Abstract
The ability to utilize oxygen has been shown to affect a wide variety of physiological factors often considered beneficial for survival. As the ability to learn can be seen as one of the core factors of survival in mammals, we studied whether selective breeding for endurance running, an indication of aerobic capacity, also has an effect on learning. Rats selectively bred over 23 generations for their ability to perform forced treadmill running were trained in an appetitively motivated discrimination-reversal classical conditioning task, an alternating T-maze task followed by a rule change (from a shift-win to stay-win rule) and motor learning task. In the discrimination-reversal and T-maze tasks, the high-capacity runner (HCR) rats outperformed the low-capacity runner (LCR) rats, most notably in the phases requiring flexible cognition. In the Rotarod (motor-learning) task, the HCR animals were overall more agile but learned at a similar rate with the LCR group as a function of training. We conclude that the intrinsic ability to utilize oxygen is associated especially with tasks requiring plasticity of the brain structures implicated in flexible cognition.
Keywords: aerobic capacity, classical conditioning, t-maze
1 Introduction
The ability to utilize oxygen forms a continuum between health and sickness. In rats selectively bred for running capacity, HCR rats have shown to be superior in many ways that are important for survival and fitness[16, 36]. In the evolutionary time scale, flexible cognition can also be seen as a factor that, especially in mammals, has made the difference between survival and extinction[19].
Aerobic capacity has been shown to be a complex but inherited trait in an animal model of selectively bred rats[17]. Acquired aerobic capacity is related to a host of health promoting factors and also to cognitive abilities. For instance, physically active elderly people perform better at cognitive tasks[5, 22] and rats given physical exercise learn various tasks more readily than their sedentary controls[11, 25]. Increased cognitive function in physically active subjects may rely on the increased capacity of the organism to utilize oxygen and glucose, elements necessary for the aerobic metabolism on which brain tissue relies. On the other hand, the explanation for better learning in physically active human and animal subjects might simply be due to a more stimulated nervous system, as it has been well established that environmental enrichment can significantly enhance learning[2, 14, 25, 33].
As the brain consumes a relatively large proportion of the oxygen in the blood, we expected to see differences in difficult learning tasks between HCR and LCR rats. In this study, we trained rats selectively bred for their endurance running capacity, but not given exercise, in order to find out whether the presumed better learning is due to innate differences in the ability to utilize oxygen. The animals were trained in relatively complex discrimination-reversal learning tasks requiring flexible cognition and also in a simpler motor learning task (Rotarod), in which improvement in performance is related to adjustment of neural function related to balance rather than to forming novel associations If the HCR animals outperform their LCR controls, this would support the idea that innate aerobic capacity also affects learning abilities. Should this be the case, we might conclude that inherited factors linked to aerobic metabolism also have a beneficial effect on learning.
2 METHODS
2.1 Subjects
The development of the rat models for aerobic exercise capacity has been described in detail earlier[17]. Briefly, selective breeding for intrinsic aerobic treadmill running capacity was started in 1996 using a founder population of a widely heterogeneous N:NIH rat stock. At each generation, young adult rats (11 weeks of age) were tested daily over five consecutive days for their inherent ability to perform forced speed-ramped treadmill running until exhausted. The greatest distance in meters achieved out of the five trials was considered the best estimate of an individual's aerobic exercise capacity. The highest scoring female and male from each of the thirteen families were selected as breeders for the next generation of high capacity runners (HCR). The same process was used with lowest scoring females and males to generate low capacity runners (LCR).
The rats in the current experiment were 19 females derived from generation 23 of selection. Distance run to exhaustion (± SEM) was 1840 ± 45.6 m for the HCR animals and 329 ± 22.4 m for the LCR rats. At the onset of the experiment, the rats were ~6 months old.
Prior to the start of the appetitive conditioning experiments the animals were gradually reduced to 85 % of their free-feeding weights. They were maintained at these weights throughout the experiment by being fed a restricted amount after each experimental session. The rats were housed in pairs in a light-proof room in which the lights were on for 12 hr/day. The animals were tested at the same time on successive days during the period when the lights were on in their holding room.
2.2 Behavioral experiments
2.2.1 Appetitive discrimination – reversal conditioning
For appetitive conditioning, four conditioning chambers (24.5 × 23.0 × 20.0 cm), made of aluminum, were housed in a cabinet with four independently ventilated and dimly lit compartments arranged in 2 × 2 fashion. One wall of each chamber was equipped with a pellet magazine. A speaker located behind the magazine, immediately outside the chamber, delivered the auditory stimuli at an intensity of 80 dB (A scale). An infrared transmitter and receiver pair formed an infrared beam inside the pellet magazine and its breakage was measured. Interruptions of these beams were continuously recorded by a self-designed protocol using MeasureFoundry software (Data Translation, inc., Marlboro, MA, USA) running on a PC clone. Another computer, equipped with E-Prime 1.2 software (Psychological Software Tools, inc., Sharpsburg, PA, USA), controlled the experiment.
For two days before the experiments, a few reward pellets (45 mg) were provided to the rats in their home cages. The rats were initially given 2 days of magazine training by placing them in the chambers for 30 min, during which the reward pellets were dispensed two at a time into the food magazine at regular 60-s intervals. Eight rats from each group were then selected for the experiment based on their magazine behaviour.
The conditioning procedure was administered so that in the discrimination phase, the animals received food (2 pellets) following one conditioned stimulus (CS+) while another conditioned stimulus was followed by nothing (CS−). The CSs (12 s in duration) were either a continuous white noise or bursts of 50-ms sine tones repeated at 200 ms intervals for the duration of the CS. The conditioned stimuli were counterbalanced across animals so that the white noise was the CS+ and the tone bursts CS− for half of the HCR and half of the LCR animals, and vice versa for the other halves. The intertrial interval varied between 90 and 180 s. A total of 20 trials per day was administered (10 CS+ and 10 CS− trials) in random order.
The discrimination training was continued for 10 days, after which the reversal phase commenced. The reversal training was exactly as the discrimination training except that the assignment of stimuli as either CS+ or CS− was the opposite. In other words, the reinforcement schedule was reversed; for example, if the continuous noise in the discrimination phase predicted food, it came to predict nothing in the reversal phase. The reversal phase consisted of 15 daily sessions.
2.2.2 Alternating T-maze
The floor of the elevated T-maze was made of wood and painted white. Each arm was 70 cm long and 10 cm wide. The side walls were made of clear acrylic and were 17 cm high. At both ends of the `horizontal' arms of the T-maze there was a 1-cm deep conical recession in the floor for the pellet to be placed in. The maze rested on three 100-cm high supports and was located orthogonally to the longitudinal axis of a 2-m × 4-m enclosed space. From the maze, the animals had a full view of distal wall cues (1 picture/wall) and some extra maze cues such as furniture and elements of the air conditioning system. A web-camera was attached to the ceiling above the maze and the rats' behavior was recorded to be analyzed afterwards.
The animals were familiarized with the T-maze in three pre-training sessions where both recesses were baited and the rats were free to explore the maze for 2 min at a time.
Initially, the rats were trained to adopt a shift-win strategy in 10 sessions on consecutive days. A trial consisted of two runs: the first one with a forced turn (the other arm was blocked by a clear acrylic wall) and the second run with both arms accessible. In the first run, the animal always found food in the food recess in the forced choice arm while in the second run the food was placed in the recess opposite to the previous one. After the first run, the animal was allowed to eat the pellet found and was then returned to the start arm. Each session contained six trials, with an equal number of forced right or left turns in a pseudorandom sequence. The experimenter baited the appropriate arm before each run. At the start of each session four rats were taken from the holding room to the experimental room in a sealed carry box from which they were unable to see the room. All four rats were tested in the same session, one at a time, with one trial (two runs) each, hence, the inter-trial interval ranged approximately from 2 to 4 min.
In the second phase of the experiment, the shift-win rule was replaced with the stay-win rule. In other words, the food in the second run was to be found in the same arm as in the first run. This training was continued for 10 days.
2.2.3 Motor learning
Motor learning was assessed using a Rotarod apparatus (Ugo Basile, Italy) consisting of a rotating drum with a grooved surface for gripping. The moving drum was set to accelerate from 4 to 40 rpm over 300 sec. The animals (n = 19) had 2 familiarization trials on the rotating drum at 4 rpm for 1 min each with an interval (between the trials) of 10 min. The interval between the second familiarization and first test session was 30 min. Rats were subjected to 7 trials with a 15-min interval between each trial. The latency to fall off the Rotarod device was assessed in each trial.
2.3 Data analysis
2.3.1 Discrimination ratio
Responses to the CSs in the appetitive discrimination-reversal conditioning were determined as the total time during which the IR beam was broken during the last 9 s of the CS period. Learning per animal per day was assessed as discrimination ratio (DR):
where R+ is the response duration to the reinforced tone and R− is the response duration to the non-reinforced tone. Thus, the DR will receive a value between 0 and 1 where 0.5 represents equal responding to both stimuli.
2.3.2 Statistical analysis
Analysis of variance (ANOVA) for repeated measures was used to examine the effects of conditioning and group on the dependent variable of learning (discrimination ratio in classical conditioning task, correct choices in T-maze test or time spent on the Rotarod beam). Greenhouse-Geisser corrected degrees of freedom were applied whenever the sphericity assumption was violated. Additional one-sample t-tests were run for both groups separately against chance levels in order to investigate whether the rats were successfully discriminating between stimuli (discrimination-reversal task) and T-maze turns. Two-tailed statistics were used.
3 RESULTS
3.1 Discrimination-reversal
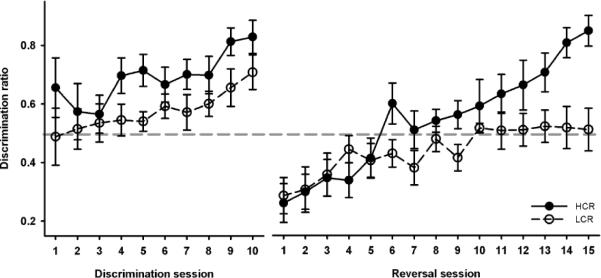
In the discrimination phase (Fig 1), the main effects of both session [F(9,126) = 3.16; p < 0.05, η2partial = 0.18] and group [F(1,14) = 5.79; p < 0.05, η2partial = 0.29] on the discrimination ratio were significant, indicating that both groups learned to discriminate between the tones, but the HCR group performed at a better level overall. One-sample t-tests of discrimination ratio against chance level (= 0.5) revealed that HCR animals were successfully discriminating between the stimuli from session 4 onwards [t(7) = 2.80 – 6.64, p < 0.05 – 0.001] while successful discrimination was not seen in LCR animals until the last two sessions of discrimination training [t(7) = 2.44, p < 0.05 and t(7) = 3.52, p < 0.01, respectively]
FIGURE 1.
Discrimination ratio (+/− SEM) as a function of training session appetitive discrimination-reversal training. In the discrimination phase, both groups learned to discriminate between the CSs, but in the reversal phase, the HCR rats outperformed the LCR rats. In fact, the LCR rats were not able to exceed the chance level of responding even after 15 days of training.
In the reversal phase (Fig 1), the HCR rats were quicker to acquire the new rule, as indicated by a significant interaction of session and group [F(14,196) = 2.53, p < 0.05; η2partial = 0.15]. The main effects of both session [F(14,196) = 11.03, p < 0.01; η2partial= 0.44] and group [F(1,14) = 4.67, p < 0.05; η2partial= 0.25] were also statistically significant. Separate analyses revealed statistically significant increases in the discrimination ratio in both groups as a function of session [HCR: F(14,98) = 10.48; p < 0.001; η2partial = 0.60; LCR: F(14,98) = 2.40; p < 0.01; η2partial = 0.25]. However, even at their best the LCR rats did not perform better than the chance level, as indicated by not even nearly significant differences between discrimination ratio and chance level [in sessions 10 to 15 t(7) = 0.12 – 0.41, p = 0.70 – 0.90]. In contrast, the difference between discrimination ratio and chance level was significant in the HCR group in the last three session of the reversal training [t(7) = 3.11 – 6.59, p < 0.05 – 0.001].
3.2 Alternating T-maze
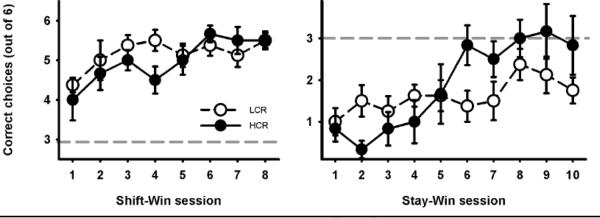
Two animals from the HCR group were omitted from the analyses because they engaged in freezing behavior when placed in the elevated T-maze making it impossible to obtain task-relevant behavioral data. The results of the T-maze test are presented in Fig. 2. In the first phase, when the animals had to learn the rule that the pellet was in the arm opposite to where it had been in the forced run, the animals quickly learned to make correct responses, as indicated by the significant main effect of session on the number of correct choices [F(7,84) = 4.14; p < 0.01; η2partial = 0.26]. Neither the group nor group x block interaction was significant. One-sample t-tests of correct choices against the chance level (= 3) revealed that both groups were successfully making correct choices very early in training [LCR: t(7) = 4.00 – 13.23, p < 0.01 – 0.001 and HCR: t(5) = 3.95 – 12.65].
FIGURE 2.
Number of correct choices (+/− SEM) as a function of T-maze training. There were no significant differences between the groups in the T-maze test in acquiring the shift-win rule (left pane): both groups learned to a similar extent at a similar rate. However, when the rule was changed to the stay-win rule (right pane), the HCR rats performed significantly better, but were still unable to reach the chance level.
When the rule was changed and the rats had to acquire the new one, according to which the pellet was found at the end of the same arm as in the forced run, there was a significant group x session interaction [F(9,108) = 3.84; p < 0.001; η2partial = 0.256] as well as significant effect of session [F(9,108) = 9.46; P < 0.001; η2partial = 0.441]. When the groups were analyzed separately, a significant increase in the correct choices was found in the HCR rats only [F(9,45) = 11.21; p < 0.001; η2partial = 0.692]. The LCR rats continued to follow the rule they initially learned in the alternating T-maze training phase, as indicated by the significant differences between the incorrect choices and chance level in almost all sessions [t(7) = 3.24 – 6.11, p < 0.05 – 0.001]. In sessions 8 and 9 the difference was almost significant [t(7) = 1.67, p = 0.14 and t(7) = 1.99, p = 0.087, respectively]. In contrast, the HCR rats, although able to abandon this rule nevertheless did not perform above the chance level; thus the task proved to be very difficult. Comparison of incorrect choices against chance level in HCR rats revealed significant differences in the first 4 sessions [t(5) = 3.87 – 12.65, p < 0.05 – 0.001] and no significant differences from session 5 to session 10 [t(5) = 0.00 – 1.86, p = 0.12 – 1.00].
3.3 Motor learning
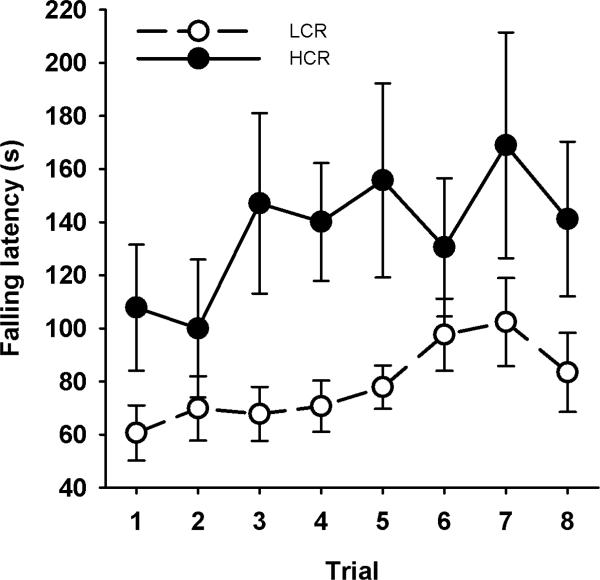
The HCR animals were overall better at the Rotarod task, as indicated by a significant main effect of group [F(1,29) = 4.66; p < 0.05; η2partial = 0.138]. Time on the beam increased as a function of trial [F(7,203) = 4.14; η2partial = 0.125], but there was no significant interaction of trial and group (Fig. 3). While the training programme had an equal effect on both groups, the HCR animals had overall better balance.
FIGURE 3.
Time the animals could stay on the Rotarod device. The overall performance of the HCR rats was better, but both groups achieved a significant increase as a function of trials.
4 DISCUSSION
We demonstrated that rats with higher intrinsic aerobic capacity outperformed those with lower capacity in a task requiring acquisition of rule change. No such difference was found when the learning task strictly involved enhancement of motor abilities (Rotarod), although the HCR animals showed better motor skills, as such, throughout the training. Thus, it seems that if there is any beneficial effect of aerobic capacity, it is biased towards flexible cognition.
4.1. Innate endurance running capacity is related to flexible cognition but not to motor learning
Rats have a tendency to alternate their choices in the T-maze[32], which facilitates learning of the shift-win rule. In contrast, because of this predisposition and, further, because of reinforcement of the alternating behavior, the following rule change requiring the adoption of a stay-win strategy should be challenging, which is exactly what the present experiment revealed. As required by the task, it seems that the HCR rats were able to abandon the (innate and previously reinforced) shift-win strategy, and in 9 sessions trained, they reached at least to the chance level of responding, while the LCR animals continued responding according to the shift-win strategy. As the order of shift-win and stay-win treatments was not counterbalanced we cannot, however, rule out the alternative interpretation that the HCR animals were simply better in learning the stay-win strategy. Still, the alternating T-maze paradigm with a rule change requiring a turn from a shift-win to stay-win strategy proved a simple and useful paradigm for future efforts to study flexible cognition in rats. The present results indicate that more extensive training would be needed to show complete mastery of the stay-win strategy. On the other hand, it would be of interest to study whether better learning of the HCR animals in the T-maze results from hippocampal dependent place learning or striatum-dependent response learning[26].This could be achieved by either using a plus-maze or rotating the T-maze between trials.
Similar results were obtained using the appetitive discrimination-reversal training, where the rats were first trained to discriminate between auditory stimuli by reinforcing one stimulus and then, by reversing the rule, reinforcing another, previously non-reinforced, stimulus. It has been shown that hippocampal lesions as well as lesions of the related cortical areas lead to specific deficit in reversal learning while having less or no effect on the initial discrimination learning in eyeblink conditioning[35]. Further, lesions to the frontostriatal areas produce perseverative behavior and deficits in flexible cognition[13]. Together these results imply differences in plasticity in the neural areas that are related to flexible cognition and learning of relational information, that is, the frontostriatal and hippocampal areas, respectively[13].
Motor learning was evident in both groups, as seen in the ability to remain longer on the Rotarod beam as a function of trial. It was also evident that the HCR animals performed better than the LCR animals, even without any training. This result echoes that of the earlier study by Koch and Britton[17]. However, the rate of learning itself was similar in both groups, suggesting that the neural plasticity required for motor learning was equally effective. HCR animals only seem to be intrinsically better performers in motor tasks.
The HCR/LCR strains were developed to contrast for the effect of innate endurance running capacity (allegedly aerobic capacity) on various measures for health and sickness. Consequently, in the absence of non-manipulated control strain we cannot determine whether the observed difference between the HCR and LCR strains in flexible cognition was because of beneficial effect of high aerobic capacity, detrimental effect of low aerobic capacity, or both. However, our results strongly support the idea that genome regulating the aerobic capacity, is linked to flexible cognition. Another alternative explanation of the present results is that the strains also differ in motivation which might contribute to differences in learning. However, the difference between strains seems to be selective to cognitive but not motor learning, whereas possible motivational aspects would probably affect all kinds of learning and performance in general.
4.2. Possible roles of oxygen delivery and glucose utilization
Delivery of oxygenated blood into metabolically demanding organs is of great importance in mammals. Data from the 15th generation of rats selectively bred for their aerobic capacity[17] showed increased peripheral oxygen delivery to muscles in the high intrinsic aerobic capacity group[12], suggesting a genetic component of this physiological trait. This evidence could prompt the further hypothesis that learning differences observed between LCR/HCR, especially in demanding learning tasks (rule reversal), could rely on enhanced oxygen delivery to the brain, a highly oxidative tissue.
The glucose-induced regulation of brain processes related to learning and memory have been well described over the past decades[9]. Evidence has emerged from animal studies on the beneficial elevation of glucose transporter proteins by both forced and voluntary running[15]. Increments of glucose metabolism-related substrates could explain the beneficial effects of physical activity on brain metabolism. Data from studies on non-diabetic women have shown a negative correlation between plasma glucose and episodic memory[29], strengthening the notion that energy metabolism plays an important role in cognitive function in humans[31]. Similarly, insulin resistance has shown a correlation with reduced brain volume in women at risk for Alzheimer's Disease (AD)[27], verifying the glucose metabolism-based hypothesis for aging-induced cognitive decline[21]. DNA microarray data from our lab on this animal model have revealed down-regulation of genes involved in metabolic syndrome, which correlates with low intrinsic aerobic capacity[16]. Similarly, Mootha et al. have shown down-regulation of functionally related genes implicated in oxidative phosphorylation in diabetic human skeletal muscle[23]. A recent study showed that LCR animals display a reduced ability for mitochondrial regeneration, decreased metabolic control in the heart, a reduced antioxidant status and decreased longevity[20]. These data indicate that metabolic properties related to energy metabolism in skeletal muscle have a genetic component. This could be of great importance with respect to cognitive performance, as shown by data from studies indicating a relationship between diabetes and cognitive impairments[1, 6, 27, 31].
The proposed mediation of BDNF action by IGF-I[7] points to the importance of its quantification in the areas of the brain where BDNF action is under investigation. Light has been shed on Blood Brain Barrier (BBB) permeability by IGF-I and the enhanced binding of BDNF to TrkB (its high affinity receptor) in the presence of IGF-I[3, 7, 24, 28, 34]. The above-mentioned properties of IGF-I combined with its exercise-induced upregulation[30] suggest the importance of conducting further experiments on the HCR/LCR model. Additionally, as exercise increases BDNF gene transcription[10], it would be of functional importance to investigate BDNF mRNA expression and TrkB receptor in the learning-relevant areas. Ongoing experiments in our group try to further investigate possible molecular differences in vascularization properties and neurogenesis in hippocampus and striatum.
4.3. Conclusion
Oxygen as an effective conveyer of energy is one of the driving forces of evolution[4, 8]. It can be argued that individuals with higher aerobic capacity (i.e. able to use more oxygen) are evolutionally favored within a species. Indeed, high aerobic capacity or fitness (either inherited or acquired) is predictive for better health and longevity[18]. The present study also shows that better brain function (learning capacity) is associated with higher oxygen metabolism and that genetically these traits segregate in concert. Our findings emphasize further the importance of fitness for survival and the centrality of oxygen in biology and evolution in general.
Highlights
-
-
Aerobic capacity correlates positively with cognitive abilities
-
-
It is not known whether this is due to acquired or innate aerobic capacity
-
-
We trained rats bred for endurance running capacity in tasks requiring flexible cognition
-
-
Rats with intrinsically high running capacity outperformed those with low capacity
Acknowledgements
This study was supported by grants from Academy of Finland to JW and HK, and from Ministry of Culture and Education to HK. GM was financially supported by National Doctoral Programme of Musculoskeletal Disorders and Biomaterials (TBDP). The LCR and HCR rat resource was supported by NIH grant R24RR017718 to SLB and LGK and by NIH grant R01DK077200 to SLB. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Gilligan. We would like to thank Michael Freeman for checking the language of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abbatecola AM, Oliveri F, Corsonello A, Antonicelli R, Corica F, Lattanzio F. Genome-wide association studies: Is there a genotype for cognitive decline in older persons with type 2 diabetes? Curr Pharm Des. 2011 doi: 10.2174/138161211795164239. [DOI] [PubMed] [Google Scholar]
- [2].Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- [3].Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–33. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Catling DC, Glein CR, Zahnle KJ, McKay CP. Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology. 2005;5:415–38. doi: 10.1089/ast.2005.5.415. [DOI] [PubMed] [Google Scholar]
- [5].Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- [6].Cox D, Gonder-Frederick L, McCall A, Kovatchev B, Clarke W. The effects of glucose fluctuation on cognitive function and QOL: The functional costs of hypoglycaemia and hyperglycaemia among adults with type 1 or type 2 diabetes. Int J Clin Pract Suppl. 2002;(129):20–6. [PubMed] [Google Scholar]
- [7].Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- [8].Falkowski PG, Katz ME, Milligan AJ, Fennel K, Cramer BS, Aubry MP, Berner RA, Novacek MJ, Zapol WM. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–4. doi: 10.1126/science.1116047. [DOI] [PubMed] [Google Scholar]
- [9].Gold PE. Role of glucose in regulating the brain and cognition. Am J Clin Nutr. 1995;61:987S–95S. doi: 10.1093/ajcn/61.4.987S. [DOI] [PubMed] [Google Scholar]
- [10].Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Green JT, Chess AC, Burns M, Schachinger KM, Thanellou A. The effects of two forms of physical activity on eyeblink classical conditioning. Behav Brain Res. 2011;219:165–74. doi: 10.1016/j.bbr.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Howlett RA, Kirkton SD, Gonzalez NC, Wagner HE, Britton SL, Koch LG, Wagner PD. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J Appl Physiol. 2009;106:1819–25. doi: 10.1152/japplphysiol.00914.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- [14].Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [15].Kinni H, Guo M, Ding JY, Konakondla S, Dornbos D, 3rd, Tran R, Guthikonda M, Ding Y. Cerebral metabolism after forced or voluntary physical exercise. Brain Res. 2011 doi: 10.1016/j.brainres.2011.02.076. [DOI] [PubMed] [Google Scholar]
- [16].Kivela R, Silvennoinen M, Lehti M, Rinnankoski-Tuikka R, Purhonen T, Ketola T, Pullinen K, Vuento M, Mutanen N, Sartor MA, Reunanen H, Koch LG, Britton SL, Kainulainen H. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J. 2010 doi: 10.1096/fj.10-157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- [18].Koch LG, Britton SL. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity (Silver Spring) 2008;16(Suppl 3):S28–32. doi: 10.1038/oby.2008.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. The Journal of Physiology. 2008;586:83–95. doi: 10.1113/jphysiol.2007.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisløff H, Høydal MA, Rolim N, Abadir PM, Van Grevenhof I, Smith GL, Burant CF, Ellingsen Ø, Britton SL, Wisløff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circulation Research. 2011 doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Korol DL, Gold PE. Glucose, memory, and aging. Am J Clin Nutr. 1998;67:764S–71S. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- [22].Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: A review and future directions. Neuropsychol Rev. 2009;19:504–22. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- [24].Niblock MM, Brunso-Bechtold JK, Riddle DR. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J Neurosci. 2000;20:4165–76. doi: 10.1523/JNEUROSCI.20-11-04165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- [26].Packard MG. Exhumed from thought: Basal ganglia and response learning in the plus-maze. Behav Brain Res. 2009;199:24–31. doi: 10.1016/j.bbr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [27].Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for alzheimer's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reinhardt R, Bondy C. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–61. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- [29].Rolandsson O, Backestrom A, Eriksson S, Hallmans G, Nilsson LG. Increased glucose levels are associated with episodic memory in nondiabetic women. Diabetes. 2008;57:440–3. doi: 10.2337/db07-1215. [DOI] [PubMed] [Google Scholar]
- [30].Schwarz A, Brasel J, Hintz R, Mohan S, Cooper D. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–7. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- [31].Stranahan AM, Mattson MP. Bidirectional metabolic regulation of neurocognitive function. Neurobiol Learn Mem. 2011 doi: 10.1016/j.nlm.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Szelest I, Cohen J. Effects of forced-choice runway variations on rats' T-maze serial pattern learning. Learn Behav. 2006;34:202–14. doi: 10.3758/bf03193195. [DOI] [PubMed] [Google Scholar]
- [33].van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–8. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- [34].Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and alzheimer's disease and vascular dementia. J Am Geriatr Soc. 2005;53:1748–53. doi: 10.1111/j.1532-5415.2005.53524.x. [DOI] [PubMed] [Google Scholar]
- [35].Weikart CL, Berger TW. Hippocampal lesions disrupt classical conditioning of cross-modality reversal learning of the rabbit nictitating membrane response. Behav Brain Res. 1986;22:85–9. doi: 10.1016/0166-4328(86)90083-5. [DOI] [PubMed] [Google Scholar]
- [36].Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–20. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]