Abstract
DBA/2 mice have altered hippocampal structure and perform poorly in several hippocampus-dependent contextual/spatial learning tasks. The performance of this strain in higher cognitive tasks is less studied. Transitive inference is a hippocampus-dependent task that requires an abstraction to be made from prior rules to form a new decision matrix; performance of DBA/2 mice in this task is unknown, whereas contextual fear conditioning is a hippocampus-dependent task in which DBA/2 mice have deficits. The present study compared DBA/2J and C57BL/6J inbred mice in two different contextual fear conditioning paradigms and transitive inference to test if similar deficits are seen across these hippocampus-dependent tasks. For background fear conditioning, mice were trained with two paired presentations of an auditory conditioned stimulus (CS, 30 second, 85 dB white noise) paired with an unconditioned stimulus (US, 2 second, 0.57 mA foot shock), the context was a continuous background CS. Mice were tested for contextual learning 24 hours later. Foreground fear conditioning differed in that no auditory CS was presented. For transitive inference, separate mice were trained to acquire a series of overlapping odor discrimination problems and tested with novel odor pairings that either did or did not require the use of transitive inference. DBA/2 mice performed significantly worse than the C57BL/6 in both foreground and background fear conditioning and transitive inference. These results demonstrate that the DBA/2 mice have deficits in higher-cognitive processes and suggest that similar substrates may underlie deficits in contextual learning and transitive inference.
Keywords: hippocampus, learning, fear conditioning, genetics, schizophrenia
INTRODUCTION
The study of inbred mouse strains offers an important means of understanding the contribution of genetics to behavior. After twenty generations of breeding, mice within an inbred strain are genetically identical (Lyon & Searle, 1989); thus when environmental factors are held constant differences between strains are due to genetics and the physical manifestations of those genetic differences. Inbred strain comparisons of measures of learning and memory have demonstrated that the C57BL/6 and DBA/2 inbred strains of mice perform differently on several behavioral paradigms (Logue, Paylor, & Wehner, 1997b; Owen, Logue, Rasmussen, & Wehner, 1997). DBA/2 mice show impairments in hippocampally-mediated tasks, which has led several authors (Ammassari-Teule, Tozzi, Rossi-Arnaud, Save, & Thinus-Blanc, 1995; Douglas, 1985; Paylor, Baskall, & Wehner, 1993) to consider this strain a genetic model of hippocampal dysfunction and an animal model of the cognitive deficits associated with schizophrenia (Radek et al., 2006; Stevens et al., 1996; Stevens, Kem, Mahnir, & Freedman, 1998; Stevens & Wear, 1997). Specifically, DBA/2 mice perform poorly in the Morris water maze and contextual fear conditioning, both hippocampus-dependent tasks (Logue, et al., 1997b; Nie & Abel, 2001; Owen, et al., 1997; Upchurch & Wehner, 1988a, 1988b, 1989). In contrast, DBA/2 mice were found to do as well as the C57BL/6 mice in tasks based on the formation of simple stimulus-response associations such as auditory fear conditioning (Ammassari-Teule, Passino, Restivo, & de Marsanich, 2000; Paylor, Tracy, Wehner, & Rudy, 1994); a task that is hippocampus-independent (Kim & Fanselow, 1992; Phillips & LeDoux, 1992, 1994; Rudy, 1993). These differences in performance support the proposal that strain-based abnormalities within the hippocampus contribute to the poor performance of DBA/2 when compared to the C57BL/6 strain of mice.
DBA/2 mice exhibit several hippocampal abnormalities including reduced levels of hippocampal protein kinase C (PKC) activity (Fordyce & Wehner, 1992; Wehner, Sleight, & Upchurch, 1990), altered glutamate receptor function (Sunyer, An, Kang, Hoger, & Lubec, 2009a), and abnormal hippocampal morphology (Crusio, Genthner-Grimm, & Schwegler, 1986; Heimrich, Schwegler, & Crusio, 1985; Schopke, Wolfer, Lipp, & Leisinger-Trigona, 1991). Moreover, hippocampal long-term potentiation (LTP), a cellular model of synaptic plasticity, is less persistent in DBA/2 mice than in C57BL/6 mice (Matsuyama, Namgung, & Routtenberg, 1997; Nguyen, Abel, Kandel, & Bourtchouladze, 2000) and Schaffer collateral LTP is disrupted in the DBA/2 strain (Schimanski & Nguyen, 2005). Examining how DBA/2 mice perform in hippocampus-dependent tasks can provide a better understanding of the role of the hippocampus in learning and cognition.
Contextual fear conditioning is an extensively studied task shown to require the hippocampus for proper performance (Logue, et al., 1997b; Phillips & LeDoux, 1992). Previous studies examining the performance of the DBA/2 strain in contextual fear conditioning have primarily used background contextual fear conditioning in which a tone CS is paired with a foot shock US, reducing the context to a secondary, or background stimulus (Balogh, Radcliffe, Logue, & Wehner, 2002; Logue, et al., 1997b; Nie & Abel, 2001). When foot shocks are administered without the tone, the context is more salient and becomes a foreground or primary stimulus (Odling-Smee, 1975, 1978). For background conditioning in DBA mice, it may be that the context is not as salient a cue and poor performance is due to a lack of attention rather than an inability to form the association. If this is the case, DBA/2 mice should perform better in foreground contextual fear conditioning compared to background contextual fear conditioning. However in different studies, DBA/2 mice perform poorly in background (Balogh, et al., 2002; Gerlai, 1998; Nguyen, et al., 2000; Nie & Abel, 2001; Owen, et al., 1997; Paylor, et al., 1994; Stiedl et al., 1999) and foreground (Paylor, et al., 1994; Wilson, Brodnicki, Lawrence, & Murphy, 2011) contextual fear conditioning. The present study examined both foreground and background contextual fear conditioning with the expectation that DBA/2 mice would show a similar magnitude of deficits for both tasks.
Impaired hippocampal function might be expected to alter other higher cognitive processes. One such task is transitive inference, which requires the ability to infer a relationship based upon knowledge from other learned relationships. For example, rodents learn a series of overlapping odor discrimination problems; A+B −, B+C−, C+D−, D+E−, in which “+” is the positively rewarded scent in the pair and “−” is not (Devito, Kanter, & Eichenbaum, 2010a; Dusek & Eichenbaum, 1997; Van Elzakker, O'Reilly, & Rudy, 2003). Subjects are then tested on a novel order pairing of A vs E, which does not require inference because A was always rewarded and E was never rewarded, and on a novel pairing B vs D that requires inference because one stimulus was rewarded more than the other. This task has been suggested to be hippocampus-dependent as hippocampal lesions disrupted performance on the novel transitive inference test B vs D but not the novel control test A vs E in rodents (Devito, et al., 2010a; Dusek & Eichenbaum, 1997) and imaging studies show hippocampal activation in humans during transitive inference (Greene, Gross, Elsinger, & Rao, 2006; Heckers, Zalesak, Weiss, Ditman, & Titone, 2004; Moses, Brown, Ryan, & McIntosh, 2010; Ongur et al., 2006; Wendelken & Bunge, 2009; Zalesak & Heckers, 2009). Proponents for a role for the hippocampus in transitive inference suggest that it is the requirement of subjects to make an abstraction from prior relational information that engages the hippocampus (Eichenbaum, Yonelinas, & Ranganath, 2007). This position, however, is not without some opposition as other researchers do not believe that the hippocampus is critical for successful performance of the transitive inference task (Frank, Rudy, & O'Reilly, 2003). Based on the value transfer theory proposed by von Fersen and colleagues (1991), which states that during training each stimulus attains a value through both a direct and indirect component, Frank and colleagues suggest that the task does not involve explicitly learning an ordered hierarchy but instead assigning a reinforcement value depending on how often a selection is associated with positive versus negative reinforcement (Frank, Rudy, Levy, & O'Reilly, 2005; Frank, et al., 2003; Van Elzakker, et al., 2003). This type of problem solving may engage areas other than the hippocampus (Frank, O'Reilly, & Curran, 2006). Because DBA/2 mice are deficient in hippocampus-dependent tasks, examining how DBA/2 mice perform in transitive inference could provide better understanding of the underlying substrates involved in this task. Therefore, the present study examined the performance of this strain in comparison to the C57BL/6 strain in three tasks that may engage the hippocampus - background contextual fear conditioning, foreground contextual fear conditioning, and transitive inference to test if deficits are found across the tasks for DBA/2, which could suggest similar substrates underlie the deficits.
MATERIALS AND METHODS
Subjects
Subjects were male C57BL/6 and DBA/2 mice that were 8 weeks at the start of each experiment (Jackson Laboratory, Bar Harbor, ME). Mice in the fear conditioning paradigm had ad libitum access to food and water throughout the study. Mice in the transitive inference experiment had ad libitum access to food and water until base weights were established and then food was restricted to 85% of their free fed weight for the remainder of the study. Mice were maintained on a 12:12 light/dark cycle (lights on at 7:00 am) with behavioral procedures occurring between 8:00 am and 5:00 pm. All procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus and materials
Fear Conditioning
Training and testing of fear conditioning took place in four identical conditioning chambers (18 × 19 × 38 cm) housed in sound-attenuating boxes (MED Associates, St. Albans, VT, USA). Ventilation fans at the back of the boxes provided air exchange and background noise (69 dB). A speaker mounted to the outside right wall of each chamber produced an 85-dB white noise CS. The conditioning chambers were assembled out of clear Plexiglas walls in the front and back and stainless steel on the sides. The grid floors were connected to a shock scrambler and generator. The shock US was a 0.57 mA footshock for 2 seconds. An IBM PC-compatible computer running MED-PC software interfaced with the conditioning chamber to control stimuli administration. All chambers were cleaned with 70% ethanol before and after each use.
Transitive Inference
The training and testing chamber was a clear Plexiglas box measuring 29.2 × 20.3 × 15.2 cm. The box was divided in the center by a piece of Plexiglas measuring the width and height of the box. There were two Plexiglas lids, one for each compartment of the box. The bottom of the box was lined with corncob bedding. Mice were trained to dig in small, circular cups (3.8 cm in diameter, 1.4 cm in height) filled with scented playground sand. The sand was scented with thyme, celery salt, paprika, coffee, basil, cumin, or cocoa at a 1% weight of the sand concentration. Scent selection was chosen to match prior published work (Devito, et al., 2010a; Dusek & Eichenbaum, 1997; Van Elzakker, et al., 2003). Previous work in rodents examined if there was a scent bias. Specifically, to make sure that the selection of B=coffee over D=cumin was not based on a bias, Van Elzakkar and colleagues (2003) replaced coffee with celery seed in a separate experiment and saw the same effect. Although it is possible there is a bias in mice, it is unlikely as the pattern for the learned pairs in this study is similar to studies in both rodents and pigeons (Devito, et al., 2010a; DeVito, Lykken, Kanter, & Eichenbaum, 2010b; Dusek & Eichenbaum, 1997; Van Elzakker, et al., 2003; von Fersen, et al., 1991); that is better performance in the outer pairs than the inner pairs. Finally, personal observations during the first phase of training, which would be the phase to show preferences, did not show any biases in either strain. Digging behavior was rewarded with 45 mg chocolate precision pellets (Bio-Serv, Frenchtown, NJ).
Behavioral Procedures
Fear Conditioning
Methods for training and testing mice in contextual fear conditioning were based on previous studies (André, Gulick, Portugal, & Gould, 2008; Davis, Porter, & Gould, 2006; Gould & Higgins, 2003).
Background Training
During training mice were placed into conditioning chambers for 5 minutes and 30 seconds and freezing was used as the behavioral measure of learning. Freezing was defined as the absence of movement except for respiration during a 1 second period assessed every 10 seconds (Blanchard & Blanchard, 1969). At the start of training, baseline freezing behavior was recorded for 120 seconds. Next, the CS (85 dB white noise) was presented for 30 seconds and co-terminated with a 2-second 0.57 mA US foot shock. A second CS-US pairing was presented at 270 seconds. The mice remained in the chamber 30 seconds after the second CS-US presentation. Twenty-four hours after training, mice were placed in the training chamber for 5 minutes and freezing to the context was scored.
Foreground Training
During training mice were placed in the conditioning chambers for 5 minutes and 30 seconds. Baseline freezing behavior was recorded during the first 120 seconds of the session. At 148 seconds, a 2-second 0.57 mA foot shock US was presented. At 298 seconds, an additional 2-second foot shock US was presented. The mice remained in the chamber 30 seconds after the second US presentation. Twenty-four hours after training, freezing to the context was assessed for 5 minutes.
Transitive Inference
Mice were trained as described in DeVito, Kanter, and Eichenbaum (2010a). At each phase of training, mice were given 16 trials broken up into two sessions per day: 8 trials in the first session followed by at least a one hour break then 8 trials in the second session. During training one cup was always baited with the treat. At the start of each training trail, the mouse was placed on one side of the apparatus and two cups were placed on the other. The placement of the cups was randomized within 4 quadrants to ensure that the mouse did not learn through spatial cues. The divider was lifted and the choice was registered when the mouse first placed a paw into the cup in a digging motion. If the correct cup was chosen, the mouse was allowed to dig until it retrieved the treat. Once the treat was eaten, the mouse was placed back on the other side and the diver was replaced. If the incorrect cup was chosen, immediately the mouse was gently pushed back and the divider was replaced; the mouse was not allowed to self-correct. Each trial lasted a maximum of 3 minutes. Mice were trained to reach a criterion of 75% accuracy (6/8 trials) across four consecutive sessions in order to move on the next phase of training. The mice learned to correctly infer the series A+ to E−; A=Paprika, B=Coffee, C=Basil, D=Cumin, and E=Bitter-sweet cocoa. Phase I consisted of 8 trials of presented pair A+B− and 8 trials of presented pair B+C− followed the next day by 8 trials of presented pair C+D− and 8 trials of presented pair D+E−. During Phase II, there were 4 trials of pairs A+B− and B+C− in the first session and 4 trials of pairs C+D− and D+E− in the second session. During Phase III, there were 2 trials each of A+B−, B+C−, C+D−, and D+E− in both the first and second sessions. Phase IV consisted of a pseudorandom presentation of all presented pairs intermixed with a total of 4 trials of each pair per day.
Probe trial sessions were identical to Phase IV of training except the animals were tested for transitive inference with novel pair B vs D and non-transitive inference with novel pair A vs E. During the novel pair trials the cups had no food reward and the amount of time the mouse spent digging in each cup was recorded. Percent preference was calculated using a formula developed by Bunsey and Eichenbaum (1996); for B vs D ([B−D]/[B+D]) and A vs E ([A−E]/[A+E]) where each letter corresponds to the amount of time digging in that cup. When time spent digging in B was significantly greater than fifty percent of the total digging time, this provided support for the use of transitive inference since B and D were both rewarded 50% of the time. As a comparison, digging in A over E could be guided by processes other than transitive inference because choices of A were always rewarded and choices of E were never rewarded.
Statistical Analyses
Main effects for performance in transitive inference were analyzed using one sample and independent sample t-tests, and a 2-way ANOVA (strain X trial pairing) was used to examine performance in the presented pairs. Statistical analysis was based on appropriate tests and prior studies (Devito, et al., 2010a; DeVito, et al., 2010b; Dusek & Eichenbaum, 1997; Van der Jeugd et al., 2009). Data from foreground and background contextual fear conditioning were analyzed with independent sample t-tests. Any subjects with values more than 2.5 standard deviations away from the mean were deemed outliers and excluded from analysis, which totaled one mouse throughout all experiments. All tests were performed at the p < 0.05 level using SPSS version 16.0.
RESULTS
Fear Conditioning
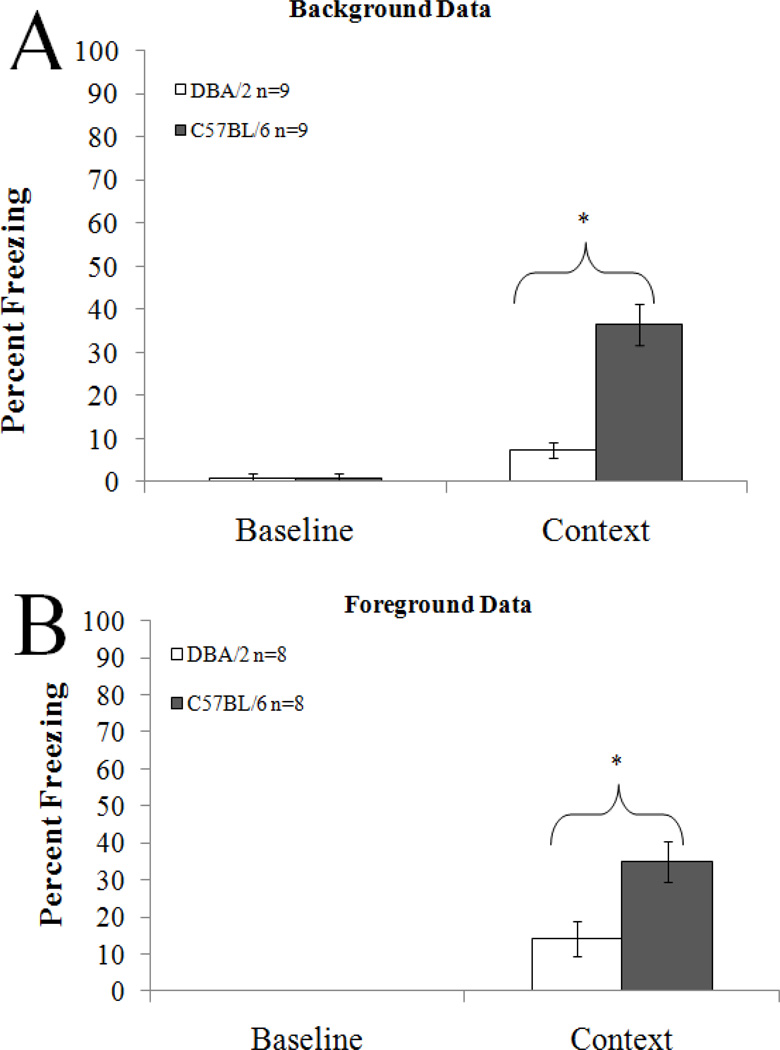
There was a significant effect of strain on background contextual fear conditioning (t(16)= 6.13 p<0.05) and on foreground contextual conditioning (t(14)=3.09 , p<0.05). In both cases, the DBA/2 mice froze significantly less to the context than the C57BL/6 mice (Figure 1). There was no difference in baseline freezing.
Figure 1.
Performance in background (A) and foreground (B) contextual fear conditioning. In both cases the DBA/2 strain performed significantly poorer than the C57BL/6 strain. Asterisks represent significance between groups at p<0.05. Error bars represent ± the SEM.
Transitive Inference
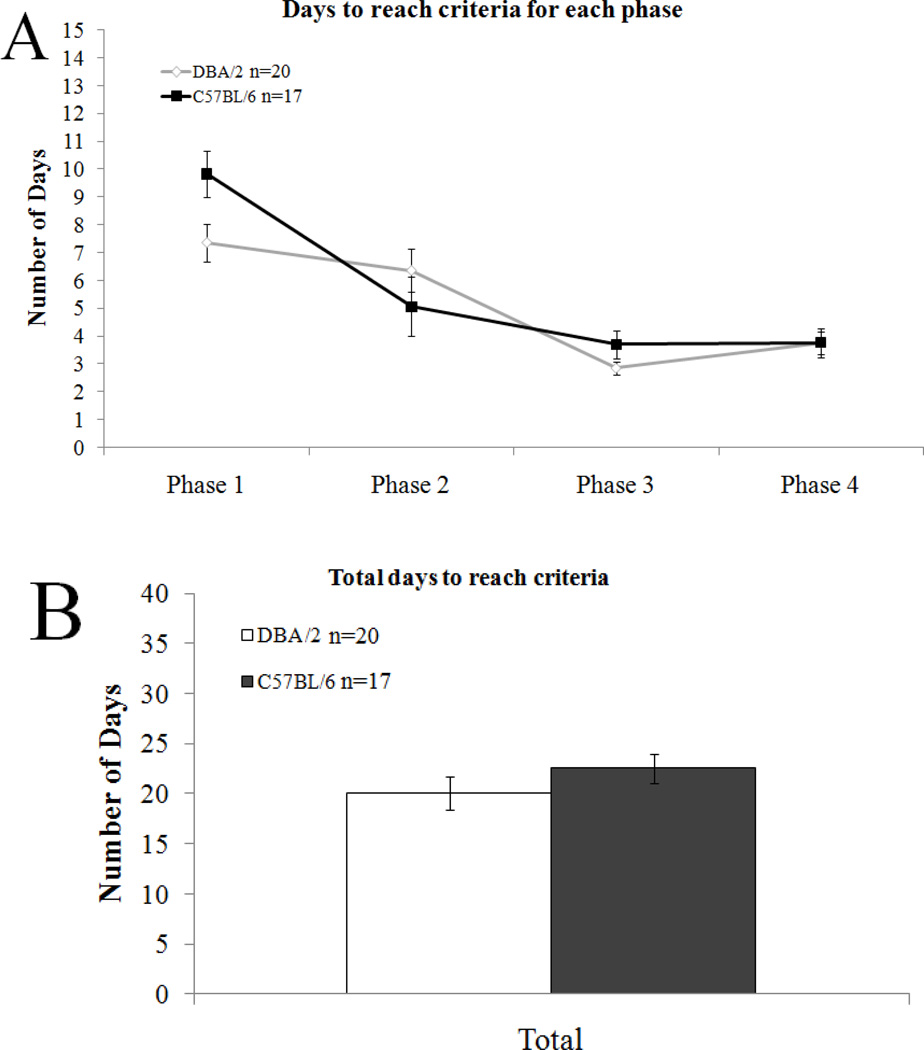
All mice successfully acquired the four presented pairs over an average of 21.27 +/− 0.78 days. For both strains, days to acquire the phase shortened as time progressed (Figure 2A). There was no significant difference between the strains in the time they took to acquire the learned pairs (C57BL/6: 22.35+/− 1.42 days, DBA/2: 20.30 +/− 0.96 days; Figure 2B).
Figure 2.
Days to criterion. A) Days to acquire each phase shortened as time progressed. B) There was no significant difference between strains in total days to reach criteria. Error bars represent ± the standard error of the mean.
Performance on presented pairs
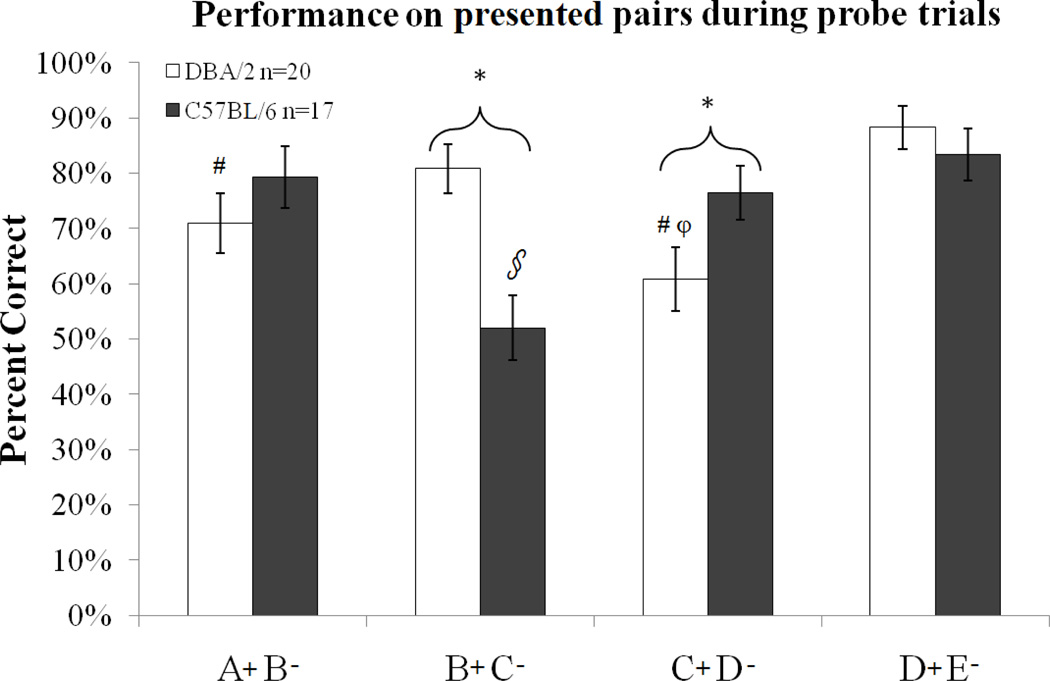
A two-way ANOVA examined the effect of strain and trial pairing on the percent correct during the probe trial sessions. There was a significant interaction between strain and pairing for percent correct (F (3,140) = 7.714, p<0.001). Simple main effects analysis showed that the DBA/2 strain performed better than the C57BL/6 strain when the pairing was B+C− (F(1,140)= 16.77, p<0.01) and worse when the pairing was C+D− ( F= (1,140)= 4.94, p<0.01) but there were no differences between strains for the outside pairs A+B−, and D+E− (p=0.236 and p=0.483, respectively). Within each strain, the C57BL/6 mice performed significantly worse on trials with presented pairs B+C− than trials with all other pairings (t(32)= 3.82, p<0.05; t(32)=3.62, p<0.05; and t(32)=4.96, p<0.05, respectively). The DBA/2 mice performed significantly better for trials with presented pairs B+C− and D+E− than ones with pair C+D− (t(38)=2.97, p<0.05) and trials with pair D+E− than ones with pair A+B− (t(38)=4.48, p<0.05, t(38)= 2.81, p<0.05; Figure 3).
Figure 3.
Performance on presented pairs during probe trials. There was a significant interaction between strain and pairing for percent correct. The DBA/2 strain performed better than the C57BL/6 strain when the pairing was B+C− and worse when the pairing was C+D−. Asterisks represent a significant main effect differences between strains. Furthermore, the C57BL/6 mice performed significantly worse on trials with pairing B+C− than trials with all other pairings, represented by the § symbol. The DBA/2 mice performed significantly better for trials with pairings B+C− and D+E− than ones with pairing C+D− and trials with pairing D+E− than ones with pairing A+B−. Within the DBA/2 strain, # represents a significant difference from D+E−; φ represents a significant difference from B+C−. All differences are at p<0.05. Error bars represent ± the standard error of the mean.
Performance on novel transitive inference pairs
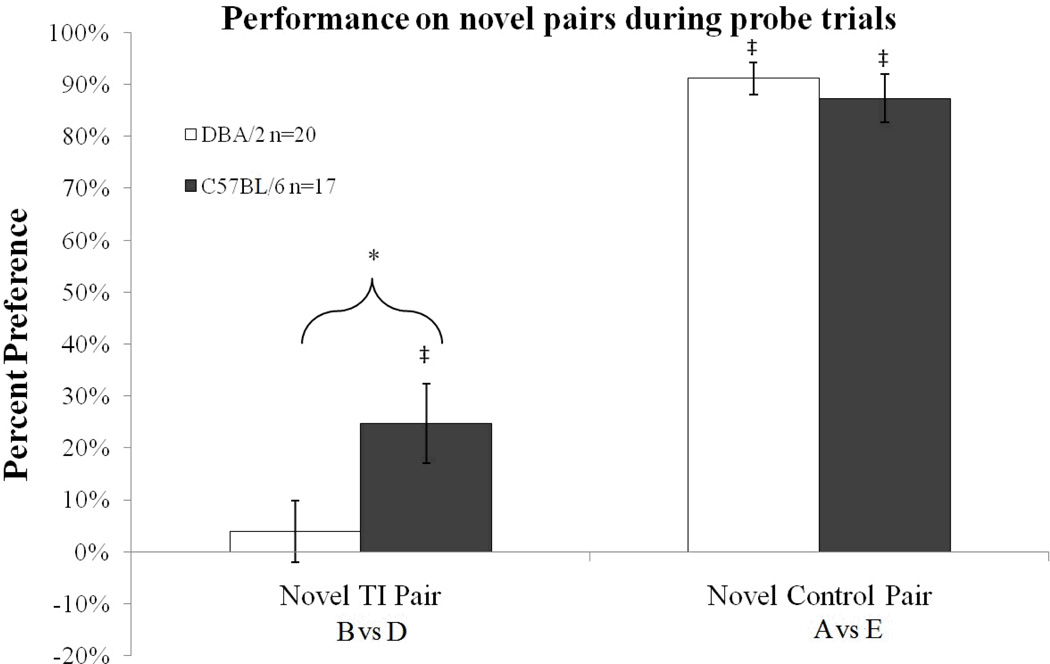
Performance on the novel transitive inference pairing B vs D and the novel control pairing A vs E was above chance for the C57BL/6 strain (t(16)=3.43, p<0.05 and t(16)=19.13, p<0.05, respectively). The DBA/2 mice performed above chance on the novel control pairing A vs E (t(19)=30.63, p<0.05) but did not perform above chance on the novel transitive inference pairing B vs D (p>0.5). Furthermore, the DBA/2 mice performed significantly worse than the C57BL/6 mice in the B vs D trials (t(35)= 2.26, p<0.05) but not trials A vs E (Figure 4).
Figure 4.
Performance on the novel pairs during probe trials. Performance was significantly different from chance for the C57BL/6 strain in both the novel transitive inference and control pairs but was only above chance for the DBA/2 strain in the novel control pair. DBA/2 strain performed significantly worse than the C57BL/6 strain in the novel transitive inference pair B vs D There were no differences between strains for the novel control pair A vs E. Error bars represent ± the standard error of the mean. Asterisks represent significance between groups at p<0.05. ‡ represents significantly different from chance.
DISCUSSION
The present study found that DBA/2 mice had deficits in both foreground and background contextual fear conditioning compared to C57BL/6 mice, and that the DBA/2 mice did not show transitive inference whereas C57BL/6 mice did. Specifically, for the novel pairing B vs D, digging in B was not significantly greater than fifty percent of the total digging time for the DBA/2 strain. These deficits do not reflect global cognitive deficits as DBA/2 mice are able to perform other cognitive tasks; DBA/2 mice preformed the novel control pairing (A vs E) similar to C57BL/6 mice and prior work has shown that DBA/2 mice learn cued fear conditioning (Paylor, et al., 1994). Both the novel control pairing and cued fear conditioning can be acquired without the hippocampus (Dusek & Eichenbaum, 1997; Logue, et al., 1997b; Phillips & LeDoux, 1992). The fact that DBA/2 mice performed poorly in all three hippocampus-dependent tasks strongly suggests that genetic-based hippocampal abnormalities in this strain may be a common underlying cause of the deficits. The present findings are consistent with theories on the role of the hippocampus in contextual learning (for reviews see (Anagnostaras, Gale, & Fanselow, 2001; Maren & Holt, 2000; Rudy, Huff, & Matus-Amat, 2004). Data suggest that the hippocampus is normally involved in contextual fear conditioning (Kim & Fanselow, 1992; Phillips & LeDoux, 1992, 1994; Rudy, 1993). However, when the hippocampus is absent, such as when the hippocampus is lesioned before training, other areas such as the cortex and amygdala may compensate allowing learning to occur (For review see (Rudy, et al., 2004; Sanders, Wiltgen, & Fanselow, 2003). While the DBA/2 mice have hippocampal dysfunction (Crusio, et al., 1986; Fordyce & Wehner, 1992; Heimrich, et al., 1985; Schopke, et al., 1991; Sunyer, et al., 2009a; Wehner, et al., 1990), a present but dysfunctional hippocampus may not result in the same behavioral outcome as an absent hippocampus. That is, a dysfunctional hippocampus may be more detrimental, but this requires further examination.
Phenotypic comparisons across inbred strains can be used to determine if common genetic and neural substrates regulate different behaviors (Logue, Owen, Rasmussen, & Wehner, 1997a; Owen, et al., 1997; Portugal, Wilkinson, Kenney, Sullivan, & Gould, 2011; Tarantino, Gould, Druhan, & Bucan, 2000). The neurochemical basis for the poor performance of the DBA/2 mice is likely to be polygenic and multifaceted, but possible mechanisms include altered hippocampal PKC activity and glutamate receptor function. Compared to C57BL/6 mice, DBA/2 mice showed deficits in a hippocampus-dependent version of the Morris water maze and reduced particulate, but not cytosolic, hippocampal PKC; and analysis of 11 C57BL/6J × DBA/2J recombinant inbred strains further revealed a significant correlation between hippocampal PKC activity and accuracy in hippocampus-dependent learning, no significant correlation was found for cortex (Wehner, et al., 1990). In further support of altered hippocampal PKC signaling contributing to deficits in learning in DBA/2 mice, administration of nootropic drugs aniracetam and oxiracetam improved hippocampus-dependent learning and increased hippocampal γ-PKC activation and membrane bound PKC (Fordyce, Clark, Paylor, & Wehner, 1995; Lucchi, Pascale, Battaini, Govoni, & Trabucchi, 1993; Smith & Wehner, 2002). Further comparisons of DBA/2 and C57BL/6 mice confirmed differences in hippocampal γ-PKC activity and found restriction fragment length polymorphisms in noncoding and nonregulatory regions of α- and γ –PKC gene (Bowers et al., 1995).
The changes in PKC signaling may lead to further disruption of plasticity-related cellular cascades causing deficits in synaptic plasticity and learning. GAP-43 is a substrate of PKC that has been implicated in synaptic plasticity. Compared to C57BL/6 mice, DBA/2 mice had reduced basal levels of phosphorylated GAP-43 and did not show changes in GAP-43 after hippocampus-dependent learning (Young, Owen, Meiri, & Wehner, 2000). Furthermore, phosphorylation of hippocampal cAMP-responsive element-binding protein (CREB), a gene transcription factor implicated in long-term memory formation (Dash, Hochner, & Kandel, 1990) and regulated by hippocampal PKC (Roberson et al., 1999), was increased in C57BL/6 but not DBA/2 mice after hippocampus-dependent learning (Hwang et al., 2010). Changes in PKC-regulated synaptic plasticity may underlie the altered learning as DBA/2 mice showed a pattern of deficits in hippocampal LTP that Matsuyama and colleagues suggested was related to altered PKC maintenance of long-term plasticity (Matsuyama, et al., 1997).
In addition to changes in PKC signaling, DBA/2 mice show other neural changes that could contribute to hippocampus-dependent learning deficits. DBA/2 mice have decreased calcium-mediated regulation of AMPA binding in the hippocampus compared to C57BL/6 mice (Menard, Valastro, Martel, Martinoli, & Massicotte, 2004). Furthermore, hippocampal protein levels of NMDA receptor subunit 1 were lower while GABAB receptor subunit 2 levels were higher in DBA/2 mice compared to C57BL/6 (Sunyer, Shim, An, Hoger, & Lubec, 2009b). Changes in glutamate signaling could alter synaptic plasticity in DBA/2 mice as DBA/2 mice showed reduced hippocampal expression of zif268, a transcription factor associated with NMDA receptor signaling and synaptic plasticity (Fordyce, Bhat, Baraban, & Wehner, 1994). Thus, genetic differences between DBA/2 mice and C57BL/6 mice result in multiple changes in hippocampal cell signaling cascades that may underlie synaptic plasticity. These changes could contribute to the hippocampus-dependent learning and cognitive deficits observed in the present study, though DBA/2 mice do show other differences such as altered dopamine receptor and mu-opioid receptor function (Bailey et al., 2010; Colelli, Fiorenza, Conversi, Orsini, & Cabib, 2010; McNamara et al., 2006; Ng, O'Dowd, & George, 1994).
The DBA/2 strain performed better than the C57BL/6 strain in the B+C− learned pair but the opposite was true for C+D− learned pair. In general for this task, a correct choice in the internal pairs B+C− and C+D− is considered more difficult than the external pairs A+B− and D+E− because all of the internal pairs yield reward fifty percent of the time. Lower performance in the internal pairs is not indicative of deficits in transitive inference as C57BL/6 mice performed at this level on the internal pairs in prior studies and also showed transitive inference (Devito, et al., 2010a; DeVito, et al., 2010b). The fact that the percent correct for the B+C− pair is higher in the DBA/2 strain and not both internal pairs was unexpected. It may be that the choices made for the learned pairs during probe trials in the DBA/2 strain are the result of choosing based on reinforcement values that are learned implicitly (Frank, et al., 2003; Van Elzakker, et al., 2003). In this manner the hierarchy is not learned; however, learning B+C− would be easier than learning C+D− and percent correct for the B+C− pair would be higher than for the C+D− pair; which is exactly what is shown. Further research is needed to uncover what exactly causes the higher percent correct in the B+C− pair in this strain.
In summary, this is the first study to examine the performance of DBA/2 mice in transitive inference. The poor performance of the DBA/2 strain, which as discussed has clear deficits in hippocampal function, combined with hippocampal lesion studies in rats and C57BL/6 mice support the position that the hippocampus is critically involved in transitive inference (Devito, et al., 2010a; Dusek & Eichenbaum, 1997). The deficit in transitive inference matches the observed deficits in both foreground and background contextual fear conditioning. Our fear conditioning results replicate prior work showing hippocampus-dependent deficits in DBA/2 mice (Balogh, et al., 2002; Logue, et al., 1997b; Nie & Abel, 2001; Stiedl, et al., 1999). By comparing DBA/2 and C57BL/6 performance in foreground contextual fear conditioning, background contextual fear conditioning, and transitive inference, the present study indicates that common neural and genetic substrates may regulate these behaviors.
Acknowledgements
We would like to acknowledge grant support from the National Institute on Drug Abuse and the National Cancer Institute (DA024787, TJG; CA143187, PI Caryn Lerman).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Ammassari-Teule, Passino E, Restivo L, de Marsanich B. Fear conditioning in c57/bl/6 and dba/2 mice: Variability in nucleus accumbens function according to the strain predisposition to show contextual- or cue-based responding. The European journal of neuroscience. 2000;12(12):4467–4474. [PubMed] [Google Scholar]
- Ammassari-Teule, Tozzi A, Rossi-Arnaud C, Save E, Thinus-Blanc C. Reactions to spatial and nonspatial change in two inbred strains of mice: Further evidence supporting the hippocampal dysfunction hypothesis in the dba:2 strain. Psychobiology. 1995;23:284–289. [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in c57bl/6 mice. Behav Brain Res. 2008;190(2):174–181. doi: 10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Al-Hasani R, Keyworth HL, Forster DM, Kitchen I. Mouse strain differences in locomotor, sensitisation and rewarding effect of heroin; association with alterations in mop-r activation and dopamine transporter binding. The European journal of neuroscience. 2010;31(4):742–753. doi: 10.1111/j.1460-9568.2010.07104.x. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Radcliffe RA, Logue SF, Wehner JM. Contextual and cued fear conditioning in c57bl/6j and dba/2j mice: Context discrimination and the effects of retention interval. Behav Neurosci. 2002;116(6):947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67(3):370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Christensen SC, Pauley JR, Paylor R, Yuva L, Dunbar SE, Wehner JM. Protein and molecular characterization of hippocampal protein kinase c in c57bl/6 and dba/2 mice. Journal of neurochemistry. 1995;64(6):2737–2746. doi: 10.1046/j.1471-4159.1995.64062737.x. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379(6562):255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Colelli V, Fiorenza MT, Conversi D, Orsini C, Cabib S. Strain-specific proportion of the two isoforms of the dopamine d2 receptor in the mouse striatum: Associated neural and behavioral phenotypes. Genes, brain, and behavior. 2010;9(7):703–711. doi: 10.1111/j.1601-183X.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Genthner-Grimm G, Schwegler H. A quantitative-genetic analysis of hippocampal variation in the mouse. J Neurogenet. 1986;3(4):203–214. doi: 10.3109/01677068609106850. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the camp-responsive element into the nucleus of aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345(6277):718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394(3):202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devito LM, Kanter BR, Eichenbaum H. The hippocampus contributes to memory expression during transitive inference in mice. Hippocampus. 2010a;20(1):208–217. doi: 10.1002/hipo.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Lykken C, Kanter BR, Eichenbaum H. Prefrontal cortex: Role in acquisition of overlapping associations and transitive inference. Learn Mem. 2010b;17(3):161–167. doi: 10.1101/lm.1685710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. The development of the hippocampal function: Implications for theory and therapy. In: Isaacson R, Pribram K, editors. The hippocampus. Vol. 2. New York: New York: Academic Press; 1985. pp. 309–326. [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci U S A. 1997;94(13):7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce DE, Bhat RV, Baraban JM, Wehner JM. Genetic and activity-dependent regulation of zif268 expression: Association with spatial learning. Hippocampus. 1994;4(5):559–568. doi: 10.1002/hipo.450040505. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Clark VJ, Paylor R, Wehner JM. Enhancement of hippocampally-mediated learning and protein kinase c activity by oxiracetam in learning-impaired dba/2 mice. Brain Res. 1995;672(1–2):170–176. doi: 10.1016/0006-8993(94)01389-y. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Determination of hippocampal protein kinase c using a frozen tissue method: Comparison of synaptosomal and total activity in c57bl/6 and dba/2 mice. Biochem Biophys Res Commun. 1992;188(2):690–694. doi: 10.1016/0006-291x(92)91111-3. [DOI] [PubMed] [Google Scholar]
- Frank MJ, O'Reilly RC, Curran T. When memory fails, intuition reigns: Midazolam enhances implicit inference in humans. Psychol Sci. 2006;17(8):700–707. doi: 10.1111/j.1467-9280.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, Levy WB, O'Reilly RC. When logic fails: Implicit transitive inference in humans. Mem Cognit. 2005;33(4):742–750. doi: 10.3758/bf03195340. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, O'Reilly RC. Transitivity, flexibility, conjunctive representations, and the hippocampus. Ii. A computational analysis. Hippocampus. 2003;13(3):341–354. doi: 10.1002/hipo.10084. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Contextual learning and cue association in fear conditioning in mice: A strain comparison and a lesion study. Behav Brain Res. 1998;95(2):191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in c57bl/6j mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80(2):147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. An fmri analysis of the human hippocampus: Inference, context, and task awareness. J Cogn Neurosci. 2006;18(7):1156–1173. doi: 10.1162/jocn.2006.18.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14(2):153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Heimrich B, Schwegler H, Crusio WE. Hippocampal variation between the inbred mouse strains c3h/hej and dba/2: A quantitative-genetic analysis. J Neurogenet. 1985;2(6):389–401. doi: 10.3109/01677068509101425. [DOI] [PubMed] [Google Scholar]
- Hwang YK, Song JC, Han SH, Cho J, Smith DR, Gallagher M, Han JS. Differences in hippocampal creb phosphorylation in trace fear conditioning of two inbred mouse strains. Brain Res. 2010;1345:156–163. doi: 10.1016/j.brainres.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and f1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997a;80(4):1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the morris water maze and conditioned-fear task. Behav Neurosci. 1997b;111(1):104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lucchi L, Pascale A, Battaini F, Govoni S, Trabucchi M. Cognition stimulating drugs modulate protein kinase c activity in cerebral cortex and hippocampus of adult rats. Life sciences. 1993;53(24):1821–1832. doi: 10.1016/0024-3205(93)90490-t. [DOI] [PubMed] [Google Scholar]
- Lyon M, Searle A. Genetic variants and strains of the laboratory mouse. 2nd ed. Oxford: Oxford University Press; 1989. [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in pavlovian conditioning. Behav Brain Res. 2000;110(1–2):97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Namgung U, Routtenberg A. Long-term potentiation persistence greater in c57bl/6 than dba/2 mice: Predicted on basis of protein kinase c levels and learning performance. Brain Res. 1997;763(1):127–130. doi: 10.1016/s0006-8993(97)00444-7. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Levant B, Taylor B, Ahlbrand R, Liu Y, Sullivan JR, Richtand NM. C57bl/6j mice exhibit reduced dopamine d3 receptor-mediated locomotor-inhibitory function relative to dba/2j mice. Neuroscience. 2006;143(1):141–153. doi: 10.1016/j.neuroscience.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Valastro B, Martel MA, Martinoli MG, Massicotte G. Strain-related variations of ampa receptor modulation by calcium-dependent mechanisms in the hippocampus: Contribution of lipoxygenase metabolites of arachidonic acid. Brain Res. 2004;1010(1–2):134–143. doi: 10.1016/j.brainres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Moses SN, Brown TM, Ryan JD, McIntosh AR. Neural system interactions underlying human transitive inference. Hippocampus. 2010;20(8):894–901. doi: 10.1002/hipo.20735. [DOI] [PubMed] [Google Scholar]
- Ng GY, O'Dowd BF, George SR. Genotypic differences in brain dopamine receptor function in the dba/2j and c57bl/6j inbred mouse strains. European journal of pharmacology. 1994;269(3):349–364. doi: 10.1016/0922-4106(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in ltp and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7(3):170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie T, Abel T. Fear conditioning in inbred mouse strains: An analysis of the time course of memory. Behav Neurosci. 2001;115(4):951–956. doi: 10.1037//0735-7044.115.4.951. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. Background stimuli and the inter-stimulus interval during pavlovian conditioning. Q J Exp Psychol. 1975;27(3):387–392. doi: 10.1080/14640747508400498. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. The overshadowing of background stimuli: Some effects of varying amounts of training and ucs intensity. Q J Exp Psychol. 1978;30(4):737–746. doi: 10.1080/14640747808400698. [DOI] [PubMed] [Google Scholar]
- Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63(4):356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the morris water task and fear conditioning in inbred mouse strains and f1 hybrids: Implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80(4):1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Paylor R, Baskall L, Wehner J. Behavioral dissociations between c57bl/6 and dba/2 mice on learning and memory tasks: A hippocampal-dysfunction hypothesis. Psychobiology. 1993;21:11–26. [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. Dba/2 and c57bl/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108(4):810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1(1):34–44. [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Kenney JW, Sullivan C, Gould TJ. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav Genet. 2011 doi: 10.1007/s10519-011-9489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decker MW, Gopalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the dba/2 mouse model of sensory gating. Psychopharmacology (Berl) 2006;187(1):47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples pka and pkc to camp response element binding protein phosphorylation in area ca1 of hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(11):4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107(5):887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: Insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. European journal of pharmacology. 2003;463(1–3):217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Schimanski LA, Nguyen PV. Impaired fear memories are correlated with subregion-specific deficits in hippocampal and amygdalar ltp. Behav Neurosci. 2005;119(1):38–54. doi: 10.1037/0735-7044.119.1.38. [DOI] [PubMed] [Google Scholar]
- Schopke R, Wolfer DP, Lipp HP, Leisinger-Trigona MC. Swimming navigation and structural variations of the infrapyramidal mossy fibers in the hippocampus of the mouse. Hippocampus. 1991;1(3):315–328. doi: 10.1002/hipo.450010322. [DOI] [PubMed] [Google Scholar]
- Smith AM, Wehner JM. Aniracetam improves contextual fear conditioning and increases hippocampal gamma-pkc activation in dba/2j mice. Hippocampus. 2002;12(1):76–85. doi: 10.1002/hipo.10008. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15(2):152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in dba mice. Psychopharmacology (Berl) 1998;136(4):320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol Biochem Behav. 1997;57(4):869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Radulovic J, Lohmann R, Birkenfeld K, Palve M, Kammermeier J, Spiess J. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav Brain Res. 1999;104(1–2):1–12. doi: 10.1016/s0166-4328(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sunyer B, An G, Kang SU, Hoger H, Lubec G. Strain-dependent hippocampal protein levels of gaba(b)-receptor subunit 2 and nmda-receptor subunit 1. Neurochemistry international. 2009a;55(4):253–256. doi: 10.1016/j.neuint.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Sunyer B, Shim KS, An G, Hoger H, Lubec G. Hippocampal levels of phosphorylated protein kinase a (phosphor-s96) are linked to spatial memory enhancement by sgs742. Hippocampus. 2009b;19(1):90–98. doi: 10.1002/hipo.20484. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: The importance of baseline analysis of inbred strains. Mammalian genome : official journal of the International Mammalian Genome Society. 2000;11(7):555–564. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- Upchurch M, Wehner JM. Dba/2ibg mice are incapable of cholinergically-based learning in the morris water task. Pharmacol Biochem Behav. 1988a;29(2):325–329. doi: 10.1016/0091-3057(88)90164-5. [DOI] [PubMed] [Google Scholar]
- Upchurch M, Wehner JM. Differences between inbred strains of mice in morris water maze performance. Behav Genet. 1988b;18(1):55–68. doi: 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- Upchurch M, Wehner JM. Inheritance of spatial learning ability in inbred mice: A classical genetic analysis. Behav Neurosci. 1989;103(6):1251–1258. doi: 10.1037//0735-7044.103.6.1251. [DOI] [PubMed] [Google Scholar]
- Van der Jeugd A, Goddyn H, Laeremans A, Arckens L, D'Hooge R, Verguts T. Hippocampal involvement in the acquisition of relational associations, but not in the expression of a transitive inference task in mice. Behav Neurosci. 2009;123(1):109–114. doi: 10.1037/a0013990. [DOI] [PubMed] [Google Scholar]
- Van Elzakker M, O'Reilly RC, Rudy JW. Transitivity, flexibility, conjunctive representations, and the hippocampus. I. An empirical analysis. Hippocampus. 2003;13(3):334–340. doi: 10.1002/hipo.10083. [DOI] [PubMed] [Google Scholar]
- von Fersen L, Wynne D, Delius J, Staddon J. Transitive infernence formation in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17(3):334–341. [Google Scholar]
- Wehner JM, Sleight S, Upchurch M. Hippocampal protein kinase c activity is reduced in poor spatial learners. Brain Res. 1990;523(2):181–187. doi: 10.1016/0006-8993(90)91485-y. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA. Transitive inference: Distinct contributions of rostrolateral prefrontal cortex and the hippocampus. J Cogn Neurosci. 2009;22(5):837–847. doi: 10.1162/jocn.2009.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson YM, Brodnicki TC, Lawrence AJ, Murphy M. Congenic mouse strains enable discrimination of genetic determinants contributing to fear and fear memory. Behav Genet. 2011;41(2):278–287. doi: 10.1007/s10519-010-9387-4. [DOI] [PubMed] [Google Scholar]
- Young EA, Owen EH, Meiri KF, Wehner JM. Alterations in hippocampal gap-43 phosphorylation and protein level following contextual fear conditioning. Brain Res. 2000;860(1–2):95–103. doi: 10.1016/s0006-8993(00)02021-7. [DOI] [PubMed] [Google Scholar]
- Zalesak M, Heckers S. The role of the hippocampus in transitive inference. Psychiatry Res. 2009;172(1):24–30. doi: 10.1016/j.pscychresns.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]