Abstract
Objective
To report the rates of mother-to-child transmission (MTCT) of the human immunodeficiency virus (HIV), and the coverage of interventions designed to prevent such transmission, in KwaZulu-Natal.
Methods
Mothers with infants aged ≤ 16 weeks and fathers or legal guardians with infants aged 4–8 weeks who, between May 2008 and April 2009, attended immunization clinics in six districts of KwaZulu-Natal were included. The mothers’ uptake of interventions for the prevention of MTCT was explored. Blood samples from infants aged 4–8 weeks were tested for anti-HIV antibodies and, if antibody-positive, for HIV desoxyribonucleic acid (DNA).
Findings
Of the 19 494 mothers investigated, 89·9% reported having had an HIV test in their recent pregnancy. Of the 19 138 mothers who reported ever having had an HIV test, 34.4% reported that they had been found HIV-positive and, of these, 13.7% had started lifelong antiretroviral treatment and 67.2% had received zidovudine and nevirapine. Overall, 40.4% of the 7981 infants tested were found positive for anti-HIV antibodies, indicating HIV exposure. Just 7.1% of the infants checked for HIV DNA (equating to 2.8% of the infants tested for anti-HIV antibodies) were found positive.
Conclusion
The low levels of MTCT observed among the infants indicate the rapid, successful implementation of interventions for the prevention of such transmission. Sampling at immunization clinics appears to offer a robust method of estimating the impact of interventions designed to reduce such transmission. Large-scale elimination of paediatric HIV infections appears feasible, although this goal has not yet been fully achieved in KwaZulu-Natal.
Résumé
Objectif
Présenter les taux de transmission mère-enfant (TME) du virus de l’immunodéficience humaine (VIH), ainsi que la portée des mesures conçues pour prévenir cette transmission, dans le KwaZulu-Natal.
Méthodes
Ont été incluses des mères de nourrissons d’un âge ≤ à 16 semaines et des pères ou des tuteurs légaux de nourrissons âgés de 4 à 8 semaines qui, entre mai 2008 et avril 2009, avaient fréquenté des centres de vaccination dans six districts du KwaZulu-Natal. On a étudié l’adhésion à ces mesures de prévention de la TME par les mères. On a recherché les anticorps anti-VIH dans les échantillons de sang des nourrissons âgés de 4 à 8 semaines puis, en cas de présence de ces anticorps, on a procédé à la recherche de l’acide désoxyribonucléique (ADN) du VIH.
Résultats
Parmi les 19 494 mères ayant participé à l’étude, 89,9% ont signalé avoir fait réaliser un dépistage VIH lors de leur récente grossesse. Parmi les 19 138 mères ayant signalé avoir eu un dépistage VIH, 34,4% ont signalé que le résultat avait indiqué qu’elles étaient séropositives et parmi celles-ci, 13,7% avaient commencé une thérapie antirétrovirale à vie et 67,2% avaient reçu de la zidovudine et de la névirapine. Globalement, on a constaté la présence d’anticorps anti-VIH chez 40,4% des 7 981 nourrissons ayant fait l’objet de cette recherche, indiquant leur exposition au VIH. Seuls 7,1% des nourrissons ayant fait l’objet du test d’ADN du VIH (équivalent à 2,8% des nourrissons chez lesquels la présence d'anticorps VIH avait été détectée) présentaient des échantillons positifs.
Conclusion
Les faibles niveaux de TME observés chez ces nourrissons indiquent la mise en place rapide et réussie des mesures de prévention contre cette transmission. Le prélèvement d'échantillons dans les centres de vaccination semble offrir une méthode solide d'estimation de l'impact des mesures destinées à réduire cette transmission. L’élimination à grande échelle des infections à VIH chez l’enfant paraît réalisable, en dépit du fait qu’elle n’ait pas été entièrement atteinte dans le KwaZulu-Natal.
Resumen
Objetivo
Notificar los índices de transmisión maternofilial (TMT) del virus de la inmunodeficiencia humana (VIH) y la cobertura de las intervenciones diseñadas para prevenir dicha transmisión en KwaZulu-Natal.
Métodos
Se incluyeron las madres con hijos con edad igual o inferior a 16 semanas y los padres o tutores legales con niños de edades comprendidas entre 4 y 8 semanas que hubieran asistido a los centros de vacunación de seis distritos de KwaZulu-Natal entre mayo de 2008 y abril de 2009. Se evaluó la aceptación de las madres respecto a las intervenciones para la prevención de la transmisión del VIH de la madre al niño. Se analizaron las muestras de sangre de niños de entre 4 y 8 semanas para comprobar la presencia de anticuerpos anti-VIH y, en el caso de obtener un resultado positivo para dichos anticuerpos, se comprobó la presencia del ácido desoxirribonucléico (ADN) del VIH.
Resultados
El 89,9% de las 19 494 madres que participaron en el estudio afirmaron haberse realizado una prueba del VIH en su último embarazo. De las 19 138 madres que afirmaron haberse realizado alguna vez una prueba del VIH, un 34,4% aseguró que la prueba para el VIH resultó positiva en su caso y, de ese porcentaje, un 13,7% inició un tratamiento crónico con antirretrovirales y un 67,2% recibió zidovudina y nevirapina. En total, el 40,4% de los 7981 niños estudiados obtuvieron un resultado positivo de anticuerpos anti-VIH, lo que indica su exposición al VIH. Solo el 7,1% de los niños sometidos a las pruebas del ADN del VIH (equivalente al 2,8% de los niños a quienes se les realizó pruebas de anticuerpos anti-VIH) obtuvieron un resultado positivo en dicha prueba.
Conclusión
Los bajos niveles de TMT del VIH observados en los niños reflejan una puesta en práctica rápida y fructífera de las intervenciones para la prevención de dicha transmisión. Las tomas de muestras en los centros de vacunación parecen constituir un método sólido para calcular el impacto de las intervenciones diseñadas para reducir dicha transmisión. La eliminación de las infecciones pediátricas del VIH a gran escala parece viable, si bien este objetivo no está completamente conseguido en KwaZulu-Natal.
ملخص
الغرض
الإبلاغ عن معدلات انتقال فيروس نقص المناعة البشرية من الأم إلى الطفل (MTCT) وتغطية التدخلات المصممة لمنع هذا الانتقال في كوازولو ناتال.
الطريقة
تضمنت الدراسة الأمهات اللاتي يوجد لديهن أطفال رضع في عمر 16 أسبوعًا أو أكبر والآباء أو الأوصياء الشرعيين الذين يوجد لديهم أطفال رضع تتراوح أعمارهم ما بين 4 إلى 8 أسابيع ممن حضروا عيادات التحصين في المناطق الست في كوازولو ناتال في الفترة من مايو 2008 إلى إبريل 2009. وتم استكشاف استيعاب الأمهات للتدخلات لمنع انتقال فيروس نقص المناعة البشرية من الأم إلى الطفل. وتم فحص عينات دم من الرضع الذين تتراوح أعمارهم ما بين 4 إلى 8 أسابيع لاكتشاف الأجسام المضادة لفيروس نقص المناعة البشرية وما إذا كان الجسم المضاد إيجابي للحمض النووي الريبي المنزوع الأكسجين لفيروس نقص المناعة البشرية (DNA).
النتائج
أبلغت نسبة 89.9% من إجمالي 19494 أمًا تم فحصهن عن إجراء اختبار فيروس نقص المناعة البشرية في حملهن الحالي. وأبلغت نسبة 34.4% من إجمالي 19138 أمًا أجرين اختبار فيروس نقص المناعة البشرية أنهن اكتشفن أن نتيجة اختبار فيروس نقص المناعة البشرية إيجابية وبدأت نسبة 13.7% من بينهن علاجًا مضادًا للفيروسات الرجعية مدى الحياة وحصلت نسبة 67.2% منهن على عقار زيدوفودين ونيفيرابين. وبشكل عام، جاءت نتيجة الاختبار لدى 40.4% من 7981 رضيعًا إيجابية للأجسام المضادة لفيروس نقص المناعة البشرية، مما يشير إلى التعرض لفيروس نقص المناعة البشرية. وجاءت نتيجة 7.1% فقط من الرضع الذين تم فحصهم لاكتشاف الحمض النووي الريبي المنزوع الأكسجين لفيروس نقص المناعة البشرية إيجابية (ما يعادل 2.8% من الرضع الذين تم فحصهم لاكتشاف الأجسام المضادة لفيروس نقص المناعة البشرية).
الاستنتاج
تشير المستويات المنخفضة لانتقال فيروس نقص المناعة البشرية من الأم إلى الطفل (MTCT) التي لوحظت بين الأطفال إلى سرعة ونجاح تنفيذ التدخلات لمنع هذا الانتقال. ويبدو أن الحصول على العينات في عيادات التحصين يوفر أسلوبًا قويًا لتقييم تأثير التدخلات المصممة لخفض الانتقال من هذا القبيل. ويبدو أن التخلص واسع النطاق من عدوى فيروس نقص المناعة البشرية لدى الأطفال ذا جدوى على الرغم من أنه لم يتم تحقيق هذا الهدف بالكامل في كوازولو ناتال .
摘要
目的
报告夸祖鲁纳塔尔省的人类免疫缺陷病毒 (HIV) 的母婴传染 (MTCT) 率和为预防此传染而设计的干预措施的作用范围。
方法
针对 2008 年 5 月至 2009 年 4 月期间在夸祖鲁纳塔尔省六个区域接受免疫诊所治疗,其婴儿年龄不超过 16 周的父母或者其婴儿年龄为 4–8 周的法定监护人。深入调查接受 MTCT 预防干预措施的母亲。检测年龄为 4–8 周婴儿的血样的抗 HIV 抗体,如果抗体呈阳性,则检测 HIV 脱氧核糖核酸 (DNA)。
结果
接受调查的 19 494 名母亲,89·9% 报告称在最近的孕期有做 HIV 化验。在报告有做 HIV 化验的 19 138 名母亲中,34.4% 报告称发现 HIV 呈阳性,这其中 13.7% 已经开始做终身的抗逆转录病毒的治疗,67.2% 接受齐多夫定和奈韦拉平药物治疗。总体而言,7981 名接受检测的婴儿中 40.4% 发现抗 HIV 抗体呈阳性,即指示 HIV 暴露。接受 HIV DNA 检测的婴儿仅 7.1%(相当于 2.8% 接受抗 HIV 抗体检测的婴儿)发现为阳性。
结论
婴儿中观测到低 MTCT 比率显示此类传染预防干预错误得到快速、成功的实施。对于为减少此类传染而设计的干预措施,免疫诊所的抽样方法似乎是评估其影响的有力方法。大规模消除儿科 HIV 感染似乎可行,尽管此目标还未在夸祖鲁纳塔尔省完全实现。
Резюме
Цель
Предоставить отчет о частоте передачи вируса иммунодефицита человека (ВИЧ) от матери к ребенку и произвести обзор мероприятий, разработанных для целей предотвращения такого рода передачи вируса в КваЗулу-Наталь.
Методы
Исследование проводилось среди матерей с грудными детьми в возрасте ≤ 16 недель и отцов или законных опекунов с младенцами в возрасте 4–8 недель, которые в период с мая 2008 г. по апрель 2009 г. посещали клиники иммунизации в шести районах КваЗулу-Наталь. Было исследовано влияние на матерей мероприятий, направленных на предупреждение передачи вируса от матери к ребенку. Пробы крови грудных детей в возрасте 4–8 недель были исследованы на предмет наличия антител к ВИЧ и, в случае наличия антител, на наличие дезоксирибонуклеиновой кислоты ВИЧ (ДНК).
Результаты
Из 19494 матерей, принявших участие в исследовании, 89,9% сообщили, что они сдавали анализ на ВИЧ во время своей последней беременности. Из 19138 матерей, которые сообщили о том, что они когда-либо сдавали анализ на ВИЧ, 34,4% подтвердили, что они были инфицированы ВИЧ, и 13,7% из них начали пожизненное антиретровирусное лечение, в то время как 67,2% получали зидовудин и невирапин. В итоге у 40,4% из 7981 обследованных младенцев были обнаружены антитела к ВИЧ, указывающие на риск инфицирования ВИЧ. Только 7,1% младенцев, проверенных на наличие ДНК ВИЧ (что равно 2,8% младенцев, проверенных на наличие антител к ВИЧ) были признаны инфицированными.
Вывод
Низкие уровни передачи вируса от матери к ребенку, наблюдаемые среди грудных детей, указывают на быстрое и успешное внедрение мероприятий, направленных на предупреждение такого рода передачи вируса. Сбор проб крови в клиниках иммунизации, по всей видимости, представляет собой обоснованный метод оценки влияния мероприятий, разработанных для снижения количества случаев передачи вируса таким способом. Представляется целесообразным крупномасштабная ликвидация педиатрического инфицирования ВИЧ, хотя данная цель еще не полностью достигнута в КваЗулу-Наталь.
Introduction
For the public health systems of countries with high prevalences of infection with the human immunodeficiency virus (HIV), the identification of HIV-infected pregnant women and their treatment with antiretroviral drugs are among the greatest opportunities and challenges. Reliable and robust methodologies for measuring and demonstrating the success of such identification and treatment, especially when applied on a large scale, are needed.1
When combinations of antiretroviral drugs are given to HIV-positive women, either as lifelong treatment or as prophylaxis to prevent mother-to-child transmission of HIV, the rate of HIV transmission from mothers to non-breastfed infants can be reduced to < 1%.2,3 In breastfeeding communities, the additional postnatal transmission can similarly be reduced to < 1% when viral load is effectively suppressed.4 The possibility of reducing the prevalences of HIV infection among HIV-exposed infants to such exceptionally low levels has inspired the belief that the elimination of HIV infection in infants is attainable. Current goals are to reduce paediatric infections by 90% and the rates of mother-to-child transmission in breastfeeding populations to < 5% by 2015.5
Measurement of the effectiveness of interventions for the prevention of mother-to-child transmission (PMTCT) has several benefits: it encourages health workers to believe that they can change, at least for children, the course of the HIV epidemic; it helps fundraising, by providing donors with evidence that their investments are worthwhile; and it provides feedback to health managers about whether challenges to implementation have been effectively resolved.
HIV prevalence in the South African province of KwaZulu-Natal is among the highest in the world. In 2008, for example, HIV prevalence among women attending government-run antenatal clinics in the province was 38.7%.6 In April of the same year, KwaZulu-Natal’s Department of Health bolstered its PMTCT programme to provide antenatal zidovudine from 28 weeks’ gestation to HIV-infected pregnant women, in addition to the single-dose nevirapine it was already providing to such women and their babies at the time of delivery. At this time, the province’s Department of Health also commissioned an impact assessment to determine to what extent the programme’s goal, to reduce HIV infection in infants, was being achieved. We report the findings of a study, designed to determine the rates of mother-to-child transmission of HIV in KwaZulu-Natal, in which all infants attending immunization clinics for their first immunizations served as a population proxy. Since > 95% of infants in KwaZulu-Natal attend such clinics for their first immunization,7 the sample included infants whose mothers might not have participated in the province’s PMTCT programme.
Methods
Participants were enrolled in a cross-sectional study conducted between May 2008 and April 2009. The study, which was designed to assess the impact of the provincial PMTCT programme in KwaZulu-Natal, South Africa, included all primary-health-care facilities in six of the 11 districts of KwaZulu-Natal. All fixed clinics providing immunizations in the six study districts were included in the sample; mobile clinics were excluded. The study districts were primarily urban (three) or primarily rural (three) and were purposively selected in collaboration with KwaZulu-Natal’s Department of Health. At the time of the study, the six study districts had a combined population of 7.1 million, representing about 69.5% of the population of KwaZulu-Natal.8 All mothers with children aged < 6 years and all fathers and legal guardians with infants aged 4–8 weeks who attended the study immunization clinics were invited to participate in the parent study. Children attending with other caregivers were excluded because the other caregivers would be unable to provide consent for blood sampling or, it was felt, provide reliable information about the HIV status of the mother and child. Mothers were asked about their histories of HIV testing and uptake of other PMTCT services in their most recent pregnancy. Consent was requested from a parent or legal guardian of each infant aged between 4 and 8 weeks (i.e. 28–62 days) to collect a heel-prick blood sample from the infant for anonymous HIV testing, regardless of the reported HIV status of the infant’s mother or the participation of the infant’s mother in the provincial PMTCT programme. The samples were stored as dried blood spots before being tested.
Sampling
To permit detection of a point estimate for a vertical transmission frequency of 15–20% (± 3% at a 95% confidence level), or a 6% HIV prevalence among all infants, it was estimated that at least 1200 dried blood spot samples should be collected in each study district. The number of weeks required to collect this number of samples in each district was estimated from the mean number of infants who received first immunizations in the same district during each week from April to December 2007 (N. Moodley, unpublished data, 2008), allowing for up to 20% non-participation. Although the total number of weeks of data collection varied between districts, data were collected for the same number of weeks in each study clinic in a given district. In each study district, therefore, each clinic contributed dried blood spot samples in proportion to the total number of children presenting for immunization in that clinic, thereby producing a self-weighted sample.9
HIV testing
Heel-prick blood samples were collected from infants aged 4–8 weeks, using a spring-loaded lancing device (ACCU-CHEK Softclix, Roche Diagnostics, Burgess Hill, United Kingdom), onto filter paper and dried. Each resultant dried blood spot sample was first tested for anti-HIV antibodies using a commercial kit (Vironostika HIV Uni-Form II plus O, bioMérieux Boxtel, Netherlands). If HIV-specific antibodies were detected, the same dried blood spot sample was then tested for HIV DNA using a commercial PCR-based assay (HIV-1 DNA Amplicor version 1.5, Roche Diagnostics, Pleasanton, United States of America).10
HIV testing was anonymous: enrolled infants were each assigned a unique study number at enrolment, no identifying information was collected, and HIV results were added to the database after completion of the study. Linked non-anonymous HIV counselling and testing were, however, provided for the child of any caregiver who wished to receive the results of his or her child’s HIV test.
Statistical analysis
The present analyses were restricted to mothers with children aged ≤ 16 weeks, and all fathers and legal guardians with infants aged 4–8 weeks. Uptake of drugs for PMTCT in the last pregnancy was assessed for mothers whose infants were ≤ 16 weeks of age at the time of interview; this age was chosen to reduce recall bias and reflect the most current status of PMTCT coverage. HIV exposure and transmission rates were estimated from the results of the HIV testing of all infants aged 4–8 weeks, irrespective of caregiver type. The rates of mother-to-child transmission were assessed according to complete or incomplete PMTCT intervention as self-reported by mothers. A PMTCT intervention was considered complete if: (i) the mother had been on lifelong antiretroviral treatment before delivery; (ii) the mother had been on zidovudine for at least 4 weeks and the baby, for at least 7 days; or (iii) the mother had been on zidovudine for < 4 weeks and the baby, for at least 28 days. Odds ratios (ORs) and 95% confidence intervals (CIs) for associations between maternal age and PMTCT regimen in pregnancy and HIV transmission to infants were calculated using generalized estimation equations (GEE) and the proc genmod procedure in SAS version 9.2 (SAS Institute, Cary, USA). This analysis allowed consideration of the potential correlation of outcomes measured in the same clinic.11
Research ethics
The University of KwaZulu-Natal’s Biomedical Ethics Review Committee granted ethical approval for the study.
Results
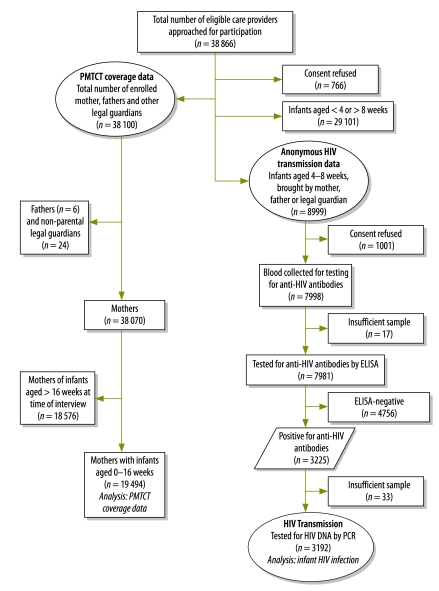
Fig. 1 shows the study profile. Data were collected from 348 (99%) of the 349 fixed immunization clinics in the six study districts between May 2008 and April 2009; one fixed clinic was excluded because of poor road access. In total, 38 866 eligible caregivers (i.e. mothers, fathers or legal guardians) were invited to participate in the parent study, of who 766 (2.0%) refused consent. Of the 38 100 caregivers who agreed to participate in the parent study, 19 494 were mothers attending the study clinics with infants aged ≤ 16 weeks, six were fathers attending with infants aged 4–8 weeks, and 24 were legal guardians attending with infants aged 4–8 weeks. Characteristics of the infants who participated in the study are shown in Table 1.
Fig. 1.
Profile of study of interventions to prevent mother-to-child HIV transmission, KwaZulu-Natal, South Africa, 2008–2009
DNA, desoxyribomucleic acid; ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
Table 1. Characteristics of mothers and childrena who participated in study of interventions to prevent mother-to-child HIV transmission, KwaZulu-Natal, South Africa, 2008–2009 (n = 19 524) .
| Characteristic | No. (%)b |
|---|---|
| Median age (IQR) | |
| Child in months (n = 19 524) | 2.3 (1.5–3.0) |
| Mother in years (n = 19 487) | 24.2 (20.4–29.3) |
| Presenting caregiver’s relationship to child (n = 19 524) | |
| Mother | 19 494 (99.8) |
| Father | 6 (0.03) |
| Legal guardian | 24 (0.12) |
| Population group (n = 19 455) | |
| African | 19 018 (97.8) |
| Indian | 375 (1.9) |
| White | 62 (0.32) |
| Father of child alive (n = 19 503) | 19 288 (98.9) |
| Mother’s education (n = 19 447) | |
| None | 312 (1.6) |
| At least some primary | 2 811 (14.5) |
| At least some secondary | 14 671 (75.4) |
| Post-secondary | 1 653 (8.5) |
| Father’s education (n = 18 401) | |
| None | 352 (1.9) |
| At least some primary | 1 911 (10.4) |
| At least some secondary | 13 534 (73.6) |
| Post-secondary | 2 604 (14.2) |
| Mother is employed (n = 19 508) | 2 799 (14.4) |
| Mother receives grant support (n = 19 523) | 7 481 (38.3) |
| Child-support grant | 7 321 (37.5) |
| Disability grant | 332 (1.7) |
| Main construction material of house (n = 19 490) | |
| Brick or cement block | 13 464 (69.1) |
| Traditional material/mud | 5 413 (27.8) |
| Informal/other (corrugated iron, zinc) | 613 (3.2) |
| Main source of drinking water for household (n = 19 494) | |
| Piped – inside home | 4 684 (24.0) |
| Piped – outside of home | 12 421 (63.7) |
| River | 2 011 (10.3) |
| Borehole or water tank | 378 (1.9) |
| Household connected to electricity (n = 19 510) | 14 065 (72.1) |
| Household has landline and/or cell phone (n = 19 514) | 17 814 (91.3) |
HIV, human immunodeficiency virus; IQR, interquartile range.
a Aged 16 weeks or less.
b All except for median ages, which are expressed in years.
Service coverage
Of the 19 494 mothers participating in the study, 15 164 (77.8%) reported having attended antenatal care at least four times during their recent pregnancy and 19 138 (98.2%) reported ever having had an HIV test. Although 17 521 (89.9%) of the mothers reported having been tested for HIV during their recent pregnancy, 12 386 (70.7%) reported that they had only been tested during the last trimester. Among those mothers who had been tested, 6586 (34.4%) said that they had known or learned that they were HIV-infected during their recent pregnancy, and, of these, 6337 (96.2%) had received either antiretroviral drugs for PMTCT or lifelong antiretroviral treatment for their own health. Among the 6594 women who reported themselves to be HIV-infected, 584 (8.9%) said that they had known they were HIV-infected before their recent pregnancy. The self-reported distribution of the various antiretroviral regimens taken is presented in Table 2.
Table 2. Self-reported coverage of interventions against mother-to-child transmission of HIV among HIV-positive mothers, KwaZulu-Natal, South Africa, 2008–2009(n = 19 494).
| Intervention | No. (%)a |
|---|---|
| Antiretroviral prophylaxis in pregnancy by women who self-report HIV positive (n = 6 586) | |
| Unknown | 3 (0.05) |
| None | 246 (3.7) |
| Nevirapine alone | 868 (13.2) |
| Zidovudine alone | 143 (2.2) |
| Nevirapine and zidovudine | 4424 (67.2) |
| Lifelong antiretroviral treatmentb | 902 (13.7) |
| CD4+ cell count monitoringc | |
| Had CD4+ cell count taken during recent pregnancy (n = 6 562) | 5242 (79.9) |
| Obtained CD4+ cell count result (n = 5 242) | 4379 (83.5) |
| Reported CD4+ cell count of < 200 cells per mm3 (n = 4 379) | 967 (22.1) |
| Among women reporting CD4+ cell counts of < 200 cells per mm3, on lifelong antiretroviral treatment at time of interview (n = 967) | 670 (69.3) |
CD4+ cell, CD4+ T lymphocyte; HIV, human immunodeficiency virus.
a The data refer to women who presented with an infant aged ≤ 16 weeks and reported that they were HIV-infected during their most recent pregnancy.
b May have been started before or during pregnancy.
c Reductions in denominators reflect either missing data or mothers who preferred not to provide a response or who did not know the relevant information.
Prevalence and transmission
Of the 8999 infants aged 4–8 weeks who were brought to the study clinics during data collection, 8969 presented with their mothers, six with their fathers, and 24 with their legal guardians. Although dried blood spot samples were collected from 7998 (88.2%) of these infants, 17 samples were excluded because they were considered inadequate. Anti-HIV antibodies, indicating HIV exposure, were detected in 3225 (40.4%) of the 7981 infants with evaluable dried blood spot samples. Overall, 17.3%, 38.2%, 54.2%, 58.3% and 44.7% of the tested infants of mothers aged < 20, 20–24, 25–29, 30–34 and > 34 years were found seropositive for HIV antibodies, respectively; infant seropositivity was positively correlated with maternal age (P < 0.0001). Complete data regarding feeding practices were available for 3143 (97.7%) of the 3217 infants who attended the study clinics with their mothers and tested positive for anti-HIV antibodies. Of these 3143, 1172 (37.3%) were exclusively breastfeeding, 1850 (58.9%) were receiving formula milk only, and 121 (3.8%) were receiving mixed feeds.
Of the 3225 dried blood spot samples found to be positive for HIV antibodies, 3192 (99%) were tested in a PCR-based assay for HIV desoxyribonucleic acid (DNA) and 225 were found positive for viral DNA. The frequency of peri-partum HIV transmission, from the HIV-positive mothers to their infants, was 7.1% overall (95% CI: 6.2–8.0%), although it varied according to the reported PMTCT regimen taken by the mother (Table 3).
Table 3. Association between mother-to-child transmission of HIV and antiretroviral regimens taken in pregnancy, KwaZulu-Natal, South Africa, 2008–2009 (n = 2858) .
| Regimena | Infants with anti-HIV antibodiesb |
OR | 95% CI | P | ||
|---|---|---|---|---|---|---|
| No. tested by PCR | Found PCR-positive for HIV DNA |
|||||
| No. | % | |||||
| No antiretroviral prophylaxisc | 68 | 11 | 16.2 | – | – | – |
| Nevirapine alone | 242 | 32 | 13.2 | 0.78 | 0.32–1.9 | 0.58 |
| Zidovudine alone | 63 | 2 | 3.2 | 0.17 | 0.04–0.78 | 0.02 |
| Nevirapine and zidovudine | 2089 | 118 | 5.7 | 0.30 | 0.14–0.66 | 0.003 |
| Lifelong ART | 396 | 20 | 5.1 | 0.27 | 0.11–0.68 | 0.006 |
ART, antiretroviral treatment; CI, confidence interval; DNA, desoxyribonucleic acid; HIV, human immunodeficiency virus; OR, odds ratio; PCR, polymerase chain reaction.
a Mothers were allocated to regimen groups irrespective of the reported duration of each antiretroviral intervention.
b The infants tested were aged 4–8 weeks.
c Reference category.
The association between the frequency of vertical transmission and the duration of drug treatment/prophylaxis for PMTCT taken by the mothers and infants was assessed. (Infant nevirapine was not included in this assessment because most mothers reported not knowing whether their infants had received nevirapine.) The rate of mother-to-child transmission was significantly reduced in infants who, with their mothers, received the full, recommended PMTCT regimen. It was 7.7% among the 480 mothers who reported taking an incomplete regimen but 4.9% among the 1912 mothers who reported taking a full regimen. Among the 527 mothers for whom duration of zidovudine or lifelong antiretroviral treatment could not be reliably assessed, the frequency of mother-to-child transmission was 9.9%.
Anti-HIV antibodies were detected in the infants of 209 mothers who reported themselves to be HIV-negative. Twenty-four of these mothers reported taking an antiretroviral regimen. Although three dried blood spot samples from infants of the remaining 185 mothers were unsuitable for testing for HIV DNA, such DNA was detected in the dried blood spot samples from 30 infants of the other 182 mothers, indicating a rate of mother-to-child transmission of 16.5% (95% CI: 11.4–22.7%) .
Discussion
Our findings show that, in the province with the highest HIV prevalences in South Africa, low rates of mother-to-child transmission at population level were achieved within a short period of more effective ATCV regimens being implemented. This major accomplishment for the provincial health authorities should be feasible elsewhere.
The present data point towards three main reasons for this success. First, almost all of the mothers investigated had been tested for HIV and knew their HIV status; almost 90% reported that they had been tested for HIV during their most recent pregnancy, and 9% knew they were infected before their most recent pregnancy began. Ensuring that women know their HIV status is the starting point for achieving low rates of mother-to-child transmission.12 Second, health facilities in KwaZulu-Natal made a rapid transition from providing only nevirapine to successfully implementing dual antiretroviral prophylaxis. Although the policy was changed only two months before the data collection for the present study began, most of the women who reported themselves to be HIV-infected said that they had received zidovudine. The overall frequency of mother-to-child transmission detected (7.1%) and, particularly, the lower value (4.9%) for the mothers who reported that they and their infants had both received prophylaxis, are consistent with the widespread uptake of dual antiretroviral prophylaxis.2 The rate of mother-to-child transmission in the province is likely to decrease further as delivery of these interventions continues to improve. Lastly, almost 14% of the HIV-positive mothers detected had started lifelong antiretroviral treatment. HIV-infected mothers with a low CD4+ T lymphocyte (CD4+ cell) count and their infants incur the greatest risk of illness and HIV transmission.13 Initiating lifelong antiretroviral treatment in such women will make the greatest contribution to reducing HIV infections in children and mortality in mothers. In a study conducted in KwaZulu-Natal in 2005 (at a time when only nevirapine was offered and lifelong antiretroviral treatment was only just starting to become available) in which similar methods were used, 20.2% of infants of HIV-positive women were HIV-infected by 6 weeks of age.14 Compared with these earlier data, the current results indicate a 66% reduction in mother-to-child transmission. While this is not quite the 90% reduction defined by the elimination agenda,5 it is an important step in that direction.
The vertical transmission to the infants who were found to have anti-HIV antibodies, even though their mothers reported themselves to be HIV-negative, was 16.5%. These mothers may have chosen not to disclose their HIV-positive status, while others may have tested negative antenatally and then only recently been infected.15 Women with incident infections are likely to have much higher transmission rates16; if the infection occurs late in pregnancy, however, the increased transmission risk may only become evident when assessing postnatal infections.
The present data reveal several weak areas of the PMTCT programme where improvement is needed. Most of the women investigated had only been tested for HIV in the last trimester of their most recent pregnancy, possibly because of their late initial attendance at an antenatal clinic. Although antenatal clinic attendance is high in South Africa, few women attend before 20 weeks’ gestation17 and many attend only in the third trimester.18 Furthermore, because they were tested for HIV late in their pregnancies, many of the HIV-infected women did not receive a CD4+ cell result during their pregnancies; one third of the women reporting CD4+ cell counts of < 200 cells per mm3 had not started lifelong antiretroviral treatment. KwaZulu-Natal’s Department of Health has already responded to these findings in an effort to improve service delivery. Maternal HIV prevalence remains extremely high, a fact that underlines the continued importance of primary prevention strategies.
Although the Global Fund to Fight AIDS, Tuberculosis and Malaria implements performance-based financing, there are, as yet, no simple approaches for directly counting the number of infant HIV infections and measuring the impact of investments in PMTCT. The method used to collect the present data is simple and replicable and can be used to monitor progress towards eliminating paediatric HIV infection and reducing child mortality. There are, however, several limitations to the methodology as well. First, the approach does not estimate postnatal HIV transmission through breastfeeding; the value of investments to promote breastfeeding may not be fully recognized because of the difficulty of estimating reliably the HIV-free survival rates of infants at 12–18 months. Second, the frequency of mother-to-child transmission may be underestimated because infants who die before they reach 6 weeks of age are not sampled and these deaths may result from HIV transmission in utero. Third, the omission of mothers and infants attending mobile clinics or refusing to participate, among whom transmission may be relatively common, can also contribute to the underestimation of the overall frequencies of mother-to-child transmission. Fourth, there may be recall bias when estimating PMTCT coverage, although in the present study an attempt was made to minimize such bias by only recording data from women with very young children (aged ≤ 16 weeks), who are likely to remember important events in their recent pregnancy. These limitations are unlikely however to significantly distort mother-to-child transmission rates and trends. Although the method was developed for a setting with high rates of infant immunization (83% of the children in KwaZulu-Natal are fully immunized at 1 year),19 it may still be helpful in settings with lower immunization rates, especially when no information about the frequency of mother-to-child transmission is available.
In conclusion, the number of HIV infections in young infants can be greatly reduced on a large scale, although major programmatic challenges must still be overcome to achieve the elimination of paediatric HIV infections. HIV testing early in pregnancy, and ensuring that > 95% of the women eligible for lifelong antiretroviral treatment actually begin such treatment, will be essential if no more than 2% of the infants born to HIV-infected women are to be HIV-positive themselves by the age of 6 weeks. Furthermore, if the overall rate of mother-to-child transmission is to be kept below the 2015 target of 5%, at least 90% of HIV-infected women will need to receive the antiretroviral interventions currently recommended by the World Health Organization throughout breastfeeding; more effective family planning, HIV counselling and support, and primary prevention of HIV will also be required. We have shown that the number of HIV infections in infants can be directly measured in populations through a simple and robust approach. The current data, and the ability to measure such data, will become even more relevant as 2015 approaches and the need to track progress towards the Millennium Development Goals becomes even more critical.
Acknowledgements
We are grateful to the staff of KwaZulu-Natal’s Department of Health, the management teams from the six study districts, and the staff at participating clinics for their cooperation and support. We also thank the data-collection teams who worked so hard, and all the mothers and infants who agreed to participate. The manuscript review by George Rutherford at the University of California San Francisco, Department of Epidemiology and Biostatistics, Global Health Sciences, is gratefully acknowledged.
Funding:
Funding for the study was provided by the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Competing interests:
None declared.
References
- 1.Stringer EM, Chi BH, Chintu N, Creek TL, Ekouevi DK, Coetzee D, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86:57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonwe-Gold B, Ekouevi DK, Viho I, Amani-Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dao H, Mofenson LM, Ekpini R, Gilks CF, Barnhart M, Bolu O, et al. International recommendations on antiretroviral drugs for treatment of HIV-infected women and prevention of mother-to-child HIV transmission in resource-limited settings: 2006 update. Am J Obstet Gynecol. 2007;197(Suppl):S42–55. doi: 10.1016/j.ajog.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.de Vincenzi I, Kesho Bora Study Group Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 5.Sidibe M. UNAIDS 2011–2015 strategy: getting to zero Geneva: Joint United Nations Programme on HIV/AIDS; 2010. Available from: http://www.unaids.org/en/resources/unaidspublications/2010/ [accessed 22 September 2011]
- 6.Antenatal survey 2008: national HIV and syphillis antenatal seroprevalence survey in South Africa 2008 Pretoria: National Department of Health, Republic of South Africa; 2009. Available from: http://www.doh.gov.za/list.php?pageNum_rsList=1&totalRows_rsList=112&type=Reports [accessed 29 September 2011].
- 7.State of the world’s children: maternal and newborn health New York: United Nations Children’s Fund; 2009. Available from: http://www.unicef.org/sowc/ [accessed 22 September 2011].
- 8.Community survey 2007: basic results – KwaZulu Natal Pretoria: Statistics South Africa; 2007. Available from: http://www.statssa.gov.za/community_new/content.asp [accessed 22 September 2011].
- 9.Kish L. Survey sampling New York: Wiley-Interscience; 1995. [Google Scholar]
- 10.Rollins NC, Dedicoat M, Danaviah S, Page T, Bishop K, Kleinschmidt I, et al. Prevalence, incidence, and mother-to-child transmission of HIV-1 in rural South Africa. Lancet. 2002;360:389. doi: 10.1016/S0140-6736(02)09599-5. [DOI] [PubMed] [Google Scholar]
- 11.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- 12.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45–8. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 13.Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: recommendations for a public health approach 2010 Geneva: World Health Organization; 2010. Available from: http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/index.html [accessed 22 September 2011]. [PubMed]
- 14.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–7. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 15.Busch MP, Lee LL, Satten GA, Henrard DR, Farzadegan H, Nelson KE, et al. Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion. 1995;35:91–7. doi: 10.1046/j.1537-2995.1995.35295125745.x. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey JH, Marinda E, Mutasa K, Moulton LH, Iliff PJ, Ntozini R, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:c6580. doi: 10.1136/bmj.c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradshaw D, Chopra M, Kerber K, Lawn JE, Bamford L, Moodley J, et al. Every death counts: use of mortality audit data for decision making to save the lives of mothers, babies, and children in South Africa. Lancet. 2008;371:1294–304. doi: 10.1016/S0140-6736(08)60564-4. [DOI] [PubMed] [Google Scholar]
- 18.Horwood C, Haskins L, Vermaak K, Phakathi S, Subbaye R, Doherty T. Prevention of mother to child transmission of HIV (PMTCT) programme in KwaZulu-Natal, South Africa: an evaluation of PMTCT implementation and integration into routine maternal, child and women's health services. Trop Med Int Health. 2010;15:992–9. doi: 10.1111/j.1365-3156.2010.02576.x. [DOI] [PubMed] [Google Scholar]
- 19.Mhlanga RE. South African Health Review: chapter 8 Durban: Health Systems Trust; 2008. Available from: http://www.hst.org.za/publications/south-african-health-review-2008 [accessed 22 September 2011].