Abstract
Objectives To determine which travellers with malaria are at greatest risk of dying, highlighting factors which can be used to target health messages to travellers.
Design Observational study based on 20 years of UK national data.
Setting National register of malaria cases.
Participants 25 054 patients notified with Plasmodium falciparum malaria, of whom 184 died, between 1987 and 2006.
Main outcome measures Comparison between those with falciparum malaria who died and non-fatal cases, including age, reason for travel, country of birth, time of year diagnosed, malaria prophylaxis used.
Results Mortality increased steadily with age, with a case fatality of 25/548 (4.6%) in people aged >65 years, adjusted odds ratio 10.68 (95% confidence interval 6.4 to 17.8), P<0.001 compared with 18–35 year olds. There were no deaths in the ≤5 year age group. Case fatality was 3.0% (81/2740 cases) in tourists compared with 0.32% (26/8077) in travellers visiting friends and relatives (adjusted odds ratio 8.2 (5.1 to 13.3), P<0.001). Those born in African countries with endemic malaria had a case fatality of 0.4% (36/8937) compared with 2.4% (142/5849) in others (adjusted odds ratio 4.6 (3.1 to 9.9), P<0.001). Case fatality was particularly high from the Gambia. There was an inverse correlation in mortality between region of presentation and number of cases seen in the region (R2=0.72, P<0.001). Most delay in fatal cases was in seeking care.
Conclusions Most travellers acquiring malaria are of African heritage visiting friends and relatives. In contrast the risks of dying from malaria once acquired are highest in the elderly, tourists, and those presenting in areas in which malaria is seldom seen. Doctors often do not think of these as high risk groups for malaria; for this reason they are important groups to target in pre-travel advice.
Introduction
Malaria is a major global infection, causing around 250 million clinical cases a year.1 It causes around 800 000 deaths a year, almost all in people living in poverty with limited access to healthcare, but it also causes avoidable deaths every year from imported malaria in non-endemic countries, mainly in people who are otherwise well. Travel to countries where malaria is endemic is increasing, and most general practitioners and acute care physicians are involved either in pre-travel advice or in diagnosing and treating cases on their return. The number of cases of imported falciparum malaria has increased over the past four years in the United Kingdom,2 3 with 5774 reported cases in the period 2007–11. The UK, other European countries, and the United States also saw increases throughout the 1990s, which stabilised over the past decade.3 4 5 6 7 8 Case fatality has remained stable at around 1%, both in the UK and in other high income countries.9 10 11
Migrants from malaria endemic countries and their descendants are at particular risk of acquiring malaria and make up over half of the UK’s imported malaria cases.3 Risk factors for acquiring malaria may, however, differ from risk factors for dying from malaria. Previously described risk factors associated with death from imported malaria include older age, European origin, travel to east Africa, failure to take chemoprophylaxis, and delays in seeking medical care and in the diagnosis and treatment of suspected malaria.9 10 12 13 Preventing malaria associated deaths is the ultimate aim in malaria prevention strategies, as those diagnosed and treated promptly generally make a full recovery.
We reviewed in detail all deaths reported to be caused by malaria infection in the UK over a 20 year period and compared them with all reported cases of malaria over the same period. The UK has extensive links with malaria endemic countries and has one of the highest rates of imported malaria in the developed world.14 We hypothesised that the groups most likely to acquire malaria may be different from the groups who die from malaria. We aimed, firstly, to identify factors associated with mortality to find high risk groups who could be targeted in prevention efforts by general practitioners and public health campaigns and, secondly, to learn clinical lessons relevant to the early identification of malaria in non-endemic countries.
Methods
Malaria is a notifiable infectious disease in the UK, so clinicians have to report cases by law and all cases of malaria or suspected malaria are treated free by the National Health Service, including among non-residents, which reduces financial barriers to accessing treatment and to being recorded. The Malaria Reference Laboratory, the national reference laboratory and part of the UK Health Protection Agency, runs a passive case detection system; the data collection system has been described previously.3 When a case is notified (via statutory notification through local authorities, clinicians sending standardised malaria reports to the Malaria Reference Laboratory, or laboratories sending blood films for diagnostic verification), the responsible clinician is contacted and asked to complete a data collection form, which covers demographic and clinical data. All cases included are parasitologically confirmed and speciated by microscopy or histology (and sometimes polymerase chain reaction) either at the referring hospital or at the Malaria Reference Laboratory. In most cases the diagnosis is confirmed by the Malaria Reference Laboratory. This system has been shown to identify 66% of cases of falciparum malaria in the UK.15 Reporting methods did not change over the period of the study.
The notifying laboratory and clinician are requested to provide further information about patients: personal details (date of birth, sex, country of birth, country of usual residence), details of travel (date of arrival in UK, country or region visited, purpose of travel, duration of visit), prophylaxis taken, and details of illness (date of onset, date of treatment, and method of diagnosis). (The data collection sheet is available from the authors on request.) Where data are not clear they are followed up at the time by telephone with the referring clinician.
All malaria associated deaths in the UK are separately directly notified to the Malaria Reference Laboratory by the Office for National Statistics. Following this, death certificates and coroner’s reports are obtained where available. In the case of a death in hospital, clinical and demographic details are requested from the responsible clinician as above. Cases are parasitologically confirmed and speciated by the above methods or by autopsy.
Data analysis
Data on malaria cases over the 20 year study period were prospectively double-entered (by VS and MB) into the Malaria Reference Laboratory malaria database. The data were rechecked for cases (by AS) and deaths (AMC) to identify duplicate or uncertain data, and any variations manually checked. Data on cases were entered into dBase IV and subsequently cleaned and validated using Microsoft Access 10, and deaths were additionally entered in Microsoft Excel. Data were analysed using Stata 11 (Statacorp, College Station TX, USA).
Almost all deaths are from falciparum malaria, and our detailed analysis was therefore restricted to falciparum cases. Bivariate methods and multivariable logistic regression were conducted to examine potential risk factors, comparing deaths from falciparum malaria with the denominator of all imported falciparum malaria cases to identify risk factors for case fatality. Unadjusted odds ratios and odds ratios adjusted for these potentially confounding factors on univariate analysis are quoted. There were no time series data.
Potential risk factors for death from imported falciparum malaria were studied by univariate analysis. These were age divided into groups (≤5, >5–18, >18–35, >35–50, >50–65, and >65 years), sex, tourist versus traveller visiting friends and relatives, presentation by calendar month, reported use of effective malaria chemoprophylaxis versus reported use of no prophylaxis, birth in an African country with endemic malaria versus birth elsewhere, and country and region visited. Countries were classified as endemic or not endemic for malaria transmission according to the World Health Organization classification.1 The biologically likely potential confounding factors age, born in a malaria endemic country, reason for travel, and reported use of prophylaxis were predetermined to be included as potential confounding factors in all logistic models. Age results are presented in clinically relevant groups (such as young adults >18–35 years old, young children 0–5 years), but adjustment for confounding in all other multivariable models was performed using age as a continuous variable. Additionally factors found to be associated with mortality with a probability value P<0.05 in univariate analysis were introduced into the logistic regression model to adjust further for confounding. Where data were missing we used multiple imputation (STATA mi command) with five imputations. An additional analysis restricted to those cases with complete data was undertaken to confirm findings; this restricted analysis led to no change in interpreting these results (data not shown but available on request).
Cases were analysed by the UK government office region from where they were reported (North East, North West, Yorkshire and The Humber, East Midlands, West Midlands, East of England, London, South East, and South West), Northern Ireland, Scotland, Wales, and non-UK regions16 to determine whether mortality was associated with number of cases of malaria seen locally, and a regression line fitted for log transformed data comparing mortality rate against number of cases seen.
This study used routine data collected within the Health Protection Agency official remit and did not require separate ethical board approval. Data were made anonymous for analysis.
Results
Between 1987 and 2006, 191 malaria associated deaths were reported to the Malaria Reference Laboratory, from a total of 39 302 cases of confirmed malaria in which the outcome was known. Of the deaths, 184 were associated with P falciparum infection. There were 25 054 cases of P falciparum malaria, giving a case fatality of 0.73%. This rate did not change over the study period,3 and adding year to models made no appreciable difference so it was excluded. Seven deaths were associated with non-falciparum malaria, (four from P vivax, and one each from P ovale and P malariae) among 13 804 cases, giving a case fatality for non-falciparum malaria of 0.05%. Among the non-falciparum cases, two patients had severe pre-existing comorbidities, and one died of splenic rupture.
The remainder of our analysis was performed on the 25 054 cases of falciparum malaria where the outcome was known, which included 483 cases of mixed infection. Completeness of data are summarised in table 1; 15 717 had full data, with use of prophylaxis the most likely to be missing.
Table 1.
Completeness of data for notified cases of Plasmodium falciparum malaria in UK between 1987 and 2006
| Factor studied | No (%) of cases with complete data |
|---|---|
| All cases of P falciparum malaria (n=25 054) | |
| Age | 24 244 (97) |
| Sex | 23 653 (94) |
| Country of birth | 14 787 (59) |
| Country visited | 21 856 (87) |
| Purpose of travel | 17 252 (69) |
| Month reported | 25 054 (100) |
| UK county where case reported | 24 751 (99) |
| Use of malaria prophylaxis | 15 933 (64) |
| All deaths from P falciparum malaria (n=184) | |
| Time from arrival in UK to onset of symptoms | 135 (73) |
| Time from arrival in UK to death | 142 (77) |
| Time from onset of symptoms to diagnosis | 146 (79) |
| Time from diagnosis to starting specific treatment | 92 (50) |
| Time from diagnosis to death | 161 (88) |
| Location of death | 177 (96) |
| Receipt of specific treatment before death | 150 (82) |
| Percentage of red blood cells parasitised | 61 (33) |
Mortality from P falciparum malaria increased steadily with increasing age (fig 1 and table 2), with a case fatality of 4.6% (25/548) in those aged >65 years. The adjusted odds ratio of dying of malaria was 10.68 (95% confidence intervals 6.4 to 17.8, P<0.001) in those >65 years old compared with the >18–35 year old group. Mortality among infants and children was low, with no deaths in the ≤5 age group, and a case fatality of 0.33% (11/3347) in those aged >5–18 years.
Fig 1 Mortality from imported P falciparum malaria in UK between 1987 and 2006 by age
Table 2.
Risk of death from imported P falciparum malaria in UK between 1987 and 2006 by age
| Age group (years) | Odds ratio (95% CI) of death | ||||
|---|---|---|---|---|---|
| Crude | P value | Adjusted* | P value | ||
| 0–5 | 0 deaths | — | — | — | |
| >5–18 | 0.73 (0.4 to 1.4) | 0.36 | 0.81 (0.4 to 1.6) | 0.54 | |
| >18–35 | 1.0 (reference group) | — | 1.0 (reference group) | — | |
| >35–50 | 1.49 (0.97 to 2.3) | 0.07 | 1.63 (1.1 to 2.5) | 0.02 | |
| >50–65 | 4.6 (3.1 to 6.8) | <0.001 | 4.6 (3.1 to 6.9) | <0.001 | |
| >65 | 10.66 (6.5 to 17.6) | <0.001 | 10.68 (6.4 to 17.8) | <0.001 | |
*Adjusted for risk factors listed in table 3.
Table 3 summarises the risk of death from P falciparum malaria associated with different factors. Case fatality varied depending on purpose of visit to a country with endemic malaria, from 2.96% (81/2740) in tourists to 0.32% (26/8077) in travellers visiting friends and relatives (table 4). The adjusted odds ratio of death associated with tourism compared with visiting friends and relatives was 8.2 (95% confidence interval 5.1 to 13.3, P<0.001). This remained true even when restricting the analysis to individuals born in African countries with endemic malaria; among these individuals, case fatality was 1.6% (3/189) in those visiting for tourism compared with 0.4% (19/5308) in those visiting friends and relatives (P=0.009). Those born in an African country with endemic malaria had a case fatality of 0.4% (36/8937) compared with 2.4% (142/5849) in those born outside such countries (χ2 test, P<0.001; adjusted odds ratio of death 4.6 (3.1 to 9.9), P<0.001). There were marked regional and national differences, with case fatality of 0.6% (86/14 174) after visiting west Africa, 1.5% (60/4064) for east Africa, and 3.3% (12/363) for southern Africa, although these reduced after adjusting for potential confounding factors such as reason for travel. Case fatality was particularly high in individuals visiting the Gambia (3.9%, (28/726)) compared with any other west African country (0.4% (58/13,448), χ2 test, P<0.001; adjusted odds ratio of death 4.7 (2.7 to 8.1), P<0.001). Restricting the analysis to tourists increased this difference, with a case fatality of 6.0% (20/333) for cases from the Gambia compared with 1.4% (8/565) for tourists visiting the rest of west Africa (χ2, P<0.001).
Table 3.
Factors other than age associated with risk of death from imported P falciparum malaria in UK between 1987 and 2006
| Risk factor | No (%) of fatal cases | Odds ratio (95% CI) of death | ||||
|---|---|---|---|---|---|---|
| Crude | P value | Adjusted* | P value | |||
| Purpose of visit to country with endemic malaria: | ||||||
| Tourism | 81/2740 (2.96) | 9.4 (6.1 to 14.7) | <0.001 | 8.2 (5.1 to 13.3) | <0.001 | |
| Visiting friends and relatives | 26/8077 (0.32) | |||||
| UK region where disease presented: | ||||||
| Where least malaria is seen | 10/119 (8.40) | 29.3 (14.5 to 59.2) | <0.001 | 18.2 (8.6 to 38.3) | <0.001 | |
| Where most malaria is seen | 50/15 993 (0.31) | |||||
| Calendar month of presentation: | ||||||
| December | 49/1922 (2.55) | 4.5 (3.2 to 6.2) | <0.001 | 3.7 (2.6 to 5.2) | <0.001 | |
| All other months | 135/23 132 (0.58) | |||||
| Reported use of effective prophylaxis: | ||||||
| No | 99/10 222 (0.97) | 1.8 (0.7 to 4.3) | 0.220 | 2.0 (0.81 to 4.9) | 0.13 | |
| Yes | 5/903 (0.55) | |||||
| Birth in African country with endemic malaria: | ||||||
| No | 142/5849 (2.43) | 6.2 (4.3 to 8.9) | <0.001 | 4.6 (3.1 to 9.9) | <0.001 | |
| Yes | 36/8937 (0.40) | |||||
*Adjusted for age (continuous) and for other listed risk factors.
Table 4.
Purpose of visiting a country with endemic malaria among cases of imported P falciparum malaria in UK between 1987 and 2006 and associated case fatality
| Purpose of travel | No (%) of fatal cases |
|---|---|
| Tourist from UK visiting malaria endemic country | 81/2740 (2.96) |
| Visiting UK from malaria endemic country | 17/3841 (0.44) |
| Visiting friends and relatives in malaria endemic country | 26/8077 (0.32) |
| Other or unstated | 60/10 396 (0.58) |
| Total | 184/25 054 (0.73) |
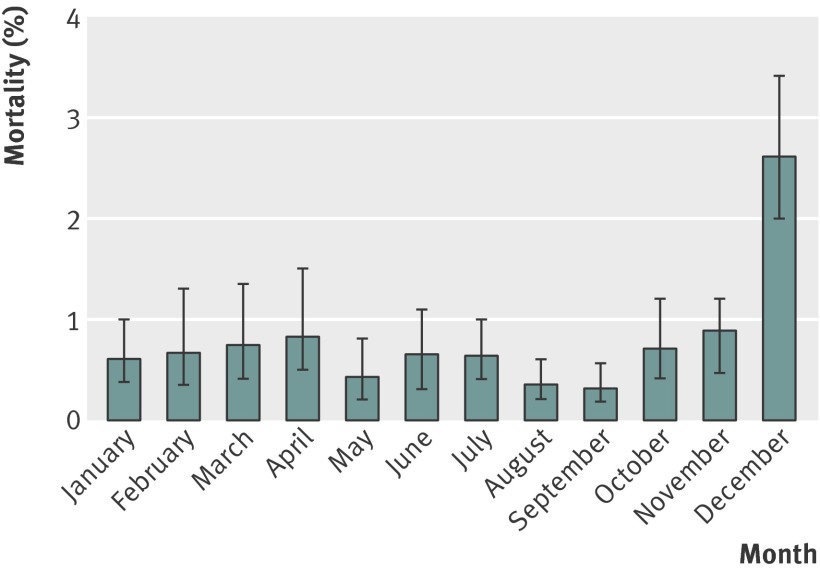
There was a striking seasonal peak in deaths from imported P falciparum malaria, with a case fatality of 2.6% (49/1922) in those notified in December compared with 0.6% across all other months (135/23 132) (χ2 test, P<0.001) (fig 2), meaning that over a quarter of deaths occurred in cases notified in December. The peak for number of imported cases is September.3 In the December cases 25.0% (11/44) of individuals died at home, compared with 17.4% (21/121) in other months (difference not significant). More tourists (9.3%) than those visiting friends and relatives (5.4%) were notified in December, although the greater mortality in December remained after correcting for purpose of visit as well as other factors (table 3).
Fig 2 Mortality from imported P falciparum malaria in UK between 1987 and 2006 by month of notification of illness
There was a clear trend in mortality based on the UK government office region where the patient presented, which was inversely correlated with number of cases seen (fig 3) (linear regression R2=0.72, P<0.001). The highest case fatality of 8.4% was in a region where only 119 patients were seen, with 10 deaths; the lowest case fatality (0.3%) was in the region where most cases were seen, with 15 993 patients seen and 50 deaths (adjusted odds ratio 18.2 (8.6 to 38.3), P<0.001). Restricting the analysis only to tourists, case fatality was 6/20 (30%) among cases presenting in the region where fewest cases were seen, compared with 7/979 (0.7%) in the region where most cases were seen (χ2 test, P<0.001).
Fig 3 Mortality from imported P falciparum malaria as a function of number of cases seen in a UK region between 1987 and 2006 (R2=0.67, P<0.001)
Data on reported chemoprophylaxis was inevitably limited and has to be treated with caution in fatal cases for reasons of reporting bias. Where the data were available, 35.8% (5711/15 933) individuals reported taking prophylaxis against malaria infection, and, among these, 5.7% (903/15 933) reported taking one of the three regimens effective in Africa (mefloquine, atovaquone-proguanil, or doxycycline). Of those taking either no prophylaxis or an ineffective regimen, 64.2% took no prophylaxis, 6.7% took chloroquine, 15.4% took chloroquine and proguanil, 4.1% proguanil, 0.7% pyrimethamine, and 3.5% another regimen. Among individuals not born in an African country with endemic malaria, case fatality was 0.6% (3/509) among those who reported taking effective prophylaxis, 1.4% (42/3046) among those who took any prophylaxis, and 3.5% (80/2304) among those who took no prophylaxis.
Median time intervals in fatal cases are summarised in fig 4. Among the fatal cases, 75.1% of deaths were in hospital, 18.1% at home, and 3.4% in an ambulance, and the median percentage of red blood cells parasitised was 8.5% (interquartile range 1–25%). These data were available only in the fatal cases.
Fig 4 Relevant time intervals in fatal cases of imported P falciparum malaria in the UK between 1987 and 2006
Discussion
This study shows that risk factors for dying from imported falciparum malaria in the UK are different from those for first acquiring the infection, and highlights groups of travellers who should be targeted for pre-travel health advice when they seek medical care. Those visiting friends and relatives in countries with endemic malaria make up the majority of cases of falciparum malaria in the UK, but the risks of this group dying from malaria are much smaller than for other travellers,3 with most deaths occurring in tourists. Older age is strongly associated with mortality, and, in contrast to cases in Africa, mortality in children is minimal. There were both seasonal and geographical risks in the UK for dying from malaria, with a clear peak in December, and consistently higher risks of dying for patients presenting in UK regions where malaria is less regularly seen and treated. Travellers to the Gambia, a popular destination for winter sun, were at particular risk. Most delays occurred before presentation to medical services. These findings are biologically plausible (see discussion below), compatible with other data, and have implications for targeting pre-travel health messages and treatment when travellers return.
The effect of previous exposure on mortality
Most cases of malaria in the UK are in individuals born in African countries with endemic malaria who are returning from visiting friends and relatives. Public health efforts have therefore concentrated on encouraging such people to take up chemoprophylaxis.3 This remains an important message and is backed up by European data showing that rates of chemoprophylaxis use are low and of malaria are high in this group.17 18 However, our study shows that, both in absolute numbers and in relative risk, tourists and those born outside African countries with endemic malaria are more likely to die once malaria is acquired. These data complement other studies from Europe and case series in the UK.4 12 19 20
There are several possible reasons for these findings, some biological and some behavioural. Those exposed to malaria in childhood develop immunity to severe malaria, with more protection the higher the degree of exposure; although they may still develop symptomatic infection, the risk of complications and death is greatly reduced.21 This protection is incomplete, and wanes with time, but studies on imported malaria and in areas where malaria occurs in occasional epidemics suggest that some degree of protection continues for a prolonged period.4 12 19 22 Other factors may also play a role; some genetic polymorphisms more prevalent in regions of malaria transmission are associated with protection from severe malaria.23 24
Immunity to malaria is however unlikely to be the full reason, as analyses restricted to those born in the UK (second or third generation migrants) or to tourists compared with those visiting friends and relatives when born in Africa still show significant differences. It is likely, although not possible to test with this study design, that differences in health seeking make up at least some of the difference. Those with an African heritage and staying with African families may be exposed to an understanding that malaria is common and dangerous, and may know the symptoms of early disease; malaria is, if anything, overdiagnosed as a cause of fever in medical practice in west and east Africa.25 26 27 Conversely most tourists to Africa (and even their healthcare providers) may be unaware that several attacks of falciparum malaria a year are typical in residents living in highly endemic areas (considerably more common than influenza in the United Kingdom), and that around 20% of fevers in those returning from Africa to the UK are due to malaria.27
The effect of age on mortality
Increasing age was strongly and independently associated with an increasing risk of death from malaria, and there was no evidence of the high case fatality seen in children in malaria endemic countries. These data are supported by previous reports of increased case fatality11 28 29 and higher levels of parasitaemia in elderly people.30 The mechanism is unclear, but it is unlikely that it is explained solely by increasing comorbidities in older age groups, as our study and others9 11 show a continuous increase in case fatality from childhood upwards.
Our findings in children are also in keeping with a smaller prospective study of children in the UK, which found a substantial number of severe cases but no deaths.31 A low case fatality in children has been documented in settings with low levels of endemic malaria, such as in the highlands of Kenya,32 KwaZulu-Natal,33 and Ceylon (Sri Lanka),34 and also in non-immune individuals migrating to an Indonesian island hyper-endemic for malaria transmission.35 These data are compatible with an explanation that the reason for high mortality from childhood malaria in Africa is limited access to health services.
The effect of region within Africa, season and number of cases seen in regions on mortality
The higher case fatality in visitors to east Africa compared with west Africa was accounted for by a greater proportion of tourists visiting east Africa. The exception in west Africa is the Gambia, which had a strikingly high case fatality. Visitors to this country typically have other risk factors, including being tourists, older, and born in the UK, but, even after adjustment for these factors, the odds ratio remained high. This previously reported high case fatality36 37 may reflect the predominance of Europeans travelling on package holidays, with low levels of use of chemoprophylaxis and little awareness of the risk of malaria on return.
Case fatality had a marked seasonal variation independent of peaks in the numbers of cases, which occurred in September and January (fig 2).3 Higher case fatality occurs over the winter in several conditions; peaks in all cause and cardiac mortality occurred on Christmas Day and New Year’s Day in a study of all deaths recorded in the United States between 1973 and 2001,38 and all cause mortality peaked on New Year’s Day in north east England.39 Delayed presentation to hospital, lower levels of hospital staffing over the holiday period, and delays in communication of the malaria diagnosis from laboratory to physician may be contributory factors. Other possibilities include initial misdiagnosis of a febrile illness as influenza-like illness, and the different demographics of travellers who travel in search of “winter sun.” The non-significant increase in the proportion of deaths occurring at home during this month may reflect delayed presentation to medical services during this time.
There was an inverse correlation between the number of cases seen per UK region and case fatality. This is partly explained by the younger age and higher proportion of African nationals presenting in London. However, after adjusting for confounding factors, we found the odds of dying from malaria remained much higher in those presenting in regions where fewer cases of malaria were seen, and the increase in case fatality with decreasing number of cases in fig 3 is striking. Less awareness of malaria among friends and neighbours may lead to travellers being less likely to seek advice from doctors before travel, or to recognise that malaria is a possibility if they experience a fever on return. Equally, in regions where travel to malarious areas is less common, general practices advising on prophylaxis and doctors seeing febrile returned travellers may be less aware of the risk of malaria, and this is an area where public health advice and medical training should be targeted.
Prophylaxis and delay to treatment
The use of effective prophylaxis was associated with lower risk of death from malaria in those not born in an African country with endemic malaria. There was no association between prophylaxis and case fatality in those born in African countries with endemic malaria. These data should, however, not be over-interpreted as a combination of reporting bias and incomplete data make it difficult to reach firm conclusions.
Delays in presentation, diagnosis, and administration of effective treatment are critical in contributing to deaths from malaria.7 10 40 The median delay from the development of symptoms to the diagnosis of malaria in our study is similar to the reported time intervals of 4–8.5 days in other studies.12 19 Reassuringly, our results suggest that treatment was usually initiated on the day of diagnosis, although we were unable to comment on individual regimens. These data also suggest that most patients who died presented late in the course of their illness, since the time interval between diagnosis and death was short. This suggestion is supported by the observation that 26% of individuals died either at home or in an ambulance. Reducing delays to presentation may be the single most important factor that could reduce mortality, although we are unable to test this from these data. There was no significant difference between time from onset of symptoms to diagnosis of malaria, from diagnosis to initiation of treatment, and from diagnosis to death, but numbers were small (data not shown). Data on delays were only available in fatal cases, so associations with mortality cannot be directly tested.
Potential limitations of the study
There are limitations associated with the use of national data. Under-reporting is inevitable in a passive case detection system, although capture-recapture data suggest that levels of reporting are high.15 The reporting of deaths known to be due to malaria is likely to be more complete than that of surviving cases, as deaths may also be reported via coroners as well as by clinical staff, although malaria may not have been suspected in all people who died at home without autopsy. Data on certain factors, such as those relating to delay in presentation, were available only for fatal cases, so their contribution to mortality cannot be directly assessed. Certain data, in particular the use of chemoprophylaxis, are likely to be associated with considerable recall bias in fatal cases. Lastly, although we adjusted for important potential confounding factors we identified, we cannot exclude the presence of residual confounding. It seems unlikely, however, that the clear associations of case fatality with age, reason for travel, being born in a non-endemic country, the calendar month, and the number of cases seen per region could be largely explained by residual confounding.
Implications for practice
These data describe two different groups of people—ethnic Africans (mainly of west African heritage), who travel mainly to west Africa to visit friends and relatives and bear the main burden of morbidity from falciparum malaria, and Europeans, who travel as tourists to east and southern Africa and to the Gambia in west Africa and have a higher case fatality. Additionally, case fatality was higher in those presenting in December, and in those presenting in UK regions where malaria is less often seen.
Our results suggest that certain groups may particularly benefit from interventions to reduce the mortality from imported malaria, in particular pre-travel advice to encourage prompt presentation to healthcare facilities on the development of a febrile illness as well as stressing the need for antimalaria measures and chemoprophylaxis. These groups include the older traveller, travellers born in countries not endemic for malaria, and those travelling on “winter sun” holidays, in particular to the Gambia. It should not be forgotten, however, that individuals born in African countries with endemic malaria still bear the brunt of morbidity from imported malaria cases, so the importance of pre-travel advice and education in this group remains. Doctors in the UK advising patients who seldom travel but take a holiday to Africa must make them aware that malaria is very common, potentially fatal, may start initially with mild symptoms and needs early diagnosis.41 Older visitors to malaria endemic countries, especially older tourists, are in particular need of targeting for pre-travel advice, both to take prophylaxis and to seek care early if unwell on return.
What is already known on this topic
Falciparum malaria is a common cause of fever in travellers returning to the UK from Africa
Individuals whose families originate from malaria endemic countries visiting friends and relatives are at greatest risk of importing malaria infection to the UK
Most deaths from malaria in endemic countries are in children, but deaths from malaria in children are rare in the UK
What this study adds
Holiday makers and tourists are at greatest risk of dying from malaria in the UK
Mortality from malaria increases steadily with age in the UK
Presentation in the month of December and to UK regions where malaria is rarely seen are associated with marked increase in mortality
Elderly travellers in particular need to be targeted for pre-travel advice
Contributors: The study was devised by AC, PLC, DB, CW. Data collection was by MB and VS. Data validation on deaths was by AC, and on cases by AS. Data analysis was by AC, AS and CW. All authors contributed to writing the paper. AC is the guarantor.
Funding: The Malaria Reference Laboratory is funded by the UK Health Protection Agency. AC has an Academic Clinical Lectureship. PLC is supported by the UCL Hospitals Comprehensive Biomedical Research Centre Infection Theme.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: PLC had support from the Malaria Reference Laboratory for the submitted work; PLC has received an honorarium from GlaxoSmithKline for a lecture on malaria in the previous 3 years. No other financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Data sharing: Data on cases imported by year available from the corresponding author.
Cite this as: BMJ 2012;344:e2116
References
- 1.World Health Organization. World Malaria Report 2010. 2010. www.who.int/malaria/publications/atoz/9789241564106/en/index.html.
- 2.HPA. Press releases, 2011. 2011. www.hpa.org.uk/NewsCentre/NationalPressReleases/2011PressReleases/110425Malaria/.
- 3.Smith AD, Bradley DJ, Smith V, Blaze M, Behrens RH, Chiodini PL, et al. Imported malaria and high risk groups: observational study using UK surveillance data 1987-2006. BMJ 2008;337:a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubos F, Dauriac A, El Mansouf L, Courouble C, Aurel M, Martinot A. Imported malaria in children: incidence and risk factors for severity. Diagn Microbiol Infect Dis 2010;66:169-74. [DOI] [PubMed] [Google Scholar]
- 5.Jensenius M, Ronning EJ, Blystad H, Bjorneklett A, Hellum KB, Bucher A, et al. Low frequency of complications in imported falciparum malaria: a review of 222 cases in south-eastern Norway. Scand J Infect Dis 1999;31:73-8. [DOI] [PubMed] [Google Scholar]
- 6.Millet JP, Garcia de Olalla P, Carrillo-Santisteve P, Gascon J, Trevino B, Munoz J, et al. Imported malaria in a cosmopolitan European city: a mirror image of the world epidemiological situation. Malar J 2008;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatinelli G, Ejov M, Joergensen P. Malaria in the WHO European Region (1971-1999). Euro Surveill 2001;6:61-5. [PubMed] [Google Scholar]
- 8.CISID. Home page. 2011. http://data.euro.who.int/cisid/?TabID=262487.
- 9.Legros F, Bouchaud O, Ancelle T, Arnaud A, Cojean S, Le Bras J, et al. Risk factors for imported fatal Plasmodium falciparum malaria, France, 1996-2003. Emerg Infect Dis 2007;13:883-8. [DOI] [PubMed] [Google Scholar]
- 10.Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among US travelers, 1963-2001. Ann Intern Med 2004;141:547-55. [DOI] [PubMed] [Google Scholar]
- 11.Bruneel F, Tubach F, Corne P, Megarbane B, Mira JP, Peytel E, et al. Severe imported falciparum malaria: a cohort study in 400 critically ill adults. PLoS One 2010;5:e13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christen D, Steffen R, Schlagenhauf P. Deaths caused by malaria in Switzerland 1988-2002. Am J Trop Med Hyg 2006;75:1188-94. [PubMed] [Google Scholar]
- 13.Jelinek T, Schulte C, Behrens R, Grobusch MP, Coulaud JP, Bisoffi Z, et al. Imported Falciparum malaria in Europe: sentinel surveillance data from the European network on surveillance of imported infectious diseases. Clin Infect Dis 2002;34:572-6. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. CASAD home page. 2011. http://data.euro.who.int/cisid/.
- 15.Cathcart SJ, Lawrence J, Grant A, Quinn D, Whitty CJ, Jones J, et al. Estimating unreported malaria cases in England: a capture-recapture study. Epidemiol Infect;138:1052-8. [DOI] [PubMed]
- 16.Office for National Statistics. Regions (former GORs). 2011. www.ons.gov.uk/ons/guide-method/geography/beginner-s-guide/administrative/england/government-office-regions/index.html.
- 17.Angell SY, Cetron MS. Health disparities among travelers visiting friends and relatives abroad. Ann Intern Med 2005;142:67-72. [DOI] [PubMed] [Google Scholar]
- 18.Mascarello M, Gobbi F, Angheben A, Concia E, Marocco S, Anselmi M, et al. Imported malaria in immigrants to Italy: a changing pattern observed in north eastern Italy. J Travel Med 2009;16:317-21. [DOI] [PubMed] [Google Scholar]
- 19.Bouchaud O, Cot M, Kony S, Durand R, Schiemann R, Ralaimazava P, et al. Do African immigrants living in France have long-term malarial immunity? Am J Trop Med Hyg 2005;72:21-5. [PubMed] [Google Scholar]
- 20.Bunn A, Escombe R, Armstrong M, Whitty CJ, Doherty JF. Falciparum malaria in malaria-naive travellers and African visitors. QJM 2004;97:645-9. [DOI] [PubMed] [Google Scholar]
- 21.Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect Dis 2010;10:51-9. [DOI] [PubMed] [Google Scholar]
- 22.Deloron P, Chougnet C. Is immunity to malaria really short-lived? Parasitol Today 1992;8:375-8. [DOI] [PubMed] [Google Scholar]
- 23.Modiano D, Luoni G, Sirima BS, Simpore J, Verra F, Konate A, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 2001;414:305-8. [DOI] [PubMed] [Google Scholar]
- 24.Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis 2005;192:178-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansah EK, Narh-Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, et al. Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ 2010;340:c930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler CI, Jones C, Boniface G, Juma K, Reyburn H, Whitty CJ. Guidelines and mindlines: why do clinical staff over-diagnose malaria in Tanzania? A qualitative study. Malar J 2008;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nic Fhogartaigh C, Hughes H, Armstrong M, Herbert S, McGregor A, Ustianowski A, et al. Falciparum malaria as a cause of fever in adult travellers returning to the United Kingdom: observational study of risk by geographical area. QJM 2008;101:649-56. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg AE, Lobel HO. Mortality from Plasmodium falciparum malaria in travelers from the United States, 1959 to 1987. Ann Intern Med 1990;113:326-7. [DOI] [PubMed] [Google Scholar]
- 29.Muhlberger N, Jelinek T, Behrens RH, Gjorup I, Coulaud JP, Clerinx J, et al. Age as a risk factor for severe manifestations and fatal outcome of falciparum malaria in European patients: observations from TropNetEurop and SIMPID Surveillance Data. Clin Infect Dis 2003;36:990-5. [DOI] [PubMed] [Google Scholar]
- 30.Gjorup IE, Ronn A. Malaria in elderly nonimmune travelers. J Travel Med 2002;9:91-3. [DOI] [PubMed] [Google Scholar]
- 31.Ladhani S, Garbash M, Whitty CJ, Chiodini PL, Aibara RJ, Riordan FA, et al. Prospective, national clinical and epidemiologic study on imported childhood malaria in the United Kingdom and the Republic of Ireland. Pediatr Infect Dis J 2010;29:434-8. [DOI] [PubMed] [Google Scholar]
- 32.Some ES. Effects and control of highland malaria epidemic in Uasin Gishu District, Kenya. East Afr Med J 1994;71:2-8. [PubMed] [Google Scholar]
- 33.Soni PN, Gouws E. Severe and complicated malaria in KwaZulu-Natal. S Afr Med J 1996;86:653-6. [PubMed] [Google Scholar]
- 34.Gill CA. Some points in the epidemiology of malaria arising out of the study of the malaria epidemic in Ceylon in 1934-35. Trans R Soc Trop Med Hyg 1936;29:429-80. [Google Scholar]
- 35.Baird JK, Masbar S, Basri H, Tirtokusumo S, Subianto B, Hoffman SL. Age-dependent susceptibility to severe disease with primary exposure to Plasmodium falciparum. J Infect Dis 1998;178:592-5. [DOI] [PubMed] [Google Scholar]
- 36.Jelinek T, Schade Larsen C, Siikamaki H, Myrvang B, Chiodini P, Gascon J, et al. European cluster of imported falciparum malaria from Gambia. Euro Surveill 2008;13:ii. [PubMed] [Google Scholar]
- 37.Moore DA, Grant AD, Armstrong M, Stumpfle R, Behrens RH. Risk factors for malaria in UK travellers. Trans R Soc Trop Med Hyg 2004;98:55-63. [DOI] [PubMed] [Google Scholar]
- 38.Phillips DP, Jarvinen JR, Abramson IS, Phillips RR. Cardiac mortality is higher around Christmas and New Year’s than at any other time: the holidays as a risk factor for death. Circulation 2004;110:3781-8. [DOI] [PubMed] [Google Scholar]
- 39.Milne EM. Mortality spike at New Year but not Christmas in North East England. Eur J Epidemiol 2005;20:849-54. [DOI] [PubMed] [Google Scholar]
- 40.De Savigny D, Mayombana C, Mwageni E, Masanja H, Minhaj A, Mkilindi Y, et al. Care-seeking patterns for fatal malaria in Tanzania. Malar J 2004;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalloo DG, Shingadia D, Pasvol G, Chiodini PL, Whitty CJ, Beeching NJ, et al. UK malaria treatment guidelines. J Infect 2007;54:111-21. [DOI] [PubMed] [Google Scholar]