Abstract
Collection of exhaled breath condensate (EBC) is a non-invasive means of sampling the airway-lining fluid of the lungs. EBC contains numerous measurable mediators, whose analysis could change the management of patients with certain pulmonary diseases.
While initially popularized in investigations involving spontaneously breathing patients, an increasing number of studies have been performed using EBC in association with mechanical ventilation. Collection of EBC in mechanically ventilated patients follows basic principles of condensation, but is influenced by multiple factors. Effective collection requires selection of a collection device, adequate minute ventilation, low cooling temperatures, and sampling times of greater than ten minutes. Condensate can be contaminated by saliva, which needs to be filtered. Dilution of samples occurs secondary to distilled water in vapors and humidification in the ventilator circuit. Dilution factors may need to be employed when investigating non-volatile biomarkers. Storage and analysis should occur promptly at −70° C to −80° C to prevent rapid degradation of samples.
The purpose of this review is to examine and describe methodologies and problems of EBC collection in mechanically ventilated patients. A straightforward and safe framework has been established to investigate disease processes in this population, yet technical aspects of EBC collection still exist that prevent clinical practicality of this technology. These include a lack of standardization of procedure and analysis of biomarkers, and of normal reference ranges for mediators in healthy individuals. Once these procedural aspects have been addressed, EBC could serve as a non-invasive alternative to invasive evaluation of lungs in mechanically ventilated patients.
Keywords: Exhaled breath condensate, Biomarkers, Inflammation, Mechanical ventilation, Airway-lining fluid
Introduction
Non-invasive methods of examining inflammatory markers of respiratory disease have been an area of interest in pulmonary research over the past few decades. EBC collection is a relatively new technique by which pulmonary specimens are obtained. EBC is the liquid form of exhaled gases and vapors collected in a portable condenser. It is derived from aerosolized non-volatile particles contained in fluid lining the airway, volatile water-soluble molecules that have been aerosolized and condensed, and distilled water from moisture within the airway itself (1). EBC can safely be collected from both actively participating patients breathing on their own, or during mechanical ventilation by placing a collection device in-line with the expiratory circuit of the ventilator (2, 3).
Collection of EBC was first described in 1980 in the former Soviet Union, but has recently been revisited as a non-invasive means of analyzing properties of the lung (4, 5). Investigations of EBC have been performed in a multitude of inflammatory conditions and connective tissue disorders including chronic obstructive pulmonary disease (COPD), acute lung injury (ALI), adult respiratory distress syndrome (ARDS), asthma, pneumonia, sarcoidosis and many more. These investigations have revealed identifiable patterns of change in an array of biomarkers that are measurable in EBC (6–10). Biomarkers include, but are not limited to nitric oxide (NO), eicosanoids such as leukotrienes, prostanoids and isoprostanes, products of lipid peroxidation, hydrogen peroxide (H2O2), and inflammatory proteins and cytokines. By examining the inflammatory biomarker profiles of specific disease processes, EBC has the potential to be helpful as a prognosticator of outcomes and may help guide treatment.
The anatomy and physiology of the pulmonary system make it difficult to obtain samples from deep within the lungs. Current methods of evaluating pathology of the lung include less invasive methods such as pulmonary function testing, imaging techniques and sputum cultures, as well as more invasive methods such as bronchoalveolar lavage (BAL), bronchoscopy, and tissue biopsy. One of the major problems with the more invasive methods is that they cannot be performed frequently secondary to risks associated with procedures. EBC is a means of monitoring biomarkers in the airways of diseased or injured patients that is quick, repeatable, and is minimal risk to the patient. The non-invasive nature of EBC is a significant advantage from the standpoint of patient safety making it a technology worth pursuing.
Mechanically ventilated patients, generally speaking, are at a greater severity of illness and therefore would benefit from less invasive means of testing. If results are found to be reproducible, EBC could replace more invasive methods of sampling airway-lining fluids. To date, significant biomarkers identified by EBC in mechanically ventilated patients focus mostly on airway inflammation. Studies have successfully monitored pH in mechanically ventilated patients, suggesting possible correlation with pro-inflammatory cytokines and overall inflammation (2, 11). Myeloperoxidase (MPO) and 8-isoprostane levels have been measured in multiple studies and were elevated in one looking at mechanically ventilated patients with severe pulmonary infection, suggesting inflammation due to increased reactive oxygen species (ROS) (3, 12, 13, 14, 15). Studies examining intubated patients with inflammatory lung diseases (ALI/ARDS) and COPD, found higher levels of all investigated cytokines (IL-1β, IL-6,IL-8, IL-10, TNFα, IL-12p70) in comparison to healthy smoking and non-smoking volunteers (9, 16). Evidence of the clinical potential of EBC has been demonstrated in this subset of patients, but remains a work in progress with potential biomarkers still being established. The purpose of this review is to examine and describe the methodology and problems of EBC collection as they pertain to the mechanically ventilated patient.
EBC Collection in Mechanical Ventilation
Collection of EBC is straightforward and is most commonly described in studies involving active participants breathing into a portable device (17), although a smaller number of studies have successfully incorporated this technology into mechanical ventilator circuits. There is currently no standardization to the use of EBC devices in a clinical setting, nor have reference ranges been established for specific biomarkers. The American Thoracic Society/European Respiratory Society (ATS/ERS) created a task force on EBC to help determine guidelines in its use. These recommendations serve as a guide for future studies (Table 1) (8).
Table 1.
ATS/ERC task force recommendation summary as pertains to mechanical ventilation
| Standardization Issue | Recommendation | Strength of Recommendation |
|---|---|---|
| General | Standardize sampling, storage and assay type within an individual study. | 3 |
| - Sampling device | Delineate device used. If commercial, note the name and manufacturer and precise modifications. If custom, clearly detail the device, materials used and provide sufficient diagrams to allow understanding of the employed equipment. | 5 |
| - Sampling temperature | Specify the collection temperature or range. | 4 |
| - Duration of collection | Duration should be recorded. | 2 |
| - Contamination | Test all materials that contact EBC and assure adequate controls are in place. | 4 |
| - Biomarker Storage | Unless proven unnecessary, store samples in the coldest temperature available. | 3 |
| Stability in storage | Data should be presented regarding marker stability in EBC, or previous publications addressing the stability of the specific marker referenced. | 4 |
| - Stabilization of marker | When possible, this should be performed. | 2 |
| - Assay | In all cases, use assays proven to be sufficiently sensitive and specific for the marker of interest in EBC. | 5 |
| Timing | Assays should be performed as soon as possible to avoid loss of marker or contamination with exogenous marker. | 4 |
| - Validation in EBC | Assay systems should be tested for utility in EBC. | 5 |
| - Immunoassays | Assure that nonspecific binding is identified and minimized, and that appropriate controls are performed in all cases. | 4 |
| - Nitrogen Oxide | Report precisely what was measured. Do not use the term nitrogen oxide (NO) without providing a definition for that term in the manuscript. Clearly note the NO that are included in the assay used. | 4 |
| - pH | Report if de-aerated (or gas-standardized) and by what means. If not de-aerated, note the timing of the measurement after collection. | 5 |
| - Spectrophotometry and other assays | Assure sufficient controls, and that the assay is in range. | 4 |
| - Dilution issues | Consider volatile and nonvolatile constituents of EBC differently. Present finding of nonvolatile cautiously in the absence of a dilution factor or relevant ratio. | 4 |
| - New markers | Skeptically consider the specificity and sensitivity of the assay. Determine the possibility of contamination. Determine stability in storage. | 3 |
EBC: exhaled breath condensate. NO: Nitrogen Oxide. Strength of Recommendations: 5) Unequivocal data and/or unanimity among Task Force experts; 4) Compelling data or when data are unnecessary, strong consensus; 3) Little data, or data unnecessary, with consensus; 2) Little or no data, majority opinion; 1) No published data, opinions of panel.
The general principle of EBC is no different than condensation of other gases. Exhaled gas is blown into the collection device and cools as heat is transferred to the cold chamber walls. Once the air reaches a temperature below its dew point, the aerosolized particles begin to condense and form droplets. The droplets are then collected in a container, which is stored or sent for analysis.
Technical considerations when performing EBC collection in mechanically ventilated patients include choice of collection device, technique of collection, storage conditions of condensate and methods of analysis of biomarkers. These elements have been described in existing studies using EBC in mechanical ventilation (Table 2) and are summarized in the following sections.
Table 2.
Summary of EBC collection methods involving mechanical ventilation.
| Device Used | Humidification | Collection Time | Collection Temp | Storage Temp | Biomarker | Authors |
|---|---|---|---|---|---|---|
| R-Tube | Disconnected | 20 min | − 20 C | − 80 C | pH, IL-10, IL-1β, IL-6, IL-8, IL 12p70, TNFα | Korovesi I et al 2011 |
| EcoScreen/R-Tube | Connected | 10–20 mm | − 14 – 7 C | − 70 C | MPO, HNL | Davidsson A et al 2010 |
| ECoScreen | Disconnected | Time needed to collect 2 ml | − 10 C | − 70 C | pH, nitrite/nitrate, 8-isoprostane | Roca O et al 2010 |
| ECoScreen | Disconnected | 25–45 mm | − 20 C | − 70 C | pH, nitrite/nitrate, 8-isoprostane, LTB4 | Roca O et al 2008 |
| ECoScreen | Connected | 30 min | NR | NR | IL-6, IL-8, Protein | Gessner C et al 2007 |
| Custom | NR | 30 min | 4 C | NR | sTREM-1 | Horonenko G et al 2007 |
| ECoScreen | Both | 30 min | − 10 C | − 80 C | 8-isoprostane | Müller WG et al 2006 |
| Anacon | Disconnected | 15 min or greater | < − 10 C | − 80 C | nitrite/nitrate, 8-isoprostane, MPO | Romero PV et al 2006 |
| ECoScreen | Connected | 20 min | 10 C | NR | IL-1β, IL-6, IL-8, IL-10, TNFα, and IL-12p70 | Sack U et al 2006 |
| R-Tube | Connected | 10 min | 0 C | N/A | pH | Walsh BK et al 2006 |
| Custom | NR | 30 – 60 mm | NR | − 80 C | H2O2 | Bruhn A et al 2005 |
| ECoScreen | Connected | 20 min | NR | NR | IL-1β, IL-6, IL-8, IL-10, TNFα, and IL-12p70 | Gessner C et al 2005 |
| R-Tube | Disconnected | 15 min | − 20 C | − 70 C | pH, H2O2, LTB4, MPO, 8-Isoprostane | Moloney ED et al 2004 |
| ECoScreen | Connected | 30 min | 10 C | NR | pH, ammonia, lactate, pCO2, HCO3−, IL-6, IL-8 | Gessner C et al 2003 |
| ECoScreen | Connected | 30 min | 10 C | 4 C | Nitrate, IL-6, IL-8 | Gessner C et al 2003 |
| Custom | NR | 20 – 30 mm | − 5 C | − 70 C | H2O2 | Heard SO et al 1999 |
| Custom | Disconnected | 30 – 60 mm | NR | − 70 C | 8-isoprostane | Carpenter CT et al 1998 |
| Custom | 15 – 30 mm | NR | NR | pCO2, pO2 | Von Pohle WR et al 1992 | |
| Custom | NR | 20 min | − 5 to 0 C | N/A | H2O2 | Sznajder JI et al 1989 |
| Custom | NR | 5 min | NR | − 70 C | H2O2 | Baldwin SR et al 1986 |
Biomarker: LTB4 - Leukotriene B4, MPO - Myeloperoxidase, HNL - Human Neutrophil Lipocalin, H2O2 - Hydrogen Peroxide, TNFα - Tumor Necrosis Factor alpha, sTREM-1 - Soluble Triggering Receptor Expressed on Myeloid Cell-1, pCO2 - Partial pressure of CO2, pO2 - Partial pressure of O2. NR – Not Recorded.
Collection Devices
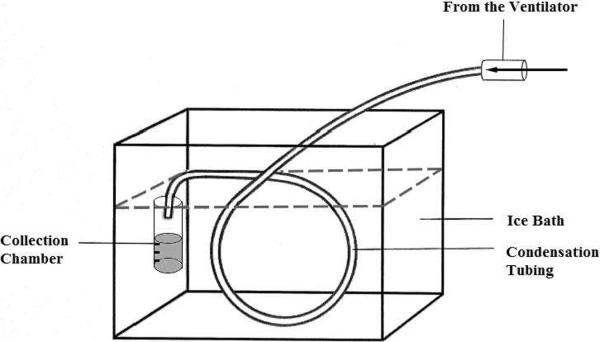
Studies looking at EBC in mechanically ventilated patients describe use of custom as well as commercially available collection devices. Custom EBC collection devices are constructed using tubing, often described as Teflon, glass, or another material, or glass chambers in line with the expiratory limb of the ventilator circuit. A long portion of the tubing is submerged in an ice bath or other cooling mechanism, with a collection tube or container at the distal end (18–24) (Figure 1).
Figure 1.
Schematic for custom EBC collection device: Tubing is submerged in ice bath with a collection chamber at the distal end. Tubing is attached to the expiratory circuit of the vent. Figure modified from Mutlu et al 2001 (46).
Recent studies have more frequently used commercial devices to collect EBC when working with mechanically ventilated patients. These devices include R-Tube (Respiratory Research, USA), ECoScreen (FILT Lung and Chest Diagnostics Ltd, Germany), TURBO-DECCS (Medivac, Italy), and ANACON (Biostec, Valencia, Spain)(5) (Table 3).
Table 3.
EBC collection devices
| EBC Collection Device | Manufacturer | Ventilator Use | Cooling Method | Temperature Control | Lowest Temperature | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| ANACON | Biostec, Valencia, Spain | Specifically designed for mechanical ventilation | Built in cooling unit | Yes | −10°C | Built in cooling unit allows for temperature control. Collection chamber connected to device, allowing for easy condensate collection. Specifically designed for use with a mechanical ventilator. | It is difficult to assess this device due to a lack of supporting literature. |
| ECoScreen I | Viasys, USA, Europe | Yes, adaptable to ventilor | Built in cooling unit | No | −20°C | Well described in literature. Built in cooling unit allows for stable temperature. | Expensive and more cumbersome device. Size makes it more difficult to transport to an ICU. Requires cleaning in between uses. Additional conduits required for use in mechanical ventilation. Doesn't allow for temperature adjustment |
| ECoScreen II | Viasys, USA, Europe | Yes, adaptable to ventilor | Built in cooling unit | Yes | −20°C | More extensive use in European center. Built in cooling unit allows for adjustable temperature control. When compared to its predecessor, it was found to collect more sample with higher concentration of biomarker. | Expensive and more cumbersome device. Size makes it more difficult to transport to an ICU. Requires cleaning in between uses. Additional conduits required for use in mechanical ventilation. |
| R-Tube | Respiratory Research, USA | Yes, adaptable to ventilor | Metal cooling sleeve stored in freezer | No | Dependent on cooling sleeve | Inexpensive and easily portable. Disposable device, therefore no cleaning necessary. Well described in the literature. | Adaptation to the mechanical ventilator requires disassembling the device. Ambient temperature affects collection. |
| TurboDeccs | Medivac, Italy | Yes, adaptable to ventilor | Built in cooling unit | No | −10°C | Multiple units can be placed in the circuit for simultaneous sample collection. Built in cooling unit with adjustable temperature. Medium sized. Disposable parts allow for easier cleaning. | Expensive. Additional conduits required for use in the mechanical ventilator. Limited use in literature. |
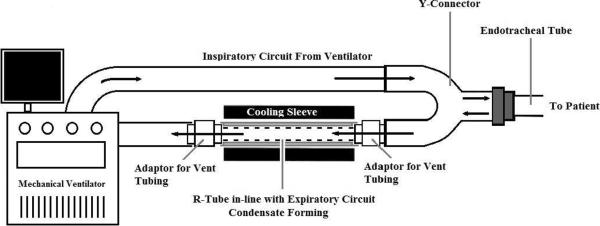
R-Tube is a handheld, single-use disposable tube that can be attached to the expiratory circuit of a mechanical ventilator in-line with the endotracheal tube and ventilator tubing. Modification by removal of the expiratory valve to decrease airway resistance has been described for this application (2, 25, 26) (Figure 2). The R-Tube is placed level on its side to prevent collected fluid from spilling back into the vent tubing. Temperature is reduced with an aluminum-cooling sleeve, which slides around the condenser tube. The circuit will need to be interrupted briefly in order to get the cooling sleeve around the attached tube. Typically the sleeve is cooled to −70 to −80° C according to studies using this device (25–29). The temperature at which EBC is collected gradually increases secondary to ambient temperature, including room temperature and that of the exhaled vapors, therefore limiting the amount of time for effective collection. To circumvent this issue, maintenance of a set condensing temperature has been described with an optional continuous cooling unit (5). Once collection is finished, the R-tube is disconnected, capped on both ends with rubber caps preventing condensate on the walls of the Teflon tube from spilling, and placed on ice. The plunger valve, which was previously removed, will need to be reinserted for collection of the fluid and processing.
Figure 2.
Schematic for R-Tube collection device: The device is connected to the expiratory circuit of a mechanical ventilator. Expiratory valve may be removed to adapt to the vent. Figure modified from Moloney et al 2004 (12).
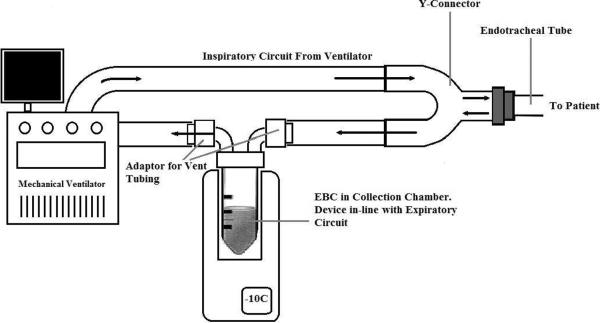
In contrast to the R-Tube, ECoScreen is a larger portable collection device that has additional features including an optional spirometer, separate collection chambers allowing for fractioning of condensate and sampling from different portions of the airway, saliva trap for removal of contaminant, and an electrical cooling system that allows for maintenance of the condenser temperature. A drawback to the ECoScreenI device was that the investigator could not alter the temperature of the cooling system due to a set temperature. Maintenance of the device is also more time consuming because the device requires cleaning between uses. In order to adapt the ECoScreen for use in mechanically ventilated patients, additional conduits were needed to attach the device to the expiratory circuit (3, 11, 13, 14, 16, 29) (Figure 3). The ECoScreenI device is no longer being manufactured. Its successor, the ECoScreenII, can be adapted for mechanical ventilation, although this is less well defined in the literature (FILT, Lung and Chest Diagnostics, LTD, Germany). The ECoScreenII device includes an adjustable thermoelectrically cooled condenser, reaching temperatures as low as −20° C. A comparison study between the ECoScreenI and II devices showed that the ECoScreenII collected larger sample volumes and greater concentrations of biomarker for analysis. Samples were also found to be more acidic (30).
Figure 3.
Schematic for ECoScreen and TURBO-DECCS devices: Temperature regulated condenser with in-line adaptor. Figure modified from Müller et al 2006 (3).
TURBO-DECCS is another portable collection device that has an electrical cooling system, ranging from −10 to 35° C. It differs from the ECoScreenI in that it has an adjustable temperature control for cooling the condenser unit. It also has disposable connectors for sample collection, making cleaning between uses easier. Use of the TURBO-DECCS in the literature is limited, but the manufacturer has designed specific adaptors for mechanical ventilators, which must be purchased separately, as well as connectors to place multiple units in line with a single vent to collect multiple specimens simultaneously (Medivac, Italy). Assembly of the TURBO-DECCS to the ventilator is not dissimilar to the ECoScreen setup with the device attached to the expiratory limb of the ventilator (Figure 3).
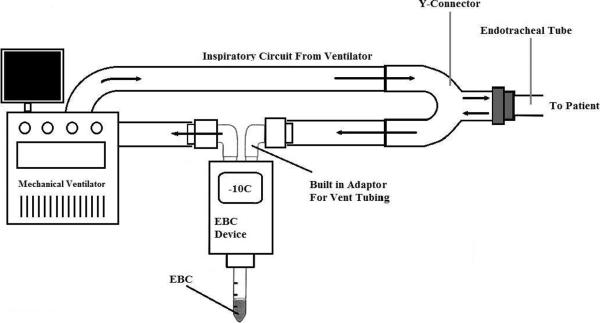
ANACON is a condensing unit that is inserted directly into the expiratory branch of the ventilator circuit via adaptors that are part of the device (Figure 4). It uses a thermoelectric pump to generate low temperatures that reach below −10° C, which can be adjusted by the investigator (15). Unfortunately, descriptions of this device in practice are limited.
Figure 4.
Schematic for ANACON collection device: Temperature regulated Condenser inserted directly into vent circuit. Figure modified from Romero et al 2006 (15).
Considerations in condensate collection
Once the collection device is attached to the ventilator, multiple factors will influence the amount and quality of condensate collected. Such factors include: minute ventilation, duration and temperature at which the specimen is collected, the presence of contaminants such as saliva (31), and diluting elements including built-in humidifiers in the ventilation circuit (3, 8).
A study by Gessner et al. focused specifically on the role of pulmonary function including minute ventilation and the effects on EBC volume (32). They compared EBC collection in healthy volunteers and COPD patients, looking at the effects that lung function may have on efficiency. Their results showed that none of the pulmonary function parameters measured (total lung capacity, vital capacity, residual volume, FEV1, and airway resistance), or patient related variables such as height or weight, correlated with EBC volume collected. EBC collection in both groups was comparable and found to be strongly dependent on minute ventilation, with larger total respired volumes resulting in greater condensate yield. The trend between minute ventilation was initially examined in an animal model using calves (33), but has been revisited in humans. A positive correlation between minute ventilation and condensate volume was shown in a study involving 30 healthy patients voluntarily breathing into the ECoScreen device at different set rates. Interestingly, these investigators found no dilution effect on EBC protein, nitrite concentrations, or pH (34). Minute ventilation is not routinely documented in existing studies involving mechanically ventilated patients. Given the trend towards lower tidal volumes in mechanical ventilation, EBC collection volumes may be lower due to decreased minute ventilation, but this has yet to be examined.
Temperature of the condenser during collection varies depending on the cooling systems of the devices used. Condensation can be achieved around 0° C, though a range of temperatures has been described. Low collection temperatures have been documented at −20° C (12, 14, 25), and the highest documented collection temperature in studies with mechanically ventilated patients was 10° C (11, 16, 35). Importantly, temperature at which EBC is performed has been shown to impact collection volumes. McCafferty et al. monitored airway temperatures in 2005, showing that less humidity in patient airways yielded less collected condensate (34). Moreover, Goldoni et al showed a trend towards higher condensate volume yield at lower condenser temperatures in 2005 (26). These findings are consistent with principles of condensation, with moisture being more abundant at higher airway temperatures, and more condensation forming in a colder collection device. The ambient temperature was also found to have an impact on pH value of EBC, with higher pH values at higher temperatures (36). To our knowledge no formal evaluation of patient airway temperature on EBC collection has been performed, but one would expect patient temperature to affect volume and pH of samples collected. The ATS/ERS task force on EBC recommends routine documentation of collection temperatures of condensate.
Collection times reflected in the literature are approximately 5 to 15 minutes for every 1 ml of EBC (1). Most studies report 1 ml to 3 ml of collected sample with collection times on mechanical ventilators ranging from 5 minutes to 1 hour (18, 19). Collection volume is dependent on duration of collection, but dilution of biomarkers becomes an issue as more of the distilled water component collects. Longer EBC collection periods have been documented with lyophilization of the condensate to concentrate biomarkers of interest (16). Current ATS/ERS recommendations include documentation of collection times as well as at least 10 minutes of collection time for most mediators.
Contamination of EBC specimens is mostly from the oral and retropharyngeal portions of the airway, with saliva being the significant source. Depending on the device used during EBC collection, there can be substantial gross or microscopic contamination by saliva (31). While some devices have built-in saliva traps to prevent contamination (ECoScreen, TURBO-DECCS), theoretically, salivary contamination should be less of an issue in mechanically ventilated patients. The endotracheal tube cuff, when properly inflated, should prevent saliva from draining into the lower airway. Oral intake of foods, liquids and medications should not be an issue in mechanically ventilated patient for the same reason, although these factors have not been thoroughly examined in studies with EBC in the general population. In mechanically ventilated patients, the assembly with the collection device is sampling the lower airways, excluding the airway above the level of the endotracheal tube. Based on experience of previous studies, some believe that monitoring for salivary contamination by condensate amylase measurement is unnecessary, but efforts to prevent salivary contamination should still be made (8).
Dilution of samples is one of the most significant problems when evaluating biomarkers through EBC collection. While most compounds collected from EBC are detectable in concentrations that are measurable by existing assays (leukotriene B4, 8-isoprostane, ammonia, hydrogen ion, and nitrate), some compounds are identified at concentrations close to their lower limits of detection (1). With additional dilution, these biomarkers may become undetectable. Dilution can come from multiple sources, including excess moisture in airway vapors, mucous formed from the airway epithelium, and in the case of mechanical ventilation, there is evidence that heated humidification systems may dilute EBC samples (3, 8). Along these lines, studies collecting EBC from mechanically ventilated patients have been performed with humidification connected (2, 9, 11, 16, 29, 37) and disconnected (12, 13, 14, 15, 16, 18). Müller et al studied the effects of humidification on EBC in mechanically ventilated children using 8-isoprostane as a biomarker. They found that levels of 8-isoprostane were not detectable in humidified EBC samples or in the sterile water used for humidification. While evidence suggests dilution, there are no clear recommendations for or against disconnecting humidification circuits during EBC collection in mechanically ventilated patients at this time.
Dilution factors have been used in order to normalize concentrations of biomarkers collected in EBC. This is justified by the assumption that the constituents of EBC (solutes and water vapors) are variable in collections, therefore substances that have known serum concentrations that diffuse through cell membranes and are not produced in the alveoli can be measured in EBC and used to estimate actual concentration of the diluted biomarkers. Dilution factors in the literature have included exhaled volume (38), exhaled ions such as sodium and chloride (39, 40, 41), urea (39, 42), protein concentration (32) and conductance of lyophilized samples (39). Despite the inherent dilemma of these dilution factors, it has not yet been convincingly demonstrated that normalization results in better reproducibility of biomarker measurement in EBC (8). The ATS/ERS task force currently recommends the use of a dilution factor when examining non-volatiles found in the airway lining fluid, whereas no dilution factor is needed for volatile biomarkers.
Other factors that could potentially influence EBC collection include patient related factors such as diseases limiting pulmonary function. For example, in patients with ARDS, lower tidal volume ventilation is routinely practiced, which would result in lower EBC collection volume. Events such as mucous plugging could potentially limit the area of the lung sampled by EBC due to blocked airway passages as well as decrease the amount of sample collected.
Storage of Samples
Once collected, depending on the biomarker of interest, the specimen can either be processed immediately (H2O2, L-Lactate, pH) or stored in a freezer for delayed processing. Freezing temperatures of −70 to −80° C are used for storing condensate in order to maintain stability of other biomarkers. Care must be taken to process samples as soon as possible or place in storage. Ambient air can interact with EBC samples, especially if they are left at room temperature, potentially changing composition of biomarkers. It is also advised to avoid multiple frosting-defrosting cycles secondary to subsequent degradation of the mediators (8). The ATS/ERS also suggest that addition of a marker-free protein to EBC samples may increase or lessen the loss of unstable markers, although this recommendation is not absolute.
Analysis of EBC Samples
Biomarkers that have been investigated in exhaled breath include non-volatile compounds that are mostly derived from the airway lining fluid as well as water-soluble volatile compounds, which are more readily assayed (1). The concentration of some volatile and non-volatile substances measured in EBC varies considerably, making it difficult to validate individual biomarkers. While dilution of biomarkers is one factor affecting consistent measurement, another is the limitation of assays used to detect mediators (27). The inability of an assay to detect low levels of certain mediators contributes significantly to variability seen. Most assay systems are not designed for use with EBC, which is a very dilute fluid that is protein and buffer poor; therefore, it is important that assay systems be tested for utility in EBC prior to standard use (8).
Biomarkers that have been studied and reported in patients on mechanical ventilators are similar to those in spontaneously breathing individuals. These include H2O2 (12,19, 20, 22, 23), NO (11, 13, 14, 15), leukotrienes (12, 13), 8-isoprostane (3, 12, 13, 14, 15, 18), pH (2, 11, 12, 13, 14, 25), ammonia (25), cytokines (9, 11, 16, 25, 29), protein (29), MPO (12, 15, 37), and other specific receptors. Continuous pH monitoring has successfully been performed in mechanically ventilated patients (2).
Currently there is no standardization of collection or processing EBC. Some studies have stressed the importance of using dilution factors for more volatile compounds collected, but this is not done consistently. At the same time, there are no reference values of normal levels of biomarkers in the general population. Attempts have been made at establishing reference values through reviewing the results of biomarkers in existing studies (43, 44). Small subject numbers as well as a lack of standard procedure limits these data. Other methods of standardization, such as comparison of EBC to BAL fluid have been attempted (9,45). Studies examining levels of inflammatory cytokines in BAL fluid and EBC in patients with severe COPD showed no significant correlation between biomarkers in the two fluid types (9). Possible reasons for observed differences include the dilute nature of EBC as well as the fact that it is collected from the entire lung as opposed to BAL, where a specific area within the lung is sampled either blindly or under direct visualization.
A significant issue facing EBC use in a clinical setting is its lack of standardization. Multiple collection devices are available and individual investigators decide upon device as well as protocol in terms of specimen collection. Variation in collection times, temperatures, and conditions (such as ventilator settings and whether or not a humidification circuit is connected) is widespread among existing studies. Until collection methods have been standardized, it will be difficult to reliably compare information between different investigations.
Methods for analysis and interpretation of relevant biomarkers also need to be standardized. There remains far too much variability in analysis of biomarkers, preventing the comparison of information between studies. Until a reliable range of reference values has been established in normal individuals, increases or decreases in values of particular biomarkers relative to their controls will provide more information than their absolute values. As previously mentioned, the use of existing assays may not be reliable given the dilute nature of EBC.
The ideal conditions for collecting specific biomarkers vary depending on the nature of the substance, volatile versus non-volatile, collection temperature, and duration of collection. Optimum assay systems also differ substantially for the markers measured in EBC. The ATS/ERS task force states it is not scientifically appropriate to standardize a broad collection technique for biomarkers. In doing so, it could greatly limit innovation in a relatively new technique. Optimization for one marker will potentially make another marker's collection or assay suboptimal. Disagreement will exist between investigators as to normal levels of a biomarker until all aspects of an EBC sampling and assay procedure are standardized, but standardization will need to be marker specific (8).
Safety
The safety of EBC has been illustrated through repeated use without significant adverse events. Over 10,000 individual collections have been performed using different devices in laboratories all over the world and no adverse events have been reported (8). Suggested risks associated with mechanical ventilation include ice formation in the tubing resulting in limited expiratory flow (46), possible accidental extubation with manipulation of the endotracheal tube, infection and the potential for seriously ill patients to become unstable during specimen collection (3).
Limitation of expiratory flow secondary to ice formation is described in the setting of collection using very low temperatures with liquid nitrogen or dry ice, and is more likely with prolonged collection times. If overlooked, this could lead to hyperinflation of the lungs (46), a potentially serious problem. Constant monitoring while performing EBC, as well as shorter collections times at low temperatures should prevent this complication.
With manipulation of the endotracheal tube (ETT), there is always a risk of accidental extubation. By connecting the ETT to a collection device, the patient's range of head motion will be restricted, and sudden movements could potentially dislodge the tube. The ETT should be safely secured and the position at the patient's lip should be noted prior to manipulation. Collection of EBC on mechanically ventilated patients should not be performed without available medical professionals such as physicians, nurses and respiratory therapists to address any potential problems. When connecting and disconnecting the device, careful attention and optimal positioning are important in minimizing risk (3).
The introduction of infection by manipulation of the ETT is also a concern when connecting or disconnecting collection devices. There is no clear association between ETT handling technique and pneumonia in the literature (47), but sterile technique should be used regardless during EBC collection. Disposable devices should be used where possible. If the collection device is not disposable then proper sterilization of the device should be performed in between uses. In the event that a patient is too unstable for EBC collection, the procedure should be aborted. While the potential for adverse events during EBC collection exists, serious adverse events have not been reported in spontaneously breathing or mechanically ventilated patients.
Impact of EBC Analysis on Mechanically Ventilated Patients
Once a standardized method of performing EBC is established, clinicians may be able to monitor specific disease processes with a simple non-invasive study that can easily be repeated, allowing for frequent reevaluation of a patient's condition. The patterns of change in exhaled volatile gases seen in previous investigations illustrate this potential for monitoring pulmonary conditions by analyzing breath composition. Inflammatory biomarkers have been identified and could serve as prognosticators of disease, guiding clinical decision making. Increased effort focusing specifically on identifying new biomarkers and associated reference values will help to determine the best clinical application of EBC.
Invasive means of studying the lungs, such as BAL, are associated with risks of interrupting ventilation and/or causing injury and infection to the patient's airway, compounding the patient's underlying condition. EBC avoids iatrogenic inflammation or injury and does not interrupt the recovery process of sick patients. Patients who have been intubated secondary to respiratory failure are already significantly ill and in some cases cannot tolerate even simple bronchoscopy to obtain a BAL sample. EBC would be useful in these patients because it has minimal effect on the ventilator circuit and has been demonstrated to be safe when performed continuously or short term with or without humidification (2, 3).
Diseases examined by EBC in mechanically ventilated patients include COPD (9), acute lung injury/acute respiratory distress syndrome (9, 11, 13, 16, 18, 19, 20, 22, 23, 29), pneumonia (11, 15, 16), as well as in healthy lungs of patients with concurrent illness such as brain injury (25) and elective thoracic surgery (12) (Table 4). This represents a small fraction of the possible disease states that could be examined using this method. Many other conditions have been examined in volunteers in an outpatient setting using EBC, which could translate to a mechanically ventilated patient in more severe disease states. There is currently limited literature examining the inflammatory profile of mechanically ventilated trauma or burn inhalation injury patients using this technique, although these patients have been included in studies looking at various aspects of EBC (2, 3). The ultimate potential of this technology is as a safe and efficient method for identifying inflammatory profiles in disease states that could help guide clinical decision making.
Table 4.
Disease processes examined with EBC during mechanical ventilation
| Disease | Patient # | Authors |
|---|---|---|
| Brain Injury | 27 | Korovesi I et al 2011 |
| Head Injury, Extra pulmonary sepsis, Infection of CNS, Burns | 30 (4 Head Injury, 4 Extra pulmonary sepsis, 1 Infection of CNS, 1 Burns, 20 Healthy) | Roca O et al 2010 |
| ALI | 6 | Roca O et al 2008 |
| ALI, ARDS | 40 (30 ALI/ARDS, 10 Control) | Gessner C et al 2007 |
| VAP | 23 (14 VAP, 9 Control) | Horonenko G et al 2007 |
| Various: PNA, aspiration PNA, persistent pulmonary hypertension of the newborn, sepsis-related respiratory failure, tracheo-esophageal fistula, CDH, CLD, lung contusion, bronchiolitis, pneumonitis, asthma, head trauma | 36 Humidification, 14 No Humidification, 27 Control (12 PNA, 16 aspiration PNA, 6 persistent pulmonary hypertension of the newborn, 4 sepsis-related respiratory failure, 3 tracheo-esophageal fistula, 1 CDH, 7 CLD, 3 lung contusion, 5 bronchiolitis, 4 pneumonitis, 8 asthma, 6 head trauma) | Müller WG et al 2006 |
| Multilobar PNA, COPD with super infection, VAP | 48 [14 Asymptomatic, 13 Multilobar PNA, 14 COPD with super infection, 7 VAP] | Romero PV et al 2006 |
| ALI, ARDS, PNA | 44 (11 ALI/ARDS/PNA, 12 Smoking, 21 Non-smoking) | Sack U et al 2006 |
| Various: Brain injury, reactive airway disease, RSV s/p heart transplant, PNA (CF), asthma exacerbation, subglottic stenosis, CDH, multiple trauma, near drowning, arteriovenous canal, lung contusions, bronchopulmonary dysplasia with pulmonary hypertension, liver failure, failure of prosthetic valve | 19 (2 Brain injury, 2 reactive airway disease, 1 RSV s/p heart transplant, 1 PNA (CF), 1 asthma exacerbation, 3 subglottic stenosis, 1 CDH, 2 multiple trauma, 1 near drowning, 1 arteriovenous canal, 1 lung contusions, 1 bronchopulmonary dysplasia with pulmonary hypertension, 1 liver failure, 1 failure of prosthetic valve) | Walsh BK et al 2006 |
| ARDS | 6 | Bruhn A et al 2005 |
| COPD | 130 (11 COPD requiring Mechanical Ventilation, 34 COPD exacerbation, 40 Stable COPD, 21 Smoking, 24 Non-smoking) | Gessner C et al 2005 |
| Elective Thoracic Surgery | 45 (26 CABG, 19 Pneumonectomy) | Moloney ED et al 2004 |
| ARDS, ALI | 35 (15 ARDS, 12 ALI, 8 Control) | Gessner C et al 2003 |
| ALI, ARDS, PNA | 35 (15 ARDS, 13 ALI, 7 Control) | Gessner C et al 2003 |
| ARDS | 14 | Heard SO et al 1999 |
| ARDS, ALI | 32 (22 ARDS/ALI, 10 Control) | Carpenter CT et al 1998 |
| Ventilator dependent (Unspecified) | 34 | Von Pohle WR et al 1992 |
| ARDS | 68 (55 ARDS, 13 Control) | Sznajder JI et al 1989 |
| ARDS | 43 | Baldwin SR et al 1986 |
Disease: ALI - Acute Lung Injury, ARDS - Adult Respiratory Distress Syndrome, CABG – Coronary Artery Bypass Graft, CDH – Congenital Diaphragmatic Hernia CF – Cystic Fibrosis, CLD – Chronic Lung Disease, CNS - Central Nervous System, COPD – Chronic Obstructive Pulmonary Disease, PNA - Pneumonia, VAP - Ventilator associated pneumonia
Summary
Methods to incorporate EBC into a mechanical ventilator circuit have been established, and studies describing this technology have been available for thirty years. Collection of EBC in mechanical ventilation follows basic principles of condensation, but is influenced by multiple factors. These factors need to be taken into consideration in order to perform effective collection. The collection and analysis of biomarkers needs to be tailored to specific compounds of interest. Analysis of biomarkers in EBC requires proper storage and choice of assay, with validation of previously unused assays.
Of the described collection devices, R-Tube and ECoSCREEN have been studied most extensively. While R-Tube is easy to use and cheap, it is not specifically designed for use in mechanical ventilation and must be adapted to fit into the ventilator circuit. It is more affected by ambient temperature as well. If the R-Tube could be redesigned for use with mechanical ventilator tubing, this would facilitate its use. ECoSCREEN and TURBO-DECCS devices are easily incorporated into a mechanical ventilation circuit, but are expensive and more cumbersome to transport, limiting their use. Device size is a significant problem, especially if one has to travel a distance to get from an ICU to a laboratory. The ANACON device is not well documented in the literature, making it difficult to comment on its utility at this time.
There remains too much variability in collection methods and analysis of biomarkers and there is a lack of normal reference values for mediators, preventing standardization. Standardization should be specific for individual biomarkers, seeing as a more general model would not optimize collection of all compounds. Collection of EBC in the ambulatory population or in volunteers undergoing mechanically ventilation for surgical procedures may serve as controls for obtaining reference values. The first step in standardization of EBC is appropriate documentation of collection conditions and processing of EBC for specific biomarkers in order to compare data between investigators. The ATS/ERS task force recommendations on EBC should serve as a guide for investigators performing studies with EBC. Through collaboration and comparison of techniques and data collection, optimal methods for studying EBC will be determined.
Adaptation of EBC to mechanical ventilators is seemingly safe through continued use without reported major adverse events. It has been applied to numerous pulmonary conditions. EBC collection and analysis of biomarkers specific to these disease processes has clinical implications in diagnosis, therapeutics and prognostication. Sample collection in mechanically ventilated patients is completely non-invasive. By comparing EBC to more invasive methods of pulmonary monitoring, there is the potential to replace or substitute for techniques that involve more risk to the patient. Avoiding invasive procedures is of benefit to all patients, especially those with greater acuity requiring mechanical ventilation.
Conclusion
The potential for EBC to function as a safe, non-invasive tool for examining the severity of lung injury is tremendous. A framework for performing EBC collection in mechanically ventilated patients has been successfully documented, but needs to be expanded to other diseases such as autoimmune and connective tissue disorders, and inflammation secondary to traumatic lung injury such as pulmonary contusions or burn inhalation injury. Documentation of patient safety by monitoring vital signs and cardiac parameters during specimen collection as well as for any adverse events will be important in clinical acceptance of this procedure. The non-invasive nature of EBC eliminates procedural risks that in some cases are not tolerated by the critically ill. Once standardization is established, EBC could serve as an alternative to more invasive procedures such as bronchoscopy, bronchoalveolar lavage and biopsy. In order to address issues of standardization, comparative studies between collection devices and techniques, modes and conditions of ventilation such as humidifier use, and biomarker processing needs to be performed. Standardization will need to be specific for each individual biomarker with future investigations focusing on optimal collection conditions.
Acknowledgements
The Dr. Ralph and Marian C. Falk Medical Research Trust supported this work. Additional thanks to Richard Gamelli MD (Loyola University Medical Center), P Marco Fisichella MD (Loyola University Medical Center), John F Hunt, MD (University of Virginia), Raffi Baddour (President, Respiratory Research Inc.), Carlos Valesi (Medivac), and Rüdinger Eichler (FILT) for technical information.
Funded by the National Institute of Health (5T32 GM008750-12), the Department of Defense (W81XWH-10-2-0172), the International Association of Fire Fighters, and the Dr. Ralph and Marian C. Falk Medical Research Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors have no conflicts of interest in relation to publishing this manuscript.
References
- 1.Hunt JF. Exhaled breath condensate: An evolving tool for noninvasive evaluation of lung disease. Journal of Allergy and Clinical Immunology. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 2.Walsh BK, Mackey DJ, Pajewski T, Yu Y, Gaston BM, Hunt JF. Exhaled-Breath Condensate pH Can Be Safely and Continuously Monitored in Mechanically Ventilated Patients. Respiratory Care. 2006;51(10):1125–1131. [PubMed] [Google Scholar]
- 3.Müller WG, Morini F, Eaton S, Peters M, Jaffe A. Safety and feasibility of exhaled breath condensate collection in ventilated infants and children. European Respiratory Journal. 2006;28:479–485. doi: 10.1183/09031936.06.00063505. [DOI] [PubMed] [Google Scholar]
- 4.Sidorenko GI, Zborovski EI, Levina DI. Surface-active properties of the exhaled air condensates (a new method of studying lung function) Terapevticheskiĭ arkhiv. 1980;52(3):65–68. [PubMed] [Google Scholar]
- 5.Hunt JF. Exhaled Breath Condensate: An Overview. Immunology and Allergy Clinics of North America. 2007;27:587–596. doi: 10.1016/j.iac.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharitonov SA, Barnes PJ. Exhaled Markers of Pulmonary Disease. CHEST. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 7.Kharitonov SA, Barnes PJ. Exhaled Biomarkers. CHEST. 2006;130:1541–1546. doi: 10.1378/chest.130.5.1541. [DOI] [PubMed] [Google Scholar]
- 8.Horváth I, Hunt J, Barnes PJ. Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 9.Gessner C, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Engelmann L, Hoheise G, Gillissen A, Sack U, Wirtz H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respiratory Medicine. 2005;99:1229–1240. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Antczak A, Górski P. Markers of pulmonary disease in exhaled breath condensate. International Journal of Occupational Medicine and Environmental Health. 2002;15(4):317–323. [PubMed] [Google Scholar]
- 11.Gessner C, Hammerschmidt S, Kuhn H, Seyfarth HJ, Sack U, Engelmann L, Schauer J, Wirtz H. Exhaled breath condensate acidification in acute lung injury. Respiratory Medicine. 2003;97:1188–1194. doi: 10.1016/s0954-6111(03)00225-7. [DOI] [PubMed] [Google Scholar]
- 12.Moloney ED, Mumby SE, Gajdocsi R, Cranshaw JH, Kharitonov SA, Quinlan GJ, Griffiths MJ. Exhaled Breath Condensate Detects Markers of Pulmonary Inflammation after Cardiothoracic Surgery. American Journal of Respiratory and Critical Care Medicine. 2004;169:64–69. doi: 10.1164/rccm.200307-1005OC. [DOI] [PubMed] [Google Scholar]
- 13.Roca O, Gómez-Ollés S, Cruz MJ, Muñoz X, Griffiths MJD, Masclans JR. Effects of salbutamol on exhaled breath condensate biomarkers in acute lung injury: prospective analysis. Critical Care. 2008;12(R72) doi: 10.1186/cc6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roca O, Gómez-Ollés S, Cruz M, Muñoz X, Griffiths MJD, Masclans JR. Mechanical ventilation induces changes in exhaled breath condensate of patients without lung injury. Respiratory Medicine. 2010;104(6):822–828. doi: 10.1016/j.rmed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Romero PV, Rodríguez B, Martínez S, Cañizares R, Sepúlveda D, Manresa F. Analysis of Oxidative Stress in Exhaled Breath Condensate From Patients With Severe Pulmonary Infections. Archivos de Bronconeumologia. 2006;42(3):113–119. doi: 10.1016/s1579-2129(06)60128-6. [DOI] [PubMed] [Google Scholar]
- 16.Sack U, Scheibe R, Wotzel M, Hammerschmidt S, Kuhn H, Emmrich F, Hoheisel G, Wirtz H, Gessner C. Multiplex Analysis of Cytokines in Exhaled Breath Condensate. Cytometry. 2006;Part A(69A):169–172. doi: 10.1002/cyto.a.20231. [DOI] [PubMed] [Google Scholar]
- 17.Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Therapeutic Advances in Respiratory Disease. 2007;1(1):5–23. doi: 10.1177/1753465807082373. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter CT, Price PV, Christman BW. Exhaled Breath Condensate Isoprostanes Are Elevated in Patients With Acute Lung Injury or ARDS. CHEST. 1998;114:1653–1659. doi: 10.1378/chest.114.6.1653. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin SR, Grum CM, Boxer LA, Simon RH, Ketai LH, Devall LJ. Oxidant Activity in Expired Breath of Patients with Adult Respiratory Distress Syndrome. The Lancet. 1986:11–14. doi: 10.1016/s0140-6736(86)91895-7. [DOI] [PubMed] [Google Scholar]
- 20.Sznajder JI, Fraiman A, Hall JB, Sanders W, Schmidt G, Crawford G, Nahum A, Factor P, Wood LD. Increased hydrogen peroxide in the expired breath of patients with acute hypoxemic respiratory failure. CHEST. 1989;96:606–612. doi: 10.1378/chest.96.3.606. [DOI] [PubMed] [Google Scholar]
- 21.Horonenko G, Hoyt JC, Robbins RA, Singarajah CU, Umar A, Pattengill J, Hayden JM. Soluble Triggering Receptor Expressed on Myeloid Cell-1 Is Increased in Patients With Ventilator-Associated Pneumonia. CHEST. 2007;132:58–63. doi: 10.1378/chest.06-2731. [DOI] [PubMed] [Google Scholar]
- 22.Bruhn A, Liberona L, Lisboa C, Borzone G. Limitations of the Technique to Determine Hydrogen Peroxide Levels in Exhaled Breath Condensate From Patients with Adult Respiratory Distress Syndrome. Archivos de Brondoneumología. 2005;41(10):542–546. doi: 10.1016/s1579-2129(06)60280-2. [DOI] [PubMed] [Google Scholar]
- 23.Heard SO, Longtine K, Toth Ildiko, Puyana JC, Potenza B, Smyrnios N. The Influence of Liposome-Encapsulated Protaglandin E1 on Hydrogen Peroxide Concentrations in the Exhaled Breath of Patients with Acute Respiratory Distress Syndrome. Anesthesia & Analgesia. 1999;89:353–357. doi: 10.1097/00000539-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Von Pohle WR, Anholm JD, McMillan J. Carbon Dioxide and Oxygen Partial Pressure in Expiratory Water Condensate Are Equivalent to Mixed Expired Carbon Dioxide and Oxygen. CHEST. 1992;101:1601–1604. doi: 10.1378/chest.101.6.1601. [DOI] [PubMed] [Google Scholar]
- 25.Korovesi I, Papadomichelakis E, Orfanos SE, Giamarellos-Bourboulis EJ, Livaditi O, Pelekanou A, Sotiropoulou C, Koutsoukou A, Dimopoulou I, Ekonomidou F, Psevdi E, Armaganidis A, Roussos C, Marczin N, Kotanidou A. Exhaled Breath Condensate in Mechanically Ventilated Brain-injured Patients with No Lung Injury or Sepsis. Anesthesiology. 2011;114(5):1118–1129. doi: 10.1097/ALN.0b013e31820d84db. [DOI] [PubMed] [Google Scholar]
- 26.Goldoni M, Caglieri A, Andreoli R, Poli D, Manini P, Vettori MV, Corradi M, Mutti A. Influence of condensation temperature on selected exhaled breath parameters. BMC Pulmonary Medicine. 2005;5(10) doi: 10.1186/1471-2466-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayley DL, Abusriwil H, Ahmad A, Stockley RA. Validation of assays for inflammatory mediators in exhaled breath condensate. European Respiratory Journal. 2008;31:943–948. doi: 10.1183/09031936.00081707. [DOI] [PubMed] [Google Scholar]
- 28.Czebe K, Barta I, Antus B, Valyon M, Horváth I, Kullmanna T. Influence of condensing equipment and temperature on exhaled breath condensate pH, total protein and leukotriene concentrations. Respiratory Medicine. 2008;102:720–725. doi: 10.1016/j.rmed.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Gessner C, Dihazi H, Brettschneider S, Hammerschmidt S, Kuhn H, Eschrich K, Keller T, Engelmann L, Sack U, Wirtz H. Presence of cytokeratins in exhaled breath condensate of mechanical ventilated patients. Respiratory Medicine. 2007;102:299–306. doi: 10.1016/j.rmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmeyer F, Raulf-Heimsoth M, Harth V, Bünger J, Brüning T. Comparative analysis of selected exhaled breath biomarkers obtained with two different temperature-controlled devices. BMC Pulmonary Medicine. 2009;9(48) doi: 10.1186/1471-2466-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaber F, Acevedo F, Delin I, Sundblad BM, Palmberg L, Larsson K, Kumlin M, Dahlén SE. Saliva is one likely source of leukotriene B4 in exhaled breath condensate. European Respiratory Journal. 2006;28:1229–1235. doi: 10.1183/09031936.00151905. [DOI] [PubMed] [Google Scholar]
- 32.Gessner C, Kuhn H, Seyfarth HJ, Pankau H, Winkler J, Schauer J, Wirtz H. Factors influencing breath condensate volume. Pneumologie. 2001;55:414–419. doi: 10.1055/s-2001-16947. [DOI] [PubMed] [Google Scholar]
- 33.Reinhold P, Langenberg A, Becher G, Rothe M. Breath condensate—a medium obtained by a noninvasive method for the detection of inflammation mediators of the lung. Berliner und Münchener Tierärztliche Wochenschrift. 1999;112(6–7):254–259. [PubMed] [Google Scholar]
- 34.McCafferty JB, Bradshaw TA, Tate S, Greening AP, Innes JA. Effects of breathing pattern and inspired air conditions on breath condensate volume, pH, nitrite, and protein concentrations. Thorax. 2004;59:694–698. doi: 10.1136/thx.2003.016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gessner C, Hammerschmidt S, Kuhn H, Lange T, Engelmann L, Schauer J, Wirtz H. Exhaled Breath Condensate Nitrite and Its Relation to Tidal Volume in Acute Lung Injury. CHEST. 2003;124:1046–1052. doi: 10.1378/chest.124.3.1046. [DOI] [PubMed] [Google Scholar]
- 36.Kockzulla AR, Noeske S, Herr C, Dette F, Pinkenburg O, Schmid S, Jörres RA, Vogelmeier C, Bals R. Ambient temperature impacts on pH of exhaled breath condensate. Respirology. 2009;15:155–159. doi: 10.1111/j.1440-1843.2009.01664.x. [DOI] [PubMed] [Google Scholar]
- 37.Davidsson A, Schmekel B. Efficacy of Two Breath Condensers. Journal of Clinical Laboratory Analysis. 2010;24:219–223. doi: 10.1002/jcla.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kietzmann D, Kahl R, Muller M, Burchardi H, Kettler D. Hydrogen peroxide in expired breath condensate of patients with acute respiratory failure and with ARDS. Intensive Care Medicine. 1993;19:78–81. doi: 10.1007/BF01708366. [DOI] [PubMed] [Google Scholar]
- 39.Effros RM, Hoagland KW, Bosbous M, Castillo D, Foss B, Dunning M, Gare M, Lin W, Sun F. Dilution of respiratory solutes in exhaled condensates. American Journal of Respiratory and Critical Care Medicine. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 40.Effros RM, Biller J, Foss B, Hoagland K, Dunning MB, Castillo D, Bosbous M, Sun F, Shaker R. A Simple Method for Estimating Respiratory Solute Dilution in Exhaled Breath Condensates. American Journal of Respiratory and Critical Care Medicine. 2003;168:1500–1503. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 41.Zacharasiewicz A, Wilson N, Lex C, Li A, Kemp M, Donovan J, Hooper J, Kharitonov SA, Bush A. Repeatability of Sodium and Chloride in Exhaled Breath Condensates. Pediatric Pulmonology. 2004;37:273–275. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 42.Dwyer TM. Sampling airway surface liquid: non-volatiles in the expired breath condensate. Lung. 2004;182:241–250. doi: 10.1007/s00408-004-2506-3. [DOI] [PubMed] [Google Scholar]
- 43.Koutsokera A, Loukides S, Gourgoulianis KI, Kostikas K. Biomarkers in the Exhaled Breath Condensate of Healthy Adults: Mapping the Path Towards Reference Values. Current Medicinal Chemistry. 2008;15:620–630. doi: 10.2174/092986708783769768. [DOI] [PubMed] [Google Scholar]
- 44.Balbi B, Pignatti P, Corradi M, Baiardi P, Bianchi L, Brunetti G, Radaeli A, Moscato G, Mutti A, Spanevello A, Malerba M. Bronchoalveolar lavage, sputum and exhaled clinically relevant inflammatory markers: values in healthy adults. European Respiratory Journal. 2007;30:769–781. doi: 10.1183/09031936.00112306. [DOI] [PubMed] [Google Scholar]
- 45.Jackson AS, Sandrini A, Campbell C, Chow S, Thomas PS, Yates DH. Comparison of Biomarkers in Exhaled Breath Condensate and Bronchoalveolar Lavage. American Journal of Respiratory and Critical Care Medicine. 2006;175:222–227. doi: 10.1164/rccm.200601-107OC. [DOI] [PubMed] [Google Scholar]
- 46.Mutlu GM, Garey KW, Robbins RA, Danziger LH, Rubinstein I. Collection and Analysis of Exhaled Breath Condensate in Humans. American Journal of Respiratory and Critical Care Medicine. 2001;164:731–737. doi: 10.1164/ajrccm.164.5.2101032. [DOI] [PubMed] [Google Scholar]
- 47.Cheung N, Betro G, Luckianow G, Napolitano L, Kaplan LJ. Endotracheal Intubation: The Role of Sterility. Surgical Infections. 2007;8(5):545–552. doi: 10.1089/sur.2006.054. [DOI] [PubMed] [Google Scholar]