Abstract
Prepulse inhibition (PPI) is an operational measure of sensorimotor gating. It is defined as a reduction in magnitude of a startle response when a startling stimulus is preceded by a weaker “prepulse”. PPI has been found to be altered in patients with schizophrenia, autism spectrum disorders and other neuropsychiatric illnesses. As such, the neural substrates regulating PPI are of particular interest. Previous studies using lesions, selective blockade of NMDA receptors and pharmacological disinhibition have demonstrated that impairment of basolateral amygdala (BLA) function disrupts PPI. However, transient GABA-mediated inactivation of BLA has not been evaluated for effects on PPI. Furthermore, the downstream projection targets that mediate BLA-evoked disruptions of PPI have not been elucidated. Thus, in the present study, we evaluated the effect on PPI of bilateral and unilateral inactivation of BLA, by microinfusion of the GABA-A receptor agonist, muscimol. We found that either bilateral or unilateral inactivation impaired PPI. Because unilateral inactivation was sufficient to impair PPI, we hypothesized that this was due to an indirect activation of a downstream target of BLA, the ventral pallidum (VP). Because VP inhibition normalizes PPI deficits evoked from nucleus accumbens (Kodsi & Swerdlow, 1994), we next tested the degree to which VP inhibition would normalize PPI deficits evoked from BLA. We unilaterally inactivated BLA with concurrent inactivation of VP and found that VP inactivation blocked BLA-evoked deficits in PPI. We suggest that BLA inactivation disrupts PPI through disinhibition of VP.
Keywords: acoustic startle response, sensorimotor gating, muscimol, GABA-A receptor, pharmacological inhibition
INTRODUCTION
Sensorimotor gating is a form of information processing, in which motor responses to sensory stimuli are inhibited. This process may allow for efficient information processing in the complex sensory world (Swerdlow, Braff, & Geyer, 2000). One measure of sensorimotor gating is prepulse inhibition of the acoustic startle reflex (PPI). PPI is defined as a reduced magnitude of the startle response; this occurs in subjects of several different species whenever a weak “prepulse” precedes a startling stimulus (pulse). It has been suggested that a deficit in PPI may reflect a more general deficit in the active filtering of irrelevant sensory stimuli in patients, a symptom present in several neuropsychiatric disorders such as schizophrenia (Braff et al., 2001, 1978), autism (William Perry, Minassian, Lopez, Maron, & Lincoln, 2007), Tourette’s syndrome (Castellanos et al., 1996), obsessive compulsive disorder (Swerdlow, Benbow, Zisook, Geyer, & Braff, 1993), Huntington’s Disease (Swerdlow et al., 1995), and post-traumatic stress disorder (Grillon, Morgan, Davis, & Southwick, 1998; Grillon, Morgan, Southwick, Davis, & Charney, 1996). Understanding the circuitry that mediates PPI may therefore help illuminate common neural substrates for these varied disorders.
A substrate of particular importance for PPI is the amygdala, a region that has received considerable attention in studies of the etiology of neuropsychiatric illnesses (Baron-Cohen et al., 2000; Benes & Berretta, 2000; Exner, Boucsein, Degner, Irle, & Weniger, 2004; Protopopescu et al., 2005; Rajarethinam et al., 2001; Rogers et al., 2009; Sugranyes, Kyriakopoulos, Corrigall, Taylor, & Frangou, 2011; Woon & Hedges, 2009; Wright et al., 2000). In the rat, electrolytic lesions of the amygdala (Decker, Curzon, & Brioni, 1995), as well as pharmacological disinhibition via the GABA-A antagonist, picrotoxin (Fendt, Schwienbacher, & Koch, 2000), blockade of NMDA-mediated neurotransmission by NMDA receptor antagonists (MK-801 or AP5) (Fendt et al., 2000; Wan & Swerdlow, 1997) or electrical impairment of amygdala function (electrical kindling of seizures) (Howland, Hannesson, Barnes, & Phillips, 2007; Koch & Ebert, 1998), disrupted PPI. More specifically, the basolateral amygdala (BLA) appears to be the key amygdala subnucleus required for PPI expression. Excitotoxic lesions confined to BLA, (Wan & Swerdlow, 1997), and blockade of adrenergic neurotransmission in BLA, but not in CeA, disrupteded PPI (Alsene, Rajbhandari, Ramaker, & Bakshi, 2011). However, the downstream structures of the PPI network that serve as the targets of BLA regulation have not been identified.
An especially compelling candidate structure for mediating BLA-evoked effects on PPI is the ventral pallidum (VP). The VP is the principal output nucleus connecting the forebrain to the brainstem circuits subserving the acoustic startle response (for a review of this circuitry see: (Koch & Schnitzler, 1997; Swerdlow, Geyer, & Braff, 2001). Disinhibition of VP neurons, by focal application of GABA-A receptor antagonist (picrotoxin), impaired PPI (Kodsi & Swerdlow, 1994, 1995; Swerdlow, Braff, & Geyer, 1990), while neither inhibition nor lesions of VP impacted PPI in otherwise normal animals (Kretschmer & Koch, 1998; Swerdlow, Braff, & Geyer, 1990). These data suggest that tonic inhibition of VP is necessary for normal PPI. The major source of this inhibition likely derives from neurons in the nucleus accumbens (NAcc), which sends GABAergic projections to VP (Conrad & Pfaff, 1976). Consistent with this finding, inactivation and lesions of NAcc impaired PPI (Kodsi & Swerdlow, 1994; Swerdlow, Braff, & Geyer, 1990). The observation that deficits in PPI resulting from inactivation of NAcc were prevented by inactivation of VP (Kodsi & Swerdlow, 1994) supports the proposal that VP mediates the influence of NAcc for controlling PPI. Because BLA provides a major source of excitatory drive to NAcc (Christie, Summers, Stephenson, Cook, & Beart, 1987; Kelley, Domesick, & Nauta, 1982; Robinson & Beart, 1988), we hypothesized that inhibition of VP would also prevent deficits in PPI that result from BLA inactivation.
To test this hypothesis, we focally applied the GABA-A receptor agonist, muscimol, to BLA, VP, or both structures concurrently and examined effects on PPI. Before examining the interaction between BLA and VP, we first compared the effect of bilateral inhibition of BLA to the effects of unilateral inhibition of BLA. Previous studies have only evaluated the effect of bilateral lesions, however because we hypothesized that BLA inactivation disrupts PPI via disinhibition of VP, and unilateral disinhibition is often sufficient to disrupt behavior (Bagri, Sandner, & Di Scala, 1989; Bakshi, Tricklebank, Neijt, Lehmann-Masten, & Geyer, 1999; Kitamura, Ikeda, Koshikawa, & Cools, 2001; Li, Priebe, & Yeomans, 1998; Malkova, Barrow, Lower, & Gale, 2003; Olpe, Schellenberg, & Koella, 1977; Périer, Tremblay, Féger, & Hirsch, 2002; Silva, Sandner, & Brandão, 2005; Wellman, 2005), we next tested the effect of unilateral inhibition of BLA. Finding that unilateral inactivation of BLA was sufficient to impair PPI, we then examined the impact of concurrent inactivation of the ipsilateral VP on this effect.
MATERIALS AND METHODS
Animals
Behavioral testing was conducted with 51 male Long Evans rats (Charles River) weighing approximately 300–350 g at the start of the study. 31 Animals were used for bilateral BLA inactivation and 18 were used for BLA-VP interaction experiments. 30 of the 51 rats were also used for unilateral BLA inactivation. Animals were housed in a temperature-controlled vivarium (22C) at Georgetown University Medical Center, and maintained on a standard 12h light-dark cycle (lights on 0600–1800h). All manipulations were performed in the light phase. All procedures were completed with approval from the Georgetown University Animal Care and Use Committee and in accordance with AALAC recommendations and the Guide for Care and Use of Laboratory Animals.
Surgery
Rats were anesthetized with equithesin (a combination of sodium pentobarbital, chloral hydrate, magnesium sulfate, ethanol, and propylene glycol) (2.5ml/kg, i.p.). For a subset of rats that were implanted with bilateral BLA cannulae (22 of the 28), isoflurane (1%) was used as an anesthetic agent due to a change in our animal care and use protocol. Animals were placed in a stereotaxic frame for implantation of cannulae. All coordinates were determined using the atlas of Paxinos and Watson (Paxinos & Watson, 2007) with animals positioned in the skull-flat plane. Cannulae were fixed to the skull with four jeweler’s screws using dental acrylic (Kooliner, GC America, Alsip, IL). Twenty-eight gauge dummy cannulae were inserted to maintain patency. Following surgery, rats were given caprofen (5 mg/kg, s.c.)as an analgesic and 1ml warm normal saline (s.c.) to maintain hydration.
BLA Cannulae
34 animals were implanted with bilateral cannulae targeting the BLA. Guide cannulae (22 gauge; Plastics One, Roanoke, VA) were fittedwith 28 gauge internal cannulae that extended 1 mm beyond the tip of the guide. Cannulae were positioned 2.8 mm posterior to bregma, 5.2 mm lateral to the midline, and 7.5 mm ventral to the dura. The sites of infusion (i.e., left vs. right hemisphere) were balanced within the group.
BLA and VP Cannulae
A separate group of 18 animals were implanted with cannulae targeting both BLA and VP. Guide cannulae (22 gauge; Plastics One, Roanoke, VA) were fittedwith 28 gauge internal cannulae that extended 1 mm beyond the tip of the guide. Cannulae targeting BLA were positioned 2.8 mm posterior to bregma, 5.2 mm lateral to the midline, and 7.5 mm ventral from dura. Cannulae targeting VP were placed 0.3 mm posterior to bregma, 2.5 mm lateral to the midline, and 6mm ventral to the dura. The sites of infusion (i.e., left vs. right hemisphere) were balanced within the group.
Drugs
For all intracerebral infusions, muscimol (Sigma, St. Louis, MO) was dissolved in saline to make a 2mM solution, and infused at a dose of 1nmol in 0.5ul per site. For BLA, this dose and volume is the same as has been previously used (Blair, Sotres-Bayon, Moita, & Ledoux, 2005). For VP, the dose is ten times greater than the dose previously used, although the volume of our infusion is half as large (Swerdlow, Braff, & Geyer, 1990).
The amount of muscimol (1nmol) in the volume used (0.5ul) has been shown to spread approximately 1mm3 over the course of an hour (Allen et al., 2008; Martin & Ghez, 1999). The time from infusion to completion of PPI experiments for any given animal was always less than 1h, suggesting that drug spread was likely restricted to the BLA or the VP, respectively, for any given infusion as our infusion sites within each structure were centrally located.
Intracerebral infusions
For infusions (as previously described in (Maggio & Gale, 1989)), internal infusion cannulae were attached to 10.0 μl Hamilton syringes via polyethylene tubing, which wasfilled with saline. A small air bubble separated thesaline from the drug or vehicle. For infusions into more than one site (i.e., bilateral BLA infusions, BLA and VP infusions), the infusions were performed simultaneously. For bilateral BLA inactivation, rats were infused bilaterally at a rate of 0.1 μl/min using a syringe pump (New Era Pump Systems, Wantagh, NY). For simultaneous BLA and VP inactivation, rats were infused in both structures concurrently at a rate of 0.1 μl/min using an infusion pump. For all experiments, cannulae were left in place for at least an additional minute to prevent spread of drug up the cannula tract. For animals with bilateral BLA cannulae that were used for both bilateral and unilateral BLA infusions, BLA infusions occurred first, followed by unilateral BLA infusions. For animals with BLA and VP cannulae that were also used for unilateral BLA infusions, unilateral BLA infusions occurred prior to BLA-VP interaction experiments. At least 48h elapsed between drug infusions and within each experiment the drug infusions followed a counterbalanced order. Animals received between 2 and 6 infusions.
Startle Chambers
All testing occurred within three sound attenuated startle chambers (SR-Lab Startle Reflex System; San Diego Instruments, San Diego, CA). Startle boxes consisted of clear non-restrictive Plexiglas cylinders resting on a platform inside a ventilated and illuminated chamber. A high-frequency loudspeaker inside the chamber produced both a continuous background noise of 70 dB and the various acoustic stimuli. The whole-body startle response of the rat caused vibrations of the Plexiglas cylinder, which were converted into signals by a piezoelectric accelerometer attached to the platform. The signals were digitized, rectified, stored, and analyzed on a Dell personal computer using SR-LAB software.
Behavioral Testing
10 minutes following intracerebral infusion, animals were placed into the startle chamber for behavior testing. Behavioral testing was performed as previously described. The test session used in all of the experiments consisted of a background noise (70 dB) that was presented alone for 5 min (acclimation period) and then continued throughout the session. All sound pressure levels were calibrated using a standard SPL meter (Radio Shack, Model 33–2050) set to the dB(A) scale. After the acclimation period, there were five presentations of a startle inducing 120 dB broadband noise pulse lasting 30 ms (“Pulse Alone”) to habituate the animals to testing in order to achieve stable baseline startle responses. These trials were excluded from data analysis.
After habituation, rats were presented with “Pulse Alone” trials and “Prepulse+Pulse” trials, with prepulses 3, 6 and 12 dB above the background noise. In the “Prepulse+Pulse trials”, the prepulse (30 ms) and the pulse (30 ms) were separated by an inter-stimulus interval (ISI) of 130 ms (onset to onset). Animals were tested on a total of 40 trials (10 Pulse-Alone trials, 10 of each of the prepulse trials) in the session. Trials were presented in a pseudorandom order; in no case did two trials of the same type occur sequentially. An average of 15s (with a range of 5–25s) separated the trials. The testing parameters employed in the current study are well established to produce robust PPI (Geyer, Wilkinson, Humby, & Robbins, 1993; Kamath, Al-Khairi, Bhardwaj, & Srivastava, 2008; Mansbach & Geyer, 1991; Reijmers & Peeters, 1994).
Data Analysis
SPSS and Graphpad Prism were used for data analysis and figure preparation. Prepulse inhibition was defined as [1–(startle amplitude on prepulse trials/startle amplitude on pulse alone trials)] × 100. Data were analyzed via ANOVA with prepulse intensity and drug treatments as within subject factors. Greenhouse-Geisser corrections for violations of sphericity were applied to all ANOVAs. Post-hoc tests (Bonferroni-Holm’s step-down) were applied following significant main effects (as specified in the results section). To verify that changes in PPI were independent of changes in ASR, we performed two additional analyses. First, we employed a median split analysis. The ratio of ASR on muscimol to ASR on saline infused trials was calculated, the median determined, and animals assigned to either “low ASR suppression” or “high ASR” suppression groups, if they fell above or below the median, respectively. This suppression variable was then entered as a factor into the original ANOVA to determine if animals with high or low ASR suppression differed with respect to PPI. An absence of an interaction between suppression grouping and treatment would indicate the independence of changes in PPI and ASR. The second analysis we performed was to correlate the ASR suppression ratio with a PPI suppression ratio (i.e., the ratio of average PPI during muscimol-infused trials to the average PPI during saline infused trials). If a decrease in ASR were responsible for impaired PPI, a positive correlation would be expected between ASR suppression ratio and PPI suppression ratio; the absence of a positive correlation would indicate the independence of changes in PPI and ASR.
Histology
Following the completion of behavioral testing, rats were overdosed with deep equithesin (4 ml/kg) anesthesia and decapitated. Brains were fixed in 4% paraformaldehyde for a minimum of 72 hours. After fixation, brains were cryoprotected in graded sucrose solutions (10%, 20% and 30%) and frozen. Coronal brain sections (40μm thick) were cut on a cryostat (Reichert Model 975C) and stained with cresyl violet acetate. Microscopic examination was performed to verify the location of cannula injection sites in the BLA or VP according the atlas of Paxinos and Watson (Paxinos & Watson, 2007).
RESULTS
Histological Verification of Infusion Sites
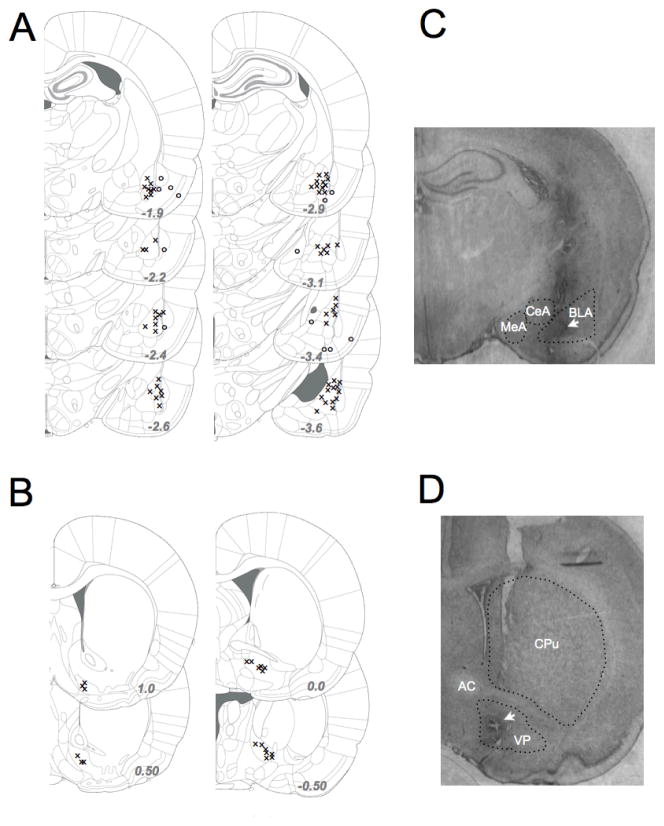
Infusion site verification resulted in the exclusion of the data from 6 animals in the BLA group, with cannula tips falling outside of the boundaries of the BLA. All cannulae positioned at VP fell within the VP. The position of cannula tips is indicated in Figure 1a (BLA) and 1c (VP) with representative photomicrographs showing localization for BLA (1b) and VP (1d).
Figure 1.
Histological verification of infusion sites (A) BLA cannula placement. “X” indicates location of cannulae from animals used for data analysis “o” indicates location of cannulae falling outside the boundaries of BLA and excluded from data analysis. (B) VP cannula placement. X” indicates location of cannulae from animals used. All cannulae aimed at VP fell within VP boundaries. (C) Representative photomicrograph showing cannula placement in BLA. MeA=medial amygdala, CeA=central nucleus of the amygdala. Outlines (dotted lines) show anatomical boundaries. (D) Representative photomicrograph showing cannula placement in VP. AC=anterior commissure, CPu=caudate-putamen. Outlines (dotted lines) show anatomical boundaries.
Bilateral infusion of muscimol into BLA impairs PPI
Of the 25 animals with correct cannula placement, 2 animals were excluded because they displayed no or negative prepulse values under control conditions (i.e., no drug present). The decision to remove these animals was made blind with respect to their performance under muscimol-infused conditions. The data from the remaining 23 animals were used for further analyses.
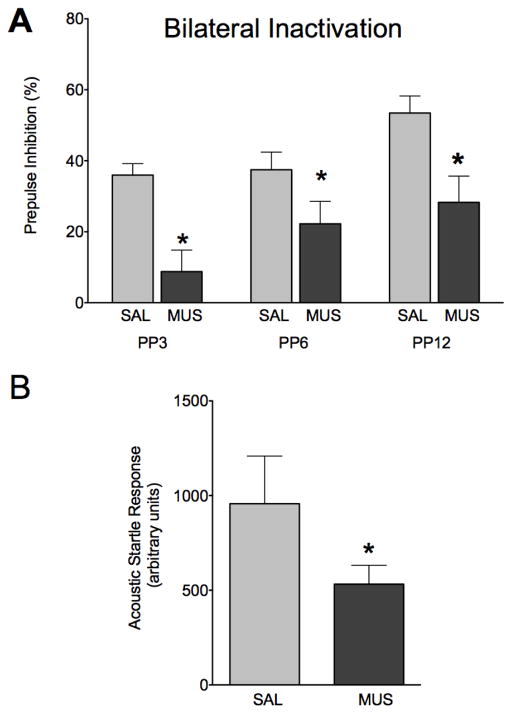
The effects of bilateral muscimol infusion into BLA (1nmol muscimol per side) on PPI are shown in Figure 2a. Under control (saline-infused) conditions, PPI increased as a function of increasing prepulse intensity (Linear Regression, R2=0.1051, P<0.01). This is consistent with prior reports (Wan & Swerdlow, 1997). Muscimol (1nmol) infused bilaterally into the BLA resulted in a significant decrease in prepulse inhibition (P<0.005) when compared within subject to a saline-infused baseline. A two-way ANOVA with drug and prepulse intensity as within subject variables yielded a main effect of drug (F1,22=18.927, P<0.001), a main effect of prepulse intensity (F2,44=4.97, P<0.05) but no drug by prepulse intensity interaction (F2,44=1.44, P=0.247). Bonferroni post-hoc tests (1-tailed) showed a significant difference between saline and muscimol infusion at each of the tested prepulse intensities (P<0.05). The effects of bilateral muscimol infusion in BLA on baseline acoustic startle response (ASR), measured as the average response on pulse-alone trials, are shown in Figure 2b. There was a significant reduction in baseline ASR under muscimol-infused conditions (paired t-test, p<0.05). Categorical (median split) and correlational analyses confirmed that the startle suppressing effects of bilateral muscimol infusion into BLA could not account for the observed changes in PPI.
Figure 2.
Bilateral infusion of muscimol in BLA impairs PPI. (A) Prepulse inhibition as a function of prepulse intensity after infusion of saline (light grey) or muscimol (dark grey) bilaterally in BLA. (B) ASR after infusion of saline (light grey) or muscimol (dark grey) bilaterally in BLA. * = significantly different than saline-infused control, p<0.05.
Finally, Supplemental Figure 1 shows the response to muscimol infusion on PPI and ASR in the 9 animals with cannulae falling outside of BLA. In these animals, muscimol infusion did not impair PPI (F1,7=0.396, P=0.055) or ASR (P=0.42, paired t-test), confirming the site specificity of our infusions.
Unilateral infusion of muscimol into BLA impairs PPI
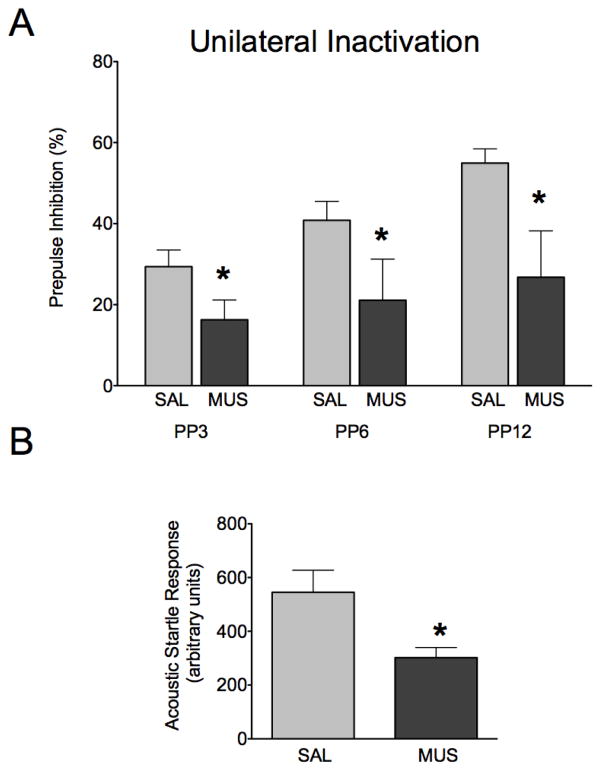
To determine whether bilateral infusion of muscimol in BLA was required for the impairment in PPI, or conversely if unilateral infusion of muscimol into BLA would be sufficient to impair PPI, we next examined the effect of unilateral inactivation (Figure 3). The pattern of response under control conditions was similar to that in Figure 2. Under control (saline-infused) conditions, animals displayed increasing PPI as prepulse magnitude increased (Linear Regression, R2=0.180, P<0.0001). Muscimol (1nmol) infused unilaterally into the BLA resulted in a significant decrease in prepulse inhibition (P<0.01) when compared within subject to a saline-infused baseline. A two-way ANOVA showed a main effect of drug treatment (F1,29=7.709, P<0.01), a main effect of prepulse intensity (F2,58=7.24, P<0.005), but no interaction between drug treatment and prepulse intensity (F2,60=1.436, P=0.247). Bonferroni post-hoc tests revealed a significant decrease in PPI following muscimol infusion at each of the tested prepulse intensities (Figure 3a, P<0.05). Baseline ASR was significantly attenuated following muscimol infusion into BLA (Figure 3b, t-test, P<0.005). Categorical (median split) and correlational analyses confirmed that the startle suppressing effects of bilateral muscimol infusion into BLA could not account for the observed changes in PPI.
Figure 3.
Unilateral infusion of muscimol in BLA impairs PPI. (A) Prepulse inhibition as a function of prepulse intensity after infusion of saline (light grey) or muscimol (dark grey) unilaterally in BLA. (B) ASR after infusion of saline (light grey) or muscimol (dark grey) unilaterally in BLA. * = significantly different than saline-infused control, p<0.05.
Infusion of muscimol in VP prevents BLA-induced deficits in PPI
To determine if concurrent infusion of muscimol in VP would prevent deficits in PPI induced by muscimol infusion into BLA, we next unilaterally infused the BLA in the presence and absence of ipsilateral infusion of the VP. Of the 18 animals implanted with cannulae in both the BLA and VP, 13 had correct cannula placement and completed all experiments; the remaining 5 animals had cannulae falling outside of BLA and/or cannulae that became clogged/dislodged preventing completion of all the necessary infusions.
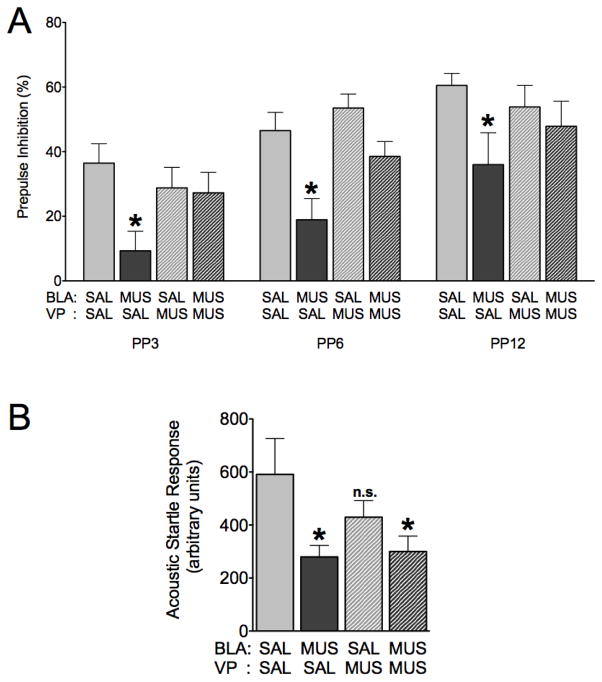
Under control conditions (saline infused into both the BLA and ventral pallidum) rats displayed increased PPI as a function of increased prepulse intensity (Linear Regression, R2=0.227, P<0.005). Similarly to the results shown in Figure 2, unilateral infusion of the BLA with muscimol (muscimol in BLA, saline in VP) reduced prepulse inhibition as compared to control conditions (Figure 4a, P<0.01). In contrast, infusion of VP (saline in BLA, muscimol in VP) had no effect on PPI; the lack of effect of muscimol in VP under baseline conditions is consistent with previous reports (Swerdlow, Braff, & Geyer, 1990). When VP was treated with muscimol concurrent with muscimol infusion into BLA, animals displayed PPI equivalent to control conditions, representing a significant blockade of BLA-evoked PPI deficits (P<0.05). Taken together, these behavioral data support our hypothesis that BLA inactivation results in disinhibition of VP.
Figure 4.
VP infusion of muscimol blocks BLA-evoked PPI deficits. (A) Prepulse inhibition as a function of prepulse intensity after infusion of saline into both BLA and VP (light grey), muscimol into BLA and saline into VP (dark grey), saline into BLA and muscimol into VP (light grey, hatched), or muscimol into both BLA and VP (dark grey, hatched). (B) ASR after infusions as described in (A). * = significantly different than saline-infused control, p<0.05.
ANOVA with BLA treatment, VP treatment and prepulse intensity as within-subject factors revealed a significant VP treatment by BLA treatment interaction (F1,12=8.181, P<0.05). Post-hoc comparisons (Bonferonni corrected) showed a significant reduction in PPI following muscimol infusion in amygdala as compared to all control (Figure 4a, P<0.05), muscimol in VP alone (P<0.05), and the VP+BLA muscimol conditions (P<0.05). No other interactions were significant.
Baseline ASR (Figure 4b) was significantly attenuated differently among treatment conditions, as revealed by a repeated measures ANOVA (F3,36=4.913, P<0.05). Startle amplitude was significantly reduced following muscimol infusion into BLA, regardless of pretreatment of VP (Bonferroni Post-hoc test, P<0.05). Categorical (median split) and correlational analyses confirmed that the startle suppressing effects of bilateral muscimol infusion into BLA could not account for the observed changes in PPI.
DISCUSSION
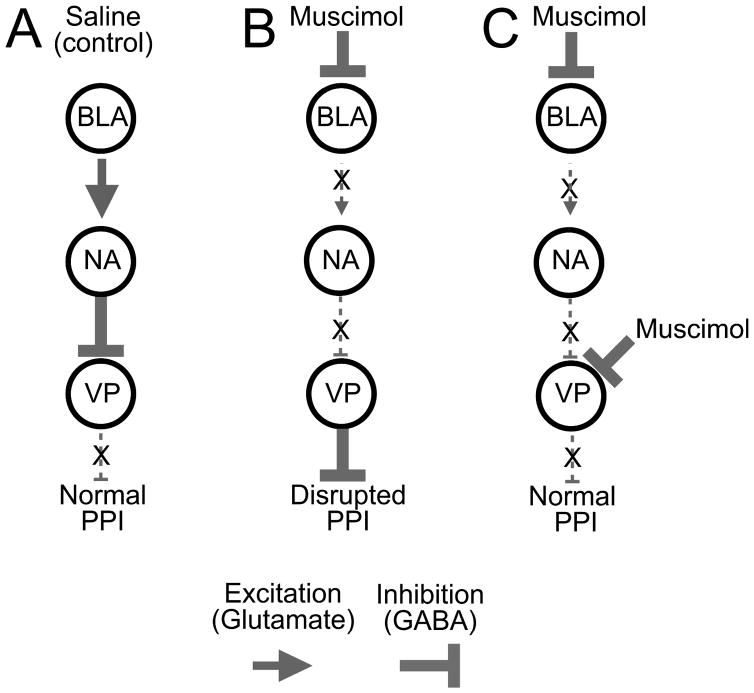
The goal of the present study was to determine the effects of transient inhibition of BLA on prepulse inhibition and to determine the degree to which deficits in PPI evoked from BLA could be normalized through inhibition of a downstream target of BLA, the VP. We have demonstrated that both bilateral and unilateral inhibition of BLA disrupted PPI. Furthermore, we found that BLA-evoked PPI deficits were blocked by pretreatment of the ipsilateral VP with muscimol. We suggest that a key downstream target of BLA in the regulation of PPI is VP (see Figure 5 for a schematic).
Figure 5.
Model of BLA-VP interactions in PPI. (A) Under baseline (or saline-infused) conditions, BLA excites NAcc which inhibits VP, decreasing VP output, allowing for normal PPI. (B) When muscimol is infused in BLA, excitatory drive to NAcc is reduced. This decreases inhibitory NAcc outflow to VP, disinhibiting VP, increasing VP output and disrupting PPI. (C) When muscimol is infused in BLA and VP, despite the decreased input to VP from NAcc, VP remains inhibited, and PPI can occur normally.
Our finding that inactivation of BLA with muscimol disrupted PPI is consistent with previous reports of a role for BLA in sensorimotor gating: both radiofrequency and excitotoxic lesions of the BLA disrupt PPI (Decker et al., 1995; Wan & Swerdlow, 1997). Furthermore, transient pharmacological manipulation of BLA function has also been tested for PPI-disruptive effects: blockade of NMDA-receptor mediated synaptic transmission, (Fendt et al., 2000; Wan & Swerdlow, 1997), blockade of D2 mediated neurotransmission (raclopride (Stevenson & Gratton, 2004)), or blockade of adrenergric transmission in BLA disrupt PPI (Alsene et al., 2011). Our finding that misplaced cannulae (Supplemental Figure 1) did not show impaired PPI increases our confidence that this effect is mediated by BLA. Our results with muscimol are particularly interesting in light of the finding that disinhibition of BLA via the GABAA channel blocker, picrotoxin (Fendt et al., 2000) also disrupts PPI. BLA is a critical integrative center of the limbic system, which interacts with many other neural substrates involved in PPI (e.g. hippocampus, nucleus accumbens, thalamus, striatum). Abnormal information processing in amygdala may therefore have the capacity to modify activity throughout much of the forebrain PPI network. We suggest that an optimal (physiological) level of activity in BLA is necessary for normal information processing, and that any manipulation that impairs BLA function is likely to disrupt PPI.
Our finding that unilateral inhibition of BLA was sufficient to disrupt PPI is consistent with a disinhibitory effect of BLA inactivation on PPI circuitry (i.e., because BLA outputs are excitatory a disinhibitory effect must be indirect - at minimum, disynaptic - to yield disinhibition). In many cases, unilateral chemical (via GABA antagonists or glutamate agonists) or electrical stimulation of a structure is sufficient to alter behavior, e.g., startle (Li et al., 1998; Silva et al., 2005), locomotor behavior (Kitamura et al., 2001; Olpe et al., 1977; Périer et al., 2002), social behavior (Malkova et al., 2003; Wellman, 2005). In contrast, in the case of inactivation, bilateral inhibition is typically required (Wellman, 2005). From a circuit-level analysis, this can be thought of as an abnormal “gain of function” effect.
Our functional data suggest that VP is likely the target functionally disinhibited following BLA inactivation. However, previous anatomical studies have failed to identify a direct connection between BLA and VP. Rather, the connection between BLA and VP is indirect, primarily through nucleus accumbens (Haber, Groenewegen, Grove, & Nauta, 1985a; Papp et al., 2011; Russchen, Bakst, Amaral, & Price, 1985), as BLA provides excitatory (glutamatergic) input to NAcc projection neurons (Christie et al., 1987; Kelley et al., 1982; Robinson & Beart, 1988), which in turn provide a principle source of GABAergic input to the VP (Johnson, Aylward, Hussain, & Totterdell, 1994).
This indirect connection between BLA and VP has been previously proposed in PPI “circuit diagrams” (cf, Swerdlow, Braff and Geyer, 2000). The proposal that NAcc mediates BLA-VP interactions is based on detailed anatomical and functional studies in several species. Russchen and Price failed to detect labeling in VP after injections of the anterograde tracer PHA-L into the basolateral amygdala of rats, while NAcc was strongly labeled (Russchen & Price, 1984). Similarly, injection of the retrograde tracers WGA-HRP or tritiated D-Aspartate into the VP did not label neurons in basolateral or lateral amygdala, while robustly labeling nucleus accumbens (Fuller, Russchen, & Price, 1987); furthermore, injections of WGA-HRP or tritiated D-Aspartate into NAcc strongly labled basolateral and lateral amygdala (Fuller et al., 1987). When Kelley, Domesick and Nauta injected radiolabeled amino acids into the rat amygdala, they found minimal labeling in VP, while there was robust labeling in NAcc. It is worth noting that the ventral caudate and putamen (dorsal to BLA) was also injected in cases where labeling was described in VP, raising the possibility that the sparsely labeled fibers in this instance may have resulted from striato-pallidal rather than amygdalo-pallidal projections (Kelley et al., 1982). Finally, this pathway has also been investigated in the monkey; Russchen, Amaral and Price demonstrated that tritiated amino acid injections confined to the basolateral amygdala failed to label the ventral pallidum, while still robustly labeling nucleus basalis of Meynert (Russchen, Amaral, & Price, 1985). The anatomical evidence, coupled with our functional data strongly suggests that the connection between BLA and VP is indirect.
This BLA-NAcc-VP circuit has also been demonstrated using in vivo electrophysiological recordings (Yim & Mogenson, 1983). Electrical stimulation (excitation) of the amygdala results in inhibition of neurons in the VP; this effect was strongly attenuated by inhibition of NAcc prior to amygdala stimulation (Yim & Mogenson, 1983). Further support for this link is derived from in vivo microdialysis, amperometric, and single-unit studies that show that stimulation of the amygdala results in increased glutamate mediated dopamine release in NAcc (Floresco, Blaha, Yang, & Phillips, 2001a, 2001b; Floresco, Yang, Phillips, & Blaha, 1998). While the present study is not directly concerned with dopamine in nucleus accumbens, the evidence that stimulation of BLA leads to increased glutamate activity in NAcc offers further evidence for the functional circuit we propose.
However, activation of NAcc disrupts PPI making it unfeasible to assess the direct interaction between NAcc and upstream brain areas like BLA (Pothuizen, Jongen-Rêlo, & Feldon, 2005; Wan & Swerdlow, 1996; Wan, Geyer, & Swerdlow, 1995). Our proposed model (Fig 5) is also consistent with the PPI-disruptive effect of NAcc lesions and pharmacological manipulations. Infusion of muscimol (Pothuizen et al., 2005), dopamine (Swerdlow, Braff, Masten, & Geyer, 1990), the D2-recepot agonist quinpirole (Wan & Swerdlow, 1993), 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA) (Wan et al., 1995), or the AMPA receptor antagonist CNQX (Wan & Swerdlow, 1996) into NAcc all disrupt PPI, as do 6-OHDA (Wan et al., 1995) or excitotoxic lesions (Kodsi & Swerdlow, 1994).
Functionally, our present findings are also incompatible with a direct monosynaptic projection from BLA to VP. BLA output neurons are excitatory, thus, if a monosynaptic direct projection existed, inactivation of BLA would result in decreased excitation of VP. Decreasing VP activity is without effect on PPI under baseline conditions (e.g., our data with muscimol in VP and saline in BLA, prior studies with VP infusion of muscimol, and VP lesions (Kretschmer & Koch, 1998; Swerdlow, Braff, & Geyer, 1990)). Therefore, decreases in VP activity cannot explain the effects of BLA inactivation on PPI. Our findings that increasing inhibition in VP via infusion of the GABA agonist muscimol normalized the PPI deficits evoked from BLA is also incompatible with a monosynaptic BLA-VP projection, as it would simply add further inhibition to that caused by the decrease in excitation from BLA. Thus, we suggest that the most parsimonious explanation of the blockade of BLA-evoked PPI deficits by VP inhibition is through a serial circuit mediated by NAcc. This suggestion is not the only possible option, as a parallel circuit could be imagined with BLA activity exerting an excitatory influence on a PPI regulatory center (e.g., pedunculopontine tegmental nucleus) and VP activity exerting an inhibitory influence on the same center, however, when constrained by the known anatomical connections (Steininger, Rye, & Wainer, 1992), a serial circuit seems more likely. Regardless of the serial or parallel nature of the circuit involved, our findings still demonstrate that BLA and VP both modulate PPI.
Several systemic drug challenges that impair prepulse inhibition are reversed by inhibition or lesions of VP including administration of the hallucinogenic serotonin receptor agonist DOI (2,5-dimethoxy-4-iodoamphetamine) or the dopamine receptor agonist apomorphine (Kretschmer & Koch, 1998; Sipes & Geyer, 1997). Furthermore, VP inhibition normalizes prepulse inhibition deficits evoked from NAcc (Kodsi & Swerdlow, 1994), and as we have shown in the present study, BLA. Thus, VP inhibition may serve as a common location from which PPI deficits induced by manipulation of limbic circuitry may be normalized.
In the present study, we selected a central location in VP for our drug infusions. This conferred the advantage of maximizing the region of VP inactivated. Previous reports have demonstrated that (Kodsi & Swerdlow, 1995) the disruptive effects of picrotoxin in the VP were strongest in the medial subregions and weakest in the lateral subregions. This regional difference may be in part due to differences in projections. The medial VP projects heavily to the pedunculopontine tegmentum, while the lateral VP projects to the SNpr and mediodorsal thalamus (Haber, Groenewegen, Grove, & Nauta, 1985b; Haber, Lynd, Klein, & Groenewegen, 1990). In our present study, it is likely we inactivated both medial and lateral VP based on our injection volume and cannula placement.
We found that inhibition of BLA not only disrupted PPI, but also attenuated baseline ASR. The reduction in ASR following BLA inactivation may represent a decrease in aversive value of the startling stimulus, a computation performed at least in part by BLA (Ebert & Koch, 1997). We found that these effects are likely independent, as in no case did the suppression of ASR correlate with the suppression of PPI. Furthermore, in the case of both the bilateral BLA inactivation experiments and the BLA-VP interaction experiments, a median split analysis showed no interaction between suppression grouping and PPI intensity or drug treatment. In the case of unilateral BLA inactivation, we found a significant interaction only between suppression grouping and drug treatment, but no correlation between suppression of PPI and ASR. We found that the disruption in ASR was not normalized by VP inhibition, suggesting that the two findings (PPI and ASR) may be mediated by different neural substrates. In fact, BLA sends projections to the central nucleus of the amygdala, which in turn projects to nucleus reticularis pontis caudalis and the pedunculopontine tegmental nucleus, key components of the brainstem startle network (Hitchcock & Davis, 1991; Lingenhöhl & Friauf, 1994; Rosen, Hitchcock, Sananes, Miserendino, & Davis, 1991). Our finding that VP inactivation did not normalize the reduction in ASR is consistent with the lack of normalization of ASR by intra-VP muscimol infusions in animals with lesions to NAcc (Kodsi & Swerdlow, 1994). The finding that transient inhibition of BLA reduced ASR contrasts with the finding that quinolinic acid lesions of BLA do not reduce ASR (Wan & Swerdlow, 1997), but is consistent with the decrease in startle amplitude seen after intra-BLA infusion of the NMDA receptor antagonist AP-5 (Wan & Swerdlow, 1997). These findings are also consistent with the well-established role for BLA in fear-potentiated startle (Campeau & Davis, 1995; Kim, Campeau, Falls, & Davis, 1993; Sananes & Davis, 1992), and startle reactivity more generally (Frankland, Josselyn, Bradwejn, Vaccarino, & Yeomans, 1997). Together with our data, these findings suggest that there may be compensatory changes following lesions that mask the BLA-mediated effect on baseline ASR seen following transient pharmacological manipulation.
In the present study, we examined PPI in Long Evans rats; Long-Evans rats have previously been used for studies of PPI, including those investigating the role of amygdala (Howland et al., 2007; Stevenson & Gratton, 2004) and ventral pallidum (Qu et al., 2009). The majority of studies of prepulse inhibition circuitry have, however, been conducted in Sprague-Dawley rats, including most of the lesion and pharmacological inactivation studies from the Swerdlow group referenced throughout this manuscript. Sprague-Dawley and Long Evans rats have differences in sensitivity to the PPI-disruptive effects of dopaminergic agents (Breier, Lewis, Shoemaker, Light, & Swerdlow, 2010; Qu et al., 2009; Shilling, Saint Marie, Shoemaker, & Swerdlow, 2008; Swerdlow, Breier, & Saint Marie, 2011; Swerdlow et al., 2004; Weber & Swerdlow, 2008; Weber, Chang, Breier, Ko, & Swerdlow, 2008), however, in the present study we did not investigate the role of dopamine. We share the opinion expressed by Swerdlow, Geyer and Braff, that, “…it will be much more difficult to study systematically the potential strain and substrain differences in the PPI-disruptive effects of a multitude of different neural circuit manipulations, from lesions to intracerebral drug infusions. Without such information, however, the generalizability of PPI “circuit maps” across substrains or strain, let alone species, must remain in doubt” (Swerdlow et al., 2001). Our present study, while not comparing strains directly, may be of use in cross-strain validation of circuit maps, as it is consistent with the role of BLA and VP previously suggested from data generated in Sprague-Dawley rats.
Our present findings demonstrate that transient inactivation of BLA (either unilateral or bilateral) disrupts PPI, and that the disruption is likely mediated by disinhibition of VP. These findings expand our current knowledge of the circuitry controlling prepulse inhibition, tying previously established anatomical and electrophysiological data to behavioral function.
Supplementary Material
Acknowledgments
Funding: Epilepsy Foundation Fellowship EFA123098 (PAF), F31NS066822 (PAF), F31DA026705 (EAW), T32DA007291, T32NS041231, the Georgetown University Undergraduate Research Opportunities Program (AM) and institutional funds from the Department of Pharmacology (LM).
ABBREVIATIONS
- PPI
prepulse inhibition
- ASR
acoustic startle response
- BLA
basolateral nucleus of the amygdale
- VP
ventral pallidum
- NAcc
nucleus accumbens
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsene KM, Rajbhandari AK, Ramaker MJ, Bakshi VP. Discrete forebrain neuronal networks supporting noradrenergic regulation of sensorimotor gating. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36(5):1003–1014. doi: 10.1038/npp.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Sandner G, Di Scala G. Effects of unilateral microinjections of GABAergic drugs into the inferior colliculus on auditory evoked potentials and on audiogenic seizure susceptibility. Experimental Neurology. 1989;104(1):82–87. doi: 10.1016/0014-4886(89)90012-5. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Tricklebank M, Neijt HC, Lehmann-Masten V, Geyer MA. Disruption of prepulse inhibition and increases in locomotor activity by competitive N-methyl-D-aspartate receptor antagonists in rats. The Journal of Pharmacology and Experimental Therapeutics. 1999;288(2):643–652. [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Annals of the New York Academy of Sciences. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- Blair HT, Sotres-Bayon F, Moita MAP, Ledoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133(2):561–569. doi: 10.1016/j.neuroscience.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophrenia Research. 2001;49(1–2):171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Stone C, Callaway E, Geyer MA, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Breier MR, Lewis B, Shoemaker J, Light G, Swerdlow NR. Sensory and sensorimotor gating-disruptive effects of apomorphine in Sprague Dawley and Long Evans rats. Behavioural Brain Research. 2010;208(2):560–565. doi: 10.1016/j.bbr.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15(3 Pt 2):2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biological Psychiatry. 1996;39(1):33–41. doi: 10.1016/0006-3223(95)00101–8. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience. 1987;22(2):425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Pfaff DW. Autoradiographic tracing of nucleus accumbens efferents in the rat. Brain Research. 1976;113(3):589–596. doi: 10.1016/0006-8993(76)90060-3. [DOI] [PubMed] [Google Scholar]
- Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiology of Learning and Memory. 1995;64(2):156–168. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- Ebert U, Koch M. Acoustic startle-evoked potentials in the rat amygdala: effect of kindling. Physiology & Behavior. 1997;62(3):557–562. doi: 10.1016/s0031-9384(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Exner C, Boucsein K, Degner D, Irle E, Weniger G. Impaired emotional learning and reduced amygdala size in schizophrenia: a 3-month follow-up. Schizophrenia Research. 2004;71(2–3):493–503. doi: 10.1016/j.schres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schwienbacher I, Koch M. Amygdaloid N-methyl-D-aspartate and gamma-aminobutyric acid(A) receptors regulate sensorimotor gating in a dopamine-dependent way in rats. Neuroscience. 2000;98(1):55–60. doi: 10.1016/s0306-4522(00)00086-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001a;21(16):6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001b;21(8):2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. The European Journal of Neuroscience. 1998;10(4):1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Bradwejn J, Vaccarino FJ, Yeomans JS. Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1997;17(5):1838–1847. doi: 10.1523/JNEUROSCI.17-05-01838.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. The Journal of Comparative Neurology. 1987;258(3):317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biological Psychiatry. 1993;34(6):361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44(10):1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Research. 1996;64(3):169–178. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. The Journal of Comparative Neurology. 1985a;235(3):322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. The Journal of Comparative Neurology. 1985b;235(3):322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. The Journal of Comparative Neurology. 1990;293(2):282–298. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behavioral Neuroscience. 1991;105(6):826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Howland JG, Hannesson DK, Barnes SJ, Phillips AG. Kindling of basolateral amygdala but not ventral hippocampus or perirhinal cortex disrupts sensorimotor gating in rats. Behavioural Brain Research. 2007;177(1):30–36. doi: 10.1016/j.bbr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61(4):851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Kamath A, Al-Khairi I, Bhardwaj S, Srivastava LK. Enhanced alpha1 adrenergic sensitivity in sensorimotor gating deficits in neonatal ventral hippocampus-lesioned rats. The International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2008;11(8):1085–1096. doi: 10.1017/S1461145708008845. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7(3):615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behavioral and Neural Biology. 1993;59(1):5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Ikeda H, Koshikawa N, Cools AR. GABA(A) agents injected into the ventral pallidum differentially affect dopaminergic pivoting and cholinergic circling elicited from the shell of the nucleus accumbens. Neuroscience. 2001;104(1):117–127. doi: 10.1016/s0306-4522(01)00053-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Ebert U. Deficient sensorimotor gating following seizures in amygdala-kindled rats. Biological Psychiatry. 1998;44(4):290–297. doi: 10.1016/s0006-3223(97)00397-1. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behavioural Brain Research. 1997;89(1–2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Quinolinic acid lesions of the ventral striatum reduce sensorimotor gating of acoustic startle in rats. Brain Research. 1994;643(1–2):59–65. doi: 10.1016/0006-8993(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Ventral pallidal GABA-A receptors regulate prepulse inhibition of acoustic startle. Brain Research. 1995;684(1):26–35. doi: 10.1016/0006-8993(95)00372-w. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD, Koch M. The ventral pallidum mediates disruption of prepulse inhibition of the acoustic startle response induced by dopamine agonists, but not by NMDA antagonists. Brain Research. 1998;798(1–2):204–210. doi: 10.1016/s0006-8993(98)00424-7. [DOI] [PubMed] [Google Scholar]
- Li L, Priebe RP, Yeomans JS. Prepulse inhibition of acoustic or trigeminal startle of rats by unilateral electrical stimulation of the inferior colliculus. Behavioral Neuroscience. 1998;112(5):1187–1198. doi: 10.1037//0735-7044.112.5.1187. [DOI] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1994;14(3 Pt 1):1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio R, Gale K. Seizures evoked from area tempestas are subject to control by GABA and glutamate receptors in substantia nigra. Exp Neurol. 1989;105(2):184–8. doi: 10.1016/0014-4886(89)90118-0. [DOI] [PubMed] [Google Scholar]
- Malkova L, Barrow K, Lower L, Gale K. Deacreased social interactions in monkeys after unilateral blockade of GABAA receptors in the basolateral amygdala. In: Shinnick-Gallagher P, Pitkanen A, Shekhar A, Cahill L, editors. The Amygdaal in Brain Function: Basic and Clinical Approaches. Vol. 985. Annals of the New York Academy of Sciences; 2003. pp. 540–1. [Google Scholar]
- Mansbach RS, Geyer MA. Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine. Psychopharmacology. 1991;105(2):162–168. doi: 10.1007/BF02244303. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. Journal of Neuroscience Methods. 1999;86(2):145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Schellenberg H, Koella WP. Rotational behavior induced in rats by intranigral application of GABA-related drugs and GABA antagonists. European Journal of Pharmacology. 1977;45(3):291–294. doi: 10.1016/0014-2999(77)90012-7. [DOI] [PubMed] [Google Scholar]
- Papp E, Borhegyi Z, Tomioka R, Rockland KS, Mody I, Freund TF. Glutamatergic input from specific sources influences the nucleus accumbens-ventral pallidum information flow. Brain Structure & Function. 2011 doi: 10.1007/s00429-011-0331-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. London, UK: Academic Press; 2007. [Google Scholar]
- Périer C, Tremblay L, Féger J, Hirsch EC. Behavioral consequences of bicuculline injection in the subthalamic nucleus and the zona incerta in rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(19):8711–8719. doi: 10.1523/JNEUROSCI.22-19-08711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry William, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biological Psychiatry. 2007;61(4):482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pothuizen HHJ, Jongen-Rêlo AL, Feldon J. The effects of temporary inactivation of the core and the shell subregions of the nucleus accumbens on prepulse inhibition of the acoustic startle reflex and activity in rats. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005;30(4):683–696. doi: 10.1038/sj.npp.1300643. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biological Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Qu Y, Saint Marie RL, Breier MR, Ko D, Stouffer D, Parsons LH, Swerdlow NR. Neural basis for a heritable phenotype: differences in the effects of apomorphine on startle gating and ventral pallidal GABA efflux in male Sprague-Dawley and Long-Evans rats. Psychopharmacology. 2009;207(2):271–280. doi: 10.1007/s00213-009-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam R, DeQuardo JR, Miedler J, Arndt S, Kirbat R, Brunberg JA, Tandon R. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Research. 2001;108(2):79–87. doi: 10.1016/s0925-4927(01)00120-2. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Peeters BW. Effects of acoustic prepulses on the startle reflex in rats: a parametric analysis. Brain Research. 1994;667(1):144–150. doi: 10.1016/0006-8993(94)91727-2. [DOI] [PubMed] [Google Scholar]
- Robinson TG, Beart PM. Excitant amino acid projections from rat amygdala and thalamus to nucleus accumbens. Brain Research Bulletin. 1988;20(4):467–471. doi: 10.1016/0361-9230(88)90136-0. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, Aoki S, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Research. 2009;174(3):210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJ, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behavioral Neuroscience. 1991;105(6):817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Price JL. Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neuroscience Letters. 1984;47(1):15–22. doi: 10.1016/0304-3940(84)90379-3. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Amaral DG, Price JL. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. The Journal of Comparative Neurology. 1985;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Research. 1985;329(1–2):241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sananes CB, Davis M. N-methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behavioral Neuroscience. 1992;106(1):72–80. doi: 10.1037//0735-7044.106.1.72. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Saint Marie RL, Shoemaker JM, Swerdlow NR. Strain differences in the gating-disruptive effects of apomorphine: relationship to gene expression in nucleus accumbens signaling pathways. Biological Psychiatry. 2008;63(8):748–758. doi: 10.1016/j.biopsych.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RCB, Sandner G, Brandão ML. Unilateral electrical stimulation of the inferior colliculus of rats modifies the prepulse modulation of the startle response (PPI): effects of ketamine and diazepam. Behavioural Brain Research. 2005;160(2):323–330. doi: 10.1016/j.bbr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disrupts prepulse inhibition of startle in rats via 5-HT2A receptors in the ventral pallidum. Brain Research. 1997;761(1):97–104. doi: 10.1016/s0006-8993(97)00316-8. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. The Journal of Comparative Neurology. 1992;321(4):515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Gratton A. Role of basolateral amygdala dopamine in modulating prepulse inhibition and latent inhibition in the rat. Psychopharmacology. 2004;176(2):139–145. doi: 10.1007/s00213-004-1879-6. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PloS One. 2011;6(10):e25322. doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biological Psychiatry. 1993;33(4):298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. GABAergic projection from nucleus accumbens to ventral pallidum mediates dopamine-induced sensorimotor gating deficits of acoustic startle in rats. Brain Research. 1990;532(1–2):146–150. doi: 10.1016/0006-8993(90)91754-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behavioural Pharmacology. 2000;11(3–4):185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Masten VL, Geyer MA. Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology. 1990;101(3):414–420. doi: 10.1007/BF02244063. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Breier MR, Saint Marie RL. Probing the molecular basis for an inherited sensitivity to the startle-gating disruptive effects of apomorphine in rats. Psychopharmacology. 2011;216(3):401–410. doi: 10.1007/s00213-011-2228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156(2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58(2):192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacology, Biochemistry, and Behavior. 2004;77(2):291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. Intra-accumbens infusion of quinpirole impairs sensorimotor gating of acoustic startle in rats. Psychopharmacology. 1993;113(1):103–109. doi: 10.1007/BF02244341. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Research. 1996;722(1–2):168–176. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. The basolateral amygdala regulates sensorimotor gating of acoustic startle in the rat. Neuroscience. 1997;76(3):715–724. doi: 10.1016/s0306-4522(96)00218-7. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Geyer MA, Swerdlow NR. Presynaptic dopamine-glutamate interactions in the nucleus accumbens regulate sensorimotor gating. Psychopharmacology. 1995;120(4):433–441. doi: 10.1007/BF02245815. [DOI] [PubMed] [Google Scholar]
- Weber M, Swerdlow NR. Rat strain differences in startle gating-disruptive effects of apomorphine occur with both acoustic and visual prepulses. Pharmacology, Biochemistry, and Behavior. 2008;88(3):306–311. doi: 10.1016/j.pbb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Chang W-li, Breier MR, Ko D, Swerdlow NR. Heritable strain differences in sensitivity to the startle gating-disruptive effects of D2 but not D3 receptor stimulation. Behavioural Pharmacology. 2008;19(8):786–795. doi: 10.1097/FBP.0b013e32831c3b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman L. Doctoral Dissertation. Georgetown University; Washington, DC: 2005. The role of the amygdala in primate socioemotional behavior. [Google Scholar]
- Woon FL, Hedges DW. Amygdala volume in adults with posttraumatic stress disorder: a meta-analysis. The Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21(1):5–12. doi: 10.1176/appi.neuropsych.21.1.5. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. The American Journal of Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of ventral pallidal neurons to amygdala stimulation and its modulation by dopamine projections to nucleus accumbens. Journal of Neurophysiology. 1983;50(1):148–161. doi: 10.1152/jn.1983.50.1.148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.