraformaldehyde, permeabilized for 15 min in 0.1% Triton-X 100 and 0.1% BSA, and subsequently blocked for 1 h at room temperature in PBS supplemented with 0.1% BSA and 1% horse serum. Dual-labeling was performed for 2 h at room temperature using rabbit antisera to MOR (1:2500; Antibodies Incorporated, Davis, CA, 61–204), mouse anti-CXCR4 (1:100; Abcam, Cambridge, MA; ab21555), or mouse anti-CCR5 (1:100; BD Pharmingen, San Diego, CA; 555991), co-localized with antibodies to either anti-microtubule associated protein-2 (MAP2; 1:1000; Millipore, Temecula, CA; MAB378 (mouse) or Abcam; ab32454 (rabbit)), or anti-glial fibrillary acidic protein (GFAP; 1:1000; Millipore; MAB360 (mouse) or AB5804 (rabbit)). Goat anti-rabbit Alexa-488, donkey anti-mouse Alexa-594, goat anti-rabbit Alexa-594 and/or goat anti-mouse Alexa-488 (Invitrogen; Eugene, OR) secondary antibodies were applied, depending on the primary antibodies, for 1 h at room temperature, followed by counterstaining for 20 min with 0.5 µg/ml Hoechst 33342 (Invitrogen), which labels DNA and identifies all cell nuclei. Coverslips were mounted in ProLong Gold Antifade reagent (Invitrogen). Cells were visualized and digital images were acquired using a Zeiss LSM 700 confocal module configured to a Axio Observer Z.1 and Zen 2010 software (Zeiss Inc., Thornwood, NY).
Assessment of neuron viability
Time-lapse digital images of cells were recorded at 20 min intervals for 60 h, using a microscope and automated, computer-controlled stage encoder with environmental control (37 °C, 95% humidity, 5% CO2) that allowed us to repeatedly track the same neurons (Axio Observer Z.1, Zeiss). Neurons possessed an ovoid cell body, extensive nuclear euchromatin, discrete nucleoli, and well-defined dendritic arbor, which was confirmed in some instances by the neuronal markers NeuN and MAP2. Repeated measures ANOVA was used to assess the effect of treatment on the survival of individual neurons followed throughout the course of the 60 h experiment (percentage of pretreatment value) (Statistica, StatSoft, Tulsa, OK). In each experiment, treatments were distributed across cells pooled from the same pups. Approximately 50 healthy neurons with well-defined dendritic and axonal arbors were identified within ≥ 8 overlapping fields (40-× magnification; MosaiX module, Zeiss AxioVision 4.6) within individual culture wells in each experiment prior to treatment (0 h). Using a computerized microscope stage encoder and tracking software (Zeiss, AxioVision 4.6; Mark&Find, MosaiX, and Time Lapse modules), the same neurons were repeatedly observed at 20 min intervals (Fig. 1), and the effect of gp120 and/or opioids on the proportion of surviving neurons determined at 4 h intervals and averaged per culture well/treatment (n = 1). Neuron death was monitored in individual cells and defined by rigorous criteria (see Fig. 1), which over time included the dissolution of Nissl substance, transient cytoplasmic swelling and vacuolization, nuclear destruction or pyknosis, and eventual destruction of the cell body (Singh et al., 2004; Singh et al., 2005; Bakalkin et al., 2010; Zou et al., 2011b). In some cases, neuron death was confirmed by viability markers such as ethidium homodimer or trypan blue (not shown); however, these markers all possess some inherent cytotoxicity with prolonged exposure, which precludes their use in experiments wherein cells are monitored continuously. The effect of each treatment on neuron survival was analyzed statistically at 4 h intervals using a repeated measures ANOVA from n = 3–7 experiments (250–350 total neurons per treatment) and reported as mean neuron survival ± the standard error of the mean (SEM).
Figure 1.
Cellular localization of CXCR4, CCR5, and/or MOR immunofluorescence in striatal neurons and astrocytes. Receptors were colocalized in neurons (a–c) or astrocytes (d–f), respectively, using antibodies against MAP2 or GFAP; cells were counterstained with Hoechst 33342 (blue). In neurons, CXCR4 (a), CCR5 (b), and MOR (c) immunoreactivity was associated with the cell body and dendrites, while CCR5 antigenicity appeared to extend into some axons (b). In GFAP-immunolabeled astrocytes, CXCR4 (d), CCR5 (e), and MOR (f) displayed a punctate pattern of labeling with some faint reactivity extending into the cell processes (d–f); scale bar = 10 µm.
Astroglial ROS
ROS was assessed using the cell-permeant dye 2’,7’-dichlorofluorescein diacetate (DCF-DA), which is hydrolyzed in the cell to DCF and fluoresces when in contact with ROS (such as OH, ONOO−, and H2O2). Measurements were performed on a Victor 3 platereader (Perkin Elmer, Waltham, MA) at λex = 488 nm and λem = 525 nm after 90 min treatments. To assess the nature of the production of ROS, pharmacological inhibitors were used. N-acetylcysteine (NAC) acts as a precursor to glutathione, which quenches reactive species; while diphenyleneiodonium chloride (DPI) reportedly inhibits NADPH oxidase, thereby reducing the conversion of O2 to superoxide (O2−•) (Williams and Griendling, 2007).
Glutamate measurements in mixed-glial cultures
Glutamate uptake was measured in confluent mixed glial cultures as previously described (Zou et al., 2011b). Briefly, adherent cells were washed once with 0.5 ml/well of 37 °C Na+-free medium containing 10 mM Hepes, 5 mM D-glucose (pH 7.4) and supplemented with 10% fetal bovine serum (FBS). Prior to assay, the cells were pre-incubated for 45–60 min at 37 °C in 500 µl of Na+-free Hank’s balanced salt solution (HBSS) alone or buffer with 500 nM morphine and/or 500 pM gp120MN. Glutamate was added to each well to a final concentration of 1.0 mM (Sigma). Sample medium was removed from individual wells at 0, 15, 30, 45, 60, 120 and 240 min. Glutamate concentration was determined using a colorimetric detection kit (BioVision, Mountain View, CA) according to the manufacturer's instructions. Absorbance was assessed at 450 nM on a Victor 3 plate reader (Perkin-Elmer, Waltham, MA).
Statistical analyses
Statistical analyses were done by repeated measures, one-way, and/or two-way analysis of variance (ANOVA) followed by Duncan’s post-hoc testing (StatSoft, Statistica, Tulsa, OK). In the time-lapse microscopy (survival) studies, the advantage of using a repeated measures (block randomized) design is that individual neurons are compared to themselves prior to and during treatment. Since the design eliminates inter-subject variability, it is a fundamentally more sensitive approach to examining cell responses. Comparing proportional changes in the same neuron before and during treatment permits the use of repeated measures ANOVA rather than regular multi-way ANOVA, and is more sensitive than statistical assessment across cell populations (Bakalkin et al., 2010; Zou et al., 2011b). For the neuron survival and glutamate determinations, we first examined for main effects of treatment, time, and/or the presence of glia, using a repeated measures ANOVA. When interaction effects were significant (p < 0.05), treatment effects and temporal differences were further analyzed by ANOVA and/or post hoc testing.
Results
MOR and chemokine receptor immunoreactivity on neurons and glia
MOR, CXCR4, and CCR5 immunofluorescence was colocalized in untreated striatal neurons and astrocytes by confocal microscopy to assess the extent to which the principal molecular targets of opioid drugs and gp120 were present and their cellular distribution in each cell type. Receptors were colocalized in neurons (Fig. 1a–c) or astrocytes (Fig. 1d–f), respectively, using antibodies against MAP2 or GFAP; cells were counterstained with Hoechst 33342. In neurons, CXCR4, CCR5, and MOR immunoreactivity was associated with the cell body and dendrites, while CCR5 immunofluorescence may continue into the axolemma (Fig. 1a–c). The proportion of CXCR4 and CCR5 immunoreactive neurons was 69.3 ± 0.7% and 77.4 ± 1.7%, respectively, which also infers that a subset of neurons co-express both chemokine receptors. Astrocytes tended to display punctate patterns of CXCR4, CCR5, and MOR immunolabeling (Fig. 1d–f); faint, diffuse receptor reactivity was occasionally evident at the cell membrane (e.g., CCR5 in Fig. 1e). The proportion of CXCR4 and CCR5 immunofluorescent astrocytes was 45.2 ± 1.6% and 43.8 ± 2.4%, respectively.
Neurotoxicity induced by gp120IIIB and morphine
Analyzing the response of individual neurons repeatedly throughout the experiment eliminates inter-subject variability and permits subtle treatment effects to be revealed through a repeated measures ANOVA analysis (Singh et al., 2004; Singh et al., 2005; Bakalkin et al., 2010; Zou et al., 2011b). The use of computer-aided, near-continuous tracking of large numbers of neurons (and glia) greatly facilitates this task, while increasing the sensitivity of the assay (Fig. 2). This strategy has been used by us to examine neuroprotective effects of silencing the phosphatase and tensin homolog on chromosome 10 (PTEN) on HIV-1 gp120IIIB-induced neurotoxicity (Zou et al., 2011a), to demonstrate the role of glia in morphine-induced exacerbation of Tat neurotoxicity (Zou et al., 2011b), and to show that mutations in dynorphin A underlie spinocerebellar ataxia type 23 (Bakalkin et al., 2010). In the present work, tracking the responses of individual neurons has revealed sometimes subtle differences in the cytotoxic effects of gp120 from different HIV-1 strains, as well as strain-specific gp120 interactions with morphine.
Figure 2.
Phase-contrast images showing time-lapse tracking of neuronal injury and death in the same neurons before and during exposure to vehicle-control medium (a), morphine (b), and/or gp120IIIB (c). Although there are some small changes in the morphology of cells in (a) and (b), there is no neuron death over this 16 h time course. A neuron that dies over the same time course during gp120IIIB treatment is followed in (c). Neuron death is often preceded by the systematic loss/degeneration of neurites (dendrite pruning; arrowheads) over a prolonged period. Death per se occurs more rapidly, as denoted here by a slight swelling and some loss of birefringence (arrow; 16 h) and DNA fragmentation thereafter (24 h and 60 h). Neuron death can be confirmed by annexin V reactivity, and the inability to exclude viability markers such as ethidium homodimer, ethidium monoazide, or trypan blue (see text). Some neurons remain viable despite morphine and gp120 co-exposure (open arrowheads “□” at 0 h and 60 h) (c); some surrounding cells, including astroglia, immature glial precursors and macrophages/microglia, are motile and can display sporadic movement, as indicated by the progenitor which moves to the top of the field between 7 h–8 h (hatched arrow) (c). Scale bar = 20 µm.
Not surprisingly, gp120IIIB alone was toxic to striatal neurons (Figs. 2; 3a–b) as previously published by our lab (Singh et al., 2004; Singh et al., 2005), which is in agreement with its neurotoxic effects in neurons from other brain regions (Meucci and Miller, 1996; Digicaylioglu et al., 2004; Kaul et al., 2007; Robinson et al., 2007). Moreover, exposure to gp120IIIB was neurotoxic irrespective of whether glia were present or absent, although there was a slight trend toward greater relative neuron losses (compared to vehicle-treated controls) when neurons were co-cultured with glia. Our analysis also revealed modest background losses of neurons in control cultures treated with vehicle alone during 60 h. When neurons were cultured in isolation, morphine did not increase neuron death beyond levels seen in controls (Fig. 3a), while in the presence of glia, morphine alone did cause significant neuron death (compare Fig 3b to 3a). Thus, our findings that morphine and Tat neurotoxic interactions are mediated by glia (Zou et al., 2011b), may also pertain to morphine and gp120, suggesting a broad role for glia in mediating opiate neurotoxicity. However, as this study’s aim was to assess strain differences in gp120-morphine interactions, and because morphine did not consistently show interactive neurotoxicity with some gp120 strain variants, the role of glia was not systematically assessed against gp120ADA or gp120MN.
Figure 3.
Time-dependent effects of morphine and/or HIV-1 gp120IIIB on the survival of medium spiny striatal neurons cultured alone (a) or with a glial bedlayer (b). There were main effects of both time and treatment, as well as a significant interaction effect (time × treatment) (a), which were further examined using Duncan’s post-hoc analysis. Exposure to gp120IIIB ± morphine was intrinsically neurotoxic, irrespective of whether neurons are cultured ± glia (a–b) (*P < 0.05 vs. control) (a). Morphine transiently and significantly accelerates gp120IIIB neurotoxicity above control levels, but only when the neurons are co-cultured with glia (**P < 0.05 vs. control, but not morphine or gp120 treatment, at 12 h; n = 6 experiments) (b). Concurrent exposure to naloxone (Nal) negated the combined neurotoxic effects of morphine and gp120IIIB at 12 h (b). Interestingly, morphine alone displayed modest, but significant neurotoxicity, but again, only in the presence of high-density glia (*P < 0.05 vs. control) (b). Neuron death is reported as the percentage dying relative to pretreatment numbers; data are the mean number of surviving neurons ± SEM from n = 4–6 experiments.
When morphine was combined with gp120IIIB in the presence of glia, it potentiated the neurotoxic effects of gp120. This interaction, although highly significant, was transient in nature, consisting of accelerated neuronal losses at both 12 h and 16 h following continuous exposure to both agents. Since neither gp120IIIB nor morphine by itself was toxic at 12 h or 16 h, the interaction was noteworthy. The mechanisms by which morphine exacerbates gp120 neurotoxicity are uncertain, but clearly they reside in either the astroglia, the microglia, or in some interactive crosstalk that occurs between these glial types since exacerbation was not observed when glia were absent (Fig. 3b). The interactive morphine and gp120-induced neurotoxicity was prevented by concurrent treatment with naloxone, suggesting the effect is mediated by opioid receptor-expressing glia (Fig. 3b), while naloxone alone has been previously shown not to effect on striatal neuronal survival in this co-culture system (Gurwell et al., 2001; Zou et al., 2011b). Finally, exposure to denatured or deglycosylated gp120 (500 pM; data not shown) was not neurotoxic suggesting the toxic effects of gp120 were selective as previously published (Singh et al., 2004; Singh et al., 2005).
Neurotoxicity induced by gp120ADA and gp120MN, and morphine
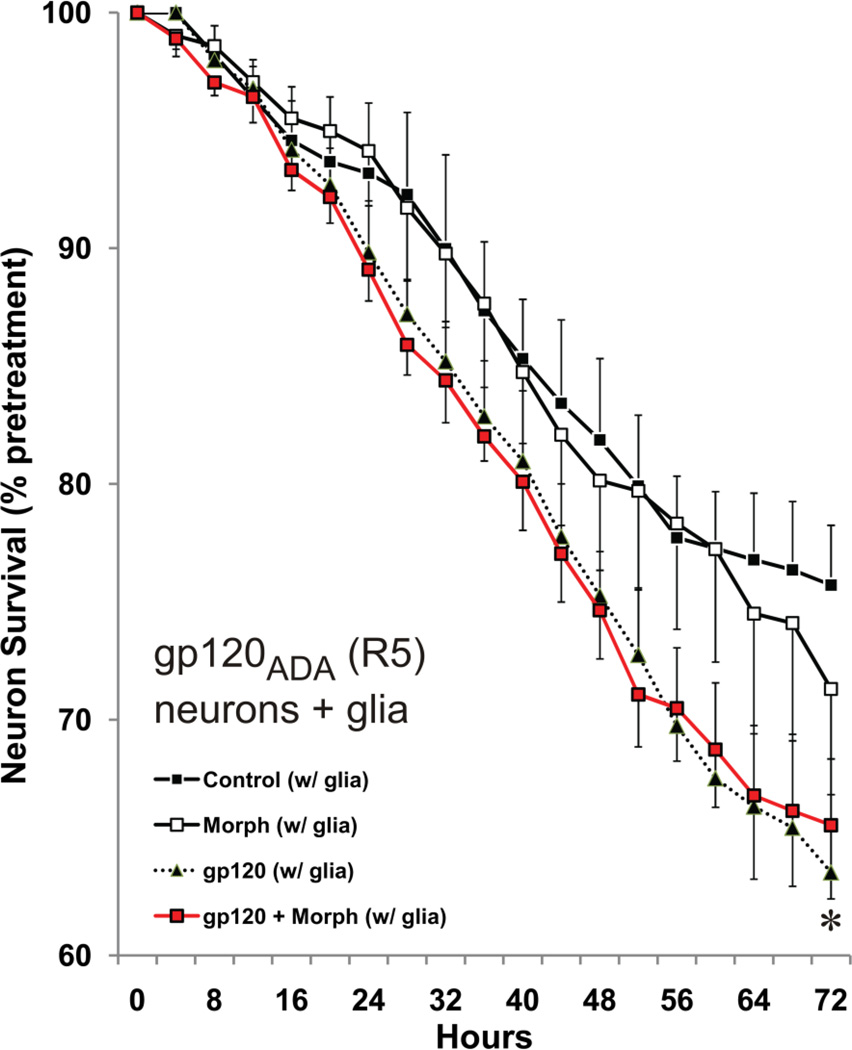
To assess whether opiate-gp120 interactions were influenced by X4 versus R5 tropism, the effects of gp120ADA, a R5-tropic strain, and gp120MN, a dual-tropic strain, were tested in the co-culture paradigm. Similar to gp120IIIB, gp120ADA (Fig. 4) and gp120MN (Fig. 5a) alone were toxic to neurons in the presence of glia, although gp120ADA was less toxic displaying significant increases in the rate of neuron death (time × treatment) compared to controls rather than mean differences at any particular time (Fig. 4). In contrast to a very transient exacerbation of gp120IIIB toxicity by morphine, morphine failed in enhance gp120ADA-induced cell death Fig. 4). Interestingly, a significant exacerbation of gp120MN toxicity was apparent at both early and later phases of the experiment (Fig. 5a). Lastly, the effects of morphine were reversed by naloxone (Fig. 5a).
Figure 4.
Time-dependent effects of exposure to morphine (Morph) and/or R5-tropic HIV-1 gp120ADA in neuron and mixed-glial co-cultures for 72 h. Analyses for R5-tropic gp120ADA showed a main effect for time, but not for treatment (a). Although there was a significant interaction effect (time × treatment), Duncan’s post-hoc testing did not reveal significance at particular times. When the analysis was simplified by eliminating the morphine group, there was decline in survival for R5-tropic gp120ADA exposed neurons at the last time point of assessment (*P < 0.05 vs. control at 72 h) (a). Otherwise, values for gp120ADA treatment alone vs. control (e.g., P = 0.056 at 68 h) or gp120ADA + morphine (e.g., P = 0.054 at 56 h) were close, but not significant; data are the mean number of surviving neurons ± SEM from n = 4–6 experiments.
Figure 5.
Time-dependent effects of exposure to morphine (Morph), X4/R5-tropic HIV-1 gp120MN and/or naloxone (Nal) in neuron and mixed-glial co-cultures for 72 h (a). There were significant time and treatment effects for dual-tropic gp120MN, as well as a significant interaction effect. Post-hoc analyses showed that all treatment groups were different from control values starting at 20 h following continuous exposure, except for morphine whose effects began at 40 h (*P < 0.05 vs. control). Additionally, morphine co-treatment enhanced the effects of gp120MN at both early time points (20–28 h), and at 56 h and thereafter (¶P ≤ 0.05), and the effects of morphine were prevented by naloxone (Nal) (#P ≤ 0.05 vs. morphine + gp120MN; *P < 0.05 vs. control). Unlike gp120ADA, co-administering morphine exacerbated the neurotoxicity of gp120MN suggesting that morphine interacts differently with gp120 variants from different HIV-1 strains. Data are the mean number of surviving neurons ± SEM from n = 4–6 experiments (a). Effects of morphine (500 nM) and/or dual-tropic gp120MN (500 pM) on glutamate buffering (b). Mixed-glial cultures were challenged with 1 mM glutamate and the levels were assessed at 0, 15, 30, 45, 60, 120 and 240 min. Exogenously administered glutamate was rapidly depleted during the first 60 min and continued to slowly decrease thereafter in untreated controls. gp120MN treated cultures ± morphine, demonstrated a significant deficit in the ability to uptake glutamate beginning at 45 min which persisted throughout the experiment. Values indicate the amount of glutamate (mM) remaining in the media over the 240 min time course ± SEM of n = 3 experiments (*P < 0.05 vs. control; §P < 0.05 control vs. gp120 alone or morphine + gp120; ¶P < 0.05 gp120 vs. morphine + gp120; ‡P < 0.05 control vs. gp120 alone) (b).
Viral protein effects on glutamate uptake
To explore whether morphine and/or gp120 might restrict the ability of astroglia within the mixed-glial cultures to buffer glutamate, an exogenous L-glutamate challenge was applied and residual glutamate was measured in the medium over 240 min (Fig. 5b). Gp120MN showed the greatest neurotoxic interaction with morphine, and was therefore anticipated to interact with morphine to affect extracellular glutamate. Extracellular glutamate was rapidly depleted during the first 45 min in untreated controls, but glutamate buffering was significantly attenuated in morphine, gp120, or morphine plus gp120-treated mixed-glial cultures at 45 min, or gp120 and/or morphine plus gp120 exposed cultures thereafter (Fig. 5b). Note that in a previous study two different inhibitors of the major glial EAAT1 and EAAT2 transporters completely blocked glutamate uptake (Zou et al., 2011b). Moreover, in the presence of the EAAT inhibitors, glutamate levels remained at pretreatment levels with no measurable glutamate release over 240 min irrespective of Tat and morphine exposure (Zou et al., 2011b). This suggests that net levels of glutamate in the medium in the present study reflect morphine and gp120-induced changes in glutamate transporter function and not glutamate release.
Effects of morphine and gp120IIIB on astroglial ROS
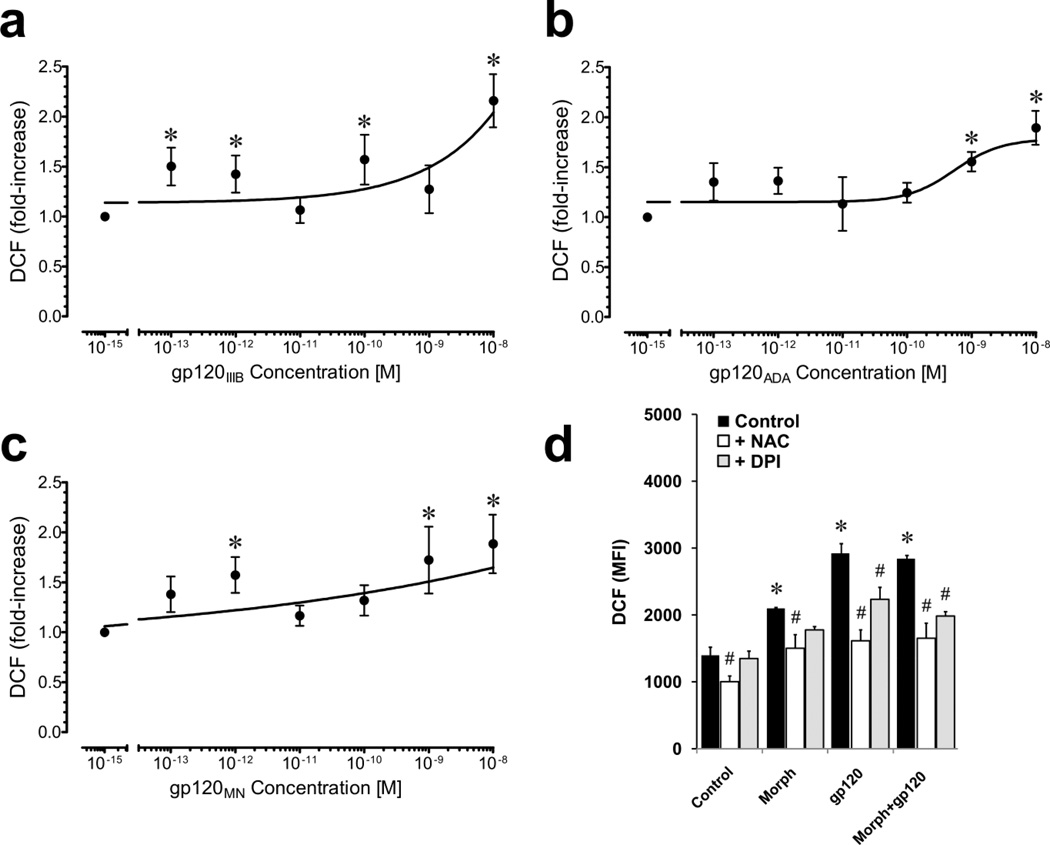
The effects of gp120 from different HIV-1 strains on ROS have not been systematically explored in our neuron mixed-glial co-culture system. Moreover, differences in gp120 neurotoxicity provided impetus to further evaluate the relationship between gp120 concentration, viral strain, and ROS (Fig. 6a–c). Morphine has previously been shown to cause concentration-dependent increases in ROS in an identical mixed-glial striatal culture model as used in the present study (Zou et al., 2011b) and was not reassessed. In all instances, gp120 caused significant, concentration-dependent increases in DCF fluorescence; however, the extent of the increase differed among gp120 variants among HIV-1 strains (Fig. 6a–c). The EC50 values for X4 gp120IIIB, R5 gp120ADA, and bitropic gp120MN were 8.4 nM, 0.54 nM, and 0.07 nM, respectively. Interestingly, the increases in ROS caused by a particular gp120 variant did not correlate well with neurotoxicity or a propensity for the variant to interact with morphine.
Figure 6.
Effects of gp120 and morphine on ROS production in mixed-glia assessed by DCF fluorescence. A concentration-effect curve revealed significant increases in DCF relative fluorescence following 45 min exposure to gp120IIIB with an EC50 = 8.39 × 10−9 (*P < 0.05) (a). Similar concentration-effect curves for gp120ADA (b) and gp120MN (c) also show ROS elevation, but with somewhat different kinetics. Gp120IIIB ± morphine significantly increased ROS formation in mixed-glial cultures but without any additive or synergistic effect (d). Cells were pretreated with vehicle, NAC or DPI for 20 min before incubation with DCF-DA and subsequently treated with gp120IIIB (500 pM) and/or morphine (500 nM) for 40 min prior to assay. Mean DCF relative fluorescence units (MFI) were used as an estimate of ROS and were compared for each treatment. Gp120IIIB and morphine both markedly increased ROS production (*P < 0.05 vs. control). NAC and/or DPI significantly decreased gp120IIIB and/or morphine-induced ROS production (#P < 0.05 vs. treatment with gp120 and/or morphine alone); data are the mean ± SEM of n = 3 experiments (d).
Exposing mixed-glial cultures to morphine and/or HIV-1 gp120IIIB significantly elevated ROS production as determined by DCF (Fig. 6d). Combined morphine plus gp120 failed to elevate ROS beyond levels seen with either substance alone. The ROS inhibitors NAC and DPI were used to confirm experimentally that morphine and/or HIV-1 gp120-induced increases in ROS were driving DCF fluorescence, rather than auto-oxidation of the probe (Bonini et al., 2006) (Fig. 6d). Pretreatment with NAC eliminated gp120 -induced increases in ROS production. Pretreatment with DPI also eliminated or significantly reduced ROS production, suggesting a specific role for NADPH oxidase in the production of ROS caused by morphine and gp120IIIB in mixed glia.
Discussion
The results provide the first direct evidence suggesting that the neurotoxic interactions of morphine and gp120 differ among HIV-1 strains. The findings additionally suggest that the timing, extent, and pattern of the morphine and gp120 insults are likely to be critical in determining the neurotoxic outcome. Lastly, our results continue to support the notion (Fig. 3) that opioids acting via MOR-expressing glia per se mediate key aspects of the neurotoxic effects of opioids alone or in combination with gp120, a concept that has gained considerable support from our recent work examining morphine and HIV-1 Tat coexposure (Zou et al., 2011b). While the nature of the opioid-induced toxic signal(s) is uncertain, clearly some aspects of opioid drug actions are not due to a direct effect on neurons, but instead originate from glia. The notion of opioid actions in astroglia (Shinoda et al., 1989; Hauser et al., 1990; Stiene-Martin and Hauser, 1991; Stiene-Martin et al., 1993; Hauser et al., 1996; Stiene-Martin et al., 1998) and microglia (Chao et al., 1994; Peterson et al., 1995; Chao et al., 1996; Turchan-Cholewo et al., 2008) or monocytic precursors (Przewlocki et al., 1992; Gaveriaux et al., 1995; Grimm et al., 1998; Rogers et al., 2000) alone or in relation to HIV-1 is not novel (Hauser et al., 2007). Indeed, it has long-been suggested that opioids modify CNS plasticity or disease through actions in astroglia (Ronnback and Hansson, 1988; Stiene-Martin et al., 1990; Stiene-Martin and Hauser, 1991; Deleo et al., 2004; Hauser et al., 2005; Narita et al., 2006; Hansson, 2006; Kim et al., 2006; Hauser et al., 2007; Hutchinson et al., 2011). However, it has been quite difficult to separate, especially in vivo, the actions of opioids on glia from their effects on neurons, since all neural cell types examined thus far can express functional opioid receptors including MOR (Stiene-Martin et al., 1991; Eriksson et al., 1991; Bem et al., 1991; Stiene-Martin and Hauser, 1991; Barg et al., 1992; Hauser et al., 1996; Stiene-Martin et al., 1998; Knapp et al., 1998; Miyatake et al., 2009). These cell types include neurons, astroglia, microglia, oligodendroglia, and glial progenitors (Hauser et al., 2009; Hahn et al., 2010), as well as support cells within the CNS such as endothelial cells and the connective tissue stroma surrounding large blood vessels, which can also express opioid receptors (see Hauser et al., 2007).
MOR and CXCR4 or CCR5 may interact at multiple levels to modify HIV-1 pathogenesis. In both human and murine models of HIV-1 encephalitis (HIVE), the neurotoxicity driven by opioids and/or gp120 appears to be mediated through the differential activation of death-promoting mixed-lineage kinases, including p38 MAPK (Yi et al., 2004; Hu et al., 2005; Singh et al., 2005; Wan et al., 2006; Kaul et al., 2007) and perhaps other cell death (Lannuzel et al., 1997; Yi et al., 2004; Bodner et al., 2004; Singh et al., 2005) or interference with pro-survival pathways (Polakiewicz et al., 1998; Kolson, 2002; Kaul et al., 2007) and ERK (Belcheva et al., 2005; Miyatake et al., 2009). Combined exposure to morphine and gp120 exacerbates apoptosis in both human peripheral blood mononuclear cells and neurons through mechanisms that likely involve β-arrestin 2 (Moorman et al., 2009) and a p38 MAPK-dependent pathway (Hu et al., 2005), respectively. Gp120, for example, has been shown to upregulate MOR in HL-60 human promyelocytic leukemia cells (Beltran et al., 2006); while morphine can increase CCR5 and CCR3 expression in U373 astrocytoma cells (Mahajan et al., 2005). This phenomenon is not restricted to gp120, as we have shown that HIV-1 Tat expression in vivo dysregulates opioid receptors, including MOR, in a regional and cell specific manner (Fitting et al., 2010). Bidirectional heterologous interactions between G-protein coupled chemokine receptors including CXCR4 or CCR5, and opioid receptors, including MOR (Rogers and Peterson, 2003; Chen et al., 2004; Finley et al., 2008), are also possible. Evidence for CXCR4-MOR heterodimers exists, although the functional significance of such direct molecular interactions is unclear (Toth et al., 2004).
Morphine alone caused modest but sustained toxicity in neurons co-cultured with glia (Fig. 3), which has been previously reported by other investigators (Hu et al., 2002; Malik et al., 2011). Interestingly, morphine appeared to interact differently with gp120 from each viral strain. Morphine transiently accelerated the neurotoxic effects of X4 gp120IIIB, had no affect on R5 gp120ADA-induced neuron death, but caused a more sustained enhancement of X4/R5 gp120MN cytotoxicity. Such findings suggest that the timing and/or duration of the opioid insult, as well as strain of HIV-1 gp120 involved, may be critical in determining the extent and pattern of neuron loss. Moreover, similar proportions of striatal neurons (and astrocytes) express both chemokine receptors, suggesting the expression of CXCR4 versus CCR5 by a disproportionate number of cells does not underlie differences in neurotoxicity. There was no clear-cut relationship between X4/R5 tropism and morphine interfacing, although further study is needed to address this issue fully. Morphine increases expression of intracellular ferritin-heavy chain, which attenuates protective CXCR4-signals in neurons (Sengupta et al., 2009); however, this mechanism is less likely to be operative in the present study since morphine had minimal interactions with X4 gp120IIIB. Although it is uncertain how morphine transiently increases gp120 neurotoxicity in the present study, the temporal selectivity and likely dependence on glia might offer some clues as to possible mechanisms and might partially explain why interactive morphine and gp120 neurotoxicity has not been consistently reported in past studies (Hu et al., 2005; Turchan-Cholewo et al., 2006). It is well established that key aspects of gp120 neurotoxicity can be mediated through microglia (Lipton et al., 1994; Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Kaul et al., 2007) by activation of either CXCR4 (Meucci et al., 1998; Zheng et al., 1999; Kaul and Lipton, 1999; Kaul et al., 2007), or CCR5 (Kaul et al., 2007). Gp120 can also affect astroglia and neurons directly through CD4-independent mechanisms, and X4 and R5 gp120 HIV-1 strain variants differ in their neurotoxic interactions among cells (Koller et al., 2002; Khan et al., 2004; Kaul et al., 2007; Medders et al., 2010; Bachis et al., 2010).
The modest neurotoxicity seen with morphine was not seen in our initial studies of mixed striatal neuronglia cultures following exposure for 16 h (Gurwell et al 2001). However, our current time-lapse, repeated measures assessments are made over an expanded time course, and are more sensitive to subtle treatment effects than previous strategies (Bakalkin et al., 2010; Zou et al., 2011b). Opioids have diverse actions that are contextual (Akil et al., 1984; Carr and Serou, 1995; Hauser and Mangoura, 1998). Despite many studies acknowledging cytoprotective actions, there are nevertheless numerous reports that MOR agonists, including morphine, can intrinsically promote regulated cell death (Fuchs and Pruett, 1993; Maneckjee and Minna, 1994; Nair et al., 1997; Yin et al., 1997; Goswami et al., 1998; Singhal et al., 1999; Malik et al., 2002; Hu et al., 2002; Madden et al., 2002; Kapasi et al., 2004; Tegeder and Geisslinger, 2004). We also previously noted a modest neurotoxicity due to morphine alone in a time-lapse study designed to examine Tat-opiate comorbidity (Zou et al., 2011b).). More recently, Alzheimer’s-like hyperphosphorylation of Tau and correlative increases in microglial activation have been reported in the brains of chronic opioid drug abusers without detectable co-infection or other disease comorbidity (Anthony et al., 2010).
Exposure to HIV-1 virus or gp120 (Wang et al., 2003), as well as morphine (Zou et al., 2011b), can inhibit glutamate uptake by astrocytes or mixed-glia, respectively. Conversely, CXCL12 (SDF-1α), the principal endogenous CXCR4 agonist, enhances glutamate release from human and murine astrocytes (reviewed by Cali and Bezzi, 2010). Together, these findings prompted an assessment of the effects of gp120 ± morphine on the time-dependent uptake of glutamate from mixed-glial cultures. The ability of morphine to exacerbate Tat-induced glutamate release from microglia (Gupta et al., 2010) provided further impetus. Although the present studies do not discern glutamate uptake from release, a previous study using this culture model revealed deficits in recovery from a glutamate challenge that were largely due to reduced uptake (Zou et al., 2011b). However, despite the ability of gp120MN and to a lesser extent morphine, to inhibit glutamate buffering by mixed-glia, the lack of significant gp120MN-morphine interactions suggests that the enhanced neuron losses are not caused by excessive extracellular glutamate. Alternatively, it is possible that gp120 variants from other HIV-1 strains might interact with morphine to affect glutamate buffering differently compared to gp120MN.
ROS increases are an important proinflammatory trigger and evident in a variety of cell types. We questioned whether morphine and/or gp120IIIB-induced increases in ROS in mixed-glia might contribute to the pro-inflammatory response (Mahajan et al., 2005). Because of reported differences in the neurotoxic effects of X4 versus R5 gp120 (Kaul et al., 2007; Medders et al., 2010; Bachis et al., 2010), and our present findings, we initially questioned whether the individual gp120 variants might differentially trigger ROS. Interestingly, despite some differences in the EC50 values, the maximum ROS response was only increased about 2-fold above untreated control values, was comparable among gp120 variants throughout the concentration-effect curve, and showed considerable variability. Similarly, at the 500 pM concentration used to examine neurotoxicity, gp120IIIB, gp120ADA, or gp120MN caused equivalent increases in ROS, suggesting that it would be difficult to differentiate among gp120 effects using ROS as an outcome measure. Next, to see whether morphine might exacerbate gp120-induced increases in ROS, we examined interactions with gp120IIIB, which showed the greatest relative increase in ROS. Despite a trend toward a near-additive increase, co-administration of morphine and gp120IIIB failed to cause even an additive increase in ROS, suggesting that increases in ROS are unlikely to play a significant role in regulating opioid drug actions in HIV-1 gp120-exposed glia. Importantly, unlike gp120, morphine + Tat show interactive increases in ROS (Zou et al., 2011b), indicating fundamental differences in the interactions of morphine with different HIV proteins in terms of ROS production. There may also be interactive effects of gp120 and morphine on ROS signaling in each of the individual cell types that are diluted in the present co-culture system. Lastly, while ROS is certainly an important component of glial inflammation, there are many other intracellular events, such as signaling through [Ca2+]i (El-Hage et al., 2005; El-Hage et al., 2008a), and bioactive intercellular compounds with potential neurotoxic effects that might be released by interactions between gp120 and morphine.
Finally, integrating prior examinations of morphine and HIV-1 Tat interactions (Nath et al., 2002; Hauser et al., 2005; Hauser et al., 2007; Zou et al., 2011a; Zou et al., 2011b), and the present findings regarding morphine-gp120 interactive neurotoxicity, several conclusions can be drawn. First, that there is considerable selectivity in morphine’s actions. Secondly, morphine interacts uniquely with each viral component resulting in distinct pathophysiologic consequences for the host. Lastly, the unique interactions with morphine extend not only to the level of the particular viral protein involved, but also to differences among viral strains, as is illustrated with gp120 in the present study. Moreover, an alternative outcome measure besides neurotoxicity, or varying the pharmacological parameters of morphine exposure or withdrawal (e.g., Bandaru et al., 2011), might reveal a different pattern of gp120-opiate drug interactions. Together with previous findings from our lab and others, the results underscore the complexity of interactions between opiate drug abuse and neuroAIDS, and the need for systematic approaches for understanding regional, temporal, cell-specific, and genotypic differences in opioid drug-HIV-1 interactions in CNS.
Acknowledgments
The support of NIH-National Institute on Drug Abuse (NIDA) grants P01 DA019398, R01 DA018633, T32 DA007072, and K02 DA027374 is gratefully acknowledged.
References
- Adler MW, Geller EB, Rogers TJ, Henderson EE, Eisenstein TK. Opioids, receptors, and immunity. Adv Exp Med Biol. 1993;335:13–20. doi: 10.1007/978-1-4615-2980-4_3. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous Opioids: Biology and Function. Ann Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, Robertson R, Bell JE. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133:3685–3698. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–578. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, Artemenko KA, Yakovleva T, Dooijes D, Van de Warrenburg BPC, Zubarev RA, Kremer B, Knapp PE, Hauser KF, Wijmenga C, Nyberg F, Sinke RJ, Verbeek DS. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Patel N, Ewaleifoh O, Haughey NJ. A failure to normalize biochemical and metabolic insults during morphine withdrawal disrupts synaptic repair in mice transgenic for HIV-gp120. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9289-0. in press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011 doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg J, Belcheva MM, Coscia CJ. Evidence for the implication of phosphoinositol signal transduction in μ-opioid inhibition of DNA synthesis. J Neurochem. 1992;59:1145–1152. doi: 10.1111/j.1471-4159.1992.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bellizzi MJ, Lu SM, Masliah E, Gelbard HA. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J Clin Invest. 2005;115:3185–3192. doi: 10.1172/JCI25444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran JA, Pallur A, Chang SL. HIV-1 gp120 up-regulation of the mu opioid receptor in TPA-differentiated HL-60 cells. Int Immunopharmacol. 2006;6:1459–1467. doi: 10.1016/j.intimp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bem WT, Yeung SJ, Belcheva M, Barg J, Coscia CJ. Age-dependent changes in the subcellular distribution of rat brain μ-opioid receptors and GTP binding regulatory proteins. J Neurochem. 1991;57:1470–1477. doi: 10.1111/j.1471-4159.1991.tb06340.x. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Hahn BH, Bubien JK, Ghosh SK, Mashburn NA, Chaikin MA, Shaw GM, Benveniste EN. Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex. Proc Natl Acad Sci U S A. 1994a;91:494–498. doi: 10.1073/pnas.91.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos DJ, McPherson S, Hahn BH, Chaikin MA, Benveniste EN. Cytokines and HIV envelope glycoprotein gp120 stimulate Na+/H+ exchange in astrocytes. J Biol Chem. 1994b;269:13811–13816. [PubMed] [Google Scholar]
- Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol. 2004;188:246–253. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2',7'-dichlorofluorescin to reactive oxygen species: a self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Cali C, Bezzi P. CXCR4-mediated glutamate exocytosis from astrocytes. J Neuroimmunol. 2010;224:13–21. doi: 10.1016/j.jneuroim.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Carr DJJ, Serou M. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology. 1995;31:59–71. doi: 10.1016/0162-3109(95)00033-6. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM, Peterson PK. kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci U S A. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-α by microglia: Implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Kaul M, Fletcher L, Dowen R, Lipton SA. Erythropoietin protects cerebrocortical neurons from HIV-1/gp120-induced damage. Neuroreport. 2004;15:761–763. doi: 10.1097/00001756-200404090-00004. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bakalkin G, Knapp PE, Hauser KF. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-κB trafficking and transcription. PLoS ONE. 2008a;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Knapp PE, Hauser KF. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 Tat exposed mice. J Neuroimmune Pharmacol. 2008b;3:275–285. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 co-infection and morphine co-exposure severely dysregulate HCV-induced hepatic pro-inflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host-defenses. J Virol. 2011 doi: 10.1128/JVI.05239-11. in press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain-coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–1239. doi: 10.1016/0028-3908(91)90170-g. [DOI] [PubMed] [Google Scholar]
- Finley MJ, Chen X, Bardi G, Davey P, Geller EB, Zhang L, Adler MW, Rogers TJ. Bi-directional heterologous desensitization between the major HIV-1 co-receptor CXCR4 and the kappa-opioid receptor. J Neuroimmunol. 2008;197:114–123. doi: 10.1016/j.jneuroim.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull CM, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: Chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177:1397–1410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BA, Pruett SB. Morphine induces apoptosis in murine thymocytes in vivo but not in vitro: involvement of both opiate and glucocorticoid receptors. J Pharmacol Exp Ther. 1993;266:417–423. [PubMed] [Google Scholar]
- Gaveriaux C, Peluso J, Simonin F, Laforet J, Kieffer B. Identification of kappa- and delta-opioid receptor transcripts in immune cells. FEBS Lett. 1995;369:272–276. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Goswami R, Dawson SA, Dawson G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Knight AG, Gupta S, Knapp PE, Hauser KF, Keller JN, Bruce-Keller AJ. HIV-Tat elicits microglial glutamate release: role of NAPDH oxidase and the cystine-glutamate antiporter. Neurosci Lett. 2010;485:233–236. doi: 10.1016/j.neulet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Vo P, Fitting S, Block ML, Hauser KF, Knapp PE. beta-Chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration. J Neurochem. 2010;114:97–109. doi: 10.1111/j.1471-4159.2010.06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf) 2006;187:321–327. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, Nath A, Bruce-Keller AJ, Knapp PE. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: Glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden CP, Nath A, Haughey NJ, Geiger JD. Involvement of Na+/H+ exchangers, Ca2+ channels, and excitatory amino acid receptors in intracellular Ca2+ responses to HIV-1 gp120 in cultured human fetal brain cells. Neuroscience. 1999;91:1369–1378. doi: 10.1016/s0306-4522(98)00714-3. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis. 2005;191:886–889. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991;65:736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi AA, Coscia SA, Pandya MP, Singhal PC. Morphine modulates HIV-1 gp160-induced murine macrophage and human monocyte apoptosis by disparate ways. J Neuroimmunol. 2004;148:86–96. doi: 10.1016/j.jneuroim.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Brandimarti R, Patel JP, Huynh N, Wang J, Huang Z, Fatatis A, Meucci O. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res Hum Retroviruses. 2004;20:1063–1071. doi: 10.1089/aid.2004.20.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu and kappa opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281:33749–33760. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: μ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Koller H, Schaal H, Rosenbaum C, Czardybon M, Von Giesen HJ, Muller HW, Arendt G. Functional CXCR4 receptor development parallels sensitivity to HIV-1 gp120 in cultured rat astroglial cells but not in cultured rat cortical neurons. J Neurovirol. 2002;8:411–419. doi: 10.1080/13550280260422712. [DOI] [PubMed] [Google Scholar]
- Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin Lab Med. 2002;22:703–717. doi: 10.1016/s0272-2712(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Yeh M, Dreyer EB. Update on current models of HIV-related neuronal injury: platelet-activating factor, arachidonic acid and nitric oxide. Adv Neuroimmunol. 1994;4:181–188. doi: 10.1016/s0960-5428(06)80255-x. [DOI] [PubMed] [Google Scholar]
- Madden JJ, Wang Y, Lankford-Turner P, Donahoe RM. Does reduced DNA repair capacity play a role in HIV infection and progression in the lymphocytes of opiate addicts? J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S78–S83. doi: 10.1097/00126334-200210012-00008. S78–S83. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr HIV Res. 2005;3:277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol. 2002;169:3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- Malik AA, Radhakrishnan N, Reddy K, Smith AD, Singhal PC. Morphine-induced macrophage apoptosis modulates migration of macrophages: use of in vitro model of urinary tract infection. J Endourol. 2002;16:605–610. doi: 10.1089/089277902320913314. [DOI] [PubMed] [Google Scholar]
- Malik S, Khalique H, Buch S, Seth P. A growth factor attenuates HIV-1 Tat and morphine induced damage to human neurons: Implication in HIV/AIDS-drug abuse cases. PLoS ONE. 2011;6:e18116. doi: 10.1371/journal.pone.0018116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994;5:1033–1040. [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol. 2010;185:4883–4895. doi: 10.4049/jimmunol.0902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misse D, Gajardo J, Oblet C, Religa A, Riquet N, Mathieu D, Yssel H, Veas F. Soluble HIV-1 gp120 enhances HIV-1 replication in non-dividing CD4+ T cells, mediated via cell signaling and Tat cofactor overexpression. AIDS. 2005;19:897–905. doi: 10.1097/01.aids.0000171403.07995.92. [DOI] [PubMed] [Google Scholar]
- Miyatake M, Rubinstein TJ, McLennan GP, Belcheva MM, Coscia CJ. Inhibition of EGF-induced ERK/MAP kinase-mediated astrocyte proliferation by mu opioids: integration of G protein and beta-arrestin 2-dependent pathways. J Neurochem. 2009;110:662–674. doi: 10.1111/j.1471-4159.2009.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP. Coreceptors: implications for HIV pathogenesis and therapy. Science. 1997;276:51–52. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- Moorman J, Zhang Y, Liu B, Lesage G, Chen Y, Stuart C, Prayther D, Yin D. HIV-1 gp120 primes lymphocytes for opioid-induced, beta-arrestin 2-dependent apoptosis. Biochim Biophys Acta. 2009;1793:1366–1371. doi: 10.1016/j.bbamcr.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MP, Schwartz SA, Polasani R, Hou J, Sweet A, Chadha KC. Immunoregulatory effects of morphine on human lymphocytes. Clin Diagn Lab Immunol. 1997;4:127–132. doi: 10.1128/cdli.4.2.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. S193–S198. [DOI] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes: A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopamine systems associated with AIDS dementia. Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Sheng WS, Molitor TW, Chao CC. Morphine stimulates phagocytosis of Mycobacterium tuberculosis by human microglial cells: involvement of a G protein-coupled opiate receptor. Adv Neuroimmunol. 1995;5:299–309. doi: 10.1016/0960-5428(95)00020-3. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr. Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Hassan AH, Lason W, Epplen C, Herz A, Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48:491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- Pulliam L, West D, Haigwood N, Swanson RA. HIV-1 envelope gp120 alters astrocytes in human brain cultures. AIDS Res Hum Retroviruses. 1993;9:439–444. doi: 10.1089/aid.1993.9.439. [DOI] [PubMed] [Google Scholar]
- Robinson B, Li Z, Nath A. Nucleoside reverse transcriptase inhibitors and human immunodeficiency virus proteins cause axonal injury in human dorsal root ganglia cultures. J Neurovirol. 2007;13:160–167. doi: 10.1080/13550280701200102. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Ronnback L, Hansson E. Are astroglial cells involved in morphine tolerance? Neurochem Res. 1988;13:87–103. doi: 10.1007/BF00973320. [DOI] [PubMed] [Google Scholar]
- Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, Rubin JB, Meucci O. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29:2534–2544. doi: 10.1523/JNEUROSCI.5865-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989;245:415–417. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–790. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G. Morphine promotes apoptosis in Jurkat cells. J Leukoc Biol. 1999;66:650–658. doi: 10.1002/jlb.66.4.650. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: Evidence for a direct effect of opiates on neural maturation. Dev Brain Res. 1991;60:1–7. doi: 10.1016/0165-3806(91)90149-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Mattson MP, Hauser KF. Opiates selectively increase intracellular calcium in developing type 1 astrocytes: Role of calcium in morphine-induced morphologic differentiation. Dev Brain Res. 1993;76:189–196. doi: 10.1016/0165-3806(93)90207-q. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Osborne JG, Hauser KF. Proenkephalin mRNA is expressed by different subpopulations of developing murine astrocytes in culture: combined in situ hybridization and GFAP immunocytochemistry. Soc Neurosci Abstr. 1990;16 [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in μ, δ, and κ—opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- Sykova E. Glia and volume transmission during physiological and pathological states. J Neural Transm. 2005;112:137–147. doi: 10.1007/s00702-004-0120-4. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004;56:351–369. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, Bruce-Keller AJ. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006;23:109–119. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Wan Q, Douglas SD, Wang X, Kolson DL, O'Donnell LA, Ho WZ. Morphine upregulates functional expression of neurokinin-1 receptor in neurons. J Neurosci Res. 2006;84:1588–1596. doi: 10.1002/jnr.21053. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- Yi Y, Lee C, Liu QH, Freedman BD, Collman RG. Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):91–96. doi: 10.1080/753312758. 91–96. [DOI] [PubMed] [Google Scholar]
- Yin DL, Ren XH, Zheng ZL, Pu L, Jiang LZ, Ma L, Pei G. Etorphine inhibits cell growth and induces apoptosis in SK-N-SH cells: involvement of pertussis toxin-sensitive G proteins. Neurosci Res. 1997;29:121–127. doi: 10.1016/s0168-0102(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zou S, El-Hage N, Podhaizer EM, Knapp PE, Hauser KF. PTEN gene silencing prevents HIV-1 gp120IIIB-induced degeneration of striatal neurons. J Neurovirol. 2011a;17:41–49. doi: 10.1007/s13365-010-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain. 2011b doi: 10.1093/brain/awr281. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]