Abstract
Background
Transgenic expression of human complement regulatory proteins (hCRPs) reduces the frequency of hyperacute rejection (HAR) in Gal-positive cardiac xenotransplantation. In this study we examine the impact of human CD55 (hCD55) expression on a Gal knock-out (GTKO) background using pig-to-primate heterotopic cardiac xenotransplantation.
Methods
Cardiac xenotransplantation was performed with GTKO (Group 1; n=6) and GTKO.hCD55 (Group 2; n=5) donor pigs using similar immunosuppression. Cardiac biopsies were obtained 30 minutes after organ reperfusion. Rejection was characterized by histology and immunohistology. Intragraft gene expression, serum non-Gal antibody and antibody recovered from rejected hearts were analyzed.
Results
HAR of a GTKO heart was observed. Remaining grafts developed delayed xenograft rejection. Median survival was 21 and 28 days for Groups 1 and 2 respectively. Vascular antibody deposition was uniformly detected 30 minutes after organ reperfusion and at explant. A higher frequency of vascular C5b deposition was seen in GTKO organs at explant. Serum non-Gal antibody, antibody recovered from the graft and intragraft gene expression were similar between the groups.
Conclusion
HAR of GTKO hearts without hCD55 may occur. Expression of hCD55 appeared to restrict local complement activation, but did not improve graft survival. Chronic vascular antibody deposition with evidence of protracted endothelial cell activation was seen. These observations suggest that non-Gal antibody-induced chronic endothelial cell activation coupled to possible haemostatic incompatibilities may be the primary stimulus for DXR of GTKO hearts. To avoid possible HAR, future clinical studies should employ donors expressing hCRPs in the GTKO background.
Keywords: Cardiac xenotransplantation, complement regulation, endothelial cell activation, antibody-mediated rejection, Xenotransplantation
Introduction
In the pig to nonhuman primate model, solid organ xenograft survival is limited by hyperacute (HAR) and delayed xenograft rejection (DXR). Both of these rejection mechanisms are antibody-mediated processes which induce organ rejection through a combination of complement mediated injury and antibody induced endothelial cell (EC) activation.(1) Disparities in porcine and primate haemostatic regulation may also contribute to DXR.(2) HAR is characterized by vascular antibody and complement deposition with widespread hemorrhage and thrombosis within the xenograft. Genetic modification of the donor, can alleviate HAR. Transgenic expression of human complement regulatory proteins (hCRPs: CD46, CD55 or CD59) enhances the xenograft’s intrinsic resistance to complement mediated injury (3, 4) and mutation of the α-galactosyl transferase gene (GTKO) eliminates galactose α 1,3, galactose (αGal) the major xeno-antigen.(5)
Transgenic hearts expressing hCRPs generally blocked HAR and extend graft survival to 3 - 7 days in the absence of immunosuppression.(6) This was not a perfect solution however as there were several reported instances where HAR despite hCRP expression.(7, 8) Elimination of the αGal antigen likewise blocks HAR although experience with GTKO transplants remains relatively limited. The initial cardiac transplants using GTKO organs reported no evidence of HAR.(9) This was not surprising given that the recipients were selected for minimal presence of non-Gal preformed antibody and most recipients were treated with cobra venom factor. In contrast, using recipients with preformed non-Gal IgM, we have reported a case of HAR after heterotopic cardiac xenotransplantation of a GTKO heart (10) and we and others have noted early antibody-mediated graft injury with complement deposition.(11, 12) These results suggest, at least in the nonhuman primate model, that the level of preformed non-Gal antibodies in the recipient and the expression of non-Gal antigens in the donor are sufficient to initiate early antibody-mediated injury which may affect GTKO xenograft survival.
When HAR is blocked, organs are rejected by delayed xenograft rejection (DXR). Despite the large reduction in immunogenicity achieved using GTKO donors, the immunopathology of DXR for both Gal-positive hCRP transgenic and GTKO donor organs is similar showing early vascular antibody deposition and myocyte vacuolization, with later microvascular thrombosis, and coagulative necrosis.(13) Vascular deposition of complement products is reported at explant for GTKO cardiac xenografts despite the use of cobra venom factor.(9, 14) Vascular complement deposition is variably observed in hCRP transgenic organs where complement restriction at the cell surface may be more efficient than systemic complement depletion.(9, 15-17) Expression of hCRP transgenes can extend xenograft survival compared to wild type organs however these transgenes do not block an induced antibody response and do not block DXR.
The effects of hCRP expression on DXR in the GTKO background has not been investigated. While expression of hCRPs is generally assumed to be beneficial there remains considerable debate on the relative contribution to DXR of complement mediated EC injury, antibody-mediated endothelial activation, and disparities in haemostatic regulation. These three processes are not completely distinct, as sublytic complement activation can exacerbate EC activation and anaphylatoxin release can attenuate innate immune responses, however, expression of hCRPs is expected to primarily limit vascular complement-mediated injury. Likewise, local vascular regulation of complement amplification does not directly address disparities in haemostatic regulation such as porcine thrombomodulin. In this study we use heterotopic pig-to-primate cardiac xenotransplantation to compare the immunohistology and graft survival of GTKO and GTKO:CD55 donor organs. The study groups were matched for immunosuppressive treatments and all recipients had preformed non-Gal reactive antibody prior to transplant.
Results
Transgenic pigs and transplant survival
Gal-deficient pigs expressing CD55 (GTKO.hCD55) were produced by standard breeding procedures starting with GT+/- females (18) and incorporating a murine H2K regulated hCD55 transgene. Intercrosses were performed to achieve GT-/- homozygosity. GTKO.hCD55 animals show no evidence of Gal expression in the heart. (Figure 1A) Expression of hCD55 is evident on primary cultured aortic ECs and on microvascular ECs in the heart. (Figure 1B and C) This line of pigs shows consistent expression of CD55 in heart RNA. (Figure 1D)
Figure 1. GTKO.CD55 donor characteristics and graft survival.
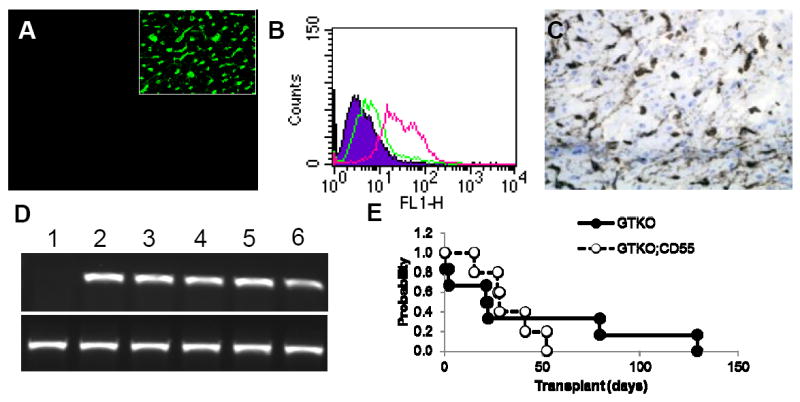
(A) FITC-conjugated GSIB-4 lectin staining of GTKO.hCD55 heart. No staining for αGal is evident in GTKO.CD55 hearts. For comparison the insert shows vascular GSIB-4 lectin staining of a Gal positive heart. (B) Flow cytometry comparison of hCD55 expression in GTKO (green) and GTKO.hCD55 (red) primary aortic endothelial cells. Expression of hCD55 was detected using a FITC-conjugated anti-hCD55. Background staining (filled) is a FITC-conjugated isotype control. (C) Immunohistology staining showing microvascular hCD55 expression in a GTKO.hCD55 heart. (D) Expression of hCD55 mRNA (top panel) in heart samples detected by semi-quantitative reverse transcriptase polymerease chain reaction. Human CD55 was amplified from cDNA using forward (5’-GTAACCATGGGCTTGCTGAC) and reverse (5’-GATCCCATTCTAAATACG) primers as described in the Methods section. Lane 1, nontransgenic heart RNA. Lanes 2 – 6, RNA from GTKO.hCD55 donor hearts. Bottom panel shows amplification of β-actin for each sample. (E) Kaplan Meier survival analysis of GTKO and GTKO;CD55 graft survival. Transplant survival was based on the day of explant or rejection without censoring.
This study used a modified immunosuppressive regime consisting of induction therapy coupled to a moderate level of Tacrolimus and Sirolimus maintenance. The levels of Tacrolimus and Sirolimus were similar to those used previously which reliably resulted in cardiac xenograft survival of 4 - 8 weeks.(15, 16) These transplant groups were well matched.(Table 1) All recipients showed anti-pig non-Gal IgM and cytotoxicity to GTKO porcine aortic endothelial cells (PAECs) prior to transplant. Minimal non-Gal IgG was present prior to transplant except in two GTKO recipients explanted on days 2 and 22. Although levels of pretransplant antibody and cytotoxicity varied between individuals there was no significant difference between the two groups (IgM, p = 0.48; IgG, p = 0.13; cytotoxicity p = 0.25) There was no significant difference in serum Tacrolimus (p=0.78) or Sirolimus (p=0.71) levels between the groups. Donor age (p = 0.42) and donor and recipient weight were not significantly different (p = 0.40 for both donor and recipient weight). Transplant survival ranged from 0 to 128 in Group 1 and 15 to 52 in Group 2. In both groups one recipient died from hemorrhage after a vascular rupture on day 2 (Group 1) and after rupture of the left atrial appendage on day 15 (Group 2). All other grafts were rejected or showed significant loss of contractility at the time of explant. There was not a significant difference in median Kaplan-Meier survival of 22 and 28 days for Groups 1 and 2 respectively (Figure 1E). Pretransplant serum IgM levels and serum cytotoxicity were strongly correlated (linear regression R = 0.82) but no strong relationship was observed between pretransplant antibody levels or cytotoxicity and graft survival (data not shown).
Table 1.
Comparison of Transplant Group Characteristics.
| Donor | GTKO | GTKO | GTKO | GTKO | GTKO | GTKO | GTKO hCD55 | GTKO hCD55 | GTKO hCD55 | GTKO hCD55 | GTKO hCD55 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survival (days) | 0 | 2 | 21 | 22 | 79 | 128 | 15 | 27 | 28 | 41 | 52 |
| Outcome* | HAR | VH | DXR | DXR | DXR | DXR | VH | DXR | DXR | DXR | DXR |
| Donor sex | M | F | F | M | M | F | F | M | F | M | F |
| Donor age (days) | 38 | 36 | 38 | 42 | 26 | 32 | 40 | 36 | 33 | 68 | 28 |
| Donor weight (kg) | 12 | 9 | 12 | 12 | 8 | 10 | 9 | 10 | 9 | 12 | 8 |
| Recipient sex | M | F | M | M | M | M | M | M | M | M | M |
| Recipient weight (kg) | 19 | 16 | 16 | 20 | 15 | 15 | 17 | 19 | 18 | 18 | 17 |
| Preformed nonGal IgM1 | 0.143 | 0.323 | 0.044 | 0.052 | 0.119 | 0.188 | 0.093 | 0.143 | 0.186 | 0.196 | 0.318 |
| Preformed nonGal IgG1 | 0.101 | 0.424 | 0.194 | 0.351 | 0.046 | 0.186 | 0.178 | 0.039 | 0.088 | 0.080 | 0.128 |
| PreTransplant cyctoxicity1 | 0.362 | 0.753 | 0.374 | 0.179 | 0.484 | 0.537 | 0.449 | 0.381 | 0.579 | 0.681 | 0.892 |
| Induced nonGal IgM2 | n.a. | n.a. | 1.5 | 1.9 | 0.9 | 1.8 | 0.9 | 3.0 | 9.7 | 3.5 | 0.9 |
| Induced nonGal IgG2 | n.a. | n.a. | 2.7 | 8.2 | 2.1 | 0.8 | 1.4 | 2.8 | 5.3 | 1.9 | 4.4 |
| Eluted nonGal IgM3 | +3 | +1 | +1 | - | +1 | - | - | +3 | +3 | +3 | - |
| Eluted nonGal IgG3 | +2 | +3 | +1 | +2 | +2 | +1 | +1 | +2 | +3 | +1 | +1 |
| Serum TAC4 | 17.5 | 24.0 | 15.5 | 15.8 | 21.1 | 16.3 | 17.9 | 15.9 | 10.5 | 13.2 | 24.8 |
| Serum SRL4 | 11.6 | 3.2 | 8.3 | 4.5 | 11.9 | 10.6 | 8.3 | 6.7 | 8.8 | 10.0 | 15.4 |
Antibody binding and cytoxicity were measured in pretransplant sera. Cytotxicity was determined by propidium iodide staining of GTKO PAECs labelled with a 2-fold dilution series (1:2 to 1:512) and incubated for 1 hours at 37C with 10% rabbit complement. IgM and IgG staining was measured at the same time. Day-to-day assay variation was controlled using a positive control sera from a sensitized recipient (1:16 dilution with average cytoxicity 60%) run with each analysis. Pretransplant antibody and cytotoxicity levels are expressed as a fraction of this positive control serum.
Induced non-Gal antibody levels were determined by flow cytometry using GTKO porcine aortic endothelial cells. Values represent the maximal post transplant fold increase in mean fluorescence intensity for each recipient compared to pretransplant levels. Values in italics are based on post explant serum, obtained within 14 days of organ removal and under continued immunosuppression. All other values come from serum obtained before organ explant. N.A., not applicable as recipient survival was too limited for an induced antibody response to occur.
Values represent the relative amounts of non-Gal antibody recovered from tissue after explant using the semi-quantitative scale described in the Materials and Methods.
Blood Tacrolimus (TAC) and Sirolimus (SRL) levels were measured 2 – 3 times per week. The table shows mean drug concentration (ng/ml) for each recipient. A comparison of all pre-rejection drug levels between Groups 1 and 2 finds no significant difference (p=0.77 Tacrolimus, p=0.71 Sirolimus)
Cause of graft failure or recipient death is indicated. HAR; hyperacute rejection, DXR; delayed xenograft rejection, VH; vascular haemorrhage.
Xenograft rejection
There was no postoperative anticoagulation in these recipients and no evidence of perioperative consumptive coagulopathy. Both groups maintained postoperative circulating platelet counts greater than 100 × 109 /liter (data not shown). Only one recipient in Group 2 showed a reduction of platelets immediately after transplant. In this recipient platelets decreased to 103 ×109/liter from 258 prior to transplant. Platelet levels rebounded however to 312 ×109/liter on postoperative day 5.
Hyperacute rejection was observed in one Group 1 recipient (Table 1, Figure 2A-C). A biopsy obtained 30 minutes after reperfusion showed normal myocardial histology, extensive vascular IgM deposition and moderate focal deposition of C5b (insert Figure 2A-C). Over a period of 90 minutes post transplant the heart became progressively discolored and dyskinetic leading to asystole. Histology at that time showed extensive intramyocardial hemorrhage, with vascular IgM deposition and increased levels of C5b deposition (Figure 2A-C). Pre transplant serum from this recipient did not have an unusually high degree of complement mediated cytotoxicity to GTKO PAECs (Table 1).
Figure 2. Histology and immunohistology of cardiac xenografts.
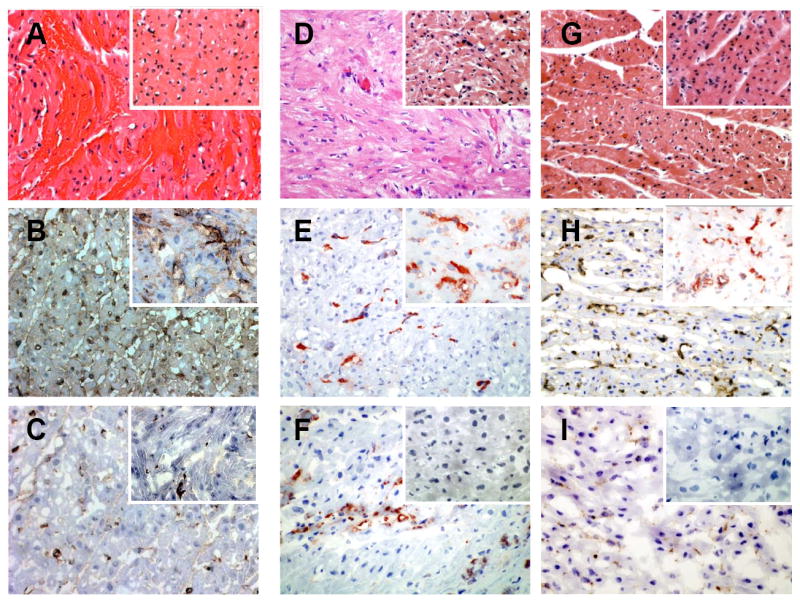
(A – C) Hyperacute rejection of Group 1 GTKO heart. (D – F) Group 1 GTKO heart. (G – I) Group 2 GTKO.hCD55 heart. Hematoxylin and eosin staining (A, D and G). Immunohistology for vascular IgM (B, E, and H) and C5b (C, F, and I). Inserts in each panel shows the corresponding staining from a representative 30 minute biopsies for each group.
Excluding the HAR in Group 1, delayed xenograft rejection (DXR) was the cause of graft failure. All grafts showed vascular antibody staining 30 minutes after reperfusion (inserts Figures 2E and 2H) with C5b deposition detected only in the GTKO graft that was hyperacutely rejected and transiently in a GTKO:CD55 graft that rejected on day 28. (Table 2). At explant all samples continued to showed vascular IgM staining (Figure 2E and 2H) and most showed IgG binding (data not shown). There was variable C5b deposition at explant (Figure 2F and I) as donor hearts expressing hCD55 showed a lower frequency of C5b deposition and appeared to effectively restrict complement activation. Steady state vascular C5b deposition was present in only one Group 2 heart (20%) which rejected on day 41. (Table 2). In contrast, Group 1 explanted GTKO hearts showed a higher frequency (80%) of vascular C5b staining (Table 2).
Table 2.
Summary of Immunohistochemical Stains.
| 30 minute biopsy | Explanted heart | |||
|---|---|---|---|---|
| GTKO (n=6) % positive | GTKO hCD55 (n=5) % positive | GTKO (n=6) % positive | GTKO hCD55 (n=5) % positive | |
| IgM | 100 | 100 | 100 | 100 |
| IgG | 100 | 100 | 100 | 100 |
| C5b | 17 (1/6) | 20 (1/5) | 80 (4/5)* | 20 (1/5) |
Values in parenthesis represent the number of recipients with positive staining in each group. For Group 1 (GTKO donors) only 5 of 6 samples at explant could be evaluated.
Immune response and intragraft gene expression
DXR was accompanied by an increased level of serum non-Gal antibody (Table 1, Figure 3A and B). Increases in non-Gal IgM ranged from minimal (< 2-fold) up to a 9-fold and induction of non-Gal IgG ranged from minimal up to 8 fold. Consistent with an induced antibody response non-Gal antibody was also recovered from explanted tissues. The qualitative recovery of non-Gal antibody reactivity from explanted tissue generally reflected the level of induced serum non-Gal antibody (Table 1).
Figure 3. Immune response and endothelial cell activation.

(A and B) Serum non-Gal immune response in a GTKO (A) and GTKO.hCD55 (B) recipient which survived organ explant. Graphs show the fold increase, compared to pretransplant, for IgM (filled) and IgG (open) binding to GTKO porcine aortic endothelial cells. Arrows indicate the day of organ rejection and explant. Immunosuppression was maintained after explant. (C) An analysis of porcine CD54 and CD106 expression in RNA extracted from explanted xenografts. The negative control (pig) is RNA from a normal not transplanted pig heart. The positive control (+cntrl) is RNA from a pig-to-baboon heterotopic heart transplant performed without immunosuppression and rejected in 7 days. Each lane indicates the duration of graft survival and corresponds to values listed in Table 1. Expression of porcine smooth muscle cell β-actin was used as a loading control.
Analysis of intragraft gene expression indicates EC activation in all grafts as evidenced by increased expression of CD54 and CD106 (Figure 3C). Elevated levels of CD54 and CD106 expression were not apparent in the GTKO graft after HAR but were evident on days 2 and 15 in the two grafts that did not reject. The early increase in CD54 and CD106 expression in these grafts prior to rejection suggests an ongoing state of EC activation.
Discussion
There have been numerous reports of cardiac xenotransplantation using various hCRP donors under a variety of conditions. While interpretation of this literature might imply that inclusion of an hCRP transgene in the GTKO background would be beneficial, there has been to our knowledge no direct study of this question. Our study compared two carefully matched transplant groups and shows that in the absence of CD55, nonhuman primates with preformed non-Gal IgM may induce HAR of GTKO cardiac xenografts. HAR of the GTKO heart displayed the classic markers of HAR observed for wild type organs namely prominent vascular deposition of antibody and complement, accompanied by rapid loss of contractility and notable intravascular hemorrhage (Figure 2A - C). Detection of a HAR for a GTKO heart is consistent with the early antibody-mediated injury, and limited GTKO graft survival reported by Ezzelarab et al.(11) Our recipients showed pre transplant cytoxicity to GTKO PAECs and 50% of human and baboon sera has been reported to be lytic for GTKO porcine cells. (19) Our study underscores the importance of utilizing nonhuman primate recipients with preformed non-Gal antibody when assessing the efficacy of new transgenic or therapeutic treatments. This study is not sufficiently powered to estimate an overall frequency of HAR for GTKO hearts or the efficacy of CD55 expression to prevent HAR. The variables that may contribute to HAR, preformed non-Gal antibody titers, specificity, and antigen density are poorly understood, making it difficult to predict the occurrence of HAR in individual recipients. We suspect, based on the generally lower titer of non-Gal IgM compared to anti-Gal IgM, that the frequency of HAR for GTKO hearts, at least in nonhuman primates, will be relatively rare. Our study also can not address the potential impact of antibody directed to sialic acid N-glycolylneuraminic acid (Neu5Gc) antigens since baboons do not make this antibody specificity. In humans anti-Neu5GC is present, and may constitutes 7 -13% of the preformed non-Gal human antibody repertoire.(20) The potential of anti-Neu5GC to affect HAR or DXR is controversial but the presence of an additional preformed non-Gal antibody specificity in humans, compared to nonhuman primates, seems unlikely to diminish the potential for early antibody-mediated graft injury. Since any future clinical application of cardiac xenotransplantation must, at the very least, be as free as possible from the risk of HAR, these observations suggest that expression of at least one hCRP in the GTKO background will minimally be required for this purpose.
This study demonstrates that expression of hCD55 in the GTKO background limited the steady state vascular deposition of C5b at the time of rejection but did not extend GTKO xenograft survival. This suggests that the mechanism(s) of DXR, under conditions where vascular antibody deposition and a mild induced non-Gal antibody response is detected, may not be dependent on complement-mediated EC injury per se but likely result from processes, such as chronic EC activation or incompatibilities in thromboregulation which would not necessarily be directly affected by vascular hCRP expression. We note that under different immunosuppressive regimens, where evidence of cellular and humoral sensitization may be minimal, DXR with vascular antibody deposition, widespread microvascular thrombosis, and coagulative necrosis still occurs.(21) This suggests that DXR of cardiac xenografts may result more from the effects of vascular EC activation, possibly coupled to disparities in thromboregulation, than the result of direct complement-induced cell injury. Consistent with this interpretation we detected increased expression of mRNA encoding EC adhesion proteins CD54 and CD106. Increased expression of these genes, evident as early as 2 days post transplantation, is indicative of EC activation. This observation, and the detection of vascular antibody deposition 30 minutes after reperfusion, strongly suggests a chronic state of EC activation, probably resultant to binding of non-Gal antibody, prior to the histological changes of DXR and organ rejection. This interpretation is consistent with our recent observation of changes in porcine gene expression after orthotopic cardiac xenotransplantation where, in the absence of graft rejection, cardiac gene expression, indicative of EC activation, was detected 2 to 57 days post transplant.(22) Given the reported specificity of induced non-gal antibody,(10, 12) with a potential to directly affect key EC inflammatory and thromboregulatory functions, and the observation of differential changes in EC gene expression in response to anti-Gal and non-Gal antibody (23) the response of vascular EC to chronic antibody binding may be complex, resulting in physiological changes which simultaneously promote and prevent EC injury and thrombosis. Haemostatic regulatory incompatibilities may eventually tip this balance resulting in microvascular thrombosis. If this is the case then immunosuppressive strategies which do not eliminate synthesis of preformed antibody may not be effective over the long term in preventing DXR. Investigation on the relative contribution of incompatibilities in haemostatic regulation and chronic EC activation to DXR awaits the development and availability of pigs expressing transgenes to regulate coagulation.
This study provides the first direct comparison of the effects of hCRP expression in GTKO cardiac xenotransplantation. Our observations show that hCD55 expression contributes to the prevention of HAR, restricts vascular complement activation within the graft, but does not extend overall graft survival. The results are consistent with the hypothesis that DXR of GTKO organs may include multiple mechanisms which are not strictly complement dependent, and further suggest that blocking of preformed nonhuman primate non-Gal antibody is needed not only to insure the absence of HAR but also to prevent chronic EC activation and DXR. Since future clinical studies would minimally require freedom from HAR, we suggest that it is important to incorporate at least one hCRP function in the donor organ in any proposed clinical studies.
Materials and Methods
Heterotopic Heart Transplantation
All transplants utilized the pig-to-baboon heterotopic abdominal cardiac xenograft model and were in compliance with standards established by the Institutional Animal Care and Use Committee of the Mayo Clinic and Foundation and consistent with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1996). Group 1 recipients (n=6) received Gal-deficient (GTKO) hearts, Group 2 (n=5) recipients received GTKO hearts expressing the human CD55 (hCD55) complement regulatory protein (GTKO.hCD55). A preliminary description of survival for group 1 recipients was previously described.(10) Immunosuppression was the same in each group consisting of induction therapy on postoperative days (POD) 1-5 with antithymocyte globulin (ATG), 1.5 mg/kg on postoperative days 1 - 5 and on POD -7, 0,7 and 14 with Rituximab (17 mg/kg). Maintenance immunosuppression consisted of Tacrolimus (target levels 10-20 ng/ml) and Sirolimus (target levels 5-15 ng/ml). A steroid taper and prophylactic antibiotics and antiviral treatments were also given. No postoperative anticoagulation therapy was used. Xenograft contractility was measured at 2-3 day intervals by echocardiography and manual palpation. Palpation was scored from 0, no contractility to 6, maximal contractility. At rejection, defined by an absence of contractility, xenografts were explanted and surviving recipients were maintained on immunosuppression for at least 20 days.
Histology
Apical left ventricular (LV) biopsies were taken 30 minutes after reperfusion. At explant full thickness mid-ventricular sections of right (RV) and LV were collected. Standard hematoxylin and eosin stained sections were made for all samples. Immunohistochemistry (IHC) samples were frozen and sections were stained to detect vascular IgM (A0425, DAKO, Carpinteria, CA), IgG (A0423, DAKO), and C5b (RDI Concord, MA, clone HCC5b.1), using the DUAL+/HRP labeled polymer (K4061, DAKO, Carpinteria, CA) and Vector Nova Red (IgM), or 3,3’-diaminobenzidine (C5b). IHC slides were counterstained with modified Schmidt’s hematoxylin. Staining was assessed for intensity (0, none; 1, minimal; 2, easily recognized, characterized by smooth linear decoration of endothelial cells (ECs); and 3, intense, granular staining of ECs) and the extent of reactivity (focal: <25 % of vessels; or diffuse: ≥25 % of vessels).
Intragraft gene expression
Intragraft gene expression was measured by qualitative RT-PCR using total RNA from left ventricle samples obtained at explant. Gene expression was assessed using 0.5 micrograms of RNA and primers for porcine adhesion proteins CD54 (forward: GGCTTGGAGGTGCTGAAATCTC, reverse: TGGCTGGCAGGACATAAGTTTG), CD106 (forward: ATACTTTGGATGGTGTTTGCCG, reverse: AACTGGGTCCTTGGGTGAGATG) and porcine β-actin (forward: AAGATCAAGATCATCGCGCCTCCA, reverse: ACTCCTGCTTGCTGATCCACATCT). Gene specific amplification was performed in a MyCycler Thermal Cycler (Bio-Rad, Hercules, CA) using one-step RT-PCR reaction (USB-Affymetrix, Santa Clara, CA) with reverse transcription performed at 42°C for 30 minutes. Amplification products were run in a 1.5% agarose gel.
Detection of Non-Gal antibody
Induced non-Gal antibody was detected by flow cytometry from serial serum dilutions (1:10 – 1:80 dilutions) to measure IgM and IgG binding to primary GTKO porcine aortic ECs. Antibody binding was detected using goat anti-Human IgG-FITC (Zymed Laboratories, San Francisco, CA) or goat anti-human IgM-FITC (Invitrogen, Life Technologies Corp. Carlsbad, CA). All flow cytometry was performed using a FACScalibur cytometer and Cellquest software (BD Pharmingen, San Diego, CA). The fold increase in antibody binding was calculated as the ratio of mean fluorescence in postoperative and pretransplant serum. Antibody bound to explanted tissue antibody was recovered as previously described.(16) The amount of IgG and IgM in the eluted material was determined by ELISA (Bethyl labs). Dilutions of recovered materials (50, 25 and 12.5%) were tested for non-Gal antibody using flow cytometry as described. Recovered non-Gal antibody reactivity was scored qualitatively with mean fluorescent antibody binding intensity less than 1.5-fold above background scored as negative, intensities between 1.5 and 2.5 scored +1, between 2.5 and 3.5 fold scored +2, and greater than 3.5 fold scored as +3. Background was set using only the secondary reagents.
Statistics
The comparison of immunosuppressive drug levels, donor age, and donor and recipient weights was performed using a Student’s t-test. P-values < 0.05 were considered statistically significant.
Acknowledgments
We wish to acknowledge the contributions of Scott Suddendorf, Paul Henke, Michael Timmons, Merilyn Walters and Junice Thompson for technical help and Karen Schumacher for writing assistance. This work was supported by the National Institute of Health Immunobiology of Xenotransplantation cooperative grant AI66310.
Funding Source: NIH Grant AI66310
Abbreviations
- CD106
vascular cell adhesion protein 1, VCAM-1
- CD54
inter-cellular adhesion molecule-1, ICAM-1
- DXR
delayed xenograft rejection
- EC
endothelial cell
- GTKO
Gal knockout
- HAR
hyperacute rejection
- hCRP
human complement regulatory proteins
- IHC
immunohistochemical
- LV
left ventricular
- PAEC
porcine aortic endothelial cell
- RV
right ventricular
Footnotes
Author Contributions:
Christopher G. A. McGregor - Participated in research design and performance; Participated in the writing of the paper. No conflict of interest.
Paul G. Stalboerger – Participated in the performance of the research; Participated in the writing of the paper. No conflict of interest.
Zeji Du - Participated in the performance of the research; Participated in the writing of the paper. No conflict of interest.
Davide Ricci - Participated in the performance of the research. No conflict of interest.
Naoto Miyagi - Participated in the performance of the research. No conflict of interest.
Elise A. Oehler - Participated in the performance of the research. No conflict of interest.
Henry D. Tazelaar- Participated in the performance of the research, data analysis and writing of the paper. No conflict of interest.
Guerard W. Byrne – Participated in research design and performance; Participated in the writing of the paper; Participated in data analysis. No conflict of interest.
References
- 1.Platt JL, Lin SS, McGregor CGA. Acute vascular rejection. Xenotransplantation. 1998;5(3):169. doi: 10.1111/j.1399-3089.1998.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 2.Cowan PJ, Roussel JC, d’Apice AJ. The vascular and coagulation issues in xenotransplantation. Curr Opin Organ Transplant. 2009;14(2):161. doi: 10.1097/mot.0b013e3283279591. [DOI] [PubMed] [Google Scholar]
- 3.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63(1):149. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- 4.Cozzi E, Yannoutsos N, Langford GA, Pino-Chavez G, Wallwork J, White DJG. Effect of transgenic expression of human decay-accelerating factor on the inhibition of hyperacute rejection of pig organs. In: Cooper DKC, Kemp E, Platt JL, White DJG, editors. Xenotransplantation. Heidelberg: Springer-Verlag; 1997. p. 665. [Google Scholar]
- 5.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logan JS, Sharma A. Potential use of genetically modified pigs as organ donors for transplantation into humans. Clin Exp Pharmacol Physiol. 1999;26:1020. doi: 10.1046/j.1440-1681.1999.03185.x. [DOI] [PubMed] [Google Scholar]
- 7.Schuurman H-J, Pino-Chavez G, Phillips MJ, Thomas L, White DJG, Cozzi E. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation. 2002;73(7):1146. doi: 10.1097/00007890-200204150-00024. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Pfeiffer S, Schroder C, et al. Coagulation cascade activation triggers early failure of pig hearts expressing human complement regulatory genes. Xenotransplantation. 2007;14(1):34. doi: 10.1111/j.1399-3089.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 10.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15(4):268. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87(6):805. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91(3):287. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and Non-Gal-Mediated Cardiac Xenograft Rejection. Transplantation. 2011 doi: 10.1097/TP.0b013e318212c7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplanted from alpha1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 2008;8(12):2516. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schirmer JM, Fass DN, Byrne GW, Tazelaar HD, Logan JS, McGregor CG. Effective antiplatelet therapy does not prolong transgenic pig to baboon cardiac xenograft survival. Xenotransplantation. 2004;11(5):436. doi: 10.1111/j.1399-3089.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 16.Byrne GW, Schirmer JM, Fass DN, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant. 2005;5(5):1011. doi: 10.1111/j.1600-6143.2005.00792.x. [DOI] [PubMed] [Google Scholar]
- 17.McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg. 2005;130(3):844. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Naziruddin B, Cui C, et al. Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75(4):430. doi: 10.1097/01.TP.0000053615.98201.77. [DOI] [PubMed] [Google Scholar]
- 19.Rood PP, Hara H, Ezzelarab M, et al. Preformed antibodies to alpha1,3-galactosyltransferase gene-knockout (GT-KO) pig cells in humans, baboons, and monkeys: implications for xenotransplantation. Transplant Proc. 2005;37(8):3514. doi: 10.1016/j.transproceed.2005.09.082. [DOI] [PubMed] [Google Scholar]
- 20.Saethre M, Baumann BC, Fung M, Seebach JD, Mollnes TE. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal-deficient endothelial cells. Transplantation. 2007;84(2):244. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172(6):1471. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne GW, Du Z, Sun Z, Asmann YW, McGregor CG. Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation. Xenotransplantation. 2011;18(1):14. doi: 10.1111/j.1399-3089.2010.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulday G, Coupel S, Coulon F, Soulillou JP, Charreau B. Antigraft antibody-mediated expression of metalloproteinases on endothelial cells. Differential expression of TIMP-1 and ADAM-10 depends on antibody specificity and isotype. Circ Res. 2001;88(4):430. doi: 10.1161/01.res.88.4.430. [DOI] [PubMed] [Google Scholar]


