Abstract
Administration of intravenous iron to supplement erythropoiesis stimulating agents (ESAs) has become a common practice in the management of anemia in patients with end-stage renal disease. Randomized clinical trials of anemia correction in this population have shown more adverse outcomes in CKD and ESRD patients assigned to the higher hemoglobin targets. Retrospective analysis of these trials suggests that morbidity is higher in subjects who fail to achieve the designated hemoglobin target and are typically exposed to higher doses of ESAs and iron than those that easily achieve the intended targets. Intravenous iron administration circumvents the natural biologic mechanisms for handling and utilization of iron. There is in vitro and in vivo evidence that intravenous iron preparations can cause oxidative stress, endothelial dysfunction, inflammation, impaired immunity, and renal injury. Since iron overload is known to promote endothelial dysfunction, cardiovascular disease, and immune dysfunction which are the leading causes of premature mortality in CKD and ESRD patients, it is imperative to exercise caution with the use of IV iron preparations in this population. The present review is intended to provide a brief overview of the potential adverse effects of the overzealous use of these agents.
Keywords: Anemia, Iron, Hemodialysis, End-stage renal disease, Chronic kidney disease
Introduction
Anemia is highly prevalent in chronic kidney disease (CKD), and its treatment is a key component of CKD management. Response to erythropoiesis-stimulating agents (ESAs) is largely dependent on adequacy of iron stores. In fact, intravenous (IV) administration of iron usually increases hemoglobin (Hgb) and lowers ESAs dose requirements. There is mounting evidence that pharmacological interventions aimed at increasing Hgb levels toward normal values can result in increased morbidity and mortality in patients with CKD. Consequently, the optimal Hgb target for anemia treatment in CKD has become controversial. The potential role of high doses of ESAs in the adverse outcomes from randomized clinical trials (RCT) of anemia correction in CKD and end-stage renal disease (ESRD) patients is reviewed elsewhere [1, 2]. The purpose of this article is to provide a brief overview of the potential adverse effects related to excessive use of IV iron preparations in this population.
Anemia correction in chronic kidney disease
The prevalence of anemia increases with each stage of CKD and is almost universal in ESRD. Erythropoietin deficiency and frequently coexistent iron deficiency are among the major causes of the CKD-associated anemia. In addition to the usual risk factors for iron deficiency anemia as seen in the general population, impaired intestinal iron absorption, blood loss during hemodialysis (HD), and enhanced incorporation of iron stores into Hgb by ESAs contribute to iron deficiency in the CKD and ESRD patients. Observational studies in CKD patients have consistently shown correlations between severity of anemia and the risk of cardiovascular (CV) morbidity and mortality [3–10]. Based on the assumption that anemia may contribute to adverse CV outcomes, in 2006 the National Kidney Foundation (NKF) panel of the Dialysis Outcomes Quality Initiative (K/DOQI) expanded the target Hgb level from 11–12 to 11–13 g/dl for ESRD patients [11]. However, evidence has accumulated from large RCT showing no benefit or trends toward increased morbidity and mortality in CKD and ESRD patients assigned to normal or near-normal Hgb [12–14]. The potential implications were that anemia correction may have caused the adverse outcomes seen in groups assigned to higher Hgb targets. This supposition prompted a change in K/DOQI guidelines to reduce the target Hgb back to 11–12 g/dl [15]. On June 24, 2011, FDA released new warnings and prescription information/guidelines for use of ESAs in patients with CKD/ESRD which may prompt changes in future anemia treatment protocols.
Oxidative stress and inflammation play an important part in the pathogenesis of anemia by promoting ESA resistance, shortening erythrocyte life span, and limiting iron availability. CKD is invariably associated with oxidative stress and inflammation [16]. The effect of CKD is compounded by comorbid conditions, such as diabetes, hypertension, autoimmune diseases, and infections, which themselves cause oxidative stress and inflammation and are commonly present in CKD patients [17, 18]. Thus, the burden of oxidative stress, inflammation, and the severity of treatment-resistant anemia differ among CKD patients with different comorbidities. Consequently, varying ESA and iron doses may have varying effects on this heterogeneous population. While many subjects assigned to lower Hgb targets readily achieved the intended target, subjects assigned to higher Hgb levels consistently did not (21% in Correction of Hemoglobin and Outcomes in Renal Insufficiency [CHOIR] and 38% in Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta [CREATE]) [13, 14]. In fact, heightened morbidity and mortality were found primarily in patients whose Hgb failed to reach the designated target. In contrast, patients whose Hgb reached the target had more favorable outcomes [12, 14, 19]. Additionally, observations from patients with CKD/ESRD who maintain normal Hgb without receiving ESAs do not support a theoretical risk of normal Hgb in CKD patients [20, 21]. Assuming similar distribution of patients with oxidative stress and inflammation in groups randomized to normal and low Hgb targets in RCT, the greater adverse outcomes seen in the former than in the latter suggest the possibility that the larger doses of IV iron and ESA used to achieve higher Hgb targets are potentially culpable. Specifically in the Besarab ESRD study, subjects assigned to normal-hematocrit had increased mortality (odds ratio 2.4, P < 0.001) if they received IV iron compared to those that did not receive IV iron. Survivors from the normal-hematocrit group received an average of 152 mg of iron dextran per 4-week period during the 6 months prior to censorship or death compared to 214 mg per 4-week period in those who died (P < 0.001). There was no difference in the amount of IV iron received between survivors and subjects who died in the low-hematocrit group (119 mg vs. 145 mg, P = 0.36) [12]. In another RCT of HD patients, the number of subjects receiving IV iron was significantly greater in the high versus low Hgb target group (37% vs. 25%, P = 0.001) [22]. The differences in the number of subjects receiving IV iron in the CHOIR study were not statistically significant (14.5% vs. 11.9%, P = 0.15) [23]. However, this study employed CKD patients who did not require dialysis and are less likely to need IV iron. The hypothesis that the trend for increased morbidity and mortality noted in patients randomized to higher Hgb targets in RCT being caused by excessive doses of ESAs as opposed to the higher Hgb and erythrocyte mass which could not be reached in most patients has previously been reviewed [1, 2]. Since IV iron is frequently used in the treatment of anemia in ESRD, its possible contribution to adverse outcomes is reviewed here.
Intravenous iron
Iron toxicity
Iron can donate and accept electrons and exist as either ferric (Fe3+) or ferrous (Fe2+) iron. Although, this property facilitates many biochemical and biological functions, catalytically active free iron can cause oxidative stress, tissue injury, and dysfunction which are normally prevented by tight regulation of intra-and extracellular iron. While most circulating iron is bound to transferrin as ferric iron, a minute quantity of non-transferrin-bound iron (NTBI) is weakly bound to negatively charged molecules. There is also an intra-cellular iron pool that is not bound to ferritin and is available for incorporation in iron-containing proteins or electron transfer reactions [24]. Through the Haber–Weiss reaction (Fe3+ + ·O2− → Fe2+ + O2) and Fenton reaction (Fe2+ + H2O2 → Fe3+ + ·OH+ OH−), poorly liganded iron produces hydroxyl radical which is a highly reactive free radical capable of causing oxidative injury [25]. In fact, the magnitude of iron overload in patients with hereditary hemochromatosis correlates with the extent of nucleic acid oxidative damage [26]. A detailed review of the other diseases believed to be mediated by iron-dependent oxidative stress has been presented elsewhere [27].
Metabolism of intravenous iron preparations
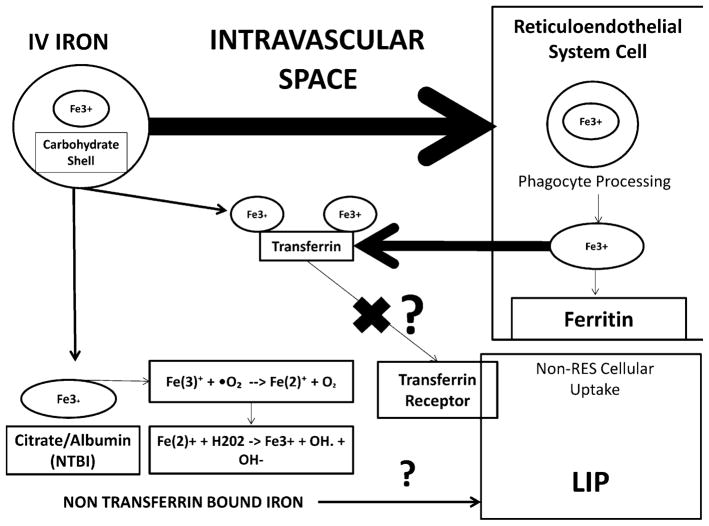
IV iron is used to manage anemia in CKD/ESRD when repletion fails with oral supplementation. IV iron is prepared as ferric iron with one of several carbohydrate complexes. Available preparations in the US include iron dextran, iron sucrose, and iron gluconate. The iron complexes are initially processed in the reticuloendothelial system (RES), where phagocytes liberate iron to be stored in ferritin or released back to the circulation as transferrin-bound iron for transport to various tissues (Fig. 1). Administration of supra-therapeutic doses of IV iron in animals has been shown to raise bone marrow and hepatic iron contents [28]. IV iron infusion with various formulations in humans results in an early increase in transferrin saturation, total serum iron, and NTBI levels followed by an increase in ferritin levels several days later [29, 30]. However, some iron can bypass the RES and directly bind to transferrin [31] with the potential for oversaturation and creation of a labile, reactive pool of NTBI (Fig. 1). In vitro studies estimate that up to 6% of IV iron bypasses RES processing [32].
Fig. 1.
Metabolism of intravenous iron preparations. IV iron complexes are taken up from the circulation by cells of the reticuloendothelial system (RES). Intracellular phagocytes process the complexes and deliver iron to intracellular ferritin stores or extracellular transferrin. IV iron infusions increase total and transferrin-bound iron, as well as ferritin and non-transferrin-bound iron. IV formulations can deliver a small amount of iron directly to transferrin without being processed in the RES. There is concern that oversaturation of transferrin results in increased extracellular iron bound to negatively charged compounds and is accessible for generation of free radicals via the Fenton/Haber–Weiss reactions. There is further concern that there is uptake of iron into non-RES cells that can (1) potentially generate intracellular free radicals and (2) limit the accessibility transferrin-bound iron to be taken up into cells. RES reticuloendothelial system, NTBI non-transferrin-bound iron, LIP labile iron pool
The concern for toxicity stems from the NTBI as a potential source of labile iron causing toxicity through iron-dependent redox reactions. Hepatoma cells (non-RES cells) exposed to IV iron preparations demonstrate increased uptake of NTBI [33]. This could either increase intracellular radical formation or increase extracellular NTBI available for redox reactions due to impaired uptake of transferrin-bound iron by iron-overloaded cells. A recently introduced IV iron formulation has undergone some investigation in this context. Ferumoxytol contains iron oxide coated with polyglucose sorbitol carboxymethylene. Compared to iron sucrose or iron gluconate, ferumoxytol has been shown to result in a lesser rise in free iron level in HD [34] and CKD patients [35] and cause less cytotoxicity in cultured cells and in experimental animals [36]. However, its effects on oxidative stress and endothelial dysfunction have yet to be evaluated in ESRD. Furthermore, ferumoxytol has been reported to cause rare episodes of hypersensitivity and hypotension [37].
Adverse effects of excessive use of IV iron preparations
Overall concerns
The leading causes of mortality in ESRD are CV disease and infection [38]. Endothelial injury and dysfunction, which are mediated by oxidative stress and inflammation, are critical steps in the pathogenesis of atherosclerosis and CV disease. This is followed by microbial infections that are due to impaired immune responses and constitute the second most common cause of death in the ESRD population. A brief overview of the possible contributions of excessive use of IV iron preparations to the pathogenesis of these adverse outcomes is provided below.
Oxidative stress
The pro-oxidant capacity of iron has been demonstrated along the spectrum of isolated cells to human studies in CKD and ESRD patients resulting in lipid, protein, and nucleic acid oxidation. Iron dextran, iron gluconate, and, to a lesser extent, iron sucrose increase malondialdehyde (MDA) levels in the renal cortical tissue [39]. In healthy subjects, IV iron acutely increases superoxide production [40], and in ESRD patients, intradialytic IV iron sucrose increases plasma MDA concentration [41, 42]. IV iron dextran given on non-HD days to ESRD patients was shown to increase serum concentration of esterified isoprostanes for several hours [43]. A randomized study in HD patients showed that IV iron administration can raise plasma oxidized DNA and advanced protein oxidation products [44, 45]. Such findings are not limited to ESRD as infusion of IV iron increases plasma MDA levels in CKD patients [46].
Endothelial dysfunction
Endothelial dysfunction is mechanistically related to oxidative stress and CKD patients exhibit both. Asymmetric dimethyl arginine (ADMA) is an endogenous inhibitor of endothelial nitric oxide synthase (eNOS), which is the major source of nitric oxide. ADMA level is increased and its elevated level is associated with increased CV events in ESRD patients [47, 48]. ADMA is degraded by dimethyl-arginine dimethylaminohydrolase (DDAH) whose expression is reduced by oxidative stress. Oxidative stress-induced reduction in DDAH activity is the primary cause of ADMA accumulation in CKD [49].
In vitro and in vivo studies have demonstrated the association of elevated iron levels with endothelial injury and dysfunction. For instance, IV iron preparations (particularly iron sucrose) inhibit proliferation and promote apoptosis in cultured endothelial cells [50]. Infusion of iron sucrose in healthy subjects is shown to increase NTBI and ROS production and cause acute impairment of endothelium-dependent flow-mediated vasodilation [40] (Table 1). However, intra-arterial injection of iron sucrose did not significantly change acetylcholine-induced increase in forearm blood flow in peritoneal dialysis patients [51]. A cross-sectional study in HD patients showed a direct association between carotid artery thickness and the cumulative dose of IV iron and plasma ferritin level. After adjusting for age and smoking status, dose of elemental iron remained a predictor of increased carotid artery media thickness [52]. These findings suggest a role of catalytically active iron in the pathogenesis of endothelial dysfunction which is the primary step in the pathogenesis of arteriosclerosis and CV disease. This is further supported by the observation that administration of the iron chelator deferoxamine improves endothelial function in patients with coronary artery disease [53].
Table 1.
Effects of intravenous iron on endothelial cell dysfunction in human studies
| Author | Study design/population | Primary analysis | Conclusions |
|---|---|---|---|
| Rooyakers (2002) [40] | Time series n = 20 healthy male subjects 100 mg IV iron saccharate + saline versus saline |
Between group difference in flow-mediated vasodilatation at 10 min and 4 h post-infusion | Greater impairment in flow-mediated vasodilatation at 10 min with IV iron (P < 0.01) No difference at 4 h |
| Schaller (2005) [51] | Randomized, placebo-controlled clinical trial n = 38 peritoneal dialysis patients 300 mg IV iron sucrose versus placebo |
Between group difference in forearm blood flow reactivity following infusion | No difference in forearm blood flow immediately following infusion |
| Garcia-Fernandez (2010) [42] | Randomized cross-over clinical trial n = 40 hemodialysis patients 50 mg IV iron sucrose 50 mg IV iron sucrose + NAC 100 mg IV iron sucrose 100 mg IV iron sucrose + NAC |
Between group differences in plasma von Willebrand factor and soluble intercellular adhesion molecule-1 at 120 and 300 min after the beginning of HD | No significant differences between groups at either time point (Hemodialysis procedure itself induced increase in both markers in all groups) |
NAC N-acetyl cysteine
Inflammation
IV iron preparations can promote inflammation via oxidative stress-dependent and oxidative stress-independent manners. Baseline elevations in both ferritin and C-reactive protein have been associated with greater increases in oxidative stress in response to IV iron infusion in HD patients, suggesting that presence of inflammation amplifies the oxidative response to iron administration [45]. However, the interpretation of the systemic effects of IV iron may be confounded by the fact that serum ferritin may reflect either iron storage status or the underlying inflammation as an acute phase reactant.
IV iron increases plasma and tissue levels of monocyte chemotactic protein-1 (MCP-1), a major pro-inflammatory mediator, in mice [54], and can cause significant but transient increases in plasma MCP-1 level in CKD patients. Repeated infusion results in a lower peak but a more persistent elevation [55]. It should be noted, however, that treatment of anemia with coadministration of IV iron with ESAs in iron-deficient ESRD patients can actually ameliorate inflammation and oxidative stress [56].
Immune dysfunction
Infection is the second leading cause of mortality in ESRD patients [38, 57]. Moreover, by promoting inflammation, infection can contribute to accelerated atherosclerosis and CV disease [58]. Presence of NTBI has been shown to result in increased iron uptake by lymphocytes and impaired lymphocyte proliferation [59]. Since lymphocytes are poorly equipped to sequester iron in ferritin, excess iron delivered from hydrophilic chelates can be toxic for these cells and may, in part, account for immune dysfunction in iron-overloaded patients. High doses of IV iron preparations can impair phagocytic activity and reduce hydrogen peroxide production capacity and microbial killing capability of polymorphonuclear leukocytes [60, 61]. Incubation of peripheral blood leukocytes in media containing therapeutic concentrations of IV iron induces time-dependent increases in intracellular ROS and diminished CD4+ lymphocyte survival [62]. The adverse effects of excess iron on lymphocytes are most likely mediated by intracellular oxidative stress which can lead to lysosomal and mitochondrial membrane destabilization and apoptosis [63].
Renal function
As yet dialysis independent CKD patients are occasionally treated with IV iron when oral iron fails to restore iron stores. This raises the concern that IV iron may accelerate progression of renal disease by intensifying oxidative stress. In this context, incubation of isolated mouse and human tubular epithelial cells in media with different IV iron preparations has been shown to induce marked lipid peroxidation and cell death. Among the studied compounds iron sucrose induced the greatest cell injury and intracellular iron accumulation [39, 64]. Additionally, studies in CKD patients (Table 2) have shown marked increases in urinary excretion of lipid peroxidation products, N-acetyl-glucosaminidase, fragmented and carboxylated albumin, and MCP-1 following administration of IV iron compounds [46, 65–67]. However, differences existed depending on the type of IV iron used. Prospective studies with renal function as an outcome are required to fully address this.
Table 2.
Effects of intravenous iron on renal function in human studies
| Author | Study design/population | Primary analysis | Conclusions |
|---|---|---|---|
| Agarwal (2004) [46] | Randomized, open label, parallel group clinical trial n = 20 subjects with Stage III or IV CKD Iron sucrose 100 mg × 1, NAC 600 mg po bid × 1 week, then iron sucrose 100 mg × 1 versus iron sucrose 100 mg × 1, no intervention × 1 week, then iron sucrose 100 mg × 1 |
Change in urinary NAG and protein excretion following iron infusion | Peak urinary NAG excretion at 30 min post-infusion (P < 0.0001 compared to other collection points) Peak urinary protein excretion at 30 min post-infusion (P < 0.0001 vs other collection points, but similar to 15 and 60 min post-infusion) No effect from NAC and no difference between visits |
| Leehey (2005) [67] | Randomized cross-over study n = 8 CKD patients (GFR < 60) with anemia Sodium ferric gluconate 125 mg + NAC 125 mg + placebo 250 mg + NAC 250 mg + placebo |
Pre/post-treatment change in MDA, albuminuria, enzymuria, or proteinuria | Plasma MDA increased in all groups (no difference between groups) No increase in albuminuria or proteinuria (no difference between groups) Non-significant increase in NAG following 250 mg dose (no difference between groups) Pre-treatment with NAC had no effect on levels |
| Agarwal (2007) [67] | Randomized crossover study n = 12 subjects with stage III or IV CKD 100 mg IV iron sucrose then 100 mg IV ferric gluconate (7 days later) versus 100 mg IV ferric gluconate then 100 mg IV iron sucrose (7 days later) |
Between group difference in pre/post-infusion urine protein/creatinine ratio | Greater increase in urine protein/creatinine after iron sucrose (P = 0.002) Greater increase when iron sucrose administered first (P < 0.01) |
NAC N-acetyl cysteine, GFR glomerular filtration rate, MDA malondialdehyde, NAG N-acetyl-β-D-glucosaminidase
Conclusions
Managing anemia in CKD/ESRD patients remains an important but controversial aspect of clinical care. Clinical trials have shown increased morbidity and mortality in patients assigned to the higher Hgb targets. Although, higher doses of ESAs have been considered as a potential cause of the adverse outcomes, the contribution of IV iron has not been fully addressed. While IV iron is highly effective in repleting iron stores, its excessive use in pursuit of high Hgb targets can be hazardous. As noted in this review, by promoting oxidative stress catalytically active iron can promote inflammation, cardiovascular disease, immune dysfunction, and progression of renal disease. It should be noted that the adverse effects of iron overload, oxidative stress, and inflammation are chronic and insidious in nature; clinical trials of IV iron preparations have been of relatively short-term durations. Therefore, long-term RCT are required to explore the effects of IV iron on outcomes in CKD and ESRD populations. In addition, the recent release of FDA new warnings for the use of ESAs may potentially have an unintended consequence of higher use of IV iron. Thus, it is imperative to aware the potential adverse effects of iron overdose and to excise caution with the use of IV iron preparations.
Acknowledgments
Funding sources include NIH F32DK 085965-O1A1 (PVB), UT Southwestern O’Brien Kidney Research Core (NIH P30DK079328), and UT Southwestern Clinical Translational Science Award (NIH UL 1RR024982).
Contributor Information
Peter Van Buren, Departments of Internal Medicine/Nephrology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Ruben L. Velez, Dallas Nephrology Associates, Dallas, TX, USA
Nosratola D. Vaziri, Email: ndvaziri@uci.edu, Division of Nephrology and Hypertension, University of California Irvine, Irvine, CA, USA. Division of Nephrology and Hypertension, University of California Irvine Medical Center, Suite 400, City Tower, 101 City Drive, Orange, CA 92868, USA
Xin J. Zhou, Email: joseph.zhou@utsouthwestern.edu, Departments of Internal Medicine/Nephrology, University of Texas Southwestern Medical Center, Dallas, TX, USA. Department of Pathology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9073, USA
References
- 1.Vaziri N. Anemia and anemia correction: surrogate markers or causes of mortality in chronic kidney disease. Nat Clin Pract Nephrol. 2008;8:436–445. doi: 10.1038/ncpneph0847. [DOI] [PubMed] [Google Scholar]
- 2.Vaziri N, Zhou X. Potential mechanisms of adverse outcomes in trials of anemia correction with erythropoietin in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1082–1088. doi: 10.1093/ndt/gfn601. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Ebben J, Xia H, Collins A. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10:610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 4.Collins A, Li S, Peter W, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36–39% J Am Soc Nephrol. 2001;12:2465–2473. doi: 10.1681/ASN.V12112465. [DOI] [PubMed] [Google Scholar]
- 5.Robinson B, Joffe M, Berns J, Pisoni R, Port F, Feldman H. Anemia and mortality in hemodialysis patients: accounting for morbidity and treatment variables updated over time. Kidney Int. 2005;68:2323–2330. doi: 10.1111/j.1523-1755.2005.00693.x. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Pisoni R, Combe C, et al. Anemia in haemodialysis patients of five European countries: association with morbidity and mortality in the dialysis outcomes and practice patterns study (DOPPS) Nephrol Dial Transplant. 2004;19:121–132. doi: 10.1093/ndt/gfg458. [DOI] [PubMed] [Google Scholar]
- 7.Xia H, Ebben J, Ma J, Collins A. Hematocrit levels and hospitalization risks in hemodialysis patients. J Am Soc Nephrol. 1999;10:1309–1316. doi: 10.1681/ASN.V1061309. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Collins A. Association of hematocrit value with cardiovascular morbidity and mortality in incident hemodialysis patients. Kidney Int. 2004;65:626–633. doi: 10.1111/j.1523-1755.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 9.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus J. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63:1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe R, Hulbert-Shearon T, Ashby V, Mahadevan S, Port F. Improvements in dialysis patient mortality are associated with improvements in urea reduction ration and hematocrit, 1999–2002. Am J Kidney Dis. 2002;45:127–135. doi: 10.1053/j.ajkd.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 11.NKF-K/DOQI . Clinical practice guidelines for anemia of chronic kidney disease. Am J Kidney Dis. 2006;47(Suppl 4):S1. [Google Scholar]
- 12.Besarab A, Bolton W, Browne J, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 13.Drueke T, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. New Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 14.Singh A, Szczech L, Tang K, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 15.NKF-K/DOQI . Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:474. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Spittle M, Hoenich N, Handelman G, Adhikarla R, Homel P, Levin N. Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis. 2001;38:1408–1413. doi: 10.1053/ajkd.2001.29280. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 18.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 19.Szczech L, Barnhart H, Inrig J, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo C, Lee C, Chuang C, Su Y, Chen J. Recombinant human erythropoietin independence in chronic hemodialysis patients: clinical features, iron homeostasis and erythropoiesis. Clin Nephrol. 2005;63:92–97. doi: 10.5414/cnp63092. [DOI] [PubMed] [Google Scholar]
- 21.Goodkin DF, Fuller DS, Robinson B, et al. Naturally occurring higher hemoglobin concentration does not increase mortality among hemodialysis patients. J Am Soc Nephrol. 2011;22:358–365. doi: 10.1681/ASN.2010020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parfrey P, Foley R, Wittreich B, et al. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 23.Goodkin D. The normal hematocrit cardiac trial revisited. Semin Dial. 2009;22:495–502. doi: 10.1111/j.1525-139X.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977;50:433–439. [PubMed] [Google Scholar]
- 25.Burkitt M, Mason R. Direct evidence for in vivo hydroxyl-radical generation in experimental iron overload: An ESR spin-trapping investigation. Proc Natl Acad Sci USA. 1991;88:8440–8444. doi: 10.1073/pnas.88.19.8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broedbaek K, Poulsen H, Weimann A, et al. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radic Biol Med. 2009;47:1230–1233. doi: 10.1016/j.freeradbiomed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Kell D. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beshara S, Lundquist H, Sundin J, et al. Kinetic analysis of 52 Fe-labelled iron (III) hydroxide-sucrose complex following bolus administration using positron emission tomography. Br J Hematol. 1999;104:288–295. doi: 10.1046/j.1365-2141.1999.01170.x. [DOI] [PubMed] [Google Scholar]
- 29.Zanen A, Adriaansen H, van Bommel E, Posthuma R, de Jong G. Oversaturation of transferrin after intravenous ferric gluconate (ferrlecit) in haemodialysis patients. Nephrol Dial Transplant. 1996;11:820–824. doi: 10.1093/oxfordjournals.ndt.a027405. [DOI] [PubMed] [Google Scholar]
- 30.Kooistra M, Kersting S, Lu G, et al. Nontransferrin-bound iron in the plasma of the haemodialysis patients after intravenous iron saccharate infusion. Eur J Clin Investig. 2002;32(Suppl 1):36–41. doi: 10.1046/j.1365-2362.2002.0320s1036.x. [DOI] [PubMed] [Google Scholar]
- 31.Henderson P, Hillman R. Characteristics of iron dextran utilization in man. Blood. 1969;34:357–375. [PubMed] [Google Scholar]
- 32.Van Wyck D, Anderson J, Johnson K. Labile iron in parenteral iron formulations: a quantitative and comparative study. Nephrol Dial Transplant. 2004;19:561–565. doi: 10.1093/ndt/gfg579. [DOI] [PubMed] [Google Scholar]
- 33.Scheiber-Mojdehkar B, Sturm B, Plank L, Kryzer I, Goldenberg H. Influence of parenteral iron preparations on non-transferrin bound iron uptake, the iron regulatory protein and the expression of ferritin and the divalent metal transporter DMT-1 in HepG2 human hepatoma cells. Biochem Pharmacol. 2003;65:1973–1978. doi: 10.1016/s0006-2952(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 34.Provenzano R, Schiller B, Rao M, et al. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinowitz B, Kausz A, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson A, Becker K, Zager R. Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. Am J Renal Phys. 2010;299:F426–F435. doi: 10.1152/ajprenal.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu M, Suh K, Lee H, Cohen M, Rieves D, Pazdur R. FDA review of ferumoxytol (feraheme) for the treatment of iron deficiency anemia in adults with chronic kidney disease. Am J Hematol. 2010;85:315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 38.US Renal Data System. USRDS 2010 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health; Bethesda: 2010. [Google Scholar]
- 39.Zager R, Johnson C, Hanson S, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40:90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 40.Rooyakers T, Stroes E, Kooistra M, et al. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Investig. 2002;32(Suppl 1):9–16. doi: 10.1046/j.1365-2362.2002.0320s1009.x. [DOI] [PubMed] [Google Scholar]
- 41.Roob J, Khoschsorur G, Tiran A, et al. Vitamin E attenuates oxidative stress induced by intravenous iron in patients on hemodialysis. J Am Soc Nephrol. 2000;11:539–549. doi: 10.1681/ASN.V113539. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Fernandez N, Echevarria A, Sanchez-Ibarrola A, Paramo J, Coma-Cannela I. Randomized clinical trial on acute effects of i.v. iron sucrose during hemodialysis. Nephrology. 2010;15:178–183. doi: 10.1111/j.1440-1797.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 43.Salahudeen A, Oliver B, Bower J, Roberts L. Increase in plasma esterified F2-isoprostanes following intravenous iron infusion in patients on hemodialysis. Kidney Int. 2001;60:1525–1531. doi: 10.1046/j.1523-1755.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuo K, Hung S, Wei Y, Tarng D. Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol. 2008;19:1817–1826. doi: 10.1681/ASN.2007101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tovbin D, Mazour D, Voroblov M, Chalmovitz C, Meyerstein N. Induction of protein oxidation by intravenous iron in hemodialysis patients: role of inflammation. Am J Kidney Dis. 2002;40:1005–1012. doi: 10.1053/ajkd.2002.36334. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal R, Vasavada N, Sachs N, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 47.Kielstein J, Boger R, Bode-Boger S. Asymmetric dimethyl arginine concentrations differ in patients with end stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol. 1999;10:594–600. doi: 10.1681/ASN.V103594. [DOI] [PubMed] [Google Scholar]
- 48.Zoccali C, Bode-Boger S, Mallamaci F, et al. Plasma concentrations of asymmetrical dimethyl arginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]
- 49.Matsuguma K, Ueda S, Yamagishi S, et al. Molecular mechanisms for elevation of asymmetric dimethyl arginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17:2176–2183. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]
- 50.Kartikasari A, Georgiou N, Visseren F, van Kats-Renaud H, van Sweder A, Marx J. Endothelial activation and induction of monocyte adhesion by nontransferrin-bound iron present in human sera. FASEB J. 2006;20:353–355. doi: 10.1096/fj.05-4700fje. [DOI] [PubMed] [Google Scholar]
- 51.Schaller G, Scheibert-Mohdehkar B, Wolzt M, et al. Intravenous iron increases labile serum iron but does not impair blood flow reactivity in dialysis patients. Kidney Int. 2005;68:2814–2822. doi: 10.1111/j.1523-1755.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 52.Reis K, Guz G, Ozdemir H, et al. Intravenous iron therapy as a possible risk factor for atherosclerosis in end stage renal disease. Int Heart J. 2005;46:255–264. doi: 10.1536/ihj.46.255. [DOI] [PubMed] [Google Scholar]
- 53.Duffy S, Biegelsen E, Holbrook M, et al. Iron chelation improves endothelial cell function in patients with coronary artery disease. Circulation. 2001;103:2799–2804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- 54.Zager R. Parenteral iron treatment induces MCP-1 accumulation in plasma, normal kidneys, and in experimental nephropathy. Kidney Int. 2005;68:1533–1542. doi: 10.1111/j.1523-1755.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R. Proinflammatory effects of iron sucrose in chronic kidney disease. Kidney Int. 2006;69:1259–1263. doi: 10.1038/sj.ki.5000164. [DOI] [PubMed] [Google Scholar]
- 56.Weiss G, Meusberger E, Radacher G, Garimorth K, Neyer U, Myaer G. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int. 2003;64:572–578. doi: 10.1046/j.1523-1755.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 57.Sarnak M, Jaber B. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 58.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djeha A, Brock J. Uptake and intracellular handling of iron from transferrin and iron chelates by mitogen stimulated mouse lymphocytes. Biochem Biopys Acta. 1992;1133:147–152. doi: 10.1016/0167-4889(92)90062-g. [DOI] [PubMed] [Google Scholar]
- 60.Deicher R, Ziai F, Cohen G, Mullner M, Horl W. High dose parenteral iron sucrose depresses neutrophil intracellular killing capacity. Kidney Int. 2003;64:728–736. doi: 10.1046/j.1523-1755.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 61.Guo D, Jaber B, Lee S, et al. Impact of iron dextran on polymorphonuclear cell function among hemodialysis patients. Clin Nephrol. 2002;58:134–142. doi: 10.5414/cnp58134. [DOI] [PubMed] [Google Scholar]
- 62.Gupta A, Zhuo J, Zha J, Reddy S, Olp J, Pai A. Effect of different intravenous iron preparations on lymphocyte intracellular reactive oxygen species generation and subpopulation survival. BMC Nephrol. 2010;17:11–16. doi: 10.1186/1471-2369-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenopoulou M, Doulias P, Barbouti A, Brunk U, Galaris D. Role of compartmentalized redox-active iron in hydrogen peroxide-induced DNA damage and apoptosis. Biochem J. 2005;387:703–710. doi: 10.1042/BJ20041650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zager R, Johnson A, Hanson S. Parenteral iron nephrotoxicity: potential mechanisms and consequences. Kidney Int. 2004;66:144–156. doi: 10.1111/j.1523-1755.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal R. On the nature of proteinuria with acute renal injury in patients with chronic kidney disease. Am J Physiol Renal Physiol. 2005;288:F265–F271. doi: 10.1152/ajprenal.00318.2004. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal R, Rizkala A, Kaskas M, Minasian R, Trout J. Iron sucrose causes greater proteinuria than ferric gluconate in non-dialysis chronic kidney disease. Kidney Int. 2007;72:638–642. doi: 10.1038/sj.ki.5002422. [DOI] [PubMed] [Google Scholar]
- 67.Leehey D, Palubiak D, Chebrolu S, Agarwal R. Sodium ferric gluconate causes oxidative stress but not acute renal injury in patients with chronic kidney disease: a pilot study. Nephrol Dial Transplant. 2005;20:135–140. doi: 10.1093/ndt/gfh565. [DOI] [PubMed] [Google Scholar]