Abstract
Objective
Previous studies have suggested that delayed gastric emptying and abnormal postprandial release of hormones that influence satiation, particularly cholecystokinin (CCK), may play an important role in the pathophysiology of bulimia nervosa (BN). This study was designed to test these hypotheses as well as the efficacy of the prokinetic agent erythromycin in patients with BN.
Method
Thirty-two normal weight women with BN and 24 control participants consumed a large liquid test meal. Gastric emptying and pre- and postprandial release of CCK, peptide YY (PYY), and ghrelin were determined. Participants with BN were then recruited for double-blind treatment with erythromycin up to 500 mg three times daily vs. placebo for six weeks, following which they consumed a repeat test meal with gastric emptying and appetitive hormone measurements.
Results
CCK release at 15 minutes following the meal was marginally lower(p = 0.1) in BN than in control participants. Rate of gastric emptying and postprandial hormone release were similar in BN and controls. BN patients assigned to erythromycin compared to those assigned to placebo had more rapid gastric emptying following treatment, but there were no differences in release of CCK, PYY, or ghrelin following the post-treatment test meal. Moreover, treatment with erythromycin was not associated with clinical response.
Discussion
The current study does not support the clinical utility of moderate dose erythromycin in treating BN. Furthermore, the findings suggest that a modest increase in gastric emptying rate is associated neither with altered postprandial hormonal release nor with clinical benefit in these patients. While providing no evidence for the effectiveness of prokinetic agents in this setting, our findings do not preclude the possibility that a greater increase in gastric emptying rate might prove beneficial.
Keywords: Bulimia nervosa, Eating laboratory, Eating disorders, Pharmacotherapy, Gastric emptying, Cholecystokinin
1. INTRODUCTION
The characteristic eating behavior of individuals with bulimia nervosa (BN), i.e. regularly occurring episodes of uncontrolled eating followed by vomiting or other forms of attempted compensation, may reflect an abnormality in the development of satiation over the course of a binge meal, particularly during its latter portion [1]. Investigations of the gastrointestinal physiology of individuals with BN have revealed abnormalities in postprandial release of cholecystokinin (CCK) and other appetitive hormones [2], gastric emptying [3,4], and gastric motility [5] following ingestion that may underlie the observed abnormality in satiation. We and others have proposed a model in which slowed gastric emptying contributes to decreased postprandial CCK release and blunted subjective sense of satiety, leading to uncontrolled overconsumption and possibly to compensatory purging [3,4,6]. The link between overconsumption and compensatory purging is at least in part attributable to overconcern with shape and weight, i.e. fear of weight-gain resulting from overconsumption, and the extreme discomfort that often follows binge eating [7]. If these behaviors directly or indirectly lead to further slowing of gastric emptying, a positive feedback loop may work to maintain the disorder.
This model suggests a crucial role for delayed gastric emptying in the causal chain that leads to maintenance of the disorder, and predicts that increasing gastric emptying in individuals with bulimia nervosa would be helpful in restoring normal satiation and in reducing binge-purge behavior. There has been one controlled study of the prokinetic agent cisapride for patients with anorexia nervosa, some with concurrent bulimic symptoms, that suggested at least a marginal effect of cisapride on subjective symptoms [8]. However, a definitive test of the model, particularly of the prediction that increasing gastric emptying would have therapeutic benefit in patients with bulimia nervosa, has not yet been conducted.
The current study was designed to assess the efficacy in patients with BN of a treatment that increases gastric emptying. We hypothesized that a medication that increased gastric emptying would: (1) partially or completely normalize postprandial appetitive hormone responses and (2) lead to clinical improvement over time. To increase gastric emptying, we used erythromycin, an antibiotic that is also an agonist of motilin and has been used to increase gastric empyting in patients with delayed gastric emptying due to gastroparesis in diabetes mellitus or postsurgical states [9]. We chose not to use cisapride due to its association with QT prolongation [10]. Additional aims of the study were to replicate previous findings regarding postprandial gastric emptying and CCK release in individuals with BN, and to examine postprandial release patterns of peptide YY (PYY) and ghrelin, appetitive hormones that have received relatively little study in these individuals [11,12].
2. MATERIALS AND METHODS
2.1 Participants
We recruited 32 normal-weight women with BN and 24 normal-weight female control participants matched for age and ethnicity. Participants with BN were recruited from the Eating Disorders Research Clinic at New York State Psychiatric Institute. All participants with BN met the following inclusion criteria: (1) DSM-IV criteria for BN [7] ascertained via the Eating Disorder Examination (EDE) [13] and clinical interview; (2) duration of BN at least 1 year; (3) purging via self-induced vomiting; (4) age 18–45 years; (5) 85–120% of ideal body weight. We excluded patients with major Axis I psychiatric disorders, ascertained via the Structured Clinical Interview for DSM-IV (SCID) [14], other than depression as well as those with recent suicide attempts. Patients taking antidepressant or other psychotropic medications known to affect appetite were excluded, as were patients whose depression warranted immediate treatment with antidepressants. We also excluded those with known intolerance to erythromycin, current pregnancy or lactation, unwillingness to use an effective method of birth control, or conduction delay on electrocardiogram. Normal-weight controls were recruited from the Columbia University and the Columbia Presbyterian Medical Center campuses, and were paid for their participation. All control participants met the following inclusion criteria: (1) no current or past psychiatric disorder; (2) 85–120% of ideal body weight for all of adult life excluding pregnancy based on 1959 Metropolitan Life Insurance standards [REF – See Zimmerli et al., 2006, Ref #18]; (3) age 18–45 years; (4) no history of binge eating or self-induced vomiting. We excluded subjects who were taking medication, who had recent histories of abusing alcohol or other drugs, or who were medically ill. In addition to clinical interview, SCID, and EDE, baseline assessment included measurement of vital signs, completion of the Beck Depression Inventory (BDI) [15] and Eating Inventory (EI) [16] and, for BN patients, electrocardiogram. Recruitment of participants for this study was approved by the New York State Psychiatric Institute and St. Luke’s-Roosevelt Hospital Center Institutional Review Boards. The study is registered with ClinicalTrials.gov (NCT ID: NCT00304187).
2.2 Gastric Emptying and Appetitive Hormone Levels
BN and control participants underwent one day of gastric emptying and appetitive hormone testing at baseline, and BN participants underwent a second day of testing following treatment. The end of treatment study was scheduled during the sixth week of the medication trial (see below).
On the evening before the test day, participants were instructed to eat a standardized dinner that did not include alcohol, no later than 7:00 P.M., and not to eat or drink after 9:00 P.M. Participants reported to the nuclear medicine department at 9:00 AM. An indwelling catheter was placed in a forearm vein for periodic blood sampling. Participants lay in a semi-reclining position with the gamma camera placed directly over the stomach and were asked to consume 600 ml Ensure-Plus (1.5 kcal/g) labeled with 50 μCuries of Technetium-99-DTPA in 5 minutes. This meal has been used in our previous studies of gastric emptying and CCK release, which found significant differences in CCK release between patients with BN and normal controls [4]. Gastric emptying was determined by gamma camera scintigraphy using standard procedures as described previously [4]. Blood samples were be drawn at 20 minutes, 10 minutes, and immediately before food consumption and then at 5 minutes, 10 minutes, 15 minutes and every 15 minutes thereafter until 90 minutes after food consumption. Blood samples were collected in tubes prepared with EDTA and aprotinin (Trasylol) and kept on ice for 2 minutes. The samples were cold centrifuged for 15 minutes, and plasma stored at −80°C.
Plasma CCK, PYY, and ghrelin concentrations were obtained by radioimmunoassay [3]. CCK was analyzed using an RIA kit from ALPCO (standard range 0.4 – 25.0 pmol/L, intra-assay CV = 5.1, interassay CV = 6.1), based on Rehfield [17] that detects the bioactive forms of human CCK, including CCK-58, CCK-32, CCK-22, and CCK-8. Total PYY (1–36 and 3–36) concentrations were measured using an RIA kit from Millipore (standard range 2.6 – 337.5 pmol/L, intra-assay CV = 3.94, interassay CV = 5.89), and total ghrelin (acylated and desacylated) was measured using an assay from Phoenix Pharmaceuticals (standard range 3.0 – 379.0 pmol/L, intra-assay CV = 4.77, interassay CV = 4.53). All hormone assays were performed in duplicate and values were averaged. Preprandial baseline levels of hormones were calculated by averaging the levels obtained at 20 minutes, 10 minutes, and immediately preceding meal consumption. Area under the curve for 0–90 minutes following the meal was determined using the trapezoidal rule. For CCK, in addition to area under the curve, the 15-minute time point was selected for comparison between patients and control participants to capture the presumed peak postprandial response based on previous findings [4].
2.3 Randomized Double-Blind Treatment with Erythromycin or Placebo
Within one week of completing the pre-treatment measures, patients were seen by a psychiatrist at the Eating Disorders Clinic at NYS Psychiatric Institute who initiated treatment with either erythromycin or matching placebo in randomized double-blind fashion. Patients were treated for six weeks. At session #1, patients were instructed to begin erythromycin stearate 250 mg or matching placebo three times a day one half hour before meals. They were instructed by the research psychiatrist to eat three meals per day in order to maximize the effectiveness of the medication on gastric emptying. Patients were seen weekly by the study psychiatrist for brief medication management sessions to assess clinical state, medication adherence, and adverse events. Dosage could be decreased if the patient reported intolerable side effects to the medication, or could be increased to 500 mg three times a day after a two-week period with no improvement of symptoms. At session #7, following the final assessment, the blind was broken by a non-study clinician and patients were informed of their medication assignment. Patients were then referred to a non-study clinician for further treatment. Following our standard procedures, all patients were asked to keep a diary of the number of daily binge eating and vomiting episodes, which was collected at each clinic visit and used to assess their clinical state.
2.4 Statistical Analyses
All comparisons between groups utilized two-tailed t-tests to compare mean values in BN vs. control groups at baseline (Experiment 1) or post-treatment values in patients assigned to erythromycin vs. placebo (Experiment 2). The SAS Mixed procedure was used to compare CCK release in BN and control groups at the 15-minute time point as part of an overall ANOVA in which all the time points were included and the standard error was based on all observations. All analyses were conducted using SAS version 9.2. Data are presented as mean ± SD in text and tables and as mean ± SE in figures.
3. RESULTS
3.1 Experiment 1: Controlled Study of Meal–Related Gastric Emptying and Appetitive Hormone Levels in Bulimia Nervosa
3.1.1 Participant Characteristics
Demographic and clinical features of BN and control participants are presented in Table 1. There were no significant differences between groups in demographic variables. Eating Inventory scores were consistent with previous reports in clinical BN samples [18]. Gastric emptying data were obtained for 32 BN participants and 22 control participants, and appetitive hormone data were obtained for 28 BN participants and 22 control participants.
Table 1.
Demographic and Clinical Characteristics of Participants (mean ± SD)
| Bulimia Nervosa (N=32) | Control (N=24) | Significance | |
|---|---|---|---|
|
| |||
| Age (yrs.) | 24.0 ± 3.0 | 26.1 ± 5.9 | NS |
|
| |||
| Height (in.) | 64.9 ± 2.4 | 64.7 ± 2.7 | NS |
|
| |||
| Weight (lbs.) | 134.4 ± 17.2 | 135.4 ± 14.2 | NS |
|
| |||
| BMI (kg/m2) | 22.4 ± 2.4 | 22.8 ± 2.2 | NS |
|
| |||
| Ethnicity (number/percent) | |||
| • Caucasian | 23 (71.9%) | 18 (75.0%) | NS |
| • Latina | 2 (6.3%) | 1 (4.2%) | |
| • Black | 1 (3.1%) | 0 (0.0%) | |
| • Asian | 2 (6.3%) | 4 (16.7%) | |
| • Mixed/Other | 4 (12.5%) | 1 (4.2%) | |
|
| |||
| BDI Score | 12.9 ± 8.0 | ||
|
| |||
| EI Restraint Score | 12.8 ± 4.2 | ||
|
| |||
| EI Hunger Score | 8.3 ± 3.8 | ||
|
| |||
| EI Disinhibition Score | 12.8 ± 2.0 | ||
|
| |||
| Binge Frequency (episodes/week) | 6.2 ± 2.1 | ||
|
| |||
| Vomit Frequency (episodes/week) | 6.3 ± 2.1 | ||
3.1.2 Gastric emptying and appetitive hormone levels
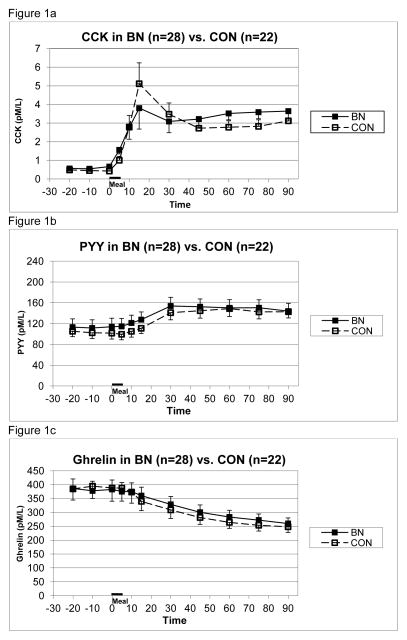
All gastric emptying data and appetitive hormone levels in text are presented as mean value ± standard deviation. There were no significant differences in the slope of gastric emptying between BN and control groups measured in percent remaining per minute (−0.24 ± 0.14 vs. −0.27 ± 0.12 respectively, difference = 0.034 ± 0.132). This corresponds to 21.6 ± 12.6% vs. 24.3 ± 10.8% emptied over 90 minutes in the two groups. There were no significant differences between BN and control groups in mean preprandial cholecystokinin level (0.60 ± 0.41 pmol/L vs. 0.45 ± 0.17 pmol/L, respectively) or postprandial CCK release measured as area under the curve (213.7 ± 156.1 pmol•min/L vs. 201.7 ± 140.0 pmol•min/L, respectively). However, at 15 minutes, mean CCK level in the BN group tended to be lower than that in the control group (3.86 ± 4.02 pmol/L vs. 5.11 ± 5.26, p=0.1) (see Figure 1a). There were no significant differences in preprandial PYY level (115.5 ± 87.0 pmol/L vs. 103.1 ± 51.0 pmol/L in BN and control groups, respectively) or in postprandial PYY area under the curve (1559.5 ± 2906.2 pmol•min/L vs. 1776.2 ± 3180.4 pmol•min/L in BN and control groups, respectively). There were no significant differences in preprandial ghrelin level (387.1 ± 185.1 pmol/L vs. 389.0 ± 205.3 pmol/L in BN and control groups, respectively) or in postprandial ghrelin area under the curve (−10211.8 ± 7065.6 pmol•min/L vs. −12001.4 ± 9489.6 pmol•min/L in BN and control groups, respectively) (see Figures 1b and 1c).
Figure 1.
Mean (± S.E.) plasma concentrations of CCK (1a), PYY (1b), and ghrelin (1c) in BN vs. control groups prior to and following test meal given from 0–5 minutes.
3.2 Experiment 2: Placebo-Controlled Study of Gastric Emptying, Appetitive Hormone Levels, and Clinical Response in Bulimia Nervosa Following Treatment with Erythromycin
3.2.1 Participant Characteristics
The study was conducted during the period August 2005 through May 2009. During this period, 250 potential participants were screened by telephone, and 160 of these were determined to be eligible for further screening and were invited to participate in this and, in some cases, additional studies. Forty-four patients were screened in person. Of these, 6 were determined to be ineligible, 3 were determined to be in need of immediate treatment due to severity of illness, and 3 withdrew. Thirty-two patients underwent baseline biological testing and 29 of these returned for randomization to treatment. Fifteen patients were assigned to treatment with erythromycin and 14 were assigned to placebo. Patients who were assigned to treatment with erythromycin did not differ from those assigned to placebo in the demographic or clinical features presented in Table 1. Of those who began treatment, 13 patients in each group completed clinical treatment. Ten participants in each group completed post-treatment testing and had gastric emptying and blood sampling for hormone analyses.
3.2.2 Gastric emptying and appetitive hormone levels
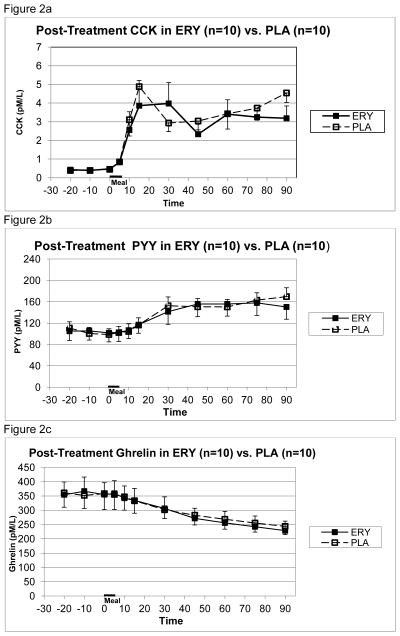
The post-treatment slope of gastric emptying in participants who had received erythromycin, measured in percent remaining per minute, was greater than that in participants randomized to receive placebo (−0.339± 0.14 vs. −0.177 ± 0.122, respectively, difference = 0.162 ± 0.131, p=.013), indicating that treatment with erythromycin was associated with more rapid gastric emptying. This corresponds to 30.5 ± 12.6% vs. 16.0 ± 11.0% emptied over 90 minutes in the erythromycin and placebo groups respectively, a difference of 87 grams between pre- and post-treatment assessments. There were no significant differences between erythromycin and placebo groups in post-treatment preprandial levels of CCK (0.43 ± 0.09pmol/L vs. 0.41 ± 0.06 pmol/L) or in postprandial CCK area under the curve (213.9 ± 127.7 pmol•min/L vs. 236.3 ± 107.2 pmol•min/L) (see Figure 2a). There were no significant differences between erythromycin and placebo groups in post-treatment preprandial PYY level (104.2 ± 54.1 pmol/L vs. 103.5 ± 35.2 pmol/L) or in postprandial PYY area under the curve 2297.1 ± 2622.2 pmol•min/L vs. 2570.7 ± 3065.0 pmol•min/L) (see Figure 2b). There were no significant differences between erythromycin and placebo groups in post-treatment preprandial ghrelin level (358.7 ± 142.1 pmol/L vs. 357.0 ± 159.2 pmol/L) or in postprandial area under the curve (−10145 ± 4362.8 pmol min/L vs. −9391.0 ± 6707.6 pmol•min/L) (see Figure 2c).
Figure 2.
Mean (± S.E.) plasma concentrations of CCK (2a), PYY (2b), and ghrelin (2c) at end of treatment in BN patients receiving erythromycin vs. placebo prior to and following test meal given from 0–5 minutes.
3.2.3 Clinical Response
Of 15 patients randomized to receive erythromycin and 14 patients randomized to receive placebo, 13 patients in the erythromycin group and 13 patients in the placebo group completed at least 5 weeks of drug treatment. Of those who completed treatment, 11 patients in the erythromycin group and 12 patients in the placebo group had their dosage increased to the higher level (500 mg. three times daily). Of the 2 patients in the erythromycin group who did not complete treatment, 1 was withdrawn for clinical worsening and 1 withdrew due to lack of improvement. The patient in the placebo group who did not complete treatment was withdrawn due to clinical worsening. No patients had dosage reduced or medication discontinued due to side effects.
There were no differences between treatment groups in baseline or post-treatment binge/vomit frequency calculated as the mean of frequencies at week 5 and week 6. Patients randomized to treatment with erythromycin had weekly binge/vomit frequencies of 9.9 ± 8.7 binge episodes/10.4 ± 8.7 vomit episodes prior to treatment and 11.4 ± 10.7 binge episodes/11.3 ± 10.9 vomit episodes following treatment. Patients randomized to treatment with placebo had weekly binge/vomit frequencies of 8.2 ± 5.4 binge episodes/9.5 ± 6.5 vomit episodes prior to treatment and 7.2 ± 4.1 binge episodes/7.6 ± 4.4 vomit episodes following treatment.
4. DISCUSSION
The major finding of this study was that treating patients with BN using the motilin agonist erythromycin did not reduce binge eating or vomiting, although it did modestly increase the rate of gastric emptying following a food load from 16% to 30.5% emptied in 90 minutes. In addition, while there were suggestions of baseline abnormalities in gastric emptying and postprandial cholecystokinin release in patients with bulimia nervosa, we did not fully replicate our previous findings. We also did not observe abnormalities in baseline or postprandial levels of PYY or ghrelin in patients with BN.
The finding that BN patients treated with erythromycin did have significantly more rapid gastric emptying than those receiving placebo suggests that the experimental manipulation provided a valid test of the hypothesis that modestly increasing gastric empting would be associated with clinical improvement. However, one explanation for the lack of predicted clinical improvement is that the magnitude of the change in rate of gastric emptying may have been insufficient to impact gastric distension to a degree that would meaningfully affect subsequent meal-related sensations or eating behavior [19]. It is possible that an intervention that more markedly increased the rate of gastric emptying or that more directly influenced postprandial levels of CCK [20,21] or other satiety-related hormones may have been more clinically effective. For example, the two cited studies suggest that the combination of CCK-8 infusion with gastric distension is much more effective than either factor alone in reducing subsequent intake. Thus, it is possible that our previously hypothesized model, in which a reduced rate of gastric emptying and/or abnormal postprandial release of satiety-related hormones are necessary for the perpetuation of the binge-vomit cycle, is applicable only when alterations in gastric emptying or satiety-related hormone release are sufficient to produce large changes in gastric distension.
An alternative explanation is that, in contrast with our previous model, alterations in gastrointestinal physiology, such as reduced rate of gastric emptying or abnormal postprandial hormone release, are not necessary causal factors in the mechanism that perpetuates binge-vomit behavior in patients with BN. This may be the case if alterations in gastrointestinal physiology do not lead to abnormal intrameal satiation or if abnormal satiation does not drive binge-vomit behavior. With regard to the former, a recent study of meal-related perceptions found that, when asked to rate fullness during the course of a large fixed-size (975 gram) meal, patients with BN did not differ significantly from matched controls [22]. This result suggested that the disturbance of intrameal satiation reported in earlier studies [1] may have been related to factors other than disturbed gastrointestinal physiology, such as the individual’s intentions and emotional state, or perceptions of meal size or amount remaining to be consumed. However, the difference between the two studies may also reflect that fact that the size of the meal in the study of Zimmerli et al. [22] was considerably smaller than the typical size of ad lib binge episodes in the earlier study [1], and it is conceivable that disturbances in gastrointestinal physiology occur only when the volume of gastric contents exceeds a relatively high threshold.
The study also attempted to replicate previous findings regarding baseline and postprandial levels of appetitive hormones in individuals with BN. A recent review and meta-analysis of studies of baseline and postprandial levels of appetitive hormones in individuals with eating disorders found that studies were relatively few in number, particularly if limited to those including patients with BN, and findings were heterogeneous [2]. However, variability in the size and composition of the food stimulus may contribute to variability in findings. With regard to studies of BN, there is no clear consensus regarding baseline or postprandial levels of CCK, PYY, or ghrelin. Two studies [11,12] reported diminished PYY response to a meal, and three studies [4,23,24] reported marginally or significantly diminished CCK postprandial response, with two studies [25,26] failing to find this. Our current findings therefore add marginal support to earlier findings of diminished postprandial CCK response, at least at the 15-minute postprandial time point, the presumed peak response point.
There are several additional limitations that must be taken into account in interpreting the findings, and that limit the generalization of these results. First, regarding our CCK assays, we employed a radioimmunoassay, rather than the bioassay used in our previous study [4]; however, the radioimmunoassay detects the bioactive forms of CCK and thus produces results comparable to a bioassay [27,28]. It is also possible that aspects of stomach functioning other than emptying rate, such as gastric relaxation following food consumption [5] or sensitivity to gastric distension [18] may play a more direct causal role. As we did not study these aspects of gastric functioning in the current study, we could not assess the effect of erythromycin treatment on them and cannot rule out the possibility that their normalization for a more sustained period might be associated with clinical improvement. Additionally, although vomiting was the predominant method of purging for all patients, we did not systematically assess the occasional use of other methods of purging that may have contributed to abnormal gastrointestinal function or may have responded differently to erythromycin treatment. Finally, since our treatment used medication and medical management only, it is possible that normalizing gastric emptying may be of adjunctive benefit but is not helpful as a sole intervention. Future studies may benefit from the inclusion of additional outcome measures including food intake or level of exercise in response to drug treatment, changes in weight or body composition, and changes in mood or in the subjective experience of eating.
4.1 Conclusions
In sum, with the quantitative limitations noted above, the current study fails to support a role for erythromycin in the treatment of BN. While these results are consistent with the inference that the abnormal intrameal satiation previously observed when individuals with BN binge eat does not result solely from delayed gastric emptying, more quantitative studies of the effect of prokinetic agents are needed, as erythromycin affected gastric emptying only modestly in this study. Moreover, interventions that directly impact CCK release or release of other satiety-inducing hormones might prove a more effective therapeutic approach. Further studies are needed to clarify the role of previously observed abnormalities in gastric functioning in the causal chain leading to binge eating and vomiting, and to better understand the interaction of cognitive and biological factors in initiating and/or maintaining these behaviors.
Highlights.
We examine gastric emptying and post-meal gastrointestinal hormone release in bulimia nervosa.
Gastric emptying is normal and early post-meal CCK release marginally low in bulimia nervosa.
Erythromycin treatment of bulimia nervosa produces a modestly increased gastric emptying rate.
Erythromycin treatment of bulimia nervosa is not associated with clinical improvement.
Acknowledgments
This work was supported by NIMH grant MH-42206, NIH grants DK-68392 and DK-74046, and the New York Obesity Research Center NIH grant DK-26687. We would like to thank Alexia Spanos for her contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kissileff HR, Wentzlaff TH, Guss JL, Walsh BT, Devlin MJ, Thornton JC. A direct measure of satiety disturbance in patients with bulimia nervosa. Physiol Behav. 1996;60:1077–85. doi: 10.1016/0031-9384(96)00086-8. [DOI] [PubMed] [Google Scholar]
- 2.Prince AC, Brooks SJ, Stahl D, Treasure J. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 2009;89:755–65. doi: 10.3945/ajcn.2008.27056. [DOI] [PubMed] [Google Scholar]
- 3.Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56:656–61. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- 4.Devlin MJ, Walsh BT, Guss JO, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;56:114–20. doi: 10.1093/ajcn/65.1.114. [DOI] [PubMed] [Google Scholar]
- 5.Walsh BT, Zimmerli E, Devlin MJ, Guss J, Kissileff HR. A disturbance of gastric function in bulimia nervosa. Biol Psychiatry. 2003;54:929–33. doi: 10.1016/s0006-3223(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 6.Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiol Behav. 2001;74:743–6. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. pp. 589–94. (Text Revision) [Google Scholar]
- 8.Szmukler GI, Young GP, Miller G, Lichtenstein M, Binns DS. A controlled trial of cisapride in anorexia nervosa. Int J Eat Disord. 1995;17:347–57. doi: 10.1002/1098-108x(199505)17:4<347::aid-eat2260170406>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Gumaste V, Baum J. Treatment of gastroparesis: an update. Digestion. 2008:173–9. doi: 10.1159/000185690. [DOI] [PubMed] [Google Scholar]
- 10.Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698–703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- 11.Kojima S, Nakahara T, Nagai N, Muranaga T, Tanaka M, Yasuhara D, Masuda A, Date Y, Ueno H, Nakazato M, Naruo T. Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clin Endocrinol (Oxf) 2005;62:74–8. doi: 10.1111/j.1365-2265.2004.02176.x. [DOI] [PubMed] [Google Scholar]
- 12.Monteleone P, Martiadis V, Rigamonti AE, Fabrazzo M, Giordani C, Muller EE, Maj M. Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol Psychiatry. 2005;57:926–31. doi: 10.1016/j.biopsych.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12. New York: The Guilford Press; 1993. pp. 317–60. [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 15.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 17.Rehfield JF. How to measure cholecystokinin in tissue, plasma and cerebrospinal fluid. Regul Pept. 1998;78:31–9. doi: 10.1016/s0167-0115(98)00133-5. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerli EJ, Walsh BT, Guss JL, Devlin MJ, Kissileff HR. Gastric compliance in bulimia nervosa. Physiol Behav. 2006;87:441–6. doi: 10.1016/j.physbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:685–8. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- 20.Muurahainen N, Kissileff HR, Derogatis AJ, Pi-Sunyer FX. Effects of cholecystokinin-octapeptide (CCK-8) on food intake and gastric emptying in man. Physiol Behav. 1988;44:645–9. doi: 10.1016/0031-9384(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 21.Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:R992–8. doi: 10.1152/ajpregu.00272.2003. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerli EJ, Devlin MJ, Kissileff HR, Walsh BT. The development of satiety in bulimia nervosa. Physiol Behav. 2010;100:346–9. doi: 10.1016/j.physbeh.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geracioti TD, Liddle RA. Impaired cholecystokinin secretion in bulimia nervosa. N Engl J Med. 1988;319:683–8. doi: 10.1056/NEJM198809153191105. [DOI] [PubMed] [Google Scholar]
- 24.Keel PK, Wolfe BE, Liddle Ra, De Young KP, Jimerson DC. Clinical features and physiological response to a test meal in purging disorder and bulimia nervosa. Arch Gen Psychiatry. 2007;64:1058–66. doi: 10.1001/archpsyc.64.9.1058. [DOI] [PubMed] [Google Scholar]
- 25.Phillip E, Pirke KM, Kellner MB, Krieg JC. Disturbed cholecystokinin secretion in patients with eating disorders. Life Sci. 1991;48:2443–50. doi: 10.1016/0024-3205(91)90379-p. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto S, Inui A, Kiyota N, Seki W, Koide K, Takamiya S, Uemoto M, Nakajima Y, Baba S, Kasuga M. Increased cholecystokinin and pancreatic polypeptide responses to a fat-rich meal in patients with restrictive but not bulimic anorexia nervosa. Biol Psychiatry. 1997;41:1068–70. doi: 10.1016/S0006-3223(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 27.Hoecker M, Schmidt WE, Cruetzfeldt W, Choudhury AR, Nustede R, Schafmayer A, Foelsch UR. Determination of plasma cholecystokinin (CCK) concentrations by bioassay and radioimmunoassay in man. A critical evaluation. Regul Pept. 1992;37:255–69. doi: 10.1016/0167-0115(92)90619-6. [DOI] [PubMed] [Google Scholar]
- 28.Hoecker M, Schmidt WE, Wilms HM, Lenhoff F, Nustede R, Schafmayer A, Foelsch UR. Measurment of tissue cholecystokinin (CCK) concentrations by bioassay and specific radioimmunoassay: characteristics of the bioactivity of CCK-58 before and afer tryptic cleavage. Eur J Clin Invest. 1990;20 (Suppl 1):S45–S50. doi: 10.1111/j.1365-2362.1990.tb01777.x. [DOI] [PubMed] [Google Scholar]