Abstract
Objective
To explore the policies for, and implementation of, the community case management (CCM) of childhood illnesses in the 68 countries that were prioritized by the “Countdown to 2015” initiative in 2008.
Methods
In 2009–2010, community approaches concerning CCM policy and implementation, the roles of community health workers (CHWs) and the availability of medicines for the treatment of malaria, diarrhoea, pneumonia and neonatal infections were investigated by sending questionnaires to implementers and policy-makers and through telephone discussions with appropriate researchers and experts.
Findings
Of the 59 countries that responded, 81%, 75%, 54% and 14% had a policy for the CCM of diarrhoea, malaria, pneumonia and neonatal infections, respectively. Only three (6%) of the 53 malaria-endemic countries providing responses had policies for all four of these conditions, although 17 (32%) had CCM policies for malaria, diarrhoea and pneumonia. Some CCM of childhood illnesses was being implemented – more commonly for diarrhoea and malaria than for pneumonia or neonatal infections – in 88% of the countries providing responses. According to the responses received, CHWs administered the recommended treatments for diarrhoea, malaria or pneumonia in 34% (17/50), 100% (41/41) and 100% (34/34) of the countries implementing CCM of these conditions, respectively. Common programme concerns were drug supplies, quality of care and CHW incentives, training and supervision.
Conclusion
Despite progress, further efforts are needed towards policy reform and the expansion of CCM programmes. Ensuring the availability of recommended medicines and operational research, to assure quality, remain priorities.
Résumé
Objectif
Etudier les politiques et la mise en œuvre de la gestion communautaire des cas (GCC) de maladies infantiles dans les 68 pays qui ont été priorisés en 2008 par l'initiative «Compte à rebours vers 2015».
Méthodes
En 2009-2010, les approches communautaires en matière de politique et de mise en œuvre de la GCC, les rôles des agents de santé communautaires (ASC) et la disponibilité des médicaments pour le traitement du paludisme, de la diarrhée, de la pneumonie et des infections néonatales ont été étudiés par l'envoi de questionnaires aux exécutants et aux décideurs et par des entretiens téléphoniques avec des chercheurs et des experts compétents.
Résultats
Sur les 59 pays ayant répondu, 81%, 75%, 54% et 14% avaient une politique de GCC pour la diarrhée, le paludisme, la pneumonie et les infections néonatales, respectivement. Seuls 3 (6%) des 53 pays d'endémie palustre ayant répondu disposaient de politiques pour chacune de ces quatre affections, bien que 17 (32%) d’entre eux disposaient de politiques de GCC pour le paludisme, la diarrhée et la pneumonie. Certaines GCC de maladies infantiles étaient mises en œuvre - plus fréquemment pour la diarrhée et le paludisme que pour la pneumonie ou les infections néonatales - dans 88% des pays ayant répondu. Selon les réponses reçues, les ASC ont administré les traitements recommandés pour la diarrhée, le paludisme ou la pneumonie dans 34% (17/50), 100% (41/41) et 100% (34/34) des pays mettant en œuvre une GCC pour ces affections, respectivement. Les préoccupations communes du programme concernaient l'approvisionnement en médicaments, la qualité des soins et les motivations, la formation et la supervision des ASC.
Conclusion
En dépit des progrès, des efforts supplémentaires sont nécessaires pour réformer la politique et développer les programmes de GCC. Les priorités restent d’assurer la disponibilité des médicaments recommandés et la recherche opérationnelle afin de garantir la qualité.
Resumen
Objetivo
Analizar los planes de acción y la puesta en marcha de la gestión comunitaria de casos (GCC) de enfermedades infantiles en los 68 países a los que se dio prioridad en la iniciativa «Cuenta Atrás para 2015» (Countdown to 2015).
Métodos
Entre 2009 y 2010 se investigaron los métodos de la comunidad en relación al plan de acción de GCC y su puesta en marcha, la función del personal sanitario de la comunidad (PSC) y la disponibilidad de los medicamentos para el tratamiento de la malaria, la diarrea, la neumonía y las infecciones neonatales. Dicha investigación se llevó a cabo mediante el envío de cuestionarios a los encargados de establecer los planes de acción y de ponerlos en marcha así como a través de conversaciones telefónicas con los investigadores y expertos pertinentes.
Resultados
De los 59 países que respondieron, el 81%, 75%, 54% y 14% contaba con planes de acción para la GCC de la diarrea, la malaria, la neumonía y las infecciones neonatales, respectivamente. Solo tres (6%) de los 53 países con malaria endémica que habían enviado sus respuestas contaban con planes de acción para esas cuatro afecciones, si bien 17 de ellos (32%) presentaban planes de acción para la GCC de malaria, diarrea y neumonía. El 88% de los países que enviaron sus respuestas estaban poniendo en práctica algunos planes de GCC de enfermedades infantiles (con más frecuencia para la diarrea y la malaria que para la neumonía y las infecciones neonatales). De acuerdo con las respuestas recibidas, el PSC administró los tratamientos recomendados para la diarrea, la malaria o la neumonía en el 34% (17/50), 100% (41/41) y 100% (34/34) de los países que pusieron en marcha la GCC para estas afecciones, respectivamente. Las preocupaciones comunes del programa fueron el suministro de medicamentos, la calidad de la asistencia así como los incentivos, la formación y la supervisión del PSC.
Conclusión
A pesar de los progresos realizados en este sentido, es necesario un esfuerzo mayor para reformar los planes de acción y ampliar los programas de GCC. Las prioridades siguen siendo garantizar la disponibilidad de los medicamentos recomendados y la investigación operativa con el fin de asegurar la calidad.
ملخص
الغرض استكشاف السياسات الخاصة بالتدبير العلاجي للحالة (CCM) في المجتمع لأمراض الطفولة وتنفيذها في 68 بلدًا التي تم منحها الأولوية وفق مبادرة "العد التنازلي إلى عام 2015".
الطريقة في الفترة ما بين 2009-2010، تم البحث في النهج المجتمعية الخاصة بسياسة التدبير العلاجي للحالة وتنفيذها، وأدوار عمال الصحة المجتمعية (CHW)، وتوافر أدوية علاج الملاريا والإسهال والالتهاب الرئوي وعدوى حديثي الولادة بإرسال الاستبيانات للمنفذين وصناع السياسة ومن خلال المناقشات الهاتفية مع الباحثين والخبراء في هذا الشأن.
النتائج من بين 59 دولة استجابت، كان نسبة 81 % و75 % و54 % و14 % لديها سياسة خاصة بالتدبير العلاجي للحالة في المجتمع بالنسبة للإسهال والملاريا والالتهاب الرئوي وعدوى حديثي الولادة على التوالي. وكانت ثلاثة فقط (6 %) من البلدان الثلاثة والخمسين الموطونة بالملاريا التي قدمت استجابات لديها سياسات لهذه الحالات الأربعة جميعها، على الرغم من أن 17 (32 %) بلدًا كانت لديها سياسات خاصة بالتدبير العلاجي للحالة في المجتمع للملاريا والإسهال والالتهاب الرئوي. وكان هناك تنفيذ لبعض التدبير العلاجي للحالة في المجتمع لأمراض الطفولة – بشكل أكثر شيوعًا فيما يخص الإسهال والملاريا مقارنة بالالتهاب الرئوي أو عدوى حديثي الولادة – في 88 % من البلدان التي قدمت استجابات. ووفقًا للاستجابات المستلمة، أعطى عمال الصحة المجتمعية العلاجات الموصى بها للإسهال أو الملاريا أو الالتهاب الرئوي في 34 % (17/50) و100 % (41/41) و100 % (34/34) من البلدان التي نفذت التدبير العلاجي للحالة في المجتمع لهذه الحالات على التوالي. وتمثلت المخاوف المشتركة في البرنامج في إمدادات الأدوية وجودة الرعاية وحوافز عمال الصحة المجتمعية وتدريبهم والإشراف عليهم.
الاستنتاج برغم التقدم، لا تزال هناك حاجة لمزيد من الجهود في اتجاه إصلاح السياسات وتوسيع برامج التدبير العلاجي للحالة في المجتمع. وتظل الأولويات هي ضمان إتاحة الأدوية الموصى بها والبحوث التشغيلية بغرض ضمان الجودة.
摘要
目的
探讨 68 个国家受 2008 年“2015 倒计时”方案而重点发展的儿童期疾病的病例管理(CCM)的政策及其实施情况。
方法
2009 年至 2010 年,通过发送问卷调查给实施者和政策制定者以及与相应研究者和专家的电话讨论调查涉及 CCM 政策和实施的社区方法、社区卫生工作者(CHW)的角色和治疗腹泻、疟疾、肺炎和新生儿感染的药品的可用性。
结果
在响应调查的 59 个国家中,制定腹泻、疟疾、肺炎和新生儿感染 CCM 政策的国家比率分别为 81%、75%、54% 和 14%。尽管 17(32%)个国家制订了疟疾、腹泻和肺炎的 CCM 政策,在 53 个提供回答的疟疾地方病国家中仅有三个国家(6%)制定了所有四种病况的政策。在提供回答的国家中有 88% 国家实施部分儿童期疾病 CCM – 腹泻和疟疾较肺炎和新生儿感染更为普遍。根据收到的回答,在实施这些病况 CCM 的国家中,CHW 对腹泻、疟疾和肺炎执行推荐治疗的比率分别为 34%(17/50)、100%(41/41)和 100%(34/34)。共同的计划关注点是药品供应、护理质量和 CHW 激励、培训和监督。
结论
尽管有所进步,还需针对政策改革和 CCM 计划推广加大工作力度。为保证质量依然需首要确保推荐药品和操作研究的可用性。
Резюме
Цель
Исследовать политику и внедрение ведения детских болезней на местном уровне в 68 странах, которым в 2008 г. был отдан приоритет в рамках инициативы «Отсчет времени до 2015 г.» .
Методы
В 2009–2010 гг. путем рассылки опросных листов лицам, ответственным за разработку и внедрение политики, а также посредством телефонных переговоров с соответствующими исследователями и экспертами были изучены подходы общин в отношении политики ведения пациентов на уровне района проживания и ее внедрения, роли местных работников здравоохранения и доступности лекарственных препаратов для лечения малярии, диареи, пневмонии и неонатальных инфекций.
Результаты
В принявших участие в обзоре 59 странах на 81%, 75%, 54% и 14% была внедрена политика ведения пациентов на местном уровне в отношении диареи, малярии, пневмонии и неонатальных инфекций, соответственно. Только в трех (6%) из 53 принявших участие в обзоре стран со вспышками малярии была внедрена политика в отношении всех четырех из указанных заболеваний, при этом в 17 (32%) странах была внедрена политика ведения пациентов на местном уровне при заболевании малярией, диареей и пневмонией. Разновидности политики ведения детских заболеванийна местном уровне внедрялась в большинстве случаев в отношении диареи и малярии, в отличие от пневмонии или неонатальных инфекций – в 88% принявших участие в обзоре стран. В соответствии с полученными отчетами работники здравоохранения обеспечивали рекомендованное лечение диареи, малярии или пневмонии в 34% (17/50), 100% (41/41) и 100% (34/34) стран, внедряя политику ведения указанных заболеваний на местном уровне, соответственно. Общими проблемами в рамках программы являлись снабжение лекарственными препаратами, качество медицинского ухода и повышение мотивации медицинских сотрудников, обучение и надзор.
Вывод
Несмотря на некоторый прогресс, необходимы дальнейшие усилия при проведении реформы политики и расширении программ в рамках политики ведения пациентов на местном уровне. Остаются приоритетными обеспечение доступности рекомендованных лекарственных препаратов и оперативные исследования, направленные для предоставление гарантии качества.
Introduction
The community case management (CCM) of childhood illnesses – defined here as the community-level provision to children of curative treatments for diarrhoea, pneumonia, malaria and/or neonatal infections by community health workers (CHWs) – is a strategy with the potential to accelerate progress towards meeting Millennium Development Goal 4 (i.e. the reduction of child mortality by two thirds between 1990 and 2015).1 Unless their current rates of decline in child mortality accelerate, most African countries will not achieve such a reduction until 2065.2 A major constraint is the weakness of national health systems,3,4 especially the shortage of human resources. CCM could increase the number of care providers at the community level. Task shifting has been used in low-resource settings since the late 1960s. After the Alma-Ata declaration in 1978, attention turned to primary-health-care services.5 The allocation of tasks to the least costly health worker has since often been applied effectively at the community level.6 Compared with other approaches to health care, the provision of services at the community level is likely to reach not only more people but also the populations most in need, thus improving equity.7–10
Although the mortality rate in children under the age of 5 years has declined by 28% since 1990,11,12 infectious diseases still contribute to 68% of deaths among such children.13 Of the 5.9 million children under 5 who die of infectious diseases each year, about 3.6 million are neonates.14 The major causes of deaths among children under 5 are pneumonia (18%), diarrhoea (15%), malaria (8%) and neonatal infections, including sepsis (6%).15 Each of these killers has, however, at least one proven effective treatment: artemisinin-based combination therapy (ACT) for malaria; low-osmolarity oral rehydration solutions (ORS) and zinc for diarrhoea; and antibiotics for pneumonia and neonatal infections. If coverage of these interventions were universal, with community-based delivery of half of the interventions, it has been estimated that the annual number of deaths among children under 5 would fall by 63%.2,16 The greatest improvements would probably be seen in sub-Saharan Africa, where pneumonia, diarrhoea and malaria account for more than half of all childhood deaths.13 If such interventions are to be delivered well, however, there have to be good national policies, including effective CCM policies.
In 2010, to identify gaps in CCM policies and understand the current status of CCM implementation, we conducted a survey on the CCM of malaria, pneumonia, diarrhoea and neonatal infections in the 68 countries prioritized by the Countdown to 2015 initiative in 2008 (list available at: http://www.countdown2015mnch.org/reports-publications/2010-country-profiles/2008-country-profiles)17; together, these Countdown priority countries account for about 97% of maternal, neonatal and child deaths worldwide each year. To our knowledge, this was the first comprehensive survey on the CCM of childhood illnesses since a survey of the CCM of pneumonia in 57 African or Asian countries in 2008.18 The results presented here provide a basis for future advocacy work, resource mobilization, programme support and evidence generation.
Methods
Survey description
A 26-item questionnaire on the CCM of malaria, pneumonia, diarrhoea and neonatal infections was pre-tested in Pakistan and Senegal and then distributed, in 2009 or 2010, to the relevant policy-makers and implementers in the 68 Countdown priority countries. The questionnaire addressed the status of CCM policies, implementation and plans; the concerns of the CCM implementers and policy-makers; CHW activities, training manuals, lengths of training; referral policies and remuneration; and drug policies and availability. In an attempt to enhance the quality of the data collected, the questionnaire included multiple questions on the same topic and was sent to two, three or four individuals in each country. Data were only included in the final analysis if they came from a country (henceforth a “responding” country) in which at least one policy-maker and one implementer had fully completed a questionnaire. Most questionnaires were completed by United Nations officers and their health ministry counterparts. For each country that provided at least one completed questionnaire, the recorded data were reviewed during one or two telephone interviews with field experts (from nongovernmental organizations [NGOs] or academic institutions working on CCM) until any ambiguities and discrepancies were resolved.
Definitions
The International Standard Classification of Occupations (ISCO-08) defines CHWs as workers who provide health education, basic preventive care, referral and follow-up, case management, and home-visit services to communities.19 In this assessment, however, we only included programmes and CHWs offering curative treatments at the community level for at least one of the four categories of illness that were of interest (i.e. diarrhoea, malaria, pneumonia or neonatal infections). A CCM policy was defined as official when there was a health ministry written policy, memorandum or letter authorizing CCM and permissive when there were only CCM guidelines, the strategy was part of the country’s national plans, or the health ministry produced or approved CCM training manuals. Either of these types of policies was considered “supportive”.
A CCM programme was considered to exist in a country when the health ministry either led CCM implementation or had an active implementing role (with or without the additional support of implementing agencies, NGOs, academic institutions or other partners). Such a programme was categorized as “national” when CCM had been introduced in more than half of the country’s districts and as “subnational” when the intervention covered fewer of the country’s districts. Some CCM implementation also occurred within pilot or research studies, without any direct health ministry leadership, and in each case this was categorized as a “district project”.
Analysis
Six aspects of the CCM approach were analysed: (i) policy; (ii) implementation; (iii) concerns; (iv) plans; (v) CHW activities, training and remuneration; and (vi) availability of drugs. Disease-specific results were given as percentages, generally using the total number of responding countries (for diarrhoea, pneumonia and neonatal infections) or the number of malaria-endemic countries from which responses were received (for malaria) as the denominator. When analysing tools or drugs used in CCM implementation, however, the denominator was the relevant number of implementing countries. The data were analysed using SPSS Statistics version 17.0 (SPSS Inc., Chicago, United States of America) and plotted on graphs using Excel 2008 (Microsoft, Redmond, USA). The degree of association between having a policy for CCM in a given country and implementation of that CCM in the same country was determined by calculating Pearson’s phi coefficient (φ). Following the guidelines of Evans (1996), φ coefficients of 0–0.19, 0.20–0.39, 0.40–0.59, 0.60–0.79 and 0.80–1.00 were considered indicative of very weak, weak, moderate, strong and very strong associations, respectively.
Results
Questionnaires were completed by at least one policy-maker and at least one implementer in each of 59 (87%) of the 68 Countdown priority countries, including 53 (95%) of the 56 where malaria is endemic.20 No responses or inadequate responses were received from Botswana, Djibouti, Egypt, Haiti, Lao People's Democratic Republic, Mexico, Morocco, Swaziland and Yemen. The combined populations of the 59 responding countries represented 98% of the total population of all 68 Countdown priority countries.
Fifty-two (88%) of the 59 responding countries had some form of CCM policy for at least one of the four health conditions of interest (i.e. diarrhoea, malaria, pneumonia and neonatal infections); in 37 (63%) of the 59, the policy was official. Of the 53 malaria-endemic countries that provided responses, 49 (92.5%) had at least one CCM policy, 17 (32%) had CCM policies for malaria, pneumonia and diarrhoea and just three (6%) – the Congo, Nepal and Niger – had such policies for diarrhoea, malaria, pneumonia and neonatal infections (Table 1).
Table 1. Community case management (CCM) policy and implementation status in 53 malaria-endemic countries, by number of health conditions covered, 2009–2010.
| Status | No. (%) of the 53 countries with policy and/or implementation against |
||||
|---|---|---|---|---|---|
| Four conditionsa | Three conditions | Two conditions | One condition | No condition | |
| Type of CCM policy | |||||
| Official | 2 (4) | 15 (28) | 10 (19) | 8 (15) | 18 (34) |
| Permissive | 1 (2) | 2 (4) | 11 (21) | 10 (19) | 29 (55) |
| None | NA | NA | NA | NA | 4 (8) |
| Type of implementation | |||||
| National programme | 1 (2) | 5 (9) | 7 (13) | 14 (26) | 26 (49) |
| Subnational programme | 2 (4) | 4 (8) | 14 (26) | 21(40) | 12 (23) |
| District project | 0 (0) | 0 (0) | 5 (9) | 5 (9) | 43 (81) |
| None | NA | NA | NA | NA | 4 (8) |
NA, not applicable.
a The four health conditions considered here are diarrhoea, malaria, pneumonia and neonatal infections.
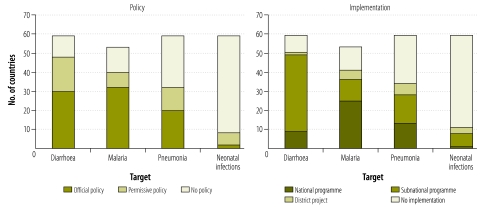
More of the responding countries had supportive (i.e. official or permissive) policies for the CCM of diarrhoea (48) than for the CCM of malaria (40), pneumonia (32) or neonatal infections (8). These supportive policies were more likely to be official for the CCM of malaria (32) and diarrhoea (30) than for the CCM of pneumonia (20) or neonatal infections (2) (Table 2 and Fig. 1). The Congo and Niger had adopted official policies for the CCM of neonatal infections and Bangladesh, Eritrea, India, Nepal, Rwanda and Togo reported corresponding permissive policies.
Table 2. Community case management policy and implementation status among countries, by target health condition, 2009–2010.
| Target and scale of implementation | No. of countries with |
||
|---|---|---|---|
| Official policy | Permissive policy | No policy | |
| Diarrhoea (n = 59 countries) | |||
| National programme (n = 9) | 8 | 1 | 0 |
| Subnational programme (n = 40) | 20 | 17 | 3 |
| District project (n = 1) | 1 | 0 | 0 |
| No implementation (n = 9) | 1 | 0 | 8 |
| Malaria (n = 53 countries) | |||
| National programme (n = 25) | 22 | 3 | 0 |
| Subnational programme (n = 11) | 7 | 4 | 0 |
| District project (n = 5) | 2 | 1 | 2 |
| No implementation (n = 12) | 1 | 0 | 11 |
| Pneumonia (n = 59 countries) | |||
| National programme (n = 13) | 10 | 2 | 1 |
| Subnational programme (n = 15) | 5 | 6 | 4 |
| District project (n = 6) | 2 | 2 | 2 |
| No Implementation (n = 25) | 3 | 2 | 20 |
| Neonatal infections (n = 59 countries) | |||
| National programme (n = 1) | 0 | 0 | 1 |
| Subnational programme (n = 7) | 0 | 3 | 4 |
| District project (n = 3) | 0 | 2 | 1 |
| No implementation (n = 48) | 2 | 1 | 45 |
Fig. 1.
Community case management (CCM) policy and implementation status, by target health condition, in 59 Countdown priority countries, 2009–2010
Note: For malaria, only the data for the 53 malaria-endemic countries are shown.
In 52 (88%) of the responding countries, CCM was implemented for at least one of the four conditions that were of interest. CCM of diarrhoea was reportedly being implemented in 50 (85%) of the responding countries and this CCM was based either on a combination of ORS and zinc (17 countries) or on ORS alone (33) (Table 3). The CCM of malaria was being implemented in 41 (77%) of the malaria-endemic countries providing responses, most frequently as a national or subnational programme led by the health ministry (Table 2 and Fig. 1). Most (34) of the Countdown priority countries providing responses were implementing CCM of pneumonia, again mostly at the programme level (Table 2 and Fig. 1). Only 11 (19%) of the responding countries were reportedly implementing CCM of neonatal infections, eight of them at the programme level. The responding countries without CCM implementation were Angola, Burundi, Comoros, Iraq, Peru, South Africa and Turkmenistan.
Table 3. Availability of the recommended drugs for community case management in Countdown priority countries from which data were collected, 2009–2010.
| Availability | No. of malaria-endemic countries (n = 53) |
No. of responding countries (n = 59) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACT | Mon | ORS + Zn | ORS | CTX | Amox | Cef | Amp | Gen | Pen | ||
| First-line treatment | 38 | 5 | 40 | 54 | 35 | 21 | 1 | 10 | 16 | 10 | |
| Essential drug list | 43 | 13 | 36 | 54 | 42 | 41 | 9 | 30 | 34 | 30 | |
| Over the counter | 26 | 16 | 16 | 49 | 25 | 29 | 12 | 18 | 17 | 18 | |
| Private sector | 38 | 24 | 22 | 49 | 41 | 43 | 30 | 33 | 33 | 32 | |
| CCM programme | 41 | 10 | 17 | 50 | 28 | 13 | 0 | 2 | 2 | 2 | |
ACT, artemisinin-based combination therapies; Amox, amoxicillin; Amp, ampicillin; CCM, community case management; Cef, cefuroxime; CTX, cotrimoxazole; Gen, gentamicin; Mon, monotherapies; ORS: oral rehydration salts; Pen, penicillin; Zn, zinc.
Most countries with supportive CCM policies were implementing the corresponding CCM (Table 2). Among the 59 responding countries were 18 that were reportedly implementing CCM in the absence of CCM policies and 10 with CCM policies in which there was no corresponding CCM implementation. The positive association seen between implementation and policy was very strong for malaria (φ = 0.845), strong for diarrhoea (φ = 0.765) and moderate for pneumonia (φ = 0.555) and neonatal infections (φ = 0.446). According to the data on the questionnaires, 33 (73%) of the 45 countries with CCM implementation at the national level (for any of the four health conditions being considered) intended to enlarge their CCM programmes (the other 12 either expected no future changes or were uncertain). Similarly, 30 (73%) of the 41 countries with CCM implementation at the subnational scale also intended to increase the scale of their CCM programmes (the other 11 did not foresee changes or were uncertain). No country with a CCM programme reported any intention to reduce the scale of that programme. Of the 25, 12, 9 and 48 countries reporting no implementation of CCM against pneumonia, malaria, diarrhoea and neonatal infections, respectively, only 10, 5, 1 and 10, respectively, reported an interest in introducing such case management.
The profiles of CHWs varied greatly among the 59 responding countries, with a total of 64 different CHW designations identified. Each of 18 countries had more than one type of CHW. The most common designation was “community health worker”, followed by “village health worker”. The CHW in 38 (73%) of the 52 countries reporting CCM implementation used algorithms to assess sick patients. The CHW in 11 (27%) of the 41 countries reporting CCM of malaria used rapid diagnostic tests (RDT) to detect malarial infections, and the CHW in 23 (68%) of the 34 countries reporting CCM of pneumonia used respiratory timers to diagnose pneumonia.
The training manuals for CHW in the 52 countries reporting CCM implementation were designed for literate (32 countries) or semi-literate individuals (16) or were mainly pictorial so that illiterate workers could follow them (11). Although each of 23 countries had more than one type of CCM training manual, none had manuals only for illiterate workers. The median length of CCM training that CHWs received was 6 days (range: 3 days to 1 year). Referrals of sick children to health facilities by CHWs were written (65% of the 52 countries with CCM implementation), verbal (44%) or via a variety of other facilitating methods (40%); CHWs commonly employed more than one referral method each. CHWs reported just to a health facility in most (87%) of the CCM-implementing countries, just to a community supervisor in 35% and to both a health facility and a community supervisor in 31%. CHWs also reported to CCM partners, village governments and NGOs. The CHWs in almost half (48%) of the CCM-implementing countries received no monetary compensation. Elsewhere, CHWs received payments from the programme implementers, or collected user fees (37% of the CCM-implementing countries) or received a mark-up on the drugs that they distributed (15%). The CHWs in 10 countries with CCM implementation (Bangladesh, China, Ethiopia, Gabon, India, Malawi, Niger, Pakistan, Philippines and South Sudan) received government salaries.
At the time of this analysis, the recommended drugs to treat malaria, pneumonia and diarrhoea were ACTs, either cotrimoxazole (CTX) or amoxicillin,21 and ORS plus zinc,22 respectively. The policy-approved availability of these treatments was categorized as follows: (i) recommended as first-line treatment; (ii) on the essential-drug list; (iii) offered by CHWs; (iv) offered over the counter; and (v) available in the private sector (Table 3). In all of the countries reportedly implementing CCM of malaria, most CHWs used ACT (although 11 of the responding countries were in the process of phasing over to ACT, and antimalarial monotherapy was still offered by some CHWs in 10 countries). Of the 34 countries implementing CCM of pneumonia, 82% used CTX and 38% used amoxicillin. All 34 of these countries had policies for the community-based delivery of CTX alone (62%), amoxicillin alone (18%) or both drugs (21%). In only 17 (34%) of the countries reporting the implementation of CCM of diarrhoea was the recommended combination of ORS and zinc distributed by CHWs; the CHWs in the other 33 countries used ORS alone. The ORS used in 33 of the countries reporting CCM of diarrhoea were still the full osmolarity formula.
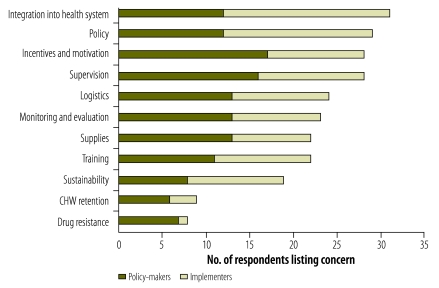
The policy-makers and implementers who completed the questionnaires had many concerns, including the low quality of the CCM services, CHW incentives and motivation, supplies, logistics and the integration of CHWs into the health system (Fig. 2). The policy-making health ministry officials who were responsible for their countries’ CCM strategies prioritized their concerns in the following order: (i) incentives and motivation of CHWs; (ii) supervision of CHWs; (iii) availability of supplies; (iv) logistics; and (v) monitoring and evaluation. For governmental implementers, nongovernmental organizations and staff of the United Nations Children’s Fund (UNICEF) and World Health Organization (WHO) involved in the CCM programmes, however, the main concerns appeared to be the integration of CHWs into the health system, policy status and CHW supervision.
Fig. 2.
Major challenges in community case management (CCM) programmes, as identified by implementers and policy-makers in 59 Countdown priority countries, 2009–2010
CHW, community health worker.
Note: The data shown are the result of asking each of 49 policy-makers and 45 implementers to list their three most important concerns regarding CCM.
Discussion
Responses were received from most of the 68 Countdown priority countries identified in 200817 and nearly all (88%) of the responding countries had (official or permissive) CCM policies in place and were implementing CCM for at least one health condition. Most of the responding countries had CCM policies (81%) and implementation (85%) for the treatment of diarrhoea. The CHWs in only 34% of such countries were distributing the recommended treatment for diarrhoea (ORS plus zinc), probably because of an international shortage of zinc,23 and the CHWs in 66% of such countries were still distributing full osmolarity ORS. These observations underscore the urgent need to strengthen both the supply of medicines to communities and the delivery of medicines at the community level.
After diarrhoea, malaria was the next most common reported target of CCM. Overall, 75% of the malaria-endemic responding countries had CCM policies for the disease and 77% were actively implementing antimalarial CCM. Encouragingly, such CCM was predominantly based on the recommended ACT (although some CHWs in 10 malaria-endemic countries were also using monotherapies).
Against pneumonia there were supportive CCM policies and CCM implementation in about half of the countries that provided responses for the present analysis. In a survey conducted in 2007 on 57 of the original 60 Countdown priority countries in Africa and Asia, only 20 (35%) of the 57 countries were found to have policies for the CCM of pneumonia.24 Three years later, in the present study, 28 (49%) of the same 57 countries had policies supporting the CCM of pneumonia, and the percentage of the 57 countries implementing such CCM had also increased, from 47% in 200724 to 56%. This change reflects less the adoption of official policies than of permissive policies and guidelines, as well as the lifting of barriers against the distribution of antibiotics by CHWs. Despite the progress made since 2007, gaps remain. Only about one third (34%) of the responding countries had official policies for the CCM of pneumonia, and fewer (22%) were implementing CCM programmes against the disease at national level. Supportive policies for the usage of antibiotics against pneumonia by CHWs generally remained sparse, although they were reported to be present in all countries with active programmes for CCM of the disease. The scarcity of zinc for the treatment of diarrhoea stood in contrast to the plentiful availability of antibiotics against pneumonia via the private sector or over the counter. However, relatively few CHWs appear to have such drugs available for distribution, and this represents a threat to programme success that could divert patients to a better-stocked private sector.
Although neonatal infections, such as sepsis, are responsible for 29% of all neonatal deaths,14 only two of the 59 responding countries (the Congo and Niger) had adopted official policies for such infections. Another six countries had permissive policies and only 11 countries (most of them in southern Asia) were implementing the CCM of neonatal infections.
Compared with countries with minimal CCM implementation, countries with CCM implementation in more than half of their districts were more likely to express an interest in increasing the scale of their CCM programmes. Respondents in countries with no current CCM implementation or with CCM limited to a “district project” were less likely to express any expectation of introducing or scaling up CCM. Together, these observations suggest that experience in the implementation of CCM improves policy-makers’ opinions of CCM, leading to recommendations and plans for increasing the coverage of CCM implementation. Unsurprisingly, the existence of a supportive CCM policy was positively associated with CCM implementation. About one in every two countries with official CCM policies had CCM in more than half of its districts, whereas countries with only permissive policies were usually implementing the interventions only at the subnational or project level. Countries with no supportive CCM policies usually had no CCM implementation, although a few countries implemented CCM (two for malaria, seven for pneumonia, three for diarrhoea and six for neonatal infections) in the apparent absence of the corresponding supportive policies. Implementation preceding policy is not so uncommon, as different factors influence policy reforms and the effectiveness of implementation.25
The programme concerns of policy-makers, which were generally similar to those of the implementers, underscore the uncertain capacity of many health systems to deliver CCM effectively. While successful CHW-based programmes could relieve the load of care at the facility level, their success will require the strengthening of the health systems that support them. Numerous ongoing lines of research are addressing the concerns of policy-makers and implementers. Benchmarks for the introduction and scaling up of CCM have been proposed. The result has been a list of indicators for monitoring and evaluating CCM that are currently being validated through implementation research. Research is also being conducted on CHW motivation and supervision, training packages for the supervisors of CHWs who are delivering CCM, and estimations of CCM programme costs. Tools to strengthen the supply chains for CCM are also being assessed and developed. Tools for programme support are also now widely available,26,27 and these and other CCM-related tools and documents can be viewed in an internet-based archive.28 A manual of community-based approaches to the management of suspected severe neonatal infection is under development (S Wall, unpublished data, 2011).
The quality of the care offered by CHWs was among the most frequent concerns of the policy-makers who were sent questionnaires, and this is clearly a problem if CCM is considered an option to address the human resource crisis in health care.29 In the integrated management of childhood illness, the duration of health worker training was not always correlated with the quality of care subsequently offered.6 For CHWs involved in integrated CCM, however, there are many points at which accurate decisions need to be made (not the least of which is the need to differentiate between malaria- and pneumonia-associated fevers). Under these circumstances, the quality of care is perhaps more likely to be closely linked to the training and supervision received.
This study, which provides the first report of the global status of CCM policy and implementation for the four leading causes of death among children aged less than 5 years, has a few limitations. First, although the desk survey employed internal cross-checks and follow-up telephone calls, implementation status was difficult to assess because it can and does change rapidly, lacks standard reporting methods and, in some cases, may reflect prescribed or intended practices more than actual routines. Second, the records of implementation did not include much detail of the coverage or any data on the use or quality of the CCM services provided. Third, we sought information only on those CHWs who provided treatments.
In conclusion, CCM is a widely endorsed strategy to save lives in settings with high child mortality. Increasingly, countries are adopting CCM policies, programmes and plans. Most of the Countdown priority countries without CCM implementation were African. The knowledge acquired and best practices identified in CCM programmes on other continents need to be discussed with African policy-makers and implementers. The supply of the medicines needed for CCM is a general challenge. This and most other concerns of the relevant policy-makers and implementers are currently the objects of active operational research and programme learning designed to further the development of more effective programme implementation.
Acknowledgements
We thank everyone who completed the questionnaires, facilitated their distribution, supported the dissemination of the results for advocacy and policy purposes, and contributed to the review of the initial report or this manuscript. These include colleagues from ministries of health, UNICEF, WHO, various NGOs, various academic institutions and the members of the CCM Operational Research Group. We especially thank Udita Patel for her valuable input and months of dedicated work.
Part of this survey was conducted while Alexandra de Sousa was a staff member at UNICEF.
Competing interests:
None declared.
References
- 1.Human development report 2003. Millennium Development Goals: a compact among nations to end human poverty New York: United Nations Development Programme; 2003. [Google Scholar]
- 2.Wagstaff A, Claeson M. The Millennium Development Goals for health: rising to the challenges Washington: The World Bank; 2004. [Google Scholar]
- 3.Task Force on Health Systems Research Informed choices for attaining the Millennium Development Goals: towards an international cooperative agenda for health-systems research. Lancet. 2004;364:997–1003. doi: 10.1016/S0140-6736(04)17026-8. [DOI] [PubMed] [Google Scholar]
- 4.Travis P, Bennett S, Haines A, Pang T, Bhutta Z, Hyder AA, et al. Overcoming health-systems constraints to achieve the Millennium Development Goals. Lancet. 2004;364:900–6. doi: 10.1016/S0140-6736(04)16987-0. [DOI] [PubMed] [Google Scholar]
- 5.Primary health care: report of the International Conference on Primary Health Care, Alma-Ata, USSR, 6–12 September, 1978 Geneva: World Health Organization; 1978.
- 6.Huicho L, Scherpbier RW, Nkowane AM, Victora CG. How much does quality of child care vary between health workers with differing durations of training? An observational multicountry study. Lancet. 2008;372:910–6. doi: 10.1016/S0140-6736(08)61401-4. [DOI] [PubMed] [Google Scholar]
- 7.Reidpath DD, Morel CM, Mecaskey JW, Allotey P. The Millennium Development Goals fail poor children: the case for equity-adjusted measures. PLoS Med. 2009;6:e1000062. doi: 10.1371/journal.pmed.1000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 9.Haines A, Sanders D, Lehmann U, Rowe AK, Lawn JE, Jan S, et al. Achieving child survival goals: potential contribution of community health workers. Lancet. 2007;369:2121–31. doi: 10.1016/S0140-6736(07)60325-0. [DOI] [PubMed] [Google Scholar]
- 10.Rudan I, Campbell H. Childhood pneumonia deaths: a new role for health workers? Lancet. 2008;372:781–2. doi: 10.1016/S0140-6736(08)61167-8. [DOI] [PubMed] [Google Scholar]
- 11.Progress on health-related Millennium Development Goals Geneva: World Health Organization; 2009. [Google Scholar]
- 12.You D, Wardlaw T, Salama P, Jones G. Levels and trends in under-5 mortality, 1990–2008. Lancet. 2010;375:100–3. doi: 10.1016/S0140-6736(09)61601-9. [DOI] [PubMed] [Google Scholar]
- 13.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 14.Lawn JE, Kerber K, Enweronu-Laryea C, Cousens S. 3.6 million neonatal deaths — what is progressing and what is not? Semin Perinatol. 2010;34:371–86. doi: 10.1053/j.semperi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Rudan I, El Arifeen S, Black RE, Campbell H. Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect Dis. 2007;7:56–61. doi: 10.1016/S1473-3099(06)70687-9. [DOI] [PubMed] [Google Scholar]
- 16.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 17.Countdown to 2015. Tracking progress in maternal, newborn and child survival. The 2008 report New York: United Nations Children’s Fund. [DOI] [PubMed]
- 18.Marsh DR, Gilroy KE, Van de Weerdt R, Wansi E, Qazi S. Community case management of pneumonia: at a tipping point? Bull World Health Organ. 2008;86:381–9. doi: 10.2471/BLT.07.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Updating the International Standard Classification of Occupations (ISCO). Draft ISCO-08 group definitions: occupations in health Geneva: International Labour Organization; 2009. [Google Scholar]
- 20.International travel and health report Geneva: World Health Organization; 2009. [Google Scholar]
- 21.WHO/UNICEF joint statement: management of pneumonia in community settings Geneva: World Health Organization; 2004. [Google Scholar]
- 22.WHO/UNICEF joint statement: clinical management of acute diarrhoea Geneva: World Health Organization; 2004. [Google Scholar]
- 23.Fischer Walker CL, Fontaine O, Young MW, Black RE. Zinc and low osmolarity oral rehydration salts for diarrhoea: a renewed call to action. Bull World Health Organ. 2009;87:780–6. doi: 10.2471/BLT.08.058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.State of the World’s children New York: United Nations Children’s Fund; 2008. [Google Scholar]
- 25.Walt G, Gilson L. Reforming the health sector in developing countries: the central role of policy analysis. Health Policy Plan. 1994;9:353–70. doi: 10.1093/heapol/9.4.353. [DOI] [PubMed] [Google Scholar]
- 26.Community case management essentials: treating common childhood illnesses in the community. A guide for program managers Washington: CORE Group; 2010. [Google Scholar]
- 27.Marsh D, Guenther T, Sadruddin S, Vijayrhagavan J, Koepsell J. Tools to introduce community case management (CCM) of serious childhood infection Washington: Save the Children; 2011. [Google Scholar]
- 28.Tools and documents Washington: United States Agency for International Development (for the Integrated Community Case Management Task Force). Available from: http://www.ccmcentral.com/?q=tools_and_documents [accessed 10 November 2011].
- 29.Task shifting: global recommendations and guidelines Geneva: World Health Organization; 2008. [Google Scholar]