Abstract
Rationale
We previously showed that muscarinic agonists with M1 and/or M4 receptor affinities attenuated cocaine discrimination and self-administration in wild type mice but not in M1/M4 double-knockout mice.
Objective
To elucidate the respective contributions of M1 and M4 receptors to this effect.
Methods
Knockout mice lacking either the M1 subtype (M1−/−) or the M4 subtype (M4−/−), and wild-type mice were trained to discriminate 10 mg/kg cocaine from saline. Muscarinic ligands were tested for modulation of cocaine discrimination: xanomeline (M1/M4-preferring agonist), VU0357017 (M1-selective partial agonist), 77-LH-28-1(M1 agonist), and BQCA (M1-selective positive allosteric modulator).
Results
Xanomeline produced rightward shifts in the cocaine dose-effect curve in all three genotypes, but most robustly in wild-type mice. VU0357017 produced rightward shifts in the cocaine dose-effect curve in wild-type and M4−/− mice, but not M1−/− mice. Response rates were suppressed by xanomeline in wild-type and M1−/−, but not in M4−/− mice, and were unaltered by VU0357017. 77-LH-28-1 and BQCA also showed evidence of attenuating cocaine’s discriminative stimulus, but at doses that suppressed responding or had other undesirable effects. Intriguingly, both VU0357017 and 77-LH-28-1 exhibited U-shaped dose-effect functions in attenuating cocaine discrimination. None of the drugs substituted for the cocaine stimulus.
Conclusions
Attenuation of the cocaine stimulus by VU0357017 depended upon M1 receptors, and full effects of xanomeline depended upon both M1 and M4 receptors. Therefore M1-selective agonists and mixed M1/M4 agonists may be promising leads for developing medications that block cocaine’s effects.
Introduction
Growing evidence implicates brain cholinergic muscarinic systems in the abuse-related effects of stimulant drugs such as cocaine, and in the development of drug addictions (for reviews see Williams and Adinoff 2008; Sofuoglu and Mooney 2009). The muscarinic receptor family consists of five subtypes (M1–M5), which regulate many important central and peripheral functions (for reviews see Wess 2004; Eglen 2006; Langmead et al. 2008b). With respect to reward systems, stimulation of muscarinic receptors in different brain regions has opposing effects. Main areas of interest regarding muscarinic receptor localization include 1) the ventral tegmental area (VTA) and substantia nigra (SN), which receive cholinergic input from the laterodorsal (LDT) and pedunculopontine tegmental nuclei (PPT), and project to the striatum, including the nucleus accumbens (NAc) medium spiny neurons, 2) striatal cholinergic interneurons, and 3) the prefrontal cortex (Loughlin and Fallon 1984; Bolam et al. 1991; Di Chiara et al. 1994; Oakman et al. 1995; Blaha et al. 1996).
Evidence from preclinical investigations indicates that muscarinic receptors in the VTA and PPT facilitate rewarding and reinforcing effects of both drug and non-drug stimuli (Yeomans et al. 1985; Bechara and van der Kooy 1989; Olmstead and Franklin 1993; Yeomans and Baptista 1997; Ikemoto and Wise 2002; Alderson et al. 2004; You et al. 2008; see also Shabani et al. 2010). Those effects are generally attributed to the M5 receptor, the only muscarinic receptor subtype detected in VTA dopaminergic neurons (Vilaro et al. 1990; Weiner et al. 1990). Thus M5 selective antagonists may prove useful in the treatment of addictions (Yeomans et al. 2000; Fink-Jensen et al. 2003; Thomsen et al. 2005; Lester et al. 2010; see Raffa et al. 2009 for review). Conversely, stimulation of striatal muscarinic receptors reduced abuse-related effects of cocaine, while pharmacological blockade of striatal muscarinic receptors or destruction of striatal cholinergic neurons increased the effects of cocaine (Hikida et al. 2001; Smith et al. 2004; Mark et al. 2006). Thus striatal muscarinic receptors appear to oppose abuse-related effects of cocaine. The striatum contains predominantly the M1, M4, and M2 subtypes, the latter mostly presynaptic inhibitory autoreceptors (Weiner et al. 1990; Bernard et al. 1992; Hersch et al. 1994; Hersch and Levey 1995; Smiley et al. 1999).
Acetylcholinesterase (AChE) inhibitors (e.g., donepezil, galantamine, tacrine) indirectly stimulate nicotinic and muscarinic receptors by increasing synaptic levels of acetylcholine. Various AChE inhibitors have been tested in laboratory animals and humans, generally decreasing stimulant drug effects. AChE inhibitors prevented the development of conditioned place preference (CPP) to morphine or cocaine in mice, and decreased cocaine self-administration in rats, although with moderate selectivity over decreases in food-maintained behavior (Hikida et al. 2003; Takamatsu et al. 2006; Grasing et al. 2008, 2009). Galantamine reduced amphetamine-induced arousal, unrest, and stereotyped behaviors in monkeys (Andersen et al. 2007). In humans, AChE inhibitors have provided mixed results but generally failed to decrease drug-taking behaviors (Winhusen et al. 2005; De La Garza et al. 2008a,b; Grasing et al. 2010). Thus the clinical usefulness of AChE inhibitors may be limited by opposing effects at different receptors (including effects at sites other than AChE due to poor selectivity) and by adverse effects that prevent high doses from being used.
Subtype-selective muscarinic agonists may represent a better alternative to AChE inhibitors for medications aimed at reducing psychostimulant use and dependence. We previously showed attenuation of cocaine’s discriminative stimulus by the M1 agonist TBPB and the M1/M4-preferring agonist xanomeline (Thomsen et al. 2010). We further found that both drugs could abolish cocaine self-administration behavior in mice (Thomsen et al. 2010). Xanomeline is moderately selective for M1 and M4 receptors over other muscarinic and non-cholinergic receptors (Shannon et al. 1994; Heinrich et al. 2009). TBPB, while lacking agonist function at M2–M5 receptors, demonstrated antagonist properties at those receptors, and also binds with low affinity to dopamine D2 receptors (Jones et al. 2008; Heinrich et al. 2009; Lebois et al. 2009). However, xanomeline failed to attenuate cocaine discrimination in knockout mice lacking both M1 and M4 receptors, indicating that M1 and/or M4 receptor stimulation was responsible for the observed “anti-cocaine”effects.
The objectives of the present experiments were 1) to confirm the cocaine-attenuating effects of M1 receptor stimulation and 2) to evaluate whether stimulation of M1, M4 or both receptors contributed to the effects of a less selective agonist. To this aim we trained and tested knockout mice lacking either M1 or M4 receptors (having the other subtype intact) in the cocaine discrimination assay, as well as intact wild-type mice and Swiss-Webster mice. In addition to xanomeline, we tested the M1-selective partial agonist VU0357017, an MLPCN probe, (Lebois et al. 2009), the M1 agonist 77-LH-28-1 (Langmead et al. 2008a), and the M1-selective positive allosteric modulator benzylquinolone carboxylic acid (BQCA; Ma et al. 2009; Shirey et al. 2009). Table 1 shows the functional M1 selectivity of each drug. Pretreatment with the dopamine D2 antagonist eticlopride was evaluated for comparison, in wild-type mice only.
Table 1.
Functional M1 selectivity in vitro of VU0357017, xanomeline, 77-LH-28-1, and BQCA
| EC50 [μM] | Ki [μM] | Reference | |||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | D2 | ||
| VU0357017 | 0.198 | >30 | >30 | >30 | >30 | >10 | Lebois et al. 2009 |
| Xanomeline | 0.020 | 1 | 0.079 | 0.040 | 0.20 | nd | Langmead et al. 2008a |
| 0.0003 | 0.0925 | 0.005 | 0.052 | 0.042 | 0.264 | Heinrich et al. 2009 | |
| 77-LH-28-1 | 0.008 | >10 | 2.51 | >10 | >10 | nd | Langmead et al. 2008a |
| 0.002 | 0.765 | 0.159 | >10 | 0.206 | 0.06 | Heinrich et al. 2009 | |
| BQCA * | 0.845 | >100 | >100 | >100 | >100 | >37.5 | Ma et al. 2009 |
VU0357017 is an allosteric partial agonist, xanomeline and 77-LH-28-1 are orthosteric agonists, BQCA a positive allosteric modulator.
Because BQCA is a positive modulator, not an agonist, no EC50 values could be determined, and M1 value represents the inflexion point (potentiation of 3 nM acetylcholine).
nd: not determined
Materials and methods
Animals
Male Swiss-Webster, C57BL/6NTac, M1−/− and M4−/− mice were acquired from Taconic Farms (Germantown, NY) at 4–8 weeks of age. M1−/− and M4−/− mice were generated as described previously (Gomeza et al. 1999; Miyakawa et al. 2001) and backcrossed 11 generations to C57BL/6NTac females. Age- and sex-matched C57BL/6NTac mice were used as wild-type controls. Mice were acclimated to the housing facilities for ≥7 days before experiments began. During this time they were handled, and were anesthetized briefly for subcutaneous implantation of an identification microchip. Mice were kept in a 12-h light/dark cycle at ~22°C and ~55% humidity, group housed up to five per cage. Water was accessible ad libitum and food (rodent diet 5001; PMI Feeds, Inc., St. Louis, MO) was provided daily after training/testing sessions, 4 g/mouse/day. Rodent “treats”, nesting material, and exercise/nesting devices were provided for enrichment. All testing was conducted during the light phase of the circadian cycle.
Training and evaluation in cocaine discrimination
Operant-conditioning chambers as well as the procedure have been described (Thomsen et al. 2010). In brief, each chamber contained two nose-poke holes each equipped with a photocell and a yellow cue light. Centered between the holes was a plate into which liquid food was delivered. Mice were trained to discriminate 10 mg/kg cocaine from saline, administered intraperitoneally (i.p.). The reinforcer was 25 μl vanilla-flavored Ensure nutrition drink (water, sucrose, corn maltodextrin, milk protein concentrate, soy oil, canola oil, flavors, vitamins, minerals), 30 reinforcers were available per 20-min session. Mice were trained initially under an FR 1 schedule, the FR was then gradually increased to a final FR 10, with increasing pretreatment time spent in the chamber (rather than the home cage). Eventually sessions were preceded by the entire 10-min pretreatment period in the chamber, during which all lights were off and responding had no scheduled consequences. Cocaine and saline were presented in pseudorandom order across daily training sessions typically five days per week, and mice were counterbalanced with cocaine trained on the left or right nose-poke. Stable discrimination was defined as at least 7 of 8 consecutive sessions satisfying the following criteria: 1) ≥10 reinforcers earned per session, 2) ≥80% correct responses for the first reinforcer, and 3) ≥90% correct total responses.
Once criteria were met, mice were tested with saline and 0.32, 1.0, 3.2, 10 and 18 mg/kg cocaine to generate dose-effect functions. In pretreatment tests, xanomeline (1.8mg/kg, selected based on previous studies; Thomsen et al. 2010), VU0357017 (1–18 mg/kg), 77-LH-28-1 (1–10 mg/kg), BQCA (1–18 mg/kg), or eticlopride (0.01–0.56 mg/kg) was administered s.c. before cocaine. VU0357017 and xanomeline were administered 15 min before cocaine, 77-LH-28-1 and eticlopride, 10 min before cocaine, and BQCA 30 min before cocaine. For each drug including cocaine, doses were tested within-subjects in a pseudorandom order, counterbalanced between subjects and genotypes. At least one training session was interspersed between each test session, and tests were only performed when mice satisfied discrimination criteria. Because of the protracted latency to stable discrimination in M4−/− mice, tests were often performed in duplicate or triplicate in this strain to ensure the data were reliable. In the few cases when responding was suppressed to the point that no reinforcers were earned, the quantity of behavior was considered insufficient to evaluate the percentage of cocaine-appropriate responses and that calculation was not included in the data presentation or analysis.
Drugs
Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD). S(−)-eticlopride was purchased from Sigma-Aldrich (St. Louis, MO). VU0357017 (ethyl 4-(2-(2-methylbenzamido)ethylamino)piperidine-1-carboxylate) and BQCA were synthesized at Vanderbilt University. Xanomeline and 77-LH-28-1 (1-[3-(4-butyl-1-piperidinyl)propyl]-3,4-dihydro-2(1H)-quinolinone) were synthesized at the McLean Hospital according to previously published methods (Kane et al. 2008; Langmead et al. 2008a). VU0357017 was dissolved in sterile water, and BQCA was dissolved in 5% β-cyclodextrin in sterile water. Eticlopride was dissolved in ethanol then diluted in sterile water (final concentration ethanol 1%). Other drugs were dissolved in 0.9% saline. Cocaine and eticlopride solutions were refrigerated, other drug solutions were prepared daily. Drug doses refer to the weights of the respective salts.
Data analysis
Acquisition of cocaine discrimination was compared between each mutant to the wild-type group using the Logrank test, with sessions to criteria as the measure. For drug discrimination results, the percentage of drug-appropriate responding (DAR) for the whole session and total response rates (i.e., in both holes) are presented. Comparable effects were always observed in %DAR for the first reinforcer (not shown). For cocaine dose-effect curves, %DAR and response rates were analyzed by ANOVA with drug dose as a repeated-measures factor and genotype as a between-subjects factor. Because we had no a priori reason to expect an interaction between M1 and M4 genotypes, we compared each mutant vs. wild-type separately, while comparisons between M1−/− and M4−/− mice were not done. For drug pretreatments, one- or two-way repeated measures ANOVA were performed with dose of pretreatment drug and/or cocaine as factors, on %DAR and response rate, within each genotype separately. Dunnett’s multiple comparisons test (cocaine dose or pretreatment dose vs. vehicle) or Bonferroni posttest (pair-wise, pretreatment vs. cocaine alone) was performed where appropriate. Missing values due to suppression of behavior by the test drug (e.g., xanomeline) sometimes precluded the use of ANOVA on %DAR data. Therefore, A50 values in cocaine dose-effect functions were also calculated in each genotype, i.e., the dose of cocaine estimated to produce 50% DAR. For pre-treatment drugs, doses estimated to produce 50% decrease in DAR, and doses estimated to produce 50% decrease in response rates were calculated. A50 values were obtained in each mouse by interpolation of the dose-effect curves, and then group means and 95% confidence intervals (95%CI) were calculated. Significance level was set at p < 0.05, and for A50 values comparisons, non-overlapping 95%CI were considered significant.
Results
Discriminative stimulus of cocaine in M1−/− and M4−/− mice
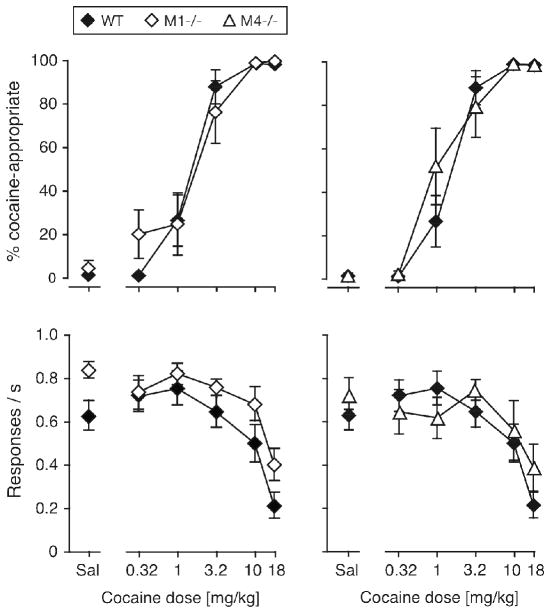
Mice were trained to discriminate 10 mg/kg cocaine from saline under an FR 10 schedule of food reinforcement. Figure 1 shows the percentage of mice meeting criteria as a function of time for each genotype. All wild-type mice met criteria within 6 months of training, but 3 of 15 M1−/− mice and 6 of 17 M4−/− mice failed to meet criteria, having had 3–9 months of training (e.g., excluded due to health complications, or failed to meet criteria after ≥9 months). A Logrank test on sessions to criteria revealed significantly longer training was needed in the M4−/− relative to wild-type (χ2 = 12.2, p < 0.001), while the M1 mutation had no significant effect.
Figure 1.
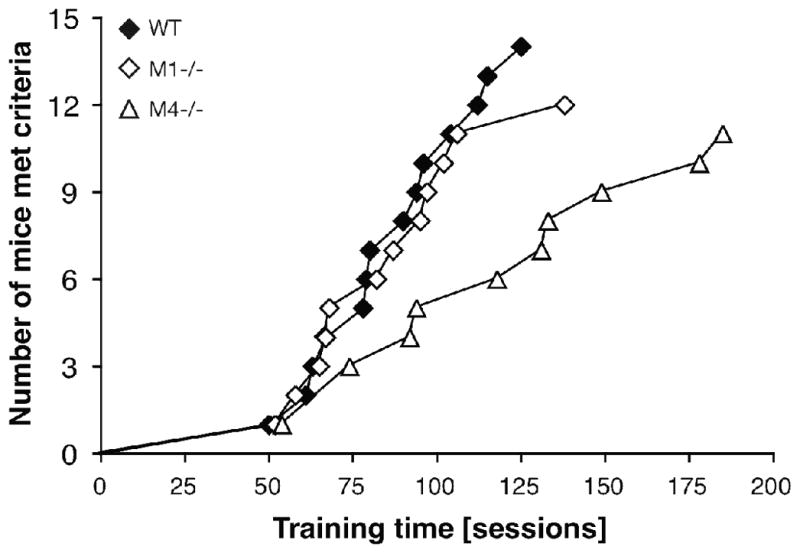
Acquisition of cocaine discrimination in wild-type, M1−/− and M4−/− mice. Abscissa: Training time in sessions; ordinate: number of mice meeting discrimination criteria. Acquisition of the procedure was significantly protracted in the M4−/− mice (p < 0.001, Logrank test vs. wild-type). N = 14–17.
Once criteria were met, mice of all three genotypes showed dose-dependent cocaine-appropriate responding, as shown in Table 2 and Figure 2. ANOVA on the percent drug-appropriate responses (DAR) between M1−/− and wild-type mice, and between M4−/− and wild-type mice, both showed an effect of cocaine dose ([F(5,100) = 77.4, p < 0.0001] and [F(5,95) = 86.2, p < 0.0001], respectively), but no effect of genotype or interaction. %DAR increased with cocaine dose in each genotype (all p < 0.0001), with 1–18 mg/kg reaching significance vs. saline in wild-type and M4−/− mice, and 3.2–18 mg/kg reaching significance in M1−/− mice (p < 0.05 to p < 0.01, Dunnett’s). Response rates decreased with increasing cocaine dose regardless of genotype in both the M1 comparison [F(5,100) = 24.1, p < 0.0001], and the M4 comparison [F(5,95) = 12.8, p < 0.0001] (see Fig. 2). One-way ANOVA in each genotype and post-hoc tests showed rates were significantly lower for 10 and 18 mg/kg cocaine relative to saline in the wild-type mice, 18 mg/kg only for M1−/− mice (p < 0.05 to p < 0.01, Dunnett’s), while the main effect of cocaine on rates just failed to reach significance in the M4−/− mice (p < 0.06). Calculated doses estimated to elicit 50% DAR, or to reduce response rates by 50% (A50 values), were also comparable between genotypes (see Table 2).
Table 2.
Cocaine discrimination accuracy at criteria and dose-effect curve
| Genotype | %DAR saline | %DAR cocaine | A50 DAR | A50 rate reduction |
|---|---|---|---|---|
| Wild-type | 1.9 ± 0.6 | 98.6 ± 0.5 | 1.49 [1.04 – 2.14] | 9.77 [8.22 – 11.6] |
| M1−/− | 1.0 ± 0.6 | 97.4 ± 0.8 | 2.05 [1.23 – 3.43] | 11.8 [7.42 – 16.6] |
| M4−/− | 1.2 ± 0.4 | 98.6 ± 0.7 | 1.49 [0.69 – 3.19] | 10.3 [6.42 – 16.5] |
%DAR saline and %DAR cocaine represent average performance over the block of sessions that satisfied discrimination criteria (group means ± s.e.m.). Group sizes: wild-type, N = 13, M1−/−, N = 9, M4−/− mice, N = 8. Reductions in response rates by at least 50% were not observed in all of the subjects, and A50 for rate reduction was calculated from 12 wild-type mice, 5 M1−/− mice, and 6 M4−/− mice.
Figure 2.
Cocaine dose-effect functions in wild-type, M1−/− and M4−/− mice trained to discriminate 10 mg/kg cocaine from saline. Abscissae: cocaine dose in mg/kg, “Sal” indicates saline; ordinates: percentage cocaine-appropriate responses (top panels), response rates in responses per second (bottom panels). Wild-type data are shown in both left (M1−/−) and right (M4−/−) panels to facilitate comparison. Data are groups means ± one S.E.M. Group sizes: wild-type, N=13; M1−/−, N=9; M4−/−, N=8.
Xanomeline pretreatment
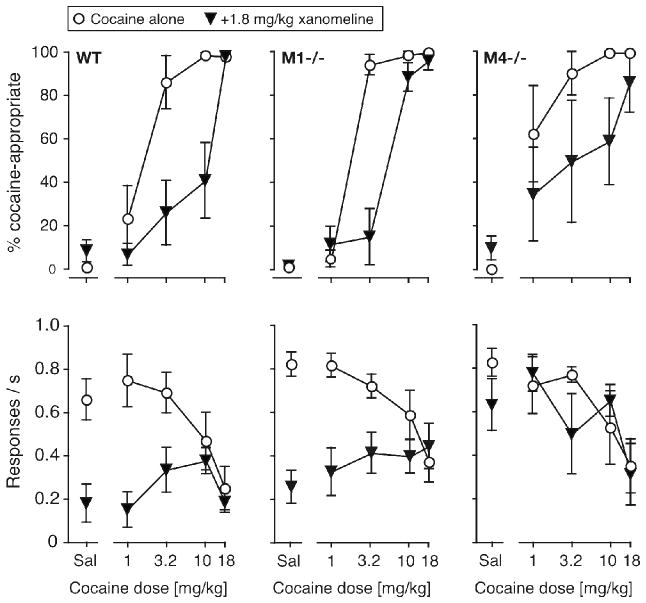
The effect of a 1.8 mg/kg xanomeline pretreatment on the cocaine dose-effect function was assessed in wild-type mice, M1−/− mice and M4−/− mice (Fig. 3). ANOVAs were not performed in the wild-type mice due to missing DAR values as xanomeline suppressed behavior in some subjects. Table 3 shows A50 values for %DAR with and without xanomeline, as well as the average -fold shift in cocaine’s potencies. Xanomeline produced a significant 8-fold rightward shift in the wild-type mice (non-overlapping 95%CI). Xanomeline also produced a significant rightward shift in the M1−/− mice, although it was only 3-fold on average (non-overlapping 95%CI). In the M4−/− mice, xanomeline produced on average an 8-fold rightward shift in the cocaine curve, but with substantial variability, so that 95%CI were just overlapping (see Table 3). ANOVA showed a significant effect of xanomeline in the M1−/− mice [F(1,40) = 21.9, p < 0.0001] and in the M4−/− mice [F(1,38) = 5.60, p < 0.05], with a xanomeline by cocaine interaction in the M1−/− mice only [F(4,40) = 18.5, p < 0.0001].
Figure 3.
Effect of pretreatment with 1.8 mg/kg xanomeline on the cocaine dose-effect function in wild-type mice (left), M1−/− mice (center) and M4−/− mice (right). Abscissae: dose cocaine in mg/kg, “Sal” indicates saline. Ordinates: percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± one S.E.M, N=5–8.
Table 3.
Pretreatment-induced shifts in cocaine dose-effect function
| Pretreatment - genotype | A50 cocaine alone | A50 pretreatment | Fold shift | Rate decrease |
|---|---|---|---|---|
| Xanomeline - Wild-type | 1.58 [0.96 – 2.61] | 8.87 [4.05 – 19.4]* | 8.1 | −36% [−10 – −62] |
| Xanomeline - M1−/− | 1.81 [1.64 – 2.00] | 5.18 [3.50 – 7.65]* | 3.1 | −36% [−19 – −54] |
| Xanomeline - M4−/− | 0.99 [0.49 – 1.99] | 5.22 [1.86 – 20.3] | 8.6 | ns |
| VU0357017 - Wild-type | 1.25 [0.74 – 2.10] | 4.95 [2.81 –8.73]* | 5.5 | ns |
| VU0357017 - M1−/− | 1.43 [1.07 – 1.90] | 1.24 [0.67 – 2.28] | 1.0 | ns |
| VU0357017 - M4−/− | 2.04 [0.85 – 4.93] | 3.49 [1.84 – 6.62] | 2.1 | ns |
| 77-LH-28-1 - SW | 0.80 [0.51 – 1.27] | 2.32 [1.03 – 5.21] | 4.1 | −18% [−4 – −31] |
non-overlapping 95% confidence intervals. Fold shift in A50 was calculated in each mouse, then averaged. Rate decrease was calculated as the % decrease from cocaine alone in each mouse, then averaged across mice and cocaine doses.
ns: non-significant.
ANOVAs on response rates showed a significant effect of both cocaine [F(4,70) = 2.69, p < 0.05] and xanomeline [F(1,70) = 27.52, p < 0.0001] in the wild-type mice, with a significant interaction [F(4,70) = 3.03, p < 0.05]. In the M1−/− mice, rate was affected by xanomeline [F(1,40) = 29.4, p < 0.0001], with a cocaine by xanomeline interaction [F(4,40) = 4.32, p < 0.01], while the main effect of cocaine did not reach significance. In the M4−/− mice, rate was affected by cocaine [F(4,40) = 3.81, p = 0.01], with no significant effect of xanomeline or interaction. Bonferroni posttests showed that in both wild-type mice and M1−/− mice, rates were significantly suppressed by xanomeline when xanomeline was administered before saline, 1.0 or 3.2 mg/kg cocaine (p < 0.01 to p < 0.001).
VU0357017 pretreatment and substitution
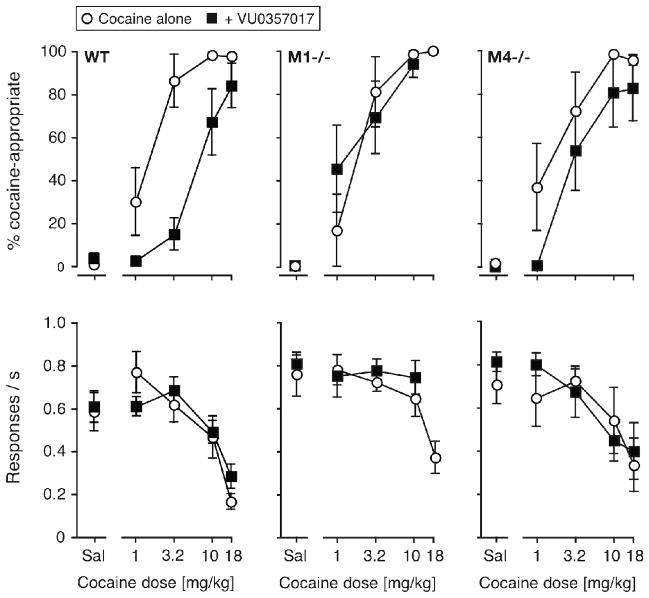
The effect of pretreatment with 3.2 mg/kg VU0357017, an M1-selective partial agonist, on the cocaine dose-effect function was similarly evaluated in each genotype (Fig. 4). In the wild-type mice, VU0357017 produced a significant rightward shift of the cocaine curve, supported by a significant effect of pretreatment [F(1,70) = 24.3, p < 0.0001], with a significant cocaine by VU0357017 interaction [F(4,70) = 4.66, p < 0.01]. In this and all subsequent comparisons of cocaine dose effect functions with and without pretreatment, the effect of cocaine dose was always significant (p < 0.0001), and is not reported further for the sake of brevity. In the M1−/− mice, VU0357017 had no significant effect on %DAR. In the M4−/− mice, ANOVA confirmed a significant rightward shift in cocaine discrimination [F(1,50) = 4.77, p < 0.05], without significant cocaine by pretreatment interaction. In all genotypes, response rates were affected by cocaine dose (p < 0.0001) but not by VU0357017. Table 3 shows calculated A50 values for %DAR with and without VU0357017, as well as the average -fold shift in cocaine’s potency in each genotype. This analysis was in general agreement with the ANOVAs, as VU0357017 produced a significant 5-fold rightward shift in the wild-type mice based on non-overlapping 95%CI. In the M1−/− mice, VU0357017 did not change cocaine’s potency as a discriminative stimulus. In the M4−/− mice, VU0357017 produced only an approximate 2-fold shift in cocaine discrimination, but with overlapping 95%CI (despite the significant ANOVA).
Figure 4.
Effect of pretreatment with 3.2 mg/kg VU0357017, an M1-selective partial agonist, on the cocaine dose-effect function in wild-type, M1−/− and M4−/− mice. Abscissae: dose cocaine in mg/kg, “Sal” indicates saline. Ordinates: percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± one S.E.M. Group sizes: N=6–8.
A range of VU0357017 doses were also tested as pretreatment to the training dose of cocaine in wild-type mice, M1−/− mice and M4−/− mice (data not shown). Over this dose range, VU0357017 reduced %DAR to 50% or lower in 5 of the 8 wild-type mice (achieving saline levels in 4 of those, 35% in the fifth). However, VU0357017 appeared effective only in a narrow dose range, which differed between animals, so that the maximum average attenuation in %DAR at any given dose was only 29%. In the M1−/− mice, VU0357017 only reduced %DAR in one of 6 mice, at the highest dose. In the M4−/− mice, VU0357017 decreased %DAR to saline-like levels in 2 of 5 mice tested, and to a small degree in one mouse. Response rates were not significantly affected by VU0357017. For comparison, we also tested the effects of the dopamine D2 antagonist eticlopride in wild-type mice (data not shown). The mean peak effect of eticlopride on %DAR was 36% (± 32% s.e.m). In contrast to VU0357017, eticlopride produced a monotonic function, up to doses that suppressed or eliminated responding. The dose of eticlopride which decreased %DAR, 0.32 mg/kg, also suppressed behavior to less than one reinforcer earned in two of the four subjects (thus ANOVA could not be performed on %DAR due to missing values; average rate reduction was 87%). The reduction in %DAR reflected an effect of eticlopride in one, but not the other, of the two mice in which at least one reinforcer was earned. ANOVA on response rates confirmed an effect of eticlopride [F(5,15) = 4.08, p < 0.05], reaching significance at 0.32 mg/kg post-hoc (p < 0.05). Only one mouse was tested up to 0.56 mg/kg, at which dose responding was suppressed completely.
We also tested the high range of VU0357017 doses (10–32 mg/kg) as a substitution for cocaine in Swiss-Webster mice, to examine the possibility of cocaine-like effects contributing to the U-shaped curve observed in %DAR modulation. We saw no evidence of substitution (Fig. 5), while these doses did decrease response rates moderately [F(3,12) = 5.05, p < 0.05], significant at 18 and 32 mg/kg (p < 0.05 and p < 0.01 vs. saline). Finally, VU0357017 (1–18 mg/kg) was also tested as a pretreatment to 10 mg/kg cocaine in five Swiss Webster mice, and decreased %DAR to <1% in two of the five, with a third showing complete blockade only for the first reinforcer but not the total session (data not shown). The compound was not tested further in this strain since it would likely only represent confirmatory data similar to the wild-type findings.
Figure 5.
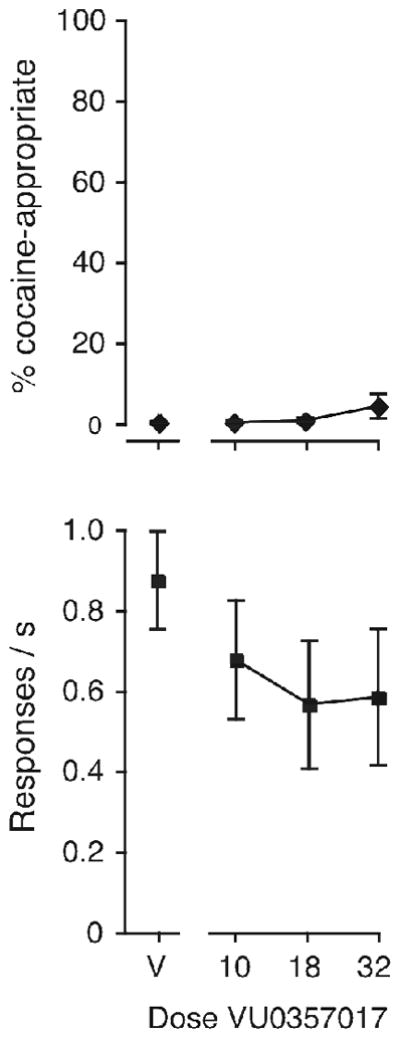
The M1-selective partial agonist VU0357017 did not substitute for cocaine in Swiss-Webster mice. Abscissae: dose VU0357017 in mg/kg, “V” indicates vehicle; ordinates: percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± one S.E.M., N=5.
77-LH-28-1 and BQCA pretreatment
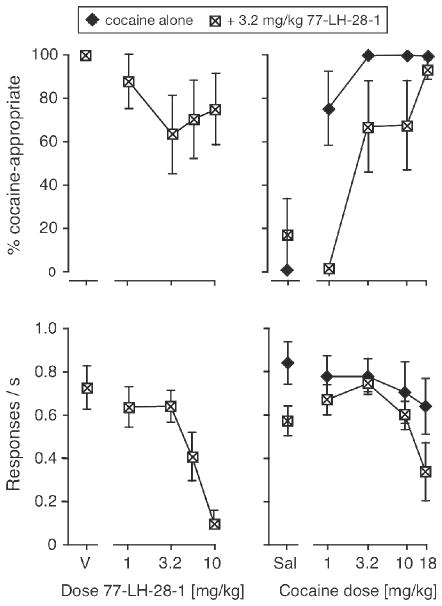
To further confirm that M1 receptor stimulation can modulate cocaine’s discriminative stimulus, another M1 agonist, 77-LH-28-1, was tested in Swiss-Webster mice. Figure 6 shows modest and statistically non-significant decreases in %DAR across a range of 77-LH-28-1 doses tested in combination with 10 mg/kg cocaine, again showing evidence of a U-shaped curve. 77-LH-28-1 also suppressed response rates [F(4,28) = 8.05, p < 0.001], significantly at the highest dose (p < 0.01). A dose of 3.2 mg/kg 77-LH-28-1 was selected as a pretreatment dose to be evaluated with the cocaine dose-effect function, and produced both a significant rightward shift in %DAR [F(1,50) = 11.4, p < 0.01] and significant decreases in response rates [F(1,50) = 6.91, p < 0.05]. Table 3 shows A50 values with overlapping 95%CI, despite a 4-fold average shift to the right.
Figure 6.
Effects of the M1 agonist 77-LH-28-1 as pretreatment to cocaine in Swiss-Webster mice. Leftmost panels show 77-LH-28-1 doses tested with 10 mg/kg cocaine, right panels show rightward shifts in the cocaine dose-effect function after administration of 3.2 mg/kg 77-LH-28-1. Abscissae, left panels: dose 77-LH-28-1 in mg/kg, “V” indicates vehicle; right panels: dose cocaine in mg/kg, “Sal” indicates saline. Ordinates: percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± one S.E.M., N=8 (left), N=6 (right).
We also tested the M1-selective positive allosteric modulator BQCA as a pre-treatment to 10 mg/kg cocaine in Swiss-Webster mice (data not shown) and wild-type C57BL/6NTac mice (Figure 7). Although BQCA produced some decrease in %DAR, it did so in a dose range that also produced marked diarrhea, despite showing only inconsistent decreases in response rate. Neither effects on DAR or on rate reached statistical significance. The Swiss-Webster strain appeared more sensitive to the diarrhea than the C57BL/6NTac strain, and testing was discontinued in the Swiss-Webster strain after 3–4 mice. While 18 mg/kg BQCA produced a rightward shift in the cocaine discrimination dose-effect curve in a few wild-type mice (data not shown), testing was discontinued due to the diarrhea. Consequently, BQCA was not tested further, including in the mutant mice.
Figure 7.
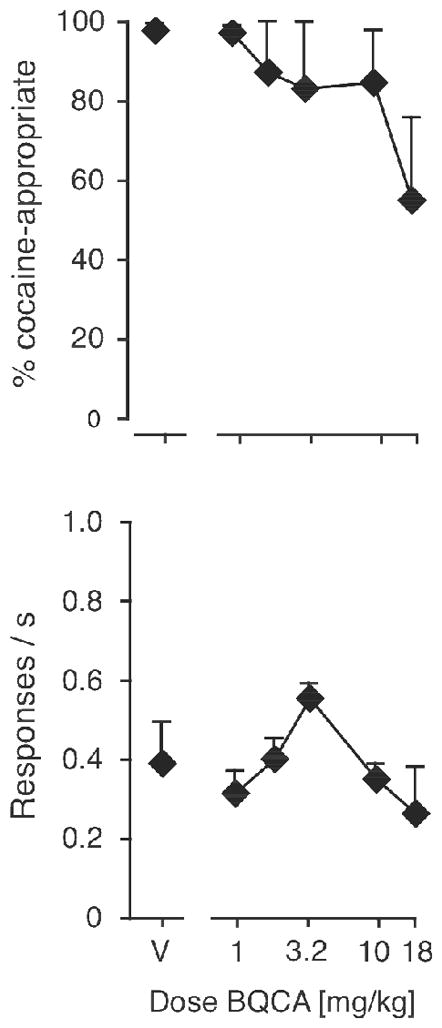
Effects of the M1-selective PAM BQCA as pretreatment to 10 mg/kg cocaine in wild-type C57BL/6NTac mice. Abscissae: dose BQCA in mg/kg, “V” indicates vehicle; ordinates: percentage cocaine-appropriate responses (top); response rate in responses per second (bottom). Data are groups means ± one S.E.M., N=6.
Discussion
The present investigation confirmed and extended our previous report on the muscarinic agonist-attenuation of abuse-related effects of cocaine in mice (Thomsen et al. 2010). Here, we combined the use of knockout mice lacking either M1 or M4 receptors with novel subtype-selective muscarinic receptor ligands to evaluate the contributions of each receptor to the “anti-cocaine” effects of muscarinic agonists. There were three main findings of the present study. First, we confirmed using structurally unrelated ligands that M1 receptor stimulation can attenuate the discriminative stimulus of cocaine, and this effect was absent in M1−/− mice. Second, as in our prior study, we observed no rate suppressing effects of the M1 selective partial agonist VU0357017 at doses that attenuated cocaine’s behavioral effects. This observation stands in contrast to the coordinate cocaine-attenuating and rate-suppressing effects of other drugs, such as dopamine D2 antagonists. Third, our data indicated that stimulation of both M1 and M4 subtypes contributed to the anti-cocaine effects of the M1/M4-preferring agonist xanomeline, while M4 receptors may mediate some of the rate-suppressing effects of xanomeline. Collectively, these data suggest that VU0357017 and other M1-selective agonists, as well as mixed M1/M4 agonists, may be promising leads for developing medications that block cocaine’s effects.
Both M1−/− mice and M4−/− mice acquired cocaine discrimination, and showed cocaine dose-effect functions comparable to wild-type mice, indicating that neither receptor is essential for cocaine to produce a discriminative stimulus. The M4−/− mice, but not the M1−/− mice, showed protracted acquisition of the procedure. This could indicate altered learning/performance, or an altered discriminative stimulus of cocaine. In agreement with their performance in the present experiment, M1−/− mice showed performance deficits only in some, but not all, tests of cognitive function, and deficits appeared attributable to the hyperactive phenotype of these mutants (Miyakawa et al. 2001; Anagnostaras et al. 2003). M1−/− mice also showed cocaine CPP comparable to wild-type mice at 10 mg/kg, although CPP was reduced at 5 mg/kg (Carrigan and Dykstra 2007). Thus it is possible that M1−/− mice may have acquired discrimination of a lower cocaine dose less readily. Cognitive function has been studied less in M4−/− mice, but electrophysiological recordings in striatal slices showed deficits in long-term depression, suggesting learning and memory could well be affected (Bonsi et al. 2008). Reminiscent of the M4−/− phenotype in the present investigation, immunotoxic destruction of striatal cholinergic neurons impaired acquisition of a food-rewarded T-maze task in mice (Kitabatake et al. 2003). We recently reported greater cocaine-induced increases in extracellular dopamine, locomotor activity, and cocaine self-administration in M4−/− mice, suggesting that the protracted acquisition of discrimination was not attributable to decreased detectability of cocaine (Schmidt et al. 2011). One caveat of the investigation is that some mutant mice were excluded from the studies because reliable discrimination was not achieved – it is possible that this selection created, by necessity, a bias in the pharmacological responses observed. The repeated cocaine administrations needed for the discrimination procedure may also have affected the mutant strains differentially (e.g., sensitization).
We tested the M1/M4-preferring agonist xanomeline, the M1-selective partial agonist VU0357017, the M1 agonist 77-LH-28-1, and the M1-selective positive allosteric modulator BQCA as pretreatments to cocaine. Each drug showed evidence of attenuating the discriminative stimulus of cocaine in intact mice. Thus we confirmed, with structurally distinct compounds, our recent report using xanomeline and the M1 agonist TBPB (Thomsen et al. 2010). These results are also in agreement with the reduction of amphetamine-induced hyperlocomotion by BQCA in mice (Ma et al. 2009). Because TBPB has modest affinity for the dopamine D2 receptor, there was some concern that D2 receptor antagonism might have contributed to its observed “anti-cocaine” effects (Jones et al. 2008). While similar potential concerns apply to xanomeline and 77-LH-28-1, VU0357017 and BQCA had no effect at D2, 5HT, and several other receptors (Heinrich et al. 2009; Lebois et al. 2009; Ma et al. 2009, Shirey et al. 2009; see Table 1). To confirm that the effects of VU0357017 were attributable to M1 receptor stimulation, we tested this compound in M1−/− mice and M4−/− mice: VU0357017 shifted the cocaine dose-effect curve to the right in the wild-type mice and the M4−/− mice, but had no effect in the M1−/− mice. Intriguingly, the effect appeared reduced or less consistent in the M4−/− mice, perhaps suggesting a permissive role of M4 receptors in the effects of M1 receptor activation. Ongoing studies in our laboratory indicate that M4−/− mice show attenuation of cocaine discrimination at least comparable to wild-type mice when pre-treated with a dopamine receptor antagonist. Therefore it is unlikely that the smaller effect observed here reflected a generally reduced responsiveness to modulation of cocaine discrimination in the M4−/− mice. Taken together with our previous report (Thomsen et al. 2010), the present findings strongly support the hypothesis that selective stimulation of M1 receptors can attenuate abuse-related effects of cocaine in mice.
We previously reported a ≈60% reduction in cocaine discrimination (10 mg/kg cocaine) in Swiss-Webster mice and C57BL/6NTac wild-type mice (Thomsen et al. 2010). In contrast, xanomeline had no effect on cocaine discrimination in mice lacking both M1 and M4 receptors up to doses that almost completely suppressed responding, indicating that activation of M1 and/or M4 receptors was responsible for these anti-cocaine effects (Thomsen et al. 2010). Here we tested xanomeline in the single gene knockout mice to evaluate the respective contributions of M1 and M4 receptor stimulation. In the C57BL/6NTac wild-type mice, xanomeline shifted the cocaine dose-effect function to the right, the present data closely matching our previous findings in Swiss-Webster mice (Thomsen et al. 2010). Xanomeline also shifted the cocaine curve to the right in M1−/− mice and in M4−/− mice, indicating that stimulation of neither receptor alone was obligatory to attenuate cocaine discrimination. However, the effect was smaller in the M1−/− mice and more variable in the M4−/− mice, indicating that both receptors contributed to the anti-cocaine effects of xanomeline.
VU0357017, 77-LH-28-1 and BQCA produced only modest reduction in cocaine discrimination (10 mg/kg cocaine), ≈29–40%. It is tempting to speculate that the apparently larger effect size of xanomeline was attributable to the combined effects of M1 and M4 activation. However, the D2 antagonist eticlopride also produced only approximately 40% attenuation of cocaine discrimination in wild-type mice, indicating that cocaine discrimination is difficult to modulate in C57BL/6 mice. Other factors may explain the modest effects of VU0357017 and BQCA. Both TBPB and VU0357017 behaved like partial agonists in some assays (Lebois et al. 2009). For BQCA, the dose range was limited by the occurrence of side effects (diarrhea). Thus activation of brain M1 receptors with higher efficacy M1 selective agonists may be able to attenuate cocaine’s effects more fully than observed here, and should be explored in future studies.
Both xanomeline and TBPB in the previous investigation, and VU0357017 and 77-LH-28-1 in the present investigation, produced U-shaped dose-effect functions, i.e., they appeared to attenuate cocaine’s effects at a fairly narrow range of doses, with effects diminishing at higher doses (Thomsen et al. 2010). This also contributed to the average maximum effect being modest, as not all mice responded at the same dose. Because TBPB showed antagonist properties at M2–M5 receptors, M4 (or other subtype) antagonism may have limited its efficacy at higher doses (Lebois et al. 2009). However, VU0357017 showed no antagonist activity at M2–M5 receptors (Lebois et al. 2009). It appears unlikely that the biphasic nature of the effect is due to a cocaine-like stimulus of the muscarinic agonists at higher doses, since VU0357017 failed to substitute for the cocaine stimulus. This type of dose-effect relationship is not uncommon for muscarinic drug effects. For example, oxotremorine reduced amphetamine-induced increases in extracellular NAc dopamine most effectively at an intermediate dose (Ichikawa et al. 2002). This phenomenon may reflect the complex nature of dopamine/cholinergic interactions at the neuronal level: a recent investigation revealed bidirectional modulation of striatal dopamine release by M2 and M4 receptors (Threlfell et al. 2010).
How, and where in the brain, M1/M4 stimulation modulates behavioral effects of cocaine remains uncertain. The AChE inhibitor rivastigmine attenuated cardiovascular effects of methamphetamine in humans, and we cannot exclude the possibility that cardiovascular or other peripheral effects contributed to the attenuation of cocaine discrimination in our studies (De La Garza et al. 2008b). However, this appears unlikely for several reasons. First, M1 receptor stimulation moderately increases heart rate in rodents, and would thus be expected to enhance cocaine’s cardiovascular effects, not attenuate them (Hardouin et al. 2002; Ma et al. 2009). Second, we previously showed that muscarinic ligands with poor brain penetration had lower potency or lacked effects in the cocaine discrimination assay, suggesting that this modulation is centrally mediated (Thomsen et al. 2010). Third, muscarinic antagonists can enhance, and muscarinic agonists attenuate, stimulant-induced increases in extracellular striatal (NAc) dopamine, consistent with at least some of the modulation of stimulant drug effects occurring in the striatum (Ichikawa et al. 2002; Tanda et al. 2007). Immunotoxic destruction of NAc cholinergic neurons enhanced cocaine-induced locomotor activity and CPP, effects that are remarkably similar to observations made with cocaine and d-amphetamine in whole-body and D1 receptor neuron-specific M4 receptor knockout mice, supporting the NAc/striatum as a site of muscarinic modulation of psychostimulant effects (Hikida et al. 2001; Jeon et al. 2010; Schmidt et al. 2011; Dencker et al. 2011).
Finally, VU0357017 in this investigation, like TBPB in a previous investigation, did not affect response rates in any genotype, and no undesirable effects were observed (Thomsen et al. 2010). Xanomeline produced no overt side effects but did decrease response rates in wild-type and M1−/− mice, while this effect was blunted in the M4−/− mice. We previously observed comparable rate-decreasing effects of xanomeline in M1−/−M4−/− double knockout mice and wild-type mice (Thomsen et al. 2010), and the contribution of M4 receptors to reductions in response rate (and thus presumably to side effects in humans), remains uncertain. 77-LH-28-1 also suppressed response rates, likely due to its lower selectivity (e.g., relative to dopamine and 5HT receptors; Heinrich et al. 2009). Unexpectedly, BQCA caused diarrhea at doses that decreased cocaine-appropriate responding. Unlike xanomeline, VU0357017 and 77-LH-28-1, which have good brain penetration, BQCA produced a brain:plasma ratio of about 1:10 in rats, which may explain its less favorable side effect profile (Sauerberg et al. 1992; Langmead et al. 2008a; Lebois et al. 2009; Ma et al. 2009; Shirey et al. 2009). No side effects were reported for BQCA in previous studies, and this may represent a species/strain difference (Ma et al. 2009; Shirey et al. 2009). Taken together, our previous and present findings are consistent with the idea that brain-penetrant, M1-selective agonists may have a low risk of side effects as medications.
In summary, we observed attenuation of cocaine’s discriminative stimulus with several M1 agonists/potentiators. Studies in M1−/− and M4−/− mice confirmed that M1 receptors mediated the effects of the M1-selective partial agonist VU0357017, and indicated that both M1 and M4 receptors contributed to the effects of the M1/M4-preferring agonist xanomeline. With selective stimulation of brain M1 receptors we observed no undesirable effects or rate suppression (the latter associated with dopamine D2 antagonists). Therefore we suggest that both the M1 and the M4 receptor may be viable drug targets for the treatment of cocaine addiction, and we will continue to evaluate new M1 and M4 agonists/potentiators. Drug combination studies may be also warranted to determine whether activation of M1 and M4 receptors produces additive, synergistic, or less-than-additive effects (both therapeutic and undesirable).
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Disorders (J.W.), by the Molecular Libraries Probe Production Centers Network (U54MH084659, P.J.C and C.W.L.), and by a grant from the National Institutes on Drug Abuse (DA027825, M.T.). All procedures were carried out in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003) and US laws. We thank Joon Y. Boon for technical assistance.
References
- Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P. An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience. 2004;125:349–58. doi: 10.1016/j.neuroscience.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–8. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Andersen MB, Werge T, Fink-Jensen A. The acetylcholinesterase inhibitor galantamine inhibits d-amphetamine-induced psychotic-like behavior in Cebus monkeys. J Pharmacol Exp Ther. 2007;321:1179–82. doi: 10.1124/jpet.107.119677. [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci. 1989;9:3400–9. doi: 10.1523/JNEUROSCI.09-10-03400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–22. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Francis CM, Henderson Z. Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience. 1991;41:483–94. doi: 10.1016/0306-4522(91)90343-m. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Martella G, Cuomo D, Platania P, Sciamanna G, Bernardi G, Wess J, Pisani A. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci. 2008;28:6258–63. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan KA, Dykstra LA. Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berl) 2007;191:985–93. doi: 10.1007/s00213-006-0671-1. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Mahoney JJ, 3rd, Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacol Biochem Behav. 2008;89:200–8. doi: 10.1016/j.pbb.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int J Neuropsychopharmacol. 2008;11:729–41. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Wortwein G, Weikop P, Jeon J, Thomsen M, Sager TN, Mork A, Woldbye DP, Wess J, Fink-Jensen A Involvement of a Subpopulation of Neuronal M4 Muscarinic Acetylcholine Receptors in the Antipsychotic-like Effects of the M1/M4 Preferring Muscarinic Receptor Agonist Xanomeline. J Neurosci. 2011;31:5905–5908. doi: 10.1523/JNEUROSCI.0370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–33. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26:219–33. doi: 10.1111/j.1474-8673.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–6. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:10483–8. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasing K, He S, Yang Y. Dose-related effects of the acetylcholinesterase inhibitor tacrine on cocaine and food self-administration in rats. Psychopharmacology (Berl) 2008;196:133–42. doi: 10.1007/s00213-007-0944-3. [DOI] [PubMed] [Google Scholar]
- Grasing K, He S, Yang Y. Long-lasting decreases in cocaine-reinforced behavior following treatment with the cholinesterase inhibitor tacrine in rats selectively bred for drug self-administration. Pharmacol Biochem Behav. 2009;94:169–78. doi: 10.1016/j.pbb.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Grasing K, Mathur D, Newton TF, DeSouza C. Donepezil treatment and the subjective effects of intravenous cocaine in dependent individuals. Drug Alcohol Depend. 2010;107:69–75. doi: 10.1016/j.drugalcdep.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Hardouin SN, Richmond KN, Zimmerman A, Hamilton SE, Feigl EO, Nathanson NM. Altered cardiovascular responses in mice lacking the M(1) muscarinic acetylcholine receptor. J Pharmacol Exp Ther. 2002;301:129–37. doi: 10.1124/jpet.301.1.129. [DOI] [PubMed] [Google Scholar]
- Heinrich JN, Butera JA, Carrick T, Kramer A, Kowal D, Lock T, Marquis KL, Pausch MH, Popiolek M, Sun SC, Tseng E, Uveges AJ, Mayer SC. Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists. Eur J Pharmacol. 2009;605:53–6. doi: 10.1016/j.ejphar.2008.12.044. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–63. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Levey AI. Diverse pre- and post-synaptic expression of m1-m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 1995;56:931–8. doi: 10.1016/0024-3205(95)00030-a. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci U S A. 2001;98:13351–4. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100:6169–73. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Chung YC, Li Z, Dai J, Meltzer HY. Cholinergic modulation of basal and amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2002;958:176–84. doi: 10.1016/s0006-8993(02)03692-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wortwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Brady AE, Davis AA, Xiang Z, Bubser M, Tantawy MN, Kane AS, Bridges TM, Kennedy JP, Bradley SR, Peterson TE, Ansari MS, Baldwin RM, Kessler RM, Deutch AY, Lah JJ, Levey AI, Lindsley CW, Conn PJ. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J Neurosci. 2008;28:10422–33. doi: 10.1523/JNEUROSCI.1850-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane BE, Grant MK, El-Fakahany EE, Ferguson DM. Synthesis and evaluation of xanomeline analogs--probing the wash-resistant phenomenon at the M1 muscarinic acetylcholine receptor. Bioorg Med Chem. 2008;16:1376–92. doi: 10.1016/j.bmc.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S. Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci U S A. 2003;100:7965–70. doi: 10.1073/pnas.1032899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead CJ, Austin NE, Branch CL, Brown JT, Buchanan KA, Davies CH, Forbes IT, Fry VA, Hagan JJ, Herdon HJ, Jones GA, Jeggo R, Kew JN, Mazzali A, Melarange R, Patel N, Pardoe J, Randall AD, Roberts C, Roopun A, Starr KR, Teriakidis A, Wood MD, Whittington M, Wu Z, Watson J. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br J Pharmacol. 2008;154:1104–15. doi: 10.1038/bjp.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–43. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Lebois EP, Bridges TM, Lewis LM, Dawson ES, Kane AS, Xiang Z, Jadhav SB, Yin H, Kennedy JP, Meiler J, Niswender CM, Jones CK, Conn PJ, Weaver CD, Lindsley CW. Discovery and Characterization of Novel Subtype-Selective Allosteric Agonists for the Investigation of M1 Receptor Function in the Central Nervous System. ACS Chemical Neurosci. 2009;1:104–121. doi: 10.1021/cn900003h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester DB, Miller AD, Blaha CD. Muscarinic receptor blockade in the ventral tegmental area attenuates cocaine enhancement of laterodorsal tegmentum stimulation-evoked accumbens dopamine efflux in the mouse. Synapse. 2010;64:216–23. doi: 10.1002/syn.20717. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Substantia nigra and ventral tegmental area projections to cortex: topography and collateralization. Neuroscience. 1984;11:425–35. doi: 10.1016/0306-4522(84)90034-4. [DOI] [PubMed] [Google Scholar]
- Ma L, Seager MA, Wittmann M, Jacobson M, Bickel D, Burno M, Jones K, Graufelds VK, Xu G, Pearson M, McCampbell A, Gaspar R, Shughrue P, Danziger A, Regan C, Flick R, Pascarella D, Garson S, Doran S, Kreatsoulas C, Veng L, Lindsley CW, Shipe W, Kuduk S, Sur C, Kinney G, Seabrook GR, Ray WJ. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A. 2009;106:15950–5. doi: 10.1073/pnas.0900903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechtholt AJ. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res. 2006;1123:51–9. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–50. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–69. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. Effects of pedunculopontine tegmental nucleus lesions on morphine-induced conditioned place preference and analgesia in the formalin test. Neuroscience. 1993;57:411–8. doi: 10.1016/0306-4522(93)90072-n. [DOI] [PubMed] [Google Scholar]
- Raffa RB. The M5 muscarinic receptor as possible target for treatment of drug abuse. J Clin Pharm Ther. 2009;34:623–9. doi: 10.1111/j.1365-2710.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Sauerberg P, Olesen PH, Nielsen S, Treppendahl S, Sheardown MJ, Honore T, Mitch CH, Ward JS, Pike AJ, Bymaster FP, et al. Novel functional M1 selective muscarinic agonists. Synthesis and structure-activity relationships of 3-(1,2,5-thiadiazolyl)-1,2,5,6-tetrahydro-1-methylpyridines. J Med Chem. 1992;35:2274–83. doi: 10.1021/jm00090a019. [DOI] [PubMed] [Google Scholar]
- Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DP, Wortwein G, Fink-Jensen A. Increased cocaine self-administration in M(4) muscarinic acetylcholine receptor knockout mice. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, Foster R, Gubner N, Phillips TJ, Mark GP. Muscarinic type 2 receptors in the lateral dorsal tegmental area modulate cocaine and food seeking behavior in rats. Neuroscience. 2010;170:559–69. doi: 10.1016/j.neuroscience.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon HE, Bymaster FP, Calligaro DO, Greenwood B, Mitch CH, Sawyer BD, Ward JS, Wong DT, Olesen PH, Sheardown MJ, et al. Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M1 receptors. J Pharmacol Exp Ther. 1994;269:271–81. [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, Christian EP, Doherty JJ, Quirk MC, Snyder DH, Lah JJ, Levey AI, Nicolle MM, Lindsley CW, Conn PJ. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci. 2009;29:14271–86. doi: 10.1523/JNEUROSCI.3930-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Mesulam MM. m2 muscarinic receptor immunolocalization in cholinergic cells of the monkey basal forebrain and striatum. Neuroscience. 1999;90:803–14. doi: 10.1016/s0306-4522(98)00527-2. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Yin X, Sizemore GM, Liguori A, Johnson WE, 3rd, Martin TJ. Involvement of cholinergic neuronal systems in intravenous cocaine self-administration. Neurosci Biobehav Rev. 2004;27:841–50. doi: 10.1016/j.neubiorev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs. 2009;23:939–52. doi: 10.2165/11310920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu Y, Yamanishi Y, Hagino Y, Yamamoto H, Ikeda K. Differential effects of donepezil on methamphetamine and cocaine dependencies. Ann N Y Acad Sci. 2006;1074:418–26. doi: 10.1196/annals.1369.042. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther. 2007;321:334–44. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, Fink-Jensen A, Caine SB. Attenuation of cocaine’s reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332:959–69. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaro MT, Palacios JM, Mengod G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett. 1990;114:154–9. doi: 10.1016/0304-3940(90)90064-g. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990;87:7050–4. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–50. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–97. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, Coleman FS, Bloch DA, Leiderman DB, Singal BM, Berger P, Elkashef A. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100(Suppl 1):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol Biochem Behav. 1997;57:915–21. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Kofman O, McFarlane V. Cholinergic involvement in lateral hypothalamic rewarding brain stimulation. Brain Res. 1985;329:19–26. doi: 10.1016/0006-8993(85)90508-6. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Takeuchi J, Baptista M, Flynn DD, Lepik K, Nobrega J, Fulton J, Ralph MR. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. J Neurosci. 2000;20:8861–7. doi: 10.1523/JNEUROSCI.20-23-08861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Wise RA. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28:9021–9. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]