Abstract
CD4 functions to enhance the sensitivity of T cells to antigenic peptide/MHC class II. However, if aggregated in isolation, e.g. in the absence of T cell receptor (TCR), CD4 can transduce yet undefined signals that lead to T cell unresponsiveness to antigen and apoptosis. In Human Immunodeficiency Virus-1 (HIV-1) disease, CD4+ T cell loss can result from gp120-induced CD4 signaling in uninfected cells. We show here that CD4 aggregation leads to Lck-dependent phosphorylation of the RasGAP adaptors Downstream of kinase-1/2 (Dok-1/2) and the inositol 5-phosphatase-1 (SHIP-1) and association of the two molecules. Studies using SHIP-1 shRNA, knockout mice and decoy inhibitors further indicate that CD4-mediated inhibition of TCR-mediated T cell activation is SHIP-1 and Dok-1/2 dependent, and involves SHIP-1 hydrolysis of Phosphatidylinositol 3,4,5-trisphosophate (PI(3,4,5)P3) needed for TCR signaling. Our studies provide evidence for a novel mechanism by which ill-timed CD4-mediated signals activated by ligands such as HIV-1 gp120 lead to disarmament of the immune system.
Keywords: CD4, SHIP-1, Dok-1/2, TCR, HIV-1
1. Introduction
CD4 is a 55 kDa single-chain transmembrane protein that is expressed primarily by MHC class II restricted T helper cells. Through its interaction with the β2 domain of MHC class II, CD4 enhances T cell activation by stabilizing the binding of the T cell to the APC (1). In addition, CD4 is directly involved in TCR signaling. CD4 is non-covalently associated with the src-family tyrosine kinase, Lck. During antigen presentation, CD4 mediates rapid autophosphorylation of Lck (2), and phosphorylation of the TCR ζ-chain. Phosphorylated ζ-chain immunoreceptor tyrosine-based activation motifs, ITAMs, and Lck act in concert to activate ZAP-70 and phosphorylation of the linker of activation of T cells (LAT). Triggering of these receptor proximal events leads to activation of several downstream signaling molecules including PLC-γ, PI3K, and MAPKs, and to calcium mobilization from intracellular stores, IL-2 transcription, and proliferation.
In contrast to its cooperative role in antigen-dependent T cell activation, TCR independent CD4 ligation by its proposed natural ligands, IL-16 (3) or HIV-1 gp120 (4–6), has been shown to initiate transmission of inhibitory signals (7, 8). Available evidence indicates that loss of functional CD4+ T cells in HIV-1 disease may result primarily from gp120-induced CD4-mediated signals that cause T cell unresponsiveness to antigen and/or death by apoptosis. Little is known regarding the molecular circuitry that links CD4 aggregation to inhibition of TCR signaling and apoptosis. CD4 aggregation by gp120 leads to phosphorylation and activation of Lck (9, 10), upregulation of Fas expression (11, 12), NF-κB (13) and Raf-1 activation (14). Consistent with a role for Lck in mediating the downstream inhibitory effects of CD4 ligation, truncation or mutation of the cytoplasmic tail to remove the Lck binding site renders CD4 unable to transduce inhibitory signals (15–17). The identity of effectors of Lck in this inhibitory signaling pathway remains unknown.
Pre-aggregation of CD4 blocks TCR-mediated activation of calcium mobilization (17, 18), suggesting that ill-timed CD4 signaling may somehow block PLCγ activation. In fact, while PLCγ phosphorylation is reportedly not affected by CD4 pre-aggregation, its induced association with other molecules (e.g. Sam68) following TCR aggregation is inhibited (19). Interestingly, this association requires PI(3,4,5)P3. Thus, CD4 mediated inhibition could reflect inhibition of PI3-kinase activation or activation of the SH2_domain-containing inositol 5 phosphatase SHIP-1 or PTEN, enzymes that dephosphorylate PI(3,4,5)P3 at inositol 5 and 3 positions, respectively (20, 21). Consistent with these possibilities is pro-apoptotic signaling by CD4, since PI(3,4,5)P3 is required for activation of Akt/PKB, a serine/threonine kinase that signals survival (22). Another potential clue comes from findings that CD4 pre-aggregation blocks TCR-mediated activation of the Ras pathway (19). Activity of the p21ras regulator rasGAP is regulated by Dok, and SHIP-1 is known to function as an adaptor for Dok-1/2 (23). Finally, in preliminary experiments we noted that CD4 aggregation leads to phosphorylation of a dominant substrate with a molecular weight consistent with that of Dok-1.
Based on these observations we explored the role of SHIP-1 and Dok-1/2 in CD4 signaling. We found that aggregation of CD4 leads to rapid tyrosine phosphorylation of SHIP-1 and Dok-1/2 and this response requires an intact CD4 cytoplasmic tail and src-family kinase activity. Importantly, Dok-1/2 are the predominant phosphorylated molecules seen following CD4 aggregation. Consistent with a role for SHIP-1 in inhibitory signaling, CD4 preligation significantly reduced TCR mediated increases in membrane PI(3,4,5)P3 as indicated by membrane translocation of the PI(3,4,5)P3 binding PH domain of Akt. Comparative analysis of T cells from SHIP-1−/− and Dok-1−/− mice indicated that inhibition of TCR-mediated calcium mobilization by prior CD4 ligation is at least partially dependent on SHIP-1 and Dok-1. Effects may be partial because of the expression of redundant effectors Dok-2 and SHIP-2. Consistent with this possibility, expression of SHIP-1 shRNA or decoy inhibitors in cells blocked the induction of SHIP-1, Dok-1, and Dok-2 phosphorylation, and rescued cells from CD4 mediated inhibition of intracellular calcium mobilization, PI(3,4,5)P3 accumulation and proliferation. Based on these studies, it appears that SHIP-1 and Dok-1 play a critical role in CD4-mediated inhibition of TCR signaling and pro-apoptotic signaling. It is noteworthy that this inhibitory signaling loop is also activated following CD4-TCR co-aggregation, and presumably acts physiologically to terminate signaling. We suggest that premature activation of the SHIP/Dok circuit via CD4 prevents effective TCR signaling.
2. Material and Methods
2.1 Antibodies and reagents
The following antibodies were purchased from Zymed (San Francisco, CA): purified rabbit anti-rat, HRP-coupled rat anti-mouse IgG1 and protein A. SHIP- and Dok-specific antibodies were generated by immunization of rabbits with aa residues 909–959 of murine SHIP and full-length murine Dok, and purified using antigen-coupled Sepharose 4B (Pharmacia). Anti-SHIP-2 antisera were generated by immunization of rabbits with a peptide corresponding to the C-terminal of SHIP-2 (24). Anti-Dok-2 antibodies were purchased from Santa Cruz (Santa Cruz, CA). Gp120 and goat anti-gp120 was a generous gift from Chiron Pharmaceuticals. The following antibodies were also used: GK1.5 (anti-mCD4 mAb), 53.6 (anti-mCD8 mAb) and M1/42.3.9.8 (anti-MHC Cl. I, H-2kall, ATCC, Manassas, VA), RA3-6B2 (anti-mCD45R, B220), H57-597 (anti-mTCR mAb) (25), 107.3 (anti-TNP mAb, Pharmingen, San Diego, CA), B7.11 (anti-TNP mAb), Ab-2 (anti-phosphotyrosine mAb, Calbiochem, La Jolla, CA), 4G10 (anti-phosphotyrosine mAb, Upstate, Charlottesville, VA). Src-family tyrosine kinase inhibitor PP2 was purchased from Calbiochem and 2,4,6 Trinitrobenzene Sulfonic Acid (TNP) from Pierce (Rockford, IL). Unless otherwise stated, all other reagents were from Sigma (St. Louis, MO).
2.2 Cells and cell culture
The murine D10 T cell clone (26) was cultured in IMDM with 10% heat-inactivated FCS (Hyclone, Logan, UT), 100 U/ml penicillin, 100 µg/ml streptomycin and 8U/ml IL-2 at 37°C with 7% CO2. Amphotropic phoenix cells (27, 28) were cultured in IMDM supplemented with 5% heat-inactivated FCS, penicillin, and streptomycin. All culture reagents were from GIBCO-RBL (Gaithersburg, MD). CD4+ T cells were isolated from lymph nodes of AND.B10BR (26), tailless CD4 (29), SHIP−/− or SHIP+/+ (30), and Dok−/− or Dok+/+ mice (31) by negative depletion. Lymphocytes were incubated for 30 min with biotinylated anti-mCD8 (53.6) plus biotinylated anti-CD45R (RA3-6B2) mAb. Cells were washed and streptavidin-conjugated magnetic beads (M280, Dynal AS, Norway) were added for 1h. CD4+ cells were isolated by removing magnetic-bead complexes with a magnet (Dynal). The resultant population was 85–95% CD4+ cells by flow cytometry. Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Amersham Pharmacia, Piscataway, NJ) density-gradient centrifugation of heparinized venous blood obtained from healthy donors. This study was approved by the University of Colorado Denver Institutional Review Board, Protocol 08-0732.
2.3 Retroviral transduction
SHIP-SH2 (residues 1–114 in SHIP) (32) and Dok-PH/PTB (residues 1–258 in murine Dok) constructs were cloned into pMXI-EGFP and pMSCV-Thy1.1 (provided by Gary Nolan, Stanford University). The Akt-PH domain (33) was cloned into the N-terminal GFP-fusion vector pONG (provided by Brian Schaefer, Uniformed Services University of the Health Sciences, Bethesda, MD). Amphotropic Phoenix cells were transfected using effectene transfection reagent (Qiagen, Valencia, CA). High titer retrovirus was produced as previously described (32) and used to infect D10 cells. GFP or Thy1.1 positive D10 cells were sorted and reanalyzed prior to experimentation.
2.4 Cell stimulation and lysis
D10 cells or CD4+ primary T cells (3–6 × 107 cells at 2 × 107/ml) were stimulated through the TCR using biotinylated anti-TCR mAb (H57-597) and avidin (50 µg/ml). Cells were incubated with biotinylated anti-TCR mAb for 10 min at RT, washed, prewarmed for 10 min at 37°C and then stimulated for the indicated period with avidin at 37°C. To aggregate CD4, cells were incubated with anti-CD4 antibodies (GK1.5, 10 µg/ml) for 10 min, washed, prewarmed and stimulated with rabbit anti-rat (50 µg/ml) for the indicated period at 37°C. CD4 aggregation by HIV-1 gp120 was performed with recombinant gp120 (20 µg/ml) plus goat anti-gp120 (1:200). CD4 pre-aggregation was carried out using anti-CD4 mAb coupled to TNP (TNP coupling ratio 1:7, 10 µg/ml) and a cocktail of 2 different anti-TNP mAbs (107.3, B7.11, 20 µg/ml). 30 min after CD4 crosslinking, cells were washed and stimulated through the TCR. After stimulation, cells were lysed in 1% NP-40, 150 mM NaCl, 10 mM Tris, pH 7.5, containing the following inhibitors: 10 mM sodium pyrophosphate, 2 mM Na3VO4, 10 mM NaF, 0.4 M EDTA, 1 mM PMSF, and 1 µg/ml each of aprotinin, antitrypsin, and leupeptin at 3 × 107 cells/ml. Lysis was performed on ice for 15 min and cleared lysates were retained for further processing.
2.5 Immunoprecipitation and immunoblotting
Cleared lysates were incubated with anti-SHIP, -SHIP-2, -Dok or –Dok-2 antibodies and protein A-Sepharose, or antibodies directly conjugated to Sepharose beads (Pharmacia). Immune complexes were analyzed by 8% SDS-PAGE, and transferred to Polyvinylidene Difluoride (PVDF) membrane (Millipore, Bedford, MA). PVDF membranes were then blotted with anti-phosphotyrosine Ab and HRP-conjugated anti-mouse IgG using the ECL Western blotting detection system (Amersham, Aylesbury, U.K.). In some cases, after blotting with anti-phosphotyrosine Ab, membranes were stripped to remove bound antibodies and subjected to sequential blotting with anti-SHIP, -SHIP-2 and -Dok.
2.6 Confocal Microscopy
D10 cells expressing Akt-PH-GFP fusion proteins alone or in the presence of the SHIP-SH2 domain were stimulated as described and 5 × 105 were placed on glass cover slips coated with poly-L-lysine (50 µg/ml, Sigma, St. Louis, MO). Cells were fixed in 1% paraformaldehyde, mounted on microscope slides with mounting solution (2 mg/ml OPDA in 90% Glycerol). Images were captured and analyzed using a Leica DMRXA microscope, a SensicamQE camera (CooKe, Auburn Hills, MI), and Slidebook imaging software (Intelligent Imaging Innovations, Denver, CO).
2.7 Analysis of intracellular calcium concentration, [Ca2+]i
For measurements of intracellular free calcium, cells (5×106 cells/ml) were loaded with Indo-1 AM (5 µM, Molecular Probes, Eugene, OR) at 37°C as previously described (34). Mean intracellular free calcium was monitored by flow cytometry (LSR, Becton Dickinson). Histograms illustrating the ratio of Indo-1 AM emission at 390/490 nm as a function of time and cell number were generated using FlowJo software (Tree Star Inc., San Carlos, CA).
2.8 In vitro proliferation assays
D10 cells were IL-2 starved for 24 hours and CD4 was pre-aggregated as described. In a 96-well plate 5×104 Dl0 cells were stimulated with 5×104 mitomycin-C treated CH12 cells expressing IAk plus conalbumin (Sigma, St. Louis, MO). For the final 6 h of culture, [3H]Thymidine (NEN, Boston, MA, 1 µCi/well) was added. Plates were harvested on a Tomtec Harvester 96 (Orange, CT) and [3H]Thymidine incorporation was quantified by a liquid scintillation counter (1450 Microbeta Plus, Wallac, Turku, Finland). Experimental values reported represent the mean of triplicate determinations.
3. Theory
Loss of CD4 T cell function during HIV-1 disease may be due to gp120 aggregation of CD4 and consequent stimulation of SHIP-1 and Dok-1/2-mediated inhibition of TCR activation.
4. Results
4.1 CD4 aggregation induces SHIP and Dok phosphorylation
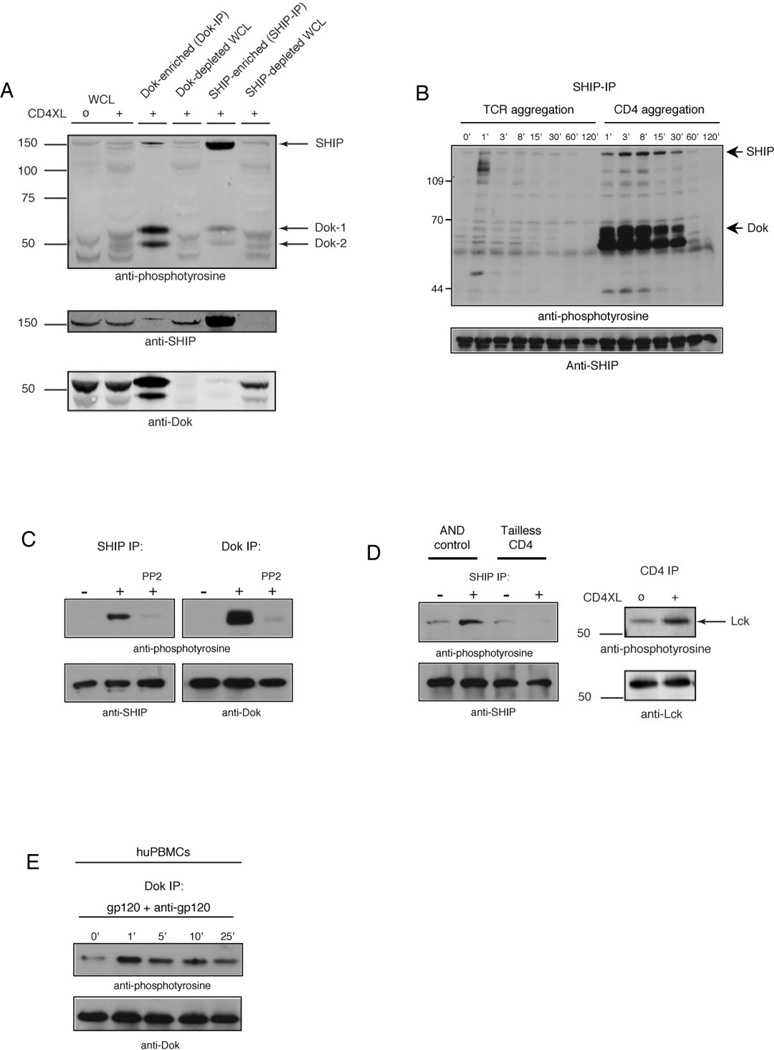
We first analyzed the spectrum of proteins phosphorylated upon CD4 aggregation. Stimulation of the IL-2-dependent murine CD4+ T cell line D10 (26) with anti-CD4 led to phosphorylation of a number of substrates, the most strongly phosphorylated of which had apparent molecular weights of ~62,000 and ~56,000 Da (Fig. 1A, lanes 1 and 2). The mass of this protein suggested that it might be the RasGAP adaptors Dok-1 and Dok-2 (35–37). To confirm the identity of these proteins, we analyzed their phosphorylation in lysates of whole cells, Dok immunoprecipitates, and anti-Dok depleted lysates. The anti-Dok antibody used in these studies was raised in rabbits by immunization using full-length recombinant Dok-1, and crossreacts with Dok-2 (23). Depletion of lysates from CD4-stimulated cells with this antibody resulted in loss of the 62k and 56kDa phosphotyrosine signals, and these phosphoproteins were enriched in Dok immunoprecipitates. Furthermore, these proteins were immunoblotted with this antibody confirming their identity as Dok-1 and Dok-2 (Fig. 1A, lanes 3 and 4).
Figure 1. CD4 aggregation induces CD4 cytoplasmic tail-dependent tyrosine phosphorylation of SHIP and Dok.
(A) D10 cells were stimulated with anti-CD4 plus rabbit anti-rat IgG antibodies (2 min, 37°C) followed by lysis and immunoprecipitation using SHIP-, or Dok-specific antibodies directly conjugated to sepharose beads. Precipitates (5×106 cell equivalents) and supernatants (1×106 cell equivalents (WCL)) were resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblotting using phosphotyrosine-specific antibodies. (B) D10 cells were treated with biotinylated anti-TCR plus avidin, or anti-CD4 plus rabbit anti-rat IgG antibodies for the indicated time, followed by lysis and immunoprecipitation with SHIP-specific antibodies. Precipitates were resolved by SDS-PAGE, transferred to PVDF membrane, and analyzed by immunoblotting using phosphotyrosine-, or SHIP-specific antibodies. (C) D10 cells were pretreated with 1µM PP2, followed by anti-CD4 plus rabbit anti-rat IgG. Cells were lysed as described, and analyzed by immunoblotting using phosphotyrosine-, Dok-, and SHIP-specific antibodies. (D) Purified CD4+ T cells from AND.B10BR or tailless CD4 mice were stimulated with anti-CD4 plus rabbit anti-rat IgG. Cells were lysed and immunoprecipitated with SHIP-specific antibodies and analyzed as described above. (E) Freshly isolated huPBMCs were stimulated with gp120 plus goat anti-gp120 Ab for the indicated time, followed by lysis and immunoprecipitation with Dok-specific antibodies. Precipitates were resolved as described above, and analyzed by immunoblotting using phosphotyrosine- or Dok-specific antibodies. All results shown in figure 1 are representative of at least three independent experiments.
Dok-1 is an established binding partner of SHIP-1, an inositol lipid phosphatase with a mass of ~150,000Da that is a mediator of inhibitory signals transduced by FcγRIIB (23, 38). A phosphoprotein with the apparent mass of SHIP-1 co-immunoprecipitated with Dok-1 and Dok-2, and a band of equivalent size blotted with anti-SHIP-1 (lane 3, figure 1A). To explore the possibility that this protein was SHIP-1, we assessed SHIP-1 tyrosine phosphorylation upon CD4 aggregation. Antibody-mediated stimulation of CD4 induced tyrosine phosphorylation of SHIP-1 (Fig. 1A, lane 5). SHIP-1 immunoprecipitates contained a significant amount of phosphorylated Dok-1 and Dok-2. As noted above, the converse was also true; Dok immunoprecipitates contained phosphorylated SHIP-1 (pSHIP-1). Thus following CD4 aggregation, SHIP-1 and Dok-1/2 become tyrosine phosphorylated and associate.
To assess whether CD4 ligation by HIV-1 gp120 induces Dok-1 phosphorylation, normal human PBMCs were treated with recombinant gp120 plus anti-gp120 to mimic the physiologic situation in patients with HIV disease (8). As shown in Figure 1E, gp120 ligation of CD4 induced transient but rapid Dok phosphorylation.
Comparative analysis of T cells from AND.B10BR and tailless CD4 transgenic mice revealed that SHIP-1 and Dok-1 phosphorylation requires the cytoplasmic tail of CD4 (Fig. 1D). Receptor aggregation results in an increase in Lck phosphorylation, Lck kinase activity and tyrosine phosphorylation of a number of cellular proteins (39, 40). We tested whether SHIP-1 and Dok-1 are downstream of Lck, by pretreating cells with the selective inhibitor of Src-family tyrosine kinases, PP2 (41). As shown in Fig. 1C, CD4 mediated SHIP-1 and Dok-1 phosphorylation was significantly reduced in the presence of this inhibitor. Under the same circumstances, IL-2 induced Jak-1 phosphorylation, which is Src-family kinase independent, was unaffected by addition of PP2 (data not shown).
4.2 Timing of activation of the SHIP/Dok circuit determines the efficacy of its inhibition of TCR signaling
Results shown above are consistent with the possibility that SHIP-1 and Dok-1/2 are effectors of CD4-mediated inhibitory signaling. Seemingly inconsistent with this idea are findings that SHIP-1 phosphorylation is also observed following stimulatory CD4-TCR co-aggregation (42–44). However, it also seems possible that while the SHIP/Dok circuit might be important in normal feedback regulation in CD4/TCR signaling, and/or for downstream signal propagation, the preliminary activation of the circuit via CD4 may prevent effective initiation of TCR signaling. To begin to test this idea we analyzed the kinetics of SHIP-1 and Dok-1/2 phosphorylation under various conditions of stimulation (Fig. 1B). Aggregation of TCR alone led to detectable but modest phosphorylation of SHIP-1 and Dok-1/2 at 60 seconds following stimulation. Much more robust and rapid phosphorylation was seen when CD4 was aggregated.
4.3 CD4-mediated inhibition of PI(3,4,5)P3 generation
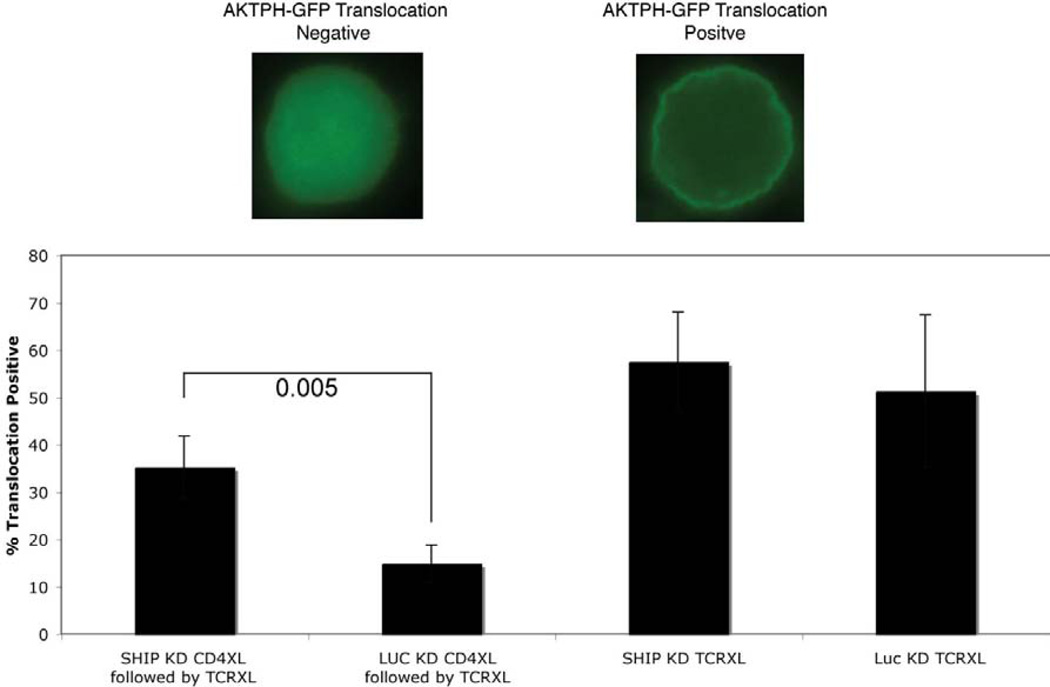
Levels of PI(3,4,5)P3 in T cells are balanced in part by the activities of PI3K, SHIP-1 and PTEN. Upon TCR stimulation, PI(3,4,5)P3 is generated from PI(4,5)P2 phosphorylation by PI3K. SHIP-1 mediates PI(3,4,5)P3 degradation (20). An expected consequence of PI(3,4,5)P3 degradation by SHIP is reduced TCR-mediated activation of Akt, a PI(3,4,5)P3-dependent serine-threonine kinase involved in signaling survival. To explore the role of SHIP-1 phosphatase activity in CD4 mediated modulation of TCR signaling, we measured PI(3,4,5)P3-mediated membrane translocation of the Akt-PH domain in response to anti-TCR mAbs with or without prior CD4 aggregation. The Akt-PH domain binds PI(3,4,5)P3 and its translocation to the plasma membrane is widely used as an indicator of PI(3,4,5)P3 levels (33). As shown in Fig. 2, TCR ligation resulted in membrane translocation of the Akt-PH domain, consistent with the generation of PI(3,4,5)P3. This response was prevented by CD4 aggregation, consistent with a role for SHIP-1 hydrolysis of PI(3,4,5)P3 in CD4-mediated inhibition of TCR signaling. In addition, SHIP-1 knockdown cells (SHIP KD) exhibited restored translocation of the Akt-PH domain following CD4 aggregation and TCR ligation further indicating SHIP-1s role in CD4-mediated inhibition of TCR signaling.
Figure 2. CD4 pre-aggregation prevents Akt membrane translocation.
SHIP deficient (SHIP KD) and control (LUC KD) D10 cells expressing GFP-tagged Akt-PH domains were stimulated through the TCR as described. CD4 was pre-aggregated using TNP-coupled anti-CD4 plus anti-TNP antibodies. Confocal images shown are representative of more than 3 experiments. Four fields containing more than 50 cells were analyzed to quantitate the amount of membrane translocation (% cells showing decreased cytoplasmic and increased membrane localization of GFP-tagged Akt-PH domain).
4.4 Effects of SHIP and Dok gene ablation on inhibitory CD4 signaling
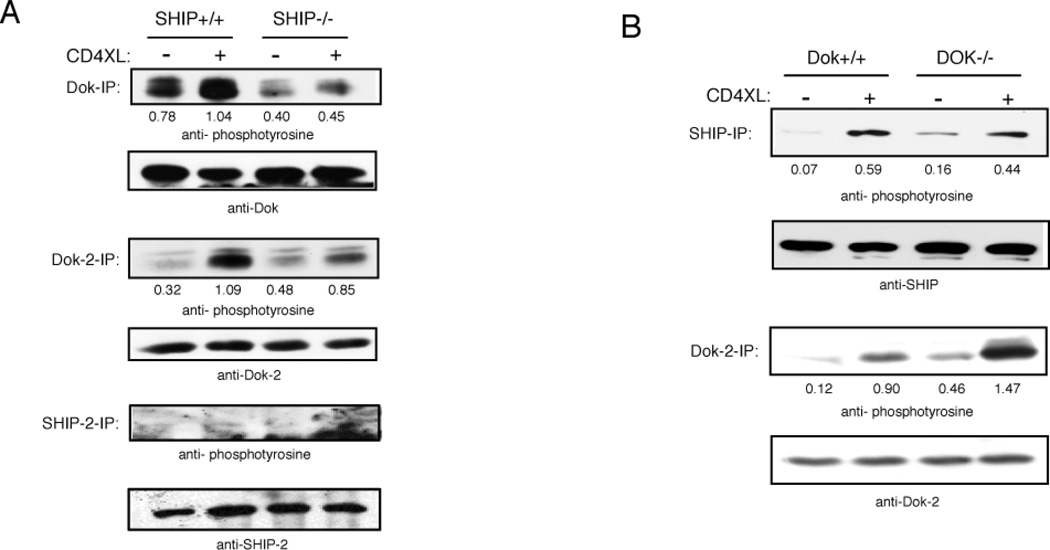
To explore more directly the role of SHIP-1 and Dok-1/2 in inhibitory CD4 signaling we studied responses of cells from mice in which the genes encoding these proteins were ablated. To first assess the interdependence of SHIP-1 and Dok-1/2 “activation”, we analyzed their phosphorylation following CD4 aggregation on ex vivo CD4+ T cells from the respective knockouts (Fig. 3A, B). CD4 aggregation on cells from SHIP−/− mice led to much reduced phosphorylation of Dok-1 and its homologue Dok-2 compared to SHIP-1 sufficient cells, suggesting that SHIP is upsteam from Dok in a linear pathway. However, induced SHIP-1 phosphorylation was also reduced in cells from Dok−/− mice (Fig. 3B). Taken together these data suggest that SHIP-1 and Dok-1/2 interact cooperatively with Lck. Interestingly, Dok-2 phosphorylation was enhanced in the absence of Dok-1 gene expression, and thus may compensate partially for the lack of Dok-1 expression, facilitating some SHIP-1 phosphorylation.
Figure 3. Effects of SHIP and Dok gene ablation on CD4 mediated inhibition of activation through the TCR.
Purified CD4+ T cells from SHIP +/+ or SHIP−/− mice (A) and Dok+/+ or Dok−/− mice (B) were stimulated through CD4 as described. In three separate experiments SHIP, SHIP-2, Dok, and Dok-2 were immunoprecipitated, and precipitates were analyzed using phosphotyrosine-, SHIP-1, SHIP-2, Dok-1, or Dok-2-specific antibodies, with similar results. Ratios (phosphorylation/protein levels) were determined using NIH Image software.
SHIP-1 and SHIP-2 have been shown to be co-expressed in T cells (45) and both are potent negative regulators of PI(3,4,5)P3-mediated signals (46, 47). As shown in Fig. 3A, SHIP-2 is expressed in CD4+ T cells from SHIP+/+ as well as SHIP−/− mice, although induction of tyrosine phosphorylation upon CD4 aggregation was not detectable in either case. Even though SHIP-2 phosphorylation was not detectable, SHIP-2 could still be enzymatically active. Phee et al. have shown that phosphorylation of SHIP-1 had minimal effect on its 5-phosphatase activity, whereas membrane targeting was required for its enzymatic activity (48). It is also remotely possible that the anti-phosphotyrosine used in this experiment does not react with phosphorylated SHIP-2. Thus, SHIP-2 expression could compensate for the lack of SHIP-1 in knockout mice, and this may explain any failure of SHIP-1 gene ablation to completely block inhibitory CD4 effects. Thus if inhibitory signaling requires SHIPs and/or Doks, the knockouts may have an incomplete phenotype.
4.5 Ectopically expressed dominant negative forms of SHIP-1 and Dok-1 block inhibitory effects of CD4 pre-aggregation
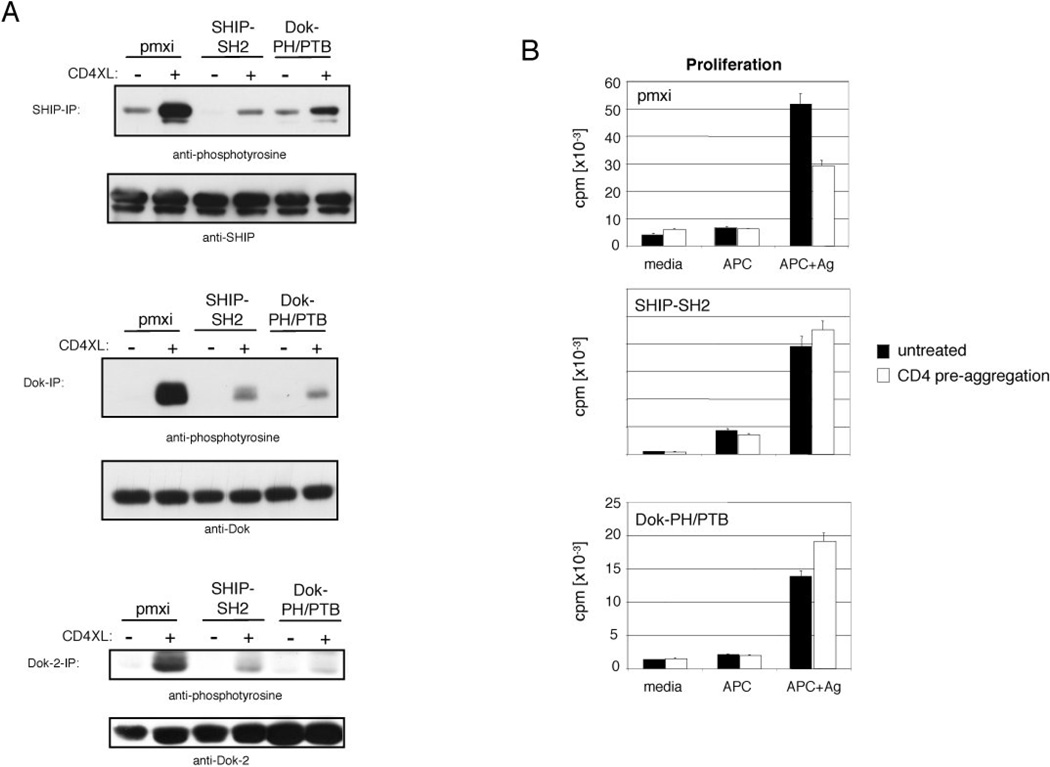
To further dissect the roles of SHIP-1 and Dok-1/2 in inhibitory CD4 signaling, we assessed the effects of decoy inhibitors that should block the function of redundant SHIP and Dok family members. The homology between SHIP-1 and SHIP-2 is the highest within the SH2 domain, suggesting that they share binding partners (49), and overexpression of the SHIP-SH2 domain in B cells has been shown to block SHIP phosphorylation and PI(3,4,5)P3 hydrolysis (32). Thus, we expected that the SHIP-SH2 decoy inhibitor would block SHIP-1 as well as SHIP-2 function in T cells. To block Dok-1 and Dok-2 function we expressed the PH+PTB domain fusions of Dok-1 (Dok-1-PH/PTB). The PH/PTB domains of Dok-1 and Dok-2 share 38%/48% sequence homology and both molecules have been shown to bind RasGAP (37). We determined expression levels of the SHIP-1-SH2 and Dok-1-PH/PTB domains by western blot using antibodies to the SHIP-1-SH2 domain and full length Dok-1. Expression levels of decoy inhibitors were 1.5 fold, or greater than 1.2 fold higher than endogenous SHIP-1 or Dok-1 protein levels, respectively (data not shown).
As shown in Fig. 4A, CD4-mediated phosphorylation of endogenous SHIP-1, Dok-1 and Dok-2 was reduced to a much greater extent in cells expressing either the SHIP-1 SH2 or Dok-1 PH/PTB domain than in cells from knockout mice. Thus the decoy inhibitors can be used to block participation of SHIP-1 and both Dok-1 and Dok-2 in signaling.
Figure 4. Effects of SHIP-SH2 and Dok-PH/PTB domains on CD4 mediated inhibition of activation through the TCR.
D10 cells expressing the indicated construct or vector alone (pmxi) were stimulated through CD4 as described. (A) In three independent experiments SHIP, Dok, and Dok-2 were immunoprecipitated using anti-SHIP, anti-Dok, or anti-Dok-2 antibodies. Precipitates were resolved as described and analyzed using phosphotyrosine-, SHIP-1, Dok-1, or Dok-2-specific antibodies. (B) Cells were pretreated with TNP coupled anti-CD4 (10 µg/ml) plus anti-TNP antibodies (20 µg/ml) and cultured in the presence of mitomycin-C treated, conalbumin pulsed antigen presenting cells (CH12). After 24 h proliferation was measured by [3H]Thymidine incorporation. Pulsed APCs cultured in the absence of D10 cells did not proliferate (140 cpms). Standard deviation of triplicate determinations are indicated in figures. Proliferation was significantly inhibited in D10 cells + pmxi (empty vector) at p<0.0006 by the Student's two-tailed t-test, and results are representative of three independent experiments.
Finally we utilized D10 cells expressing decoy inhibitors to test the role of SHIP-1, Dok-1 and Dok-2 in CD4 aggregation-mediated inhibition of TCR/CD28-mediated activation of T cell proliferation. D10 cells were stimulated with conalbumin-pulsed, CH12 B lymphoma cells. As shown in figure 4B, pre-aggregation of CD4 led to a 40% reduction in thymidine uptake induced by APC in T cells transduced with control virus. No CD4-mediated inhibition was seen in cells expressing either SHIP-1-SH2 or Dok-1-PH/PTB. Thus the SHIP/Dok pathway is required for CD4-mediated inhibition of both calcium mobilization and proliferation activated by TCR stimulation.
5. Discussion
Several studies indicate that aggregation of CD4 by agents such as gp120 and IL-16 leads to significant inhibition of subsequent antigen- and mitogen-induced T cell proliferation (6, 11, 17, 50). The mechanism of this inhibition is not well understood. Results reported here demonstrate an important role for SHIP-1 and Dok-1/2 in this process. Aggregation of CD4 by antibody or HIV-1 envelope glycoprotein gp120 resulted in rapid tyrosine phosphorylation of SHIP-1 and Dok-1/2, and inhibition of PI(3,4,5)P3 accumulation following subsequent TCR stimulation. Thus, we hypothesized that CD4 signals inhibit TCR signaling in part via activation of SHIP-1 function. Supporting of this hypothesis, in T cells from SHIP-1−/− and Dok-1−/− mice, the inhibitory effect of TCR activation via CD4 pre-aggregation was reduced (data not shown). Redundant effects of expressed SHIP-2 and Dok-2 could account for the failure to completely block CD4 signaling in knockout mice. Consistent with this possibility, blocking both SHIP-1 and SHIP-2, or Dok-1 and Dok-2 function using decoy inhibitors had a more profound effect on CD4 signaling and suggested that both of these molecules are necessary for inhibitory signaling. Finally, the SHIP-1-SH2 decoy inhibitor rescued TCR-mediated PI(3,4,5)P3 accumulation following CD4 signals confirming that SHIP-1 mediates its effect by virtue, at least in part, of hydrolysis of PI(3,4,5)P3 that is required for TCR signaling (51).
It seems paradoxical that CD4 can act as a stimulatory TCR co-receptor when engaged by MHC/peptide and an inhibitory receptor when engaged by gp120, in both situations activating SHIP-1 (Fig. 1). We believe that the answer may lie in the timing and magnitude of activation of the SHIP/Dok pathway relative to TCR signaling. The virus may subvert the immune response by, in this case, activating a feedback regulatory loop that is normally employed to turn off TCR signaling after it reaches some threshold and/or persists for a certain period of time. In patients with high viral load, the inexhaustible supply of gp120/anti-gp120 may maintain T cell insensitivity to antigen.
The molecular mechanism by which SHIP-1 and Dok-1/2 mediate inhibition has been best characterized in the FcγRIIB model of signaling (23, 36, 38, 52). When activated via FcγRIIB, these effectors mediate inhibitory signals by two bifurcating pathways. Recruitment of SHIP-1 to the phosphorylated Fc receptor leads to very efficient SHIP-1-mediated hydrolysis of PI(3,4,5)P3 needed for antigen and chemokine receptor signaling. PI(3,4,5)P3 functions in antigen receptor signaling by binding the pleckstrin homology domains of Tec family kinases, Akt, PLCγ and probably other proteins. Translocation of these molecules to the membrane is essential for their function. SHIP-1 hydrolysis of PI(3,4,5)P3 thereby inhibits the Akt survival signaling pathway as well as PLCγ-mediated phosphoinositide hydrolysis and calcium mobilization. SHIP-1 also functions as an adaptor or linker molecule. Following recruitment to the FcγRIIB, SHIP undergoes rapid phosphorylation on NPXY motifs, leading to binding of PTB domains of Dok-1/2 and Shc. We have recently shown that the C-terminal domain of Dok-1/2, which contains a RasGAP binding sequence, can recruit RasGAP and inhibit antigen receptor mediated activation of the Ras pathway (23).
Previously published findings and data reported here suggest that CD4 aggregation may activate both arms of this bifurcating pathway. TCR-mediated accumulation of PI(3,4,5)P3 is blocked by pre-aggregation of CD4. PLCγ recruitment to the plasma membrane is required for its function. This translocation appears to require binding of its (PH) domain to PI(3,4,5)P3 and SH2-domain to LAT(53). Thus, SHIP is an obvious candidate for a negative regulator of PLCγ activity and, secondarily, calcium mobilization.
The reported CD4 inhibition of Ras activation (19) could be due to the recruitment of RasGAP via pDok. Phosphorylated Dok binds downstream signaling molecules including SHIP-1, Nck, Csk, RasGAP and Crk-L (54–56). Dok-mediated RasGAP recruitment to the proximity of RasGTP likely leads to its conversion to RasGDP and possibly to termination of Erk activation. An additional possibility derives from the recent description of the Ras-guanine nucleotide exchange factor RasGRP (57). RasGRP is recruited to the membrane by binding to diacylglycerol (DAG) and mediates Ras activation. RasGRP mediated Ras activation is dependent on PLCγ activity, which is impaired after CD4 pre-aggregation.
CD4 signaling may also affect PLCγ activation via additional mechanisms that do not require SHIP-1 enzymatic activity. Co-aggregation of chimeric FcγRIIB-Dok-1 receptors with the TCR inhibited TCR mediated calcium mobilization and tyrosine phosphorylation of several molecules, including p36/p38 LAT (data not shown). LAT phosphorylation provides a direct docking site for PLCγ (see above), the Grb2-SOS complex and a number of other transducers required for downstream signaling by TCR. An SH2 mediated interaction between Dok and Csk (55) and Dok and Lck (58) has been reported. This interaction might localize Csk near Lck and provide a mechanism of downmodulation of the TCR response involving repression of Lck activity by tyrosine 505 phosphorylation. Additional experiments are required to define the molecular basis of Dok mediated inhibition of LAT phosphorylation.
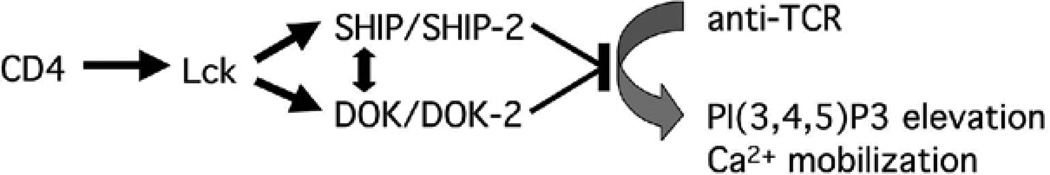
But how are SHIP-1 and Dok-1/2 function activated upon CD4 aggregation? This must involve a mechanism fundamentally different from receptors that contain SHIP-1-binding ITIMs (immunoreceptor tyrosine-based inhibitory motif) i.e. FcγRIIB and MAFA (59, 60). Tyrosine phosphorylation of these receptor’s ITIMs by src-family kinases leads to the activation and recruitment of SHIP-1. CD4 does not contain an ITIM. Studies comparing SHIP-1 and Dok-1/2 phosphorylation after FcR- and CD4-aggregation revealed that while SHIP-1 phosphorylation predominates after FcR-aggregation, CD4 aggregation leads to more robust Dok-1/2 phosphorylation (Fig.1). This suggests that SHIP-1 may be upstream of Dok-1/2 in ITIM signaling, but downstream of Dok-1/2 in CD4 signaling. However, knockouts and decoy inhibitors of SHIP-1 and Dok-1/2 inhibited CD4-mediated Dok-1/2 and SHIP-1 phosphorylation, respectively. These findings suggest that SHIP-1 and Dok-1/2 interactions with CD4/Lck are cooperative and that they do not exist in a linear pathway (Fig. 5). CD4 aggregation leads to tyrosine phosphorylation of both Dok-1/2 and SHIP-1, but especially Dok-1/2, and to dissociation of both. Interestingly some phosphorylated Dok-1/2 and SHIP-1 remain associated with each other. This presumably involves the PTB domain of Dok-1/2 binding to NPxpY motifs in SHIP-1, and the SH2 domain of SHIP-1 interacting with as yet undefined phosphotyrosines in Dok-1/2 (23). We hypothesize that in this bidentate complex, the PH domain of Dok-1/2 may, by binding PI(3,4,5)P3, target SHIP-1 to its substrate. This possibility is currently under study.
Figure 5. Schematic of CD4-mediated inhibition of TCR signaling.
6. Conclusions
Findings reported here demonstrate that CD4 aggregation activates an inhibitory signaling pathway involving SHIP-1 and Dok-1/2. This pathway inhibits subsequent TCR-mediated calcium mobilization and tyrosine phosphorylation of early signaling molecules such as LAT. Perhaps most importantly, this novel inhibitory circuit may represent an effective site for pharmacologic intervention to prevent T cell loss in HIV infected individuals after CD4 ligation by the HIV-1 glycoprotein gp120. The importance of this pathway in progression to AIDS is currently under study.
Highlights.
CD4 aggregation induces SHIP-1 and Dok-1/2 phosphorylation.
CD4 induced SHIP-1 and Dok-1/2 phosphorylation leads to inhibition of TCR signaling.
HIV-1 gp120-induced SHIP/Dok signaling may cause loss of CD4+ T cell function.
Acknowledgements
We thank Drs. Steve Swendeman and B. Clarkson for the SHIP-2-GST construct, Chiron for the generous gift of gp120 and anti-gp120, and Brian Schaefer for the pONG vector. SM was supported by grant Number 70524-28-RFI from the American Foundation for AIDS Research (AmFAR) and the Karl K. Hansen Fellowship Fund for AIDS Research. JCC is Ida and Cecil Green endowed Professor of Immunology. This work was supported by grant RO1 AI 36676-05 from the NIH institute of Health.
Abbreviations
- SHIP-1
SH2_domain-containing inositol 5 phosphatase
- Dok
Downstream of kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gay D, Maddon P, Sekaly R, Talle MA, Godfrey M, Long E, Goldstein G, Chess L, Axel R, Kappler J, Marrack P. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. 1987;328:626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- 2.Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc. Natl. Acad, Sci. USA. 1990;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruikshank WW, Kornfeld H, Center DM. Signaling and functional properties of interleukin-16. Int Rev Immunol. 1998;16:523–540. doi: 10.3109/08830189809043007. [DOI] [PubMed] [Google Scholar]
- 4.Jabado N, Le Deist F, Fisher A, Hivroz C. Interaction of HIV gp120 and anti-CD4 antibodies with the CD4 molecule on human CD4+ T cells inhibits the binding activity of NF-AT, NF-kappa B and AP-1, three nuclear factors regulating interleukin-2 gene enhancer activity. Eur J Immunol. 1994;24:2646–2652. doi: 10.1002/eji.1830241112. [DOI] [PubMed] [Google Scholar]
- 5.Oyaizu N, Chirmule N, Kalyanaraman VS, Hall WW, Good RA, Pahwa S. Human immunodeficiency virus type 1 envelope glycoprotein gp120 produces immune defects in CD4+ T lymphocytes by inhibiting interleukin 2 mRNA. Proc. Natl. Acad. Sci. USA. 1990;87:2379–2383. doi: 10.1073/pnas.87.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly R-P, Finkel TH. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond DC, Sleckman BP, Gregory T, Lasky LA, Greenstein JL, Burakoff SJ. Inhibition of CD4+ T cell function by the HIV envelope protein, gp120. J. Immunol. 1988;141:3715–3717. [PubMed] [Google Scholar]
- 8.Mittler RS, Hoffmann MK. Synergism between HIV gp120 and gp120-specific antibody in blocking human T cell activation. Science. 1989;245:1380–1382. doi: 10.1126/science.2571187. [DOI] [PubMed] [Google Scholar]
- 9.Hivroz C, Mazerolles F, Soula M, Fagard R, Graton S, Meloche S, Sekaly RP, Fischer A. Human immunodeficiency virus gp120 and derived peptides activate protein tyrosine kinase p56lck in human CD4 T lymphocytes. Eur J Immunol. 1993;23:600–607. doi: 10.1002/eji.1830230303. [DOI] [PubMed] [Google Scholar]
- 10.Goldman F, Jensen WA, Johnson GL, Heasley L, Cambier JC. Gp120 ligation of CD4 induces p56lck activation and TCR desensitization independent of TCR tyrosine phosphorylation. J. Immunol. 1994;153:2905–2917. [PubMed] [Google Scholar]
- 11.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 12.Wang Z-W, Dudhane A, Orlikowski T, Clarke K, Li X, Darzynkiewicz Z, Hoffmann MK. CD4 engagemant induces Fas antigen-dependent apoptosis of T cells in vivo. Eur. J. Immunol. 1994;24:1549–1552. doi: 10.1002/eji.1830240714. [DOI] [PubMed] [Google Scholar]
- 13.Briant L, Robert-Hebmann V, Acquaviva C, Pelchen-Matthew A, Marsh M, Devauz C. The protein tyrosine kinase p56lck is required for triggering NF-kappaB activation upon interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with cell surface CD4. Journal of Virology. 1998;72:6207–6214. doi: 10.1128/jvi.72.7.6207-6214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popik W, Pitha PM. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbeil J, Tremblay M, Richman DD. HIV-induced apoptosis requires the CD4 receptor cytoplasmic tail and is accelerated by interaction of CD4 with p56lck. J. Exp. Med. 1996;183:39–48. doi: 10.1084/jem.183.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haughn L, Gratton S, Caron L, Sekaly R-P, Veillette A, Julius M. Association of tyrosine kinase p56lck with CD4 inhibits the induction of growth through the αβ T-cell receptor. Nature. 1992;358:328–331. doi: 10.1038/358328a0. [DOI] [PubMed] [Google Scholar]
- 17.Marschner S, Hünig T, Cambier JC, Finkel TH. Ligation of the human CD4 receptor interferes with T cell activation in transgenic mice. Immunology Letters. 2002;82:131–139. doi: 10.1016/s0165-2478(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 18.Mittler RS, Hoffmann MK. Synergism between HIV gp120 and gp120-specific antibody in blocking human T cell activation. Science. 1989;245:1380–1382. doi: 10.1126/science.2571187. [DOI] [PubMed] [Google Scholar]
- 19.Jabado N, Pallier A, Le Deist F, Bernard F, Fischer A, Hivroz C. CD4 ligands inhibit the formation of multifunctional transduction complexes involved in T cell activation. J Immunol. 1997;158:94–103. [PubMed] [Google Scholar]
- 20.Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci U S A. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 23.Tamir I, Stolpa JC, Helgason CD, Nakamura K, Bruhns P, Daeron M, Cambier JC. The RasGAP-binding protein p62dok is a mediator of inhibitory FcgammaRIIB signals in B cells. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski D, Strife A, Swendeman S, Erdjument-Bromage H, Geromanos S, Kavanaugh WM, Tempst P, Clarkson B. A novel SH2-containing phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase (SHIP2) is constitutively tyrosine phosphorylated and associated with src homologous and collagen gene (SHC) in chronic myelogenous leukemia progenitor cells. Blood. 1999;93:2707–2720. [PubMed] [Google Scholar]
- 25.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J. Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 26.Kaye J, Hsa K-L, Sauron M-E, Jameson SC, Gasciogne N, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 27.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolan GP, Shatzman AR. Expression vectors and delivery systems. Curr Opin Biotechnol. 1998;9:447–450. doi: 10.1016/s0958-1669(98)80027-x. [DOI] [PubMed] [Google Scholar]
- 29.Killeen N, Littman DR. Helper T-cell development in the absence of CD4-p56lck association. Nature. 1993;364:729–732. doi: 10.1038/364729a0. [DOI] [PubMed] [Google Scholar]
- 30.Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Cristofano A, Niki M, Zhao M, Karnell FG, Clarkson B, Pear WS, Van Aelst L, Pandolfi PP. p62(dok), a Negative Regulator of Ras and Mitogen-activated Protein Kinase (MAPK) Activity, Opposes Leukemogenesis by p210(bcr-abl) J Exp Med. 2001;194:275–284. doi: 10.1084/jem.194.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Brauweiler A, Cambier JC. Effects of Src homology domain 2 (SH2)-containing inositol phosphatase (SHIP), SH2-containing phosphotyrosine phosphatase (SHP)-1, and SHP-2 SH2 decoy proteins on Fc gamma RIIB1-effector interactions and inhibitory functions. J Immunol. 2000;164:631–638. doi: 10.4049/jimmunol.164.2.631. [DOI] [PubMed] [Google Scholar]
- 33.Astoul E, Watton S, Cantrell D. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J Cell Biol. 1999;145:1511–1520. doi: 10.1083/jcb.145.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Justement LB, Campbell KS, Chien NC, Cambier JC. Regulation of B cell antigen receptor signal transduction and phosphorylation by CD45. Science. 1991;252:1839–1842. doi: 10.1126/science.1648262. [DOI] [PubMed] [Google Scholar]
- 35.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 36.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 37.Di Cristofano A, Carpino N, Dunant N, Friedland G, Kobayashi R, Strife A, Wisniewski D, Clarkson B, Pandolfi PP, Resh MD. Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins. J Biol Chem. 1998;273:4827–4830. doi: 10.1074/jbc.273.9.4827. [DOI] [PubMed] [Google Scholar]
- 38.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 39.Veillette A, Bolen JB, Bookman MA. Alterations in tyrosine protein phosphorylation induced by antibody- mediated cross-linking of the CD4 receptor of T lymphocytes. Mol Cell Biol. 1989;9:4441–4446. doi: 10.1128/mcb.9.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veillette A, Bookman MA, Horak EM, Samelson LE, Bolen JB. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 41.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 42.Lamkin TD, Walk SF, Liu L, Damen JE, Krystal G, Ravichandran KS. Shc interaction with Src homology 2 domain containing inositol phosphatase (SHIP) in vivo requires the Shc-phosphotyrosine binding domain and two specific phosphotyrosines on SHIP. J Biol Chem. 1997;272:10396–10401. doi: 10.1074/jbc.272.16.10396. [DOI] [PubMed] [Google Scholar]
- 43.Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O, Michel F. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J Exp Med. 2006;203:2509–2518. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasuda T, Bundo K, Hino A, Honda K, Inoue A, Shirakata M, Osawa M, Tamura T, Nariuchi H, Oda H, Yamamoto T, Yamanashi Y. Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int Immunol. 2007;19:487–495. doi: 10.1093/intimm/dxm015. [DOI] [PubMed] [Google Scholar]
- 45.Bruyns C, Pesesse X, Moreau C, Blero D, Erneux C. The two SH2-domain-containing inositol 5-phosphatases SHIP1 and SHIP2 are coexpressed in human T lymphocytes. Biol Chem. 1999;380:969–974. doi: 10.1515/BC.1999.120. [DOI] [PubMed] [Google Scholar]
- 46.Taylor V, Wong M, Brandts C, Reilly L, Dean NM, Cowsert LM, Moodie S, Stokoe D. 5' phospholipid phosphatase SHIP-2 causes protein kinase B inactivation and cell cycle arrest in glioblastoma cells. Mol Cell Biol. 2000;20:6860–6871. doi: 10.1128/mcb.20.18.6860-6871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brauweiler A, Tamir I, Marschner S, Helgason CD, Cambier JC. Partially distinct molecular mechanisms mediate inhibitory FcgammaRIIB signaling in resting and activated B cells. J Immunol. 2001;167:204–211. doi: 10.4049/jimmunol.167.1.204. [DOI] [PubMed] [Google Scholar]
- 48.Phee H, Jacob A, Coggeshall KM. Enzymatic activity of the Src homology 2 domain-containing inositol phosphatase is regulated by a plasma membrane location. J Biol Chem. 2000;275:19090–19097. doi: 10.1074/jbc.M001093200. [DOI] [PubMed] [Google Scholar]
- 49.March ME, Ravichandran K. Regulation of the immune response by SHIP. Semin Immunol. 2002;14:37–47. doi: 10.1006/smim.2001.0340. [DOI] [PubMed] [Google Scholar]
- 50.Liegler TJ, Stites DP. HIV-1 gp120 and anti-gp120 induce reversible unresponsiveness in peripheral CD4 T lymphocyes. J. Acquired Immune Defic. Syndr. 1994;7:340–348. [PubMed] [Google Scholar]
- 51.Eder AM, Dominguez L, Franke TF, Ashwell JD. Phosphoinositide 3-kinase regulation of T cell receptor-mediated interleukin-2 gene expression in normal T cells. J Biol Chem. 1998;273:28025–28031. doi: 10.1074/jbc.273.43.28025. [DOI] [PubMed] [Google Scholar]
- 52.Damen JE, Liu L, Ware MD, Ermolaeva M, Majerus PW, Krystal G. Multiple forms of the SH2-containing inositol phosphatase, SHIP, are generated by C-terminal truncation. Blood. 1998;92:1199–1205. [PubMed] [Google Scholar]
- 53.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 54.Lock P, Casagranda F, Dunn AR. Independent SH2-binding sites mediate interaction of Dok-related protein with RasGTPase-activating protein and Nck. J Biol Chem. 1999;274:22775–22784. doi: 10.1074/jbc.274.32.22775. [DOI] [PubMed] [Google Scholar]
- 55.Rafnar T, Peebles RS, Brummet ME, Catipovic B, Imani F, MacGlashan DW, Marsh DG. Stimulation of the high-affinity IgE receptor results in the tyrosine phosphorylation of a 60 kD protein which is associated with the protein-tyrosine kinase, Csk. Mol Immunol. 1998;35:249–257. doi: 10.1016/s0161-5890(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 56.Martelli MP, Boomer J, Bu M, Bierer BE. T cell regulation of p62(dok) (Dok1) association with Crk-L. J Biol Chem. 2001;276:45654–45661. doi: 10.1074/jbc.M105777200. [DOI] [PubMed] [Google Scholar]
- 57.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 58.Nemorin JG, Duplay P. Evidence that Lck-mediated phosphorylation of p56dok and p62dok may play a role in CD2 signaling. J Biol Chem. 2000;275:14590–14597. doi: 10.1074/jbc.275.19.14590. [DOI] [PubMed] [Google Scholar]
- 59.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 60.Xu R, Abramson J, Fridkin M, Pecht I. SH2 domain-containing inositol polyphosphate 5'-phosphatase is the main mediator of the inhibitory action of the mast cell function-associated antigen. J Immunol. 2001;167:6394–6402. doi: 10.4049/jimmunol.167.11.6394. [DOI] [PubMed] [Google Scholar]