Abstract
Background
The kappa opioid receptor (KOR) and its endogenous agonist, the neuropeptide dynorphin, are a critical component of the central stress system. Both dynorphin and KOR are expressed in the bed nucleus of the stria terminalis (BNST), a brain region associated with anxiety and stress. This suggests that KOR activation in this region may play a role in the regulation of emotional behaviors. To date, however, there has been no investigation of the ability of KOR to modulate synaptic transmission in the BNST.
Methods
We used whole-cell patch clamp recordings from acutely prepared mouse brain slices to examine the actions of KOR on inhibitory transmission in the BNST. Additionally, we used neurochemical and pathway-specific optogenetic manipulations to selectively stimulate GABAergic fibers from the central nucleus of the amygdala (CeA) to the BNST.
Results
We found that activation of KOR reduced GABAergic transmission through a presynaptic mechanism. Futhermore, we examined the signal transduction pathways that mediate this inhibition, and provide the first functional information implicating ERK in KOR-mediated presynaptic modulation. Moreover, we found that at KOR-signaling robustly reduced inhibitory synaptic transmission in the CeA to BNST pathway.
Conclusions
Together, these results demonstrate that KOR provide important inhibitory control over presynaptic GABAergic signaling within the BNST, and provide the first direct functional demonstration of KOR sensitive long-range GABAergic connections between the CeA and the BNST.
Keywords: extended amygdala, optogenetics, knock-in, genetic targeting, opioid, GABA, release
Introduction
Affective disorders, including depression, anxiety, and addiction, are widespread and take a tremendous toll on society. Recent work suggests that neuropeptide signaling systems are involved in the modulation of behaviors associated with these affective disorders and thus are potential targets for their treatment (1). In particular, the neuropeptide dynorphin, an endogenous agonist for the kappa opioid receptor (KOR), has been suggested to play a critical role in affective disorders and reward (2–5). Recent studies have shown that KOR signaling is involved in stress-induced behaviors including dysphoria (6), relapse to cocaine seeking (7), and analgesia (8), as well as in anxiety (5, 9) and depression (4). In addition, KOR signaling has been shown to modulate synaptic transmission of classic neurotransmitter systems in a variety of brain regions involved in reward, including the ventral tegmental area and the nucleus accumbens (10–13). However, KOR modulation of synaptic transmission in the extended amygdala, which is important for the regulation of both stress- and reward-related behaviors, has not yet been examined.
The extended amygdala is a series of extensively interconnected structures including the bed nucleus of the stria terminalis (BNST) and the central nucleus of the amygdala (CeA). A large body of evidence from the Davis group has demonstrated that the CRF originating in the CeA acts within the BNST, via CRF1 receptors to gate the switch from a phasic to tonic fear (14, 15). It was hypothesized that this was due to a CRF dependent increase in glutamate transmission, which was demonstrated by Kash et al in 2008 (16). However, recent reports from the Chavkin group have suggested that the actions of CRF are due in part to activation of KOR systems. Despite the important role that CRF signaling plays in this region, there is no data suggesting what KOR activation does in the BNST. Both dynorphin and KORs are expressed in neurons of both the BNST and CeA (17), and evidence suggests that there is a population of GABAergic neurons in the CeA that express dynorphin and project to the BNST (18). Further, a recent imaging study found that administration of a KOR agonist led to metabolic activation in the BNST (19). Thus, KOR activation in the BNST, potentially caused by dynorphin release from CeA neurons that terminate in the BNST, may have a significant effect on the function of BNST neurons that project to downstream targets involved in anxiety and reward-related behaviors, including the paraventricular nucleus of the hypothalamus, dorsal raphe, and ventral tegmental area. In this study, we examined the mechanism by which KOR activation alters synaptic transmission in the BNST. Further, using optogenetics in a knock-in mouse that allows targeting of genetically-defined GABAergic neurons, we directly probed the ability of KOR activation to alter GABAergic synaptic transmission from the CeA to the BNST.
Methods
Animals
Male C57BL/6J mice were acquired from Jackson Laboratories (Bar Harbor, ME) at 5 weeks of age and housed in our colony room under a 12:12 hr light cycle, with lights on at 7 a.m. daily. Mice were given ad libitum access to rodent chow and water. All procedures were performed in accordance with the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
Whole-cell voltage-clamp recordings
Brain slices containing dorsolateral BNST were obtained (see Supplemental Information for details) and stored in a heated, oxygenated holding chamber containing ACSF [(in mM) 124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3] before being transferred to a submerged recording chamber maintained at approximately 30 °C (Warner Instruments, Hamden, CT). Recording electrodes were filled with (in mM) 70 KCl , 65 K+-gluconate, 5 NaCl, 10 HEPES, 2 QX-314, 0.6 EGTA, 4 ATP, 0.4 GTP, pH 7.2, 290–295 mOsmol. Pilot data demonstrate similar effects of KOR agonists using a cesium-based internal solution (see Supplemental Information).
Cells were held at −70 mV and spontaneous (sIPSCs) and evoked IPSCs (eIPSCs) were pharmacologically isolated by adding either 3 mM kynurenic acid to block AMPA and NMDA receptor-dependent postsynaptic currents or NBQX (10 μM) to block AMPA receptor-dependent post-synaptic currents. In pilot experiments, we found no differences in the effects of U69593 or dynorphin in the presence of these different glutamate receptor antagonists. To isolate miniature IPSCs (mIPSCs), tetrodotoxin (0.5 μM) was added to the perfusing ACSF solution described above.
Electrode stimulation-evoked GABAergic IPSCs were induced via twisted bipolar nichrome wire placed dorsal to the recording electrode, 100–500 μm medial from the recorded neuron. Electric output was set to stimulate at 0.1 Hz, between 5–50 V with a 100–150 μs duration. For light-evoked IPSCs, a blue LED light (Thorlabs) routed through the epifluoresnce port and the objective was used to optically stimulate ChR2-positive fibers. Light output was set to 2 mW with 1–5 ms pulse durations. Signals were acquired via a Multiclamp 700B amplifier (Molecular Devices), digitized at 10 kHz, filtered at 3 kHz, and analyzed using Clampfit 10.2 software (Molecular Devices). Input resistance and access resistance were continuously monitored during experiments. Experiments in which changes in access resistance were greater than 20% were not included in the data analysis. Evoked IPSC experiments were analyzed by measuring the average peak amplitude of the synaptic response per minute, which was normalized to the baseline period 5 min immediately preceding application of the drug. No more than two cells per animal were included in each experiment. All drug information can be located in the Supplemental Information.
Stereotaxic surgery
Mice (heterozygous vGAT-ires-Cre for targeting GABAergic neurons (20)) received bilateral CeA injections of 0.3 – 0.5 μl of purified and concentrated AAV (~1012 infectious units/mL). Further details regarding stereotaxic surgery are included in Supplemental Information.
Statistical analysis
Effects of drugs during electrophysiological recordings were evaluated by comparing the magnitude of the dependent measure (peak eIPSC amplitude, sIPSC/mIPSC frequency, or sIPSC/mIPSC amplitude) between the baseline and washout (when drug had reached maximal effect) periods using t-tests. The effects of antagonists/blockers on the ability of drugs to modulate synaptic transmission were compared using t-tests during the washout period. All values given for drug effects throughout the paper are presented as mean ± S.E.M.
Results
We examined the effects of KOR activation on inhibitory synaptic transmission in the dorsolateral BNST, focusing our efforts on the oval subnucleus and utilizing whole-cell voltage clamp of neurons from acutely prepared brain slices of adult C57BL/6J mice. Local stimulation in the BNST produced an eIPSC that was driven by activation of GABAARs, as the selective GABAAR antagonists, SR95531 and picrotoxin (data not shown), blocked the response. Additionally, we observed spontaneous IPSCs (sIPSCs) (frequency: 1.92 ± 0.6 Hz, n = 6 cells; amplitude: 74 ± 10 pA, n = 6 cells) that were blocked by SR95531, suggesting that these events were also mediated by GABAA receptors (data not shown).
Dynorphin depresses GABAergic transmission through activation of KOR
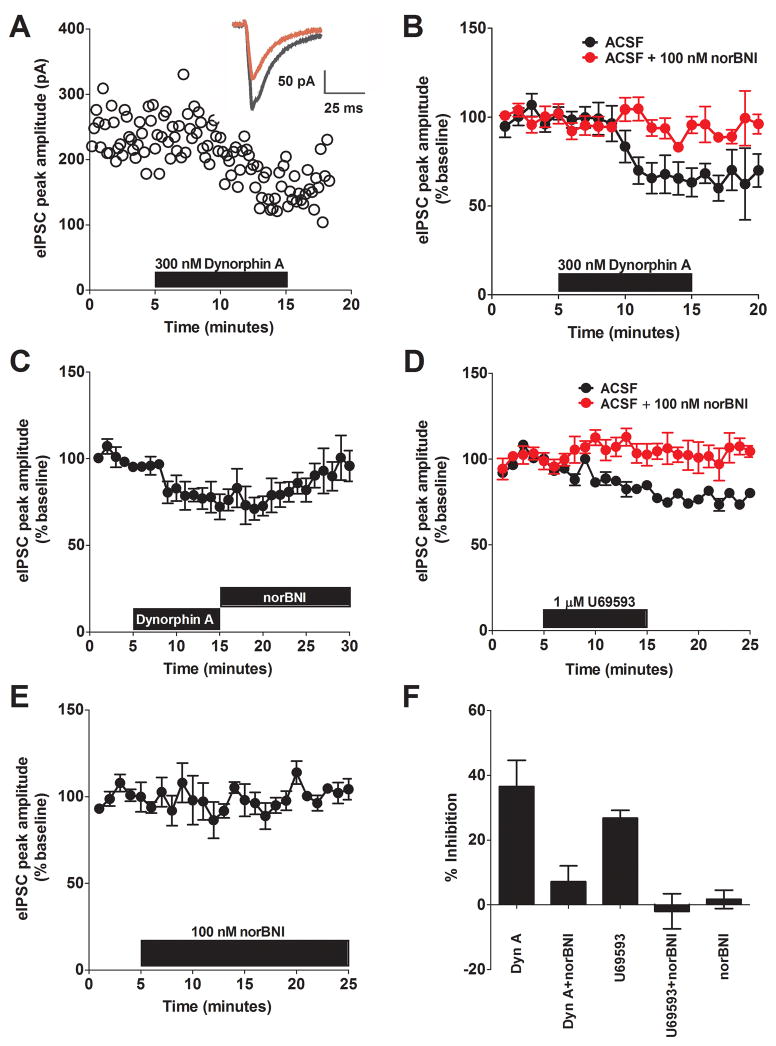
To assess the impact of KOR activation on GABAergic transmission, we first investigated the effect of dynorphin on eIPSCs. Ten min bath application of dynorphin A (300 nM) significantly decreased the peak amplitude of the eIPSC to 64 ± 8 % of baseline (p < 0.05, Figure 1A–C), which was observed in 5/5 cells. To verify that KOR is the receptor underlying the actions of dynorphin, we demonstrated that pre-application of the selective KOR antagonist norbinaltorphimine (nor-BNI, 100 nM) blocked dynorphin-induced decreases in eIPSCs in the BNST (94 ± 5 % of baseline, n =5, Figure 1B). We next examined the impact of a KOR selective agonist, U69593, on GABAergic transmission. We found that bath application of U69593 (1 μM) significantly decreased the peak amplitude of the eIPSC to 77 ± 8% of the baseline (p < 0.05, n =6, Figure 1D). Similar to what was observed with dynorphin, this effect was blocked by pre-application of 100 nM nor-BNI (Figure 1D, 102 ± 5% of the baseline, n = 5). Importantly, this effect does not appear to be a long-term depression, as application of the KOR antagonist (100 nM nor-BNI) following removal of agonist reverses the inhibition (Figure 1C). To assess tonic KOR activation by endogenous dynorphin, 100nM nor-BNI was bath-applied alone, and we found no effects on baseline transmission (104 ± 2% of the baseline, Figure 1E). Taken together, these results (summarized in Figure 1F) demonstrate that activation of KOR in the BNST leads to a reduction of GABAergic transmission.
Figure 1. Kappa Opioid Receptor (KOR) activation reduces synaptic inhibition in the BNST.
A) Representative experiment demonstrating the ability of Dynorphin A (300 nM) to reduce the peak amplitude of eIPSCs in the BNST. Inset: average traces from the representative experiment showing the effects before (black) and after (red) application of Dynorphin A. B) Pooled data demonstrating that the ability of 300 nM dynorphin to reduce eIPSC peak amplitude is blocked by pre-application of the KOR receptor antagonist, nor-BNI (100 nM). C) Pooled data demonstrating that 100 nM nor-BNI reverses the Dynorphin A effect on eIPSC amplitude. D) Pooled data demonstrating that the ability of the KOR agonist, U69593, to inhibit eIPSC amplitude is blocked by the KOR antagonist nor-BNI in the BNST. E) Pooled data demonstrating no tonic activation of KORs by endogenous dynorphin. F) Summary of magnitude of maximal inhibition produced by bath-application of drugs in A–E.
Mechanism of KOR inhibition of GABAergic transmission
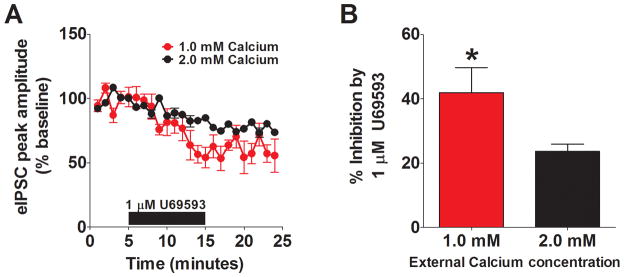
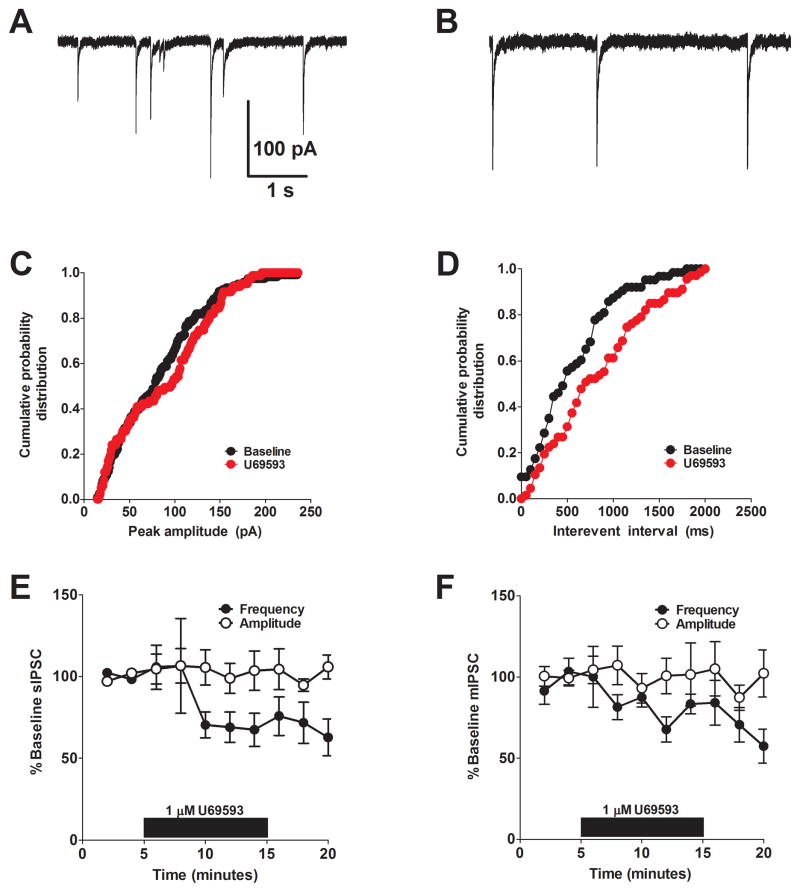
To determine whether KOR-mediated suppression of GABAergic activity occurred pre- or postsynaptically we utilized multiple well-established approaches. We first examined the impact of reducing the concentrations of extracellular calcium. Previous studies have shown that altering the external calcium concentration can increase the extent of the inhibition when it is mediated presynaptically (21). To understand the site of action for KOR-mediated inhibition of GABAergic transmission in the BNST, we examined the actions of U69593 in an external calcium concentration of 1.0 mM. We found that in reduced external calcium, the effect of 1 μM U69593 was significantly increased compared to the experiments performed in normal ACSF that contains 2.0 mM calcium (Figure 2A, B). This finding suggests that KOR-mediated inhibition of GABAergic transmission in the BNST occurs presynaptically. We next examined the impact of U69593 on spontaneous inhibitory synaptic transmission. The basal frequency and amplitude for sIPSC were 1.92 ± 0.6 Hz and 74 ± 10 pA, respectively (n = 6). In most situations, presynaptic modulation alters the frequency of events, while post-synaptic modulation alters the amplitude of events. We found that bath application of 1 μM U69593 led to a significant reduction in the frequency of sIPSCs (68 ± 12% of baseline, p < 0.05, n =6, Figure 3D, E) without an effect on sIPSC amplitude (106 ± 14% of baseline, n =6, Figure 3C, E), further supporting the possibility that KORs modulate presynaptic function at GABAergic synapses.
Figure 2. KOR inhibition is increased in reduced external calcium.
A) Pooled data demonstrating that reducing the external concentration of calcium to 1.0 mM (n = 5) leads to an increased effect of U69593 (2.0 mM calcium data replotted from Figure 1 for comparison). B) Bar chart demonstrating the significantly increase in U69593-mediated inhibition in 1.0 mM calcium.
Figure 3. KOR activation reduces sIPSC and mIPSC frequency but not amplitude.
A & B) Representative baseline (A) and post-drug (B) traces demonstrating the effect of U69593 on spontaneous GABAergic transmission in the BNST. C & D) Cumulative probability distribution of peak amplitude (C) and frequency (D) before (black) and after (red) application of U69593, demonstrating no effect on amplitude but a reduction in frequency. E) Pooled data demonstrating that U69593 reduces sIPSC frequency but not amplitude. F) Pooled data demonstrating that U69593 reduces mIPSC frequency but not amplitude.
One potential explanation for our results is that KORs inhibit the firing of a population of local GABAergic interneurons post-synaptically, leading to reduced GABA release within the BNST. We evaluated this possibility by examining the impact of U69593 on miniature inhibitory post-synaptic currents (mIPSCs). mIPSCs are similar to sIPSCs, however, the sodium channel blocker, tetrodotoxin, is added to prevent action-potential coupled GABA release. The basal frequency and amplitude for mIPSC were 1.24 ± 0.6 Hz and 37 ± 9 pA, respectively (n = 6). mIPSC amplitude, but not frequency, was significantly lower than that of sIPSC (p < 0.05). We found that bath application of 1 μM U69593 significantly reduced mIPSC frequency (67 ± 2% of baseline, p < 0.05, n =5) with no effect on mIPSC amplitude (102 ± 8% of baseline, n =5), suggesting that these effects are mediated at the synapse rather than via altering excitability of local interneurons (Figure 3F).
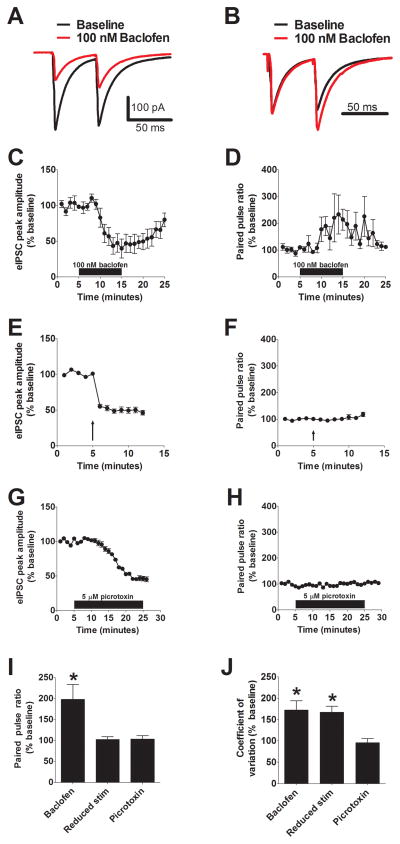
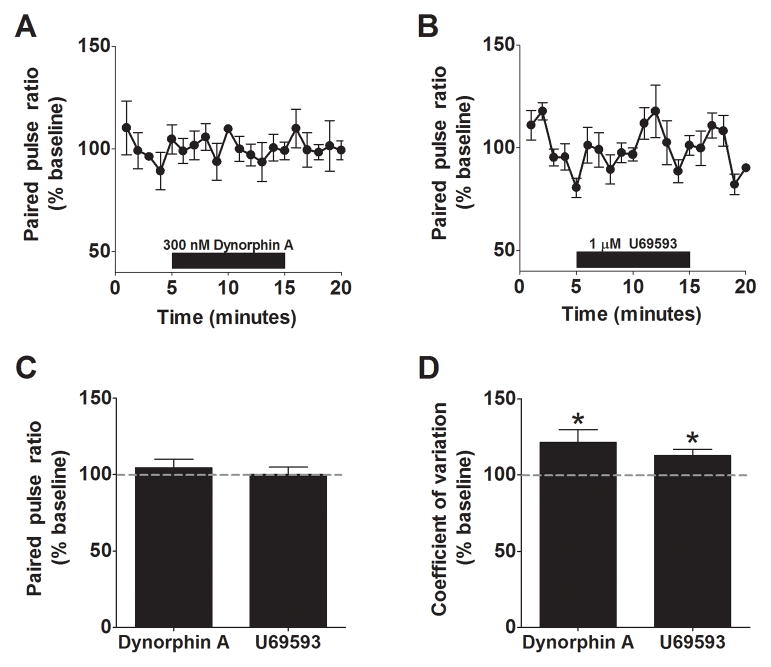
To more rigorously evaluate the mechanisms underlying this modulation, we next examined two parameters that are related to presynaptic release properties: paired pulse ratio (PPR) and coefficient of variation (CV) (22). PPR is examined by looking at the ratio of the amplitudes of two synaptic events evoked within a short time period (for example, 50 ms). CV is defined as σ/μ, where σ is the standard deviation of IPSC amplitude and μ is the mean IPSC amplitude. At most central synapses, changes in PPR are associated with modifications of the release probability, while changes in CV are associated with alterations in the release probability and the number of release sites. We tested the utility of these approaches in the BNST by performing a series of positive and negative control experiments for presynaptic modulation.
GABAB receptors act as autoreceptors in many regions of the brain at GABAergic synapses, providing negative feedback on to inhibitory terminals (23). We therefore examined the impact of GABAB receptor activation on eIPSCs recorded from BNST neurons. We found that bath application of 100 nM baclofen, a GABAB receptor agonist, lead to a similar reduction of eIPSC amplitude (58 ± 8% of baseline, p < 0.05, n =6, Figure 4A, C) as a KOR agonist. Further, this reduction was associated with a significant increase in both the PPR (198 ± 35 % of baseline, p < 0.05, n = 6, Figure 4B, I) and the CV (172 ± 21 % of baseline, p < 0.05, n = 6, Figure 4J), consistent with the ability of GABAB receptors to regulate GABA release. We next examined the impact of reducing the stimulation intensity, a manipulation that should reduce the number of release sites but have no effect on the probability of release (22). As expected, we found that reducing stimulation intensity lead to a significant reduction in eIPSC amplitude (49 ± 4% of baseline, p < 0.05, n =5, Figure 4E) with no change in PPR (103 ± 7 % of baseline, n = 5, Figure 4F, I), but a significant increase in CV (167 ± 14 % of baseline, p < 0.05, n = 5, Figure 4J). Finally, we examined the impact of a manipulation with no predicted effect on either CV or PPR – application of a sub-saturating concentration of the GABAA-R antagonist picrotoxin. Application of 5 μM picrotoxin significantly reduced eIPSC amplitude (46 ± 3% of baseline, p < 0.05, n =5, Figure 4G) but had no effect on either CV (96 ± 9 % of baseline, n = 5, Figure 4J) or PPR (104 ± 8 % of baseline, n = 5, Figure 4H, I). Taken together, the results from these experiments strongly support the use of CV and PPR as tools to evaluate presynaptic function in the BNST.
Figure 4. Evaluation of CV/PPR as means to determine presynaptic versus postsynaptic changes in synaptic transmission in the BNST.
A) Representative paired pulse traces at baseline and following application of 100 nM baclofen (red). B) The same traces from panel A normalized to highlight the effect of baclofen on the paired pulse ratio. C & D) Pooled results demonstrating the effect of baclofen on eIPSC amplitude (C) and PPR (D). E & F) Pooled results demonstrating the effect of reduced stimulation on eIPSC amplitude (E) and PPR (F). G & H) Pooled results demonstrating the effect of sub-saturating picrotoxin (5 μM) on eIPSC amplitude (G) and PPR (H). I & J) Summary bar graphs demonstrating the effects drug treatments on PPR (I) and coefficient of variation (J).
Having confirmed the utility of these approaches to measure changes in presynaptic function in our slice preparation, we next examined the effect of both dynorphin and U69593 on PPR. Surprisingly, we found that neither 300 nM dynorphin (97 ± 5 % of baseline, n = 5, Figure 5A), nor 1 μM U69593 (97 ± 4 % of baseline, n = 6, Figure 5B), had any measurable effect on the PPR of eIPSCs in the BNST (Figure 5C). In contrast, both compounds lead to small but significant increases in CV (dynorphin: 122 ± 8% of baseline, p < 0.05, n =5; U69593: 113 ± 8% of baseline, p < 0.05, n =5; Figure 5D). Collectively, these findings support the possibility that KOR activation in the BNST is altering presynaptic GABA function, possibly through a reduction in presynaptic release sites.
Figure 5. Effect of KOR activation on PPR/CV in the BNST.
A) Pooled data demonstrating the lack of an effect of Dynorphin A on PPR. (B) Pooled data demonstrating the lack of an effect of U69593 on PPR. C) Summary bar chart demonstrating the lack of an effect of either KOR agonist on PPR in the BNST. D) Bar chart demonstrating the significant effects of Dynorphin A and U69593 on CV in the BNST.
The role of MAPK signaling in KOR modulation of GABAergic transmission
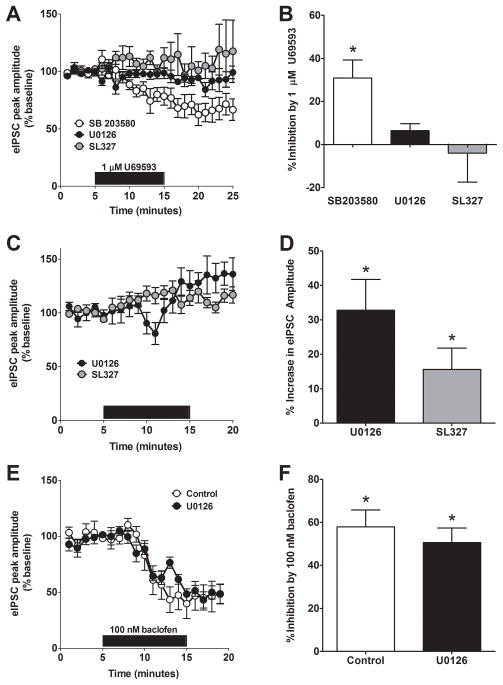
Next, we investigated the underlying signaling cascades that are involved in KOR-mediated inhibition of GABA release in the BNST. Reports have demonstrated that KOR can activate MAPK signaling pathways including p38MAPK and ERK1/2 and that these pathways could be activated by exposure to stress (24–26). To determine if KOR signaling through these pathways was responsible for the reduction of GABA release in the BNST, we investigated the impact of a series of well-characterized pharmacological probes. To determine if p38MAPK signaling was required for KOR-mediated inhibition of GABA release, we bath applied the p38MAPK inhibitor (27), SB203580 (20 μM), for at least 45 minutes prior to beginning the experiment. This compound had no effect on the ability of 1 μM U69593 to inhibit eIPSCs in the BNST (66 ± 9 % of baseline, n = 6, Figure 6A, B). To determine if ERK signaling was required for KOR-mediated inhibition of GABA release, we applied U69593 (1 μM) to BNST slices in the presence of the mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitors U0126 (20 μM) or SL327 (10 μM) while recording eIPSCs. The presence of U0126 or SL327 blocked the ability of U69593 to reduce eIPSC peak amplitude when compared to drug alone (U0126: p < 0.05, 94 ± 4 % of baseline, n = 6. SL327: p < 0.05, 104 ± 13 % of baseline, n = 6, Figure 6A, B). Interestingly, bath application of both MEK inhibitors, U0126 and SL327, alone slightly but significantly increased eIPSC amplitude (U0126: p < 0.05, 133 ± 9 % of baseline, n = 5. SL327; p < 0.05, 115 ± 6 % of baseline, n = 6 . Figure 6C, D), suggesting a critical role of ERK1/2 signaling in the regulation of GABAergic transmission. In order to determine if the effect of the MEK inhibitors generalized to other forms of presynaptic inhibition, we next evaluated the ability of U0126 to modulate baclofen induced inhibition of GABAergic transmission. In contrast to the effects on U69593, we found that U0126 had no effect on baclofen-induced inhibition (Figure 6E, F). Taken together, these results (summarized in Figure 6B, F) provide the first evidence that the ability of KOR to inhibit GABA release is dependent on ERK signaling.
Figure 6. ERK, but not p38, signaling is required for KOR-mediated inhibition of GABA release in the BNST.
A) Pre-incubation of brain slices with the p38 map kinase inhibitor SB204580 (20 μM) did not alter the ability of U69593 to reduce eIPSC peak amplitude in the BNST, while pre-incubation of the MEK inhibitors, U0126 (20 μM) and SL327 (10 μM) led to significant impairment in the ability of U69593 to modulate GABA transmission in the BNST. B) Summary of the impact of MAPK inhibitors on KOR-mediated inhibition. C) Both MEK inhibitors, U0126 (20 μM) and SL327 (10 μM), bath-applied alone increased eIPSC amplitude in the BNST. D) Summary of the effects of MEK inhibitors on GABA transmission in the BNST. E) Pre-incubation of brain slices with the MEK inhibitor, U0126 (20 μM), did not alter the ability of baclofen to modulate GABA transmission in the BNST. F) Summary of the impact of U0126 on baclofen-mediated inhibition of GABA transmission in the BNST.
Optogenetic control of the CeA-to-BNST pathway
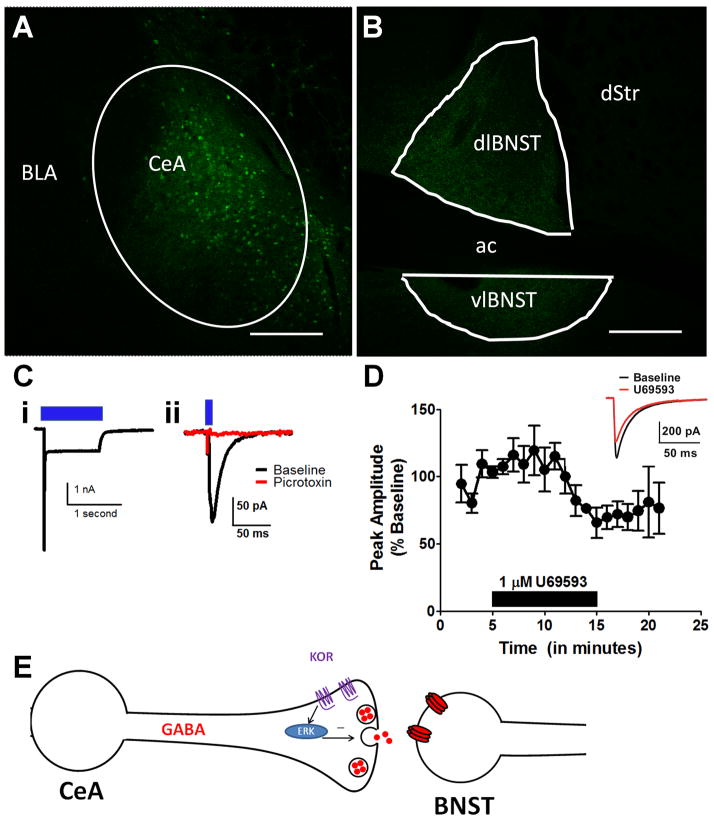
We sought to directly evaluate the sensitivity of the GABAergic projection from the CeA to the BNST using a newly generated knock-in Cre line (vGAT-ires-Cre). This pathway has been hypothesized to play a critical role in anxiety, stress-induced relapse, and alcohol abuse. In order to isolate the CeA-BNST pathway, we injected a double-floxed virus containing channelrhodopsin (ChR2) tagged to YFP (in mice used for electrophysiology data) or GFP (in the mouse used for imaging in Figure 7) into the CeA of vGat-ires-Cre mice. A pilot study using whole-cell patch-clamp recordings confirmed that blue LED light could depolarize ChR2-positive, YFP-expressing GABA neurons in the CeA (Figure 7C). Successful viral transduction of neurons in the CeA (Figure 7A) also resulted in robust expression of YFP-positive fibers in the BNST (Figure 7B). As shown in Figure 7C, a 5 ms light stimulation led to a picrotoxin-sensitive IPSC in the BNST, demonstrating that we were able to use ChR2 to specifically activate CeA to BNST GABAergic projection neurons. Using epifluorescence to visualize YFP in cell bodies in the CeA and terminals in the BNST, we confirmed that virus injections to the CeA were successful in seven of 10 hemispheres. We were able to evoke time-locked IPSCs with blue LED light in neurons from all BNST slices in these seven successfully-injected hemispheres. In total, we successfully light-evoked currents from 34 of 42 (81%) BNST neurons that we patched in these slices. Injection site “misses” that resulted in unsuccessful virus infection in the CeA yielded no fluorescent signal or light-evoked IPSCs in the BNST (data not shown). These findings confirmed that this animal model was suitable to functionally probe the CeA-BNST pathway using an optogenetic approach.
Figure 7. KOR activation inhibits CeA to BNST GABAergic transmission.
A & B) Injection of an AAV-expressing, Cre-inducible GFP in the CeA resulted in GFP positive neurons in the CeA (A) and a large number of GFP positive fibers in the dorsolateral BNST. C) A brief flash of blue-light leads to a depolarization of GABAergic cell bodies in the CeA (i), and an inhibitory post-synaptic current (IPSC) in a BNST neuron (ii), which was completely blocked by the GABAA-R receptor antagonist picrotoxin (25 μM), demonstrating that this current is mediated via GABAA receptors. D) Pooled data demonstrating that the KOR agonist, U69593, inhibits light-evoked IPSC amplitude in the CeA-BNST pathway. E) Model summarizing the results of this study.
In order to examine whether inhibitory projections from the CeA to the BNST target a specific type of cell in the BNST, we examined whether there was a relationship between any basal physiological properties (membrane capacitance, membrane resistance, hyperpolarization-activated current) and the peak amplitude of light-evoked IPSCs from each cell we patched. No cellular properties were correlated with the magnitude of the postsynaptic response, nor were there any significant differences in the magnitude of any of these properties between neurons that could be successfully light-evoked and those that could not. When we evaluated the ability of the KOR agonist, U69593, to modulate the CeA-BNST GABAergic synapse, we found that application of 1 μM U69593 led to a significant reduction in light-evoked IPSCs (71 ± 20% of baseline, p < 0.05, n =6, Figure 7D) as it did in electrically-evoked IPSCs.
Discussion
KOR signaling has been shown to be involved in stress-induced alterations in behavior, but few studies have examined the impact of KOR signaling on synaptic transmission in brain regions that regulate affective behavior. Here we provide the first evidence demonstrating that KOR signaling depresses GABAergic synaptic transmission in the BNST via a presynaptic mechanism. Further, we provide evidence suggesting that this form of modulation is mediated by ERK activation. Finally, using a knock-in mouse combined with an inducible viral optogenetic approach, we show that KOR signaling can modulate the CeA to BNST GABAergic pathway as detailed in Figure 7E.
KOR activation leads to presynaptic inhibition of GABAergic transmission in the BNST
We provide multiple lines of evidence that KOR activation modulates inhibitory transmission in the BNST via a presynaptic mechanism, consistent with previous studies demonstrating that opioid peptides, including dynorphin, modulate synaptic transmission presynaptically (28–30). First, the inhibitory effect of U69593 was increased in reduced extracellular calcium concentration, as has been shown with other forms of presynaptic modulation (21). Second, KOR activation reduced the frequency, but not amplitude, of both spontaneous and miniature IPSCs. Third, there was a significant increase in CV, a measure sensitive to both release probability and the number of neurotransmitter release sites (22). Interestingly, the effect of KOR activation on CV but not PPR suggest that this may be through a reduction in the number of release sites, rather than a reduction in the probability of GABA release. This is similar to how norepinephrine inhibits glutamatergic synaptic function in the CeA via α2A-adrenergic receptors (31). Alternatively, it is possible that the magnitude of the effect is not sufficient to cause observable changes in PPR.
A novel role for ERK signaling in KOR-mediated inhibition of GABAergic transmission
The ERK signaling cascade plays a critical role in a wide variety of cell regulatory events and behavioral responsivity (32–34). The ability of KOR to activate ERK signaling has been well characterized both in cell culture (35) and in vivo (24, 26). A large body of evidence has demonstrated that the ERK pathway plays a critical role in regulation of glutamatergic transmission and plasticity (36, 37), with a principal focus on postsynaptic modulation of function. In contrast, there has been little investigation in to the ability of ERK signaling to modulate GABAergic transmission, and to date, there is only one report of ERK signaling regulating GABA release. This study demonstrated that the behavioral deficits in a mouse model for neurofibromatosis type 1 were due to an ERK-dependent increase in GABA release in the hippocampus, potentially via phosphorylation of synapsin (38). It is currently unclear how KOR directed ERK activation is linked to inhibition of GABA release, but this may be a novel action by which ERK can influence cellular and/or network function.
Implications for KOR modulation of GABAergic transmission in the BNST
The projection from the CeA to the BNST has been proposed to play a critical role in the regulation of anxiety behavior (39). Supported by recent studies from the Winder lab (16, 40), this model suggests that neurons in the CeA that project to the BNST release CRF to enhance glutamate release, leading to increased anxiety (39). Based on evidence that a portion of CRF neurons in the CeA co-express dynorphin (18) and our results that KOR activation inhibits GABA release in the CeA to BNST pathway, we hypothesize that CRF and dynorphin act in concert to increase anxiety-like behavior. The net effect of both decreasing inhibitory transmission (KOR activation) and increasing excitatory transmission (CRF) at synapses with potential BNST projection neurons likely enhances the output of the BNST to downstream structures directly involved in the regulation of affective/anxiety-related behaviors. This hypothesized interaction between dynorphin and CRF is supported by findings from the Chavkin group that have suggested that the dysphoric actions of CRF are mediated in part through the activation of KOR (41) . While we hypothesize that the CeA serves as a source of dynorphin to the BNST, it is also possible that this dynorphin could originate within the BNST or the paraventricular nucleus of the hypothalamus.
Conclusions
The results from this study add to a growing body of evidence suggesting that the KOR system is involved in the regulation of affective neuronal circuitry. We provide the first evidence that dynorphin modulates synaptic transmission in the BNST, a brain region that has been strongly implicated in a range of affective disorders, including post-traumatic stress disorder (PTSD), anxiety and depression. Further, we provide the first evidence that ERK is involved in the actions of KOR at GABAergic synapses. Based on these findings, as well as the larger body of literature focused on the role of BNST in maintaining organismal homeostasis, we speculate that negative modulators of KOR-directed ERK signaling may prove a successful treatment strategy for stress-induced affective disorders.
Supplementary Material
Acknowledgments
Research supported by the NIH Grants R00AA017668, R01AA019454, ABMRF Young Investigator Award, and an INIA-STRESS Pilot Project to TLK, NIH Grants R01DK089044, R01DK075632, P30DK046200, and P30DK057521 to BBL. We would like to thank Dr. Zoe McElligott and Dr. Ben Philpot for comments on a previous version of this manuscript.
Footnotes
The authors have no biomedical financial interests or potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madaan V, Wilson DR. Neuropeptides: relevance in treatment of depression and anxiety disorders. Drug News Perspect. 2009;22:319–324. doi: 10.1358/dnp.2009.22.6.1395255. [DOI] [PubMed] [Google Scholar]
- 2.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2009 doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-Like Effects of kappa-Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2009 doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, et al. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luk T, Jin W, Zvonok A, Lu D, Lin XZ, Chavkin C, et al. Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist. Br J Pharmacol. 2004;142:495–500. doi: 10.1038/sj.bjp.0705792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 10.Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol. 2007;97:883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol. 2005;93:3086–3093. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- 13.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- 15.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 16.Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1356–1365. doi: 10.1016/j.pnpbp.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- 19.Hooker JM, Patel V, Kothari S, Schiffer WK. Metabolic changes in the rodent brain after acute administration of salvinorin A. Mol Imaging Biol. 2009;11:137–143. doi: 10.1007/s11307-008-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watabe AM, Carlisle HJ, O'Dell TJ. Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol. 2002;87:1395–1403. doi: 10.1152/jn.00723.2001. [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci U S A. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, et al. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated Exposure to the kappa-Opioid Receptor Agonist Salvinorin A Modulates Extracellular Signal-Regulated Kinase and Reward Sensitivity. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton CC, Xu M, Chavkin C. Tyrosine phosphorylation of Kir3 following kappa-opioid receptor activation of p38 MAPK causes heterologous desensitization. J Biol Chem. 2009;284:31872–31881. doi: 10.1074/jbc.M109.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iremonger KJ, Bains JS. Integration of asynchronously released quanta prolongs the postsynaptic spike window. J Neurosci. 2007;27:6684–6691. doi: 10.1523/JNEUROSCI.0934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherubini E, North RA. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A. 1985;82:1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delaney AJ, Crane JW, Sah P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron. 2007;56:880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 33.Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- 34.Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 35.Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, et al. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008 doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 40.Nobis WP, Kash TL, Silberman Y, Winder DG. beta-Adrenergic Receptors Enhance Excitatory Transmission in the Bed Nucleus of the Stria Terminalis Through a Corticotrophin-Releasing Factor Receptor-Dependent and Cocaine-Regulated Mechanism. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.