Abstract
Recovery from stroke engages mechanisms of neural plasticity. Here we examine a role for MHC Class I (MHCI) H2-Kb and H2-Db, and PirB receptor. These molecules restrict synaptic plasticity and motor learning in the healthy brain. Stroke elevates not only neuronal expression of H2-Kb and H2-Db, but also PirB and downstream signaling. KbDb KO or PirB KO mice have smaller infarcts and enhanced motor recovery. KO hippocampal organotypic slices, which lack an intact peripheral immune response, have less cell death after in vitro ischemia. In PirB KO mice, corticospinal projections from motor cortex are enhanced and the reactive astrocytic response is dampened following MCAO. Thus molecules that function in the immune system act not only to limit synaptic plasticity in healthy neurons, but also to exacerbate brain injury following ischemia. These results suggest novel therapies for stroke by targeting MHCI and PirB.
Keywords: MCAO, Corticospinal Tract Sprouting, Oxygen Glucose Deprivation, Plasticity, MHC class I, PirB, Stroke, Neuroprotection
Introduction
After stroke, the extent of brain and behavioral recovery is influenced by local inflammatory changes and neural circuit plasticity. Inflammation exacerbates damage through a range of mechanisms including activation of microglia, oxidative stress and infiltration by peripheral immune cells (Choe et al., 2011; Hurn et al., 2007; Offner et al., 2006). Increased functional recovery is associated with neural plasticity including axonal sprouting in corticospinal projections that occurs days to weeks following ischemic injury (Carmichael et al., 2001; Lee et al., 2004; Netz et al., 1997). Ischemia induces changes in neuronal excitability and alters dendritic spines within hours (Brown et al., 2007; Brown et al., 2008; Takatsuru et al., 2009). Sprouting and growth of intracortical axons are also thought to serve as substrates for recovery in somatosensory and visual cortex after peripheral injury or retinal lesion (Florence et al., 1998; Palagina et al., 2009), and can happen rapidly (Yamahachi et al., 2009). On the other hand, cellular correlates of synaptic plasticity such as LTP are diminished by stroke (Sopala et al., 2000; Wang et al., 2005). These observations suggest that recovery might be enhanced not only by dampening inflammation but also by enhancing synaptic and structural plasticity.
Recently we discovered that mice lacking major histocompatibility class I (MHCI) function have enhanced visual cortical and hippocampal plasticity – not only in development but also in adulthood (Corriveau et al., 1998; Datwani et al., 2009; Huh et al., 2000; Shatz, 2009). MHCI molecules are expressed in neurons and located at synapses in the healthy CNS (Datwani et al., 2009; Needleman et al., 2010), and knocking out just H2-Kb (Kb) and H2-Db (Db) (KbDb KO), two of the more than 50 MHCI genes, is sufficient to enhance plasticity in mouse visual cortex (Datwani et al., 2009) and cerebellum (McConnell et al., 2009). An innate immune receptor, PirB (Paired immunoglobulin-like receptor B) is known to bind MHCI, both in neurons (Syken et al., 2006) and in the immune system (Matsushita et al., 2011; Takai, 2005). Like Kb and Db, PirB is expressed in forebrain neurons, and PirB KO mice also have greater visual cortical plasticity (Syken et al., 2006). MHCI molecules are upregulated by inflammation, both in the immune system and in the CNS following damage or viral infection (Piehl and Lidman, 2001; Thams et al., 2008). Because these molecules play a dual role in the peripheral immune response and in neural plasticity in the CNS, they could be involved not only in the acute phases of stroke but also in subsequent recovery. Following stroke, these molecules might make a dual contribution to exacerbate damage in the context of the inflammatory response and to restrict recovery by limiting plasticity. Here, we investigate these possibilities by examining response to in vivo and in vitro models of stroke in PirB KO mice and KbDb KO mice.
Results
KbDb KO mice recover better than WT following stroke
To examine if Kb and Db contribute to damage following stroke, adult KbDb KO mice (Vugmeyster et al., 1998) received transient middle cerebral artery occlusion (MCAO; Han et al., 2009). KbDb KO mice subjected to MCAO had no significant difference from WT in infarct area at 24hr (37% vs 41%, p=0.45, Figure 1A), and their initial neurological deficit was also similar (p=0.4, Figure S1A; Han et al., 2009). However, by 7d post MCAO, infarct area in KbDb KO mice was modestly reduced (32%) compared to WT (44%; p=0.03). Physiological parameters monitored during surgery were similar between WT and KO and fell within previously reported ranges (Supplemental Table 1; Han et al., 2009).
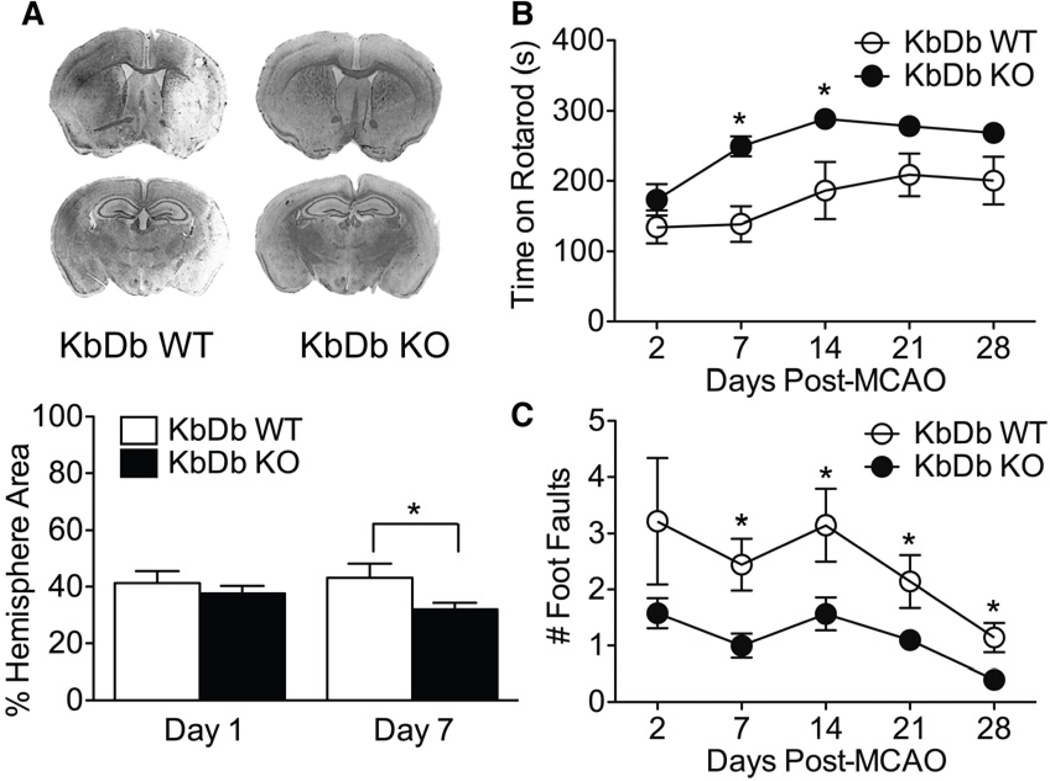
Figure 1.
KbDb KO mice recover better than WT cohorts following MCAO. (A) Top: Example of sections of Cresyl Violet stained brains 7d post MCAO. Bottom: KO animals have significantly smaller infarct areas at 7d (p=0.03), but not 24hr (p=0.45), post MCAO. (B, C) KO animals outperform WT cohorts on motor tasks post MCAO. Average Rotarod (B) and Foot Fault (C) performances were assessed on days 2 and 7 post MCAO in all animals, and cohorts of each genotype were kept through 1 month survival with additional testing on days 14, 21 and 28. P values for the comparisons between genotypes on Rotarod on days 21 and 28 were 0.056 and 0.057 respectively, reflecting the reduced numbers of surviving animals. Groups were 19–25 mice (days 2 and 7), and 7–10 mice (days 14–28). P<0.05 refers to A–C. Data are represented as mean +/− SEM. See also Figure S1.
To examine motor recovery following MCAO, KbDb KO and WT mice were tested on two motor performance tasks, Rotarod and Foot Fault. Prior to MCAO, KO and WT mice learned both tasks, improving performance over subsequent trials, as evidenced by the increased latency to fall from the Rotarod (Figure S1B) and fewer missteps on Foot Fault (Figure S1C). KO mice learned both behaviors better than WT (p< 0.001), consistent with prior observations of enhanced motor learning (McConnell et al., 2009). After stroke, performance on Rotarod and Foot Fault was significantly better in KO mice vs WT (p<0.001 for both paradigms; Figure 1B, C). Overall, KbDb KO mice had smaller infarcts, and recovered significantly faster and to a greater extent on motor performance (to 91% of pre-stroke Rotarod time compared to 75% for WT at 28 days).
Kb and Db expression increase following MCAO
The observations that KbDb KO mice have smaller infarct areas and better behavioral recovery after MCAO suggest that Kb and Db may contribute to damage in WT mice. Moreover, since mice lacking Kb and Db have enhanced synaptic plasticity, it is conceivable that increased expression would contribute to diminished plasticity, thereby compromising recovery. To examine this idea further we assessed MHCIs levels following MCAO. Quantitative real-time PCR (RT qPCR) revealed highly increased Kb and Db mRNA in the damaged hemisphere (ipsi) compared to sham control after MCAO (Figure 2A), both at 24hr (Kb mRNA: 2.5 fold increase, p<0.05; Db mRNA: 3.1 fold increase, p<0.001) and at 7d (Kb mRNA: 8.0 fold increase, p<0.01; Db mRNA: 7.0 fold increase, p<0.001). Kb and Db levels in the damaged hemisphere were also over 2 fold higher than levels in the undamaged hemisphere at 24hr post MCAO and over 5 fold higher 7d post MCAO (Figure 1A; p<0.01). Western blot analysis of Kb expression in both synaptosome-enriched samples or in synaptoneurosomes demonstrated increased protein levels after MCAO in the damaged hemisphere relative to the undamaged side or sham (Figure 2B, C).
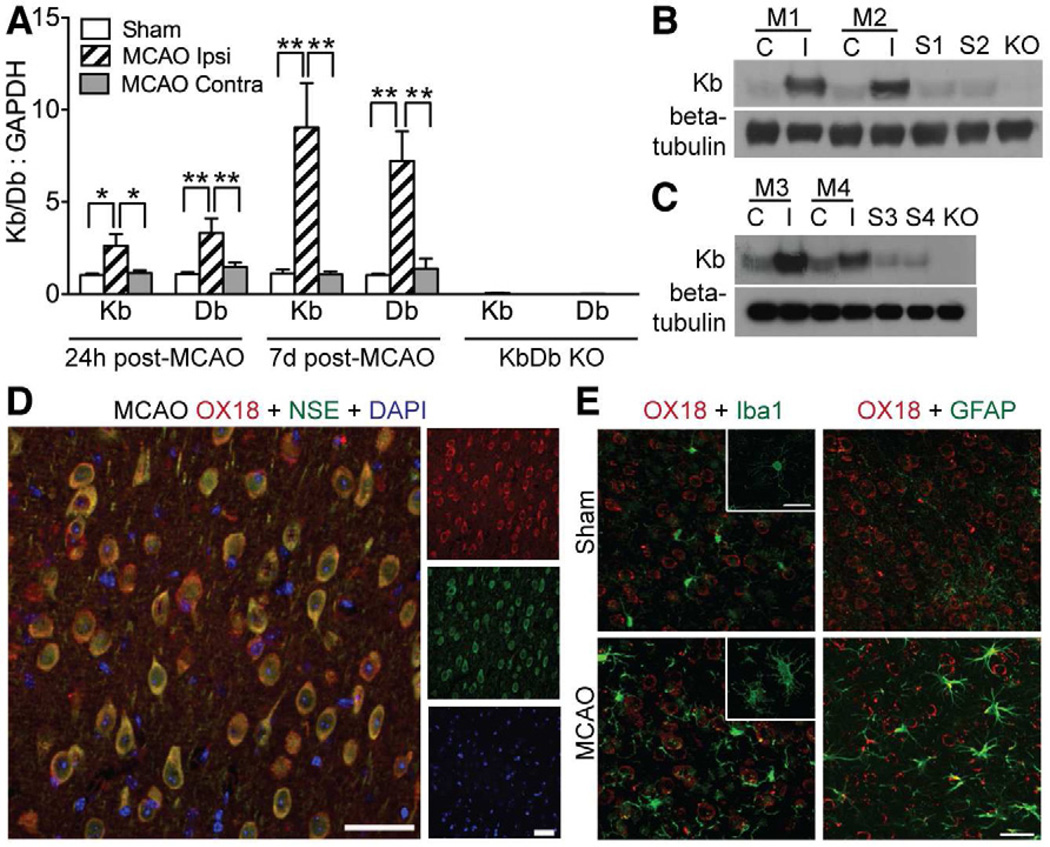
Figure 2.
Expression of Kb and Db increases in brain of WT mice following MCAO. (A) Kb and Db gene expression was measured using qPCR at 24hr (n=7 for each experimental condition) or 7d (n=7 MCAO; n=4–8 Sham) post MCAO (Ipsi=Damaged hemisphere, Contra=undamaged hemisphere). Negative control data from KbDb KO samples demonstrates primer specificity (Kb:GAPDH=0.06 control relative to sham; Db:GAPDH=0.01 control relative to sham, p<10−8 for both). *P≤0.05, **p≤0.01. (B) Western blots showing protein expression of Kb 7d post MCAO in synaptosome-enriched preparations and (C) in synaptoneurosomes C=contralateral to injury; I=ipsilateral to injury; M1-4=different MCAO animals; S1-4=shams; KO=KbDb KO. (D, E) Immunohistochemistry of L5 in cortical penumbra using OX18 antibody. D: Majority of MHCI immunostaining (OX18; red) colocalizes with neuronal marker NSE (green) 7d post MCAO. E: GFAP (green; astrocytes) or Iba1 (green; microglia) 7d post MCAO. Scale bars D, E, 50μm. qPCR data represented as mean +/− SEM. See also Figure S2.
Since synaptoneurosomes enrich for synaptic proteins (Johnson et al., 1997), following MCAO Kb protein could be upregulated at synapses and also possibly within glial processes that enwrap the synapse. Previous studies have shown that MHCI proteins are expressed in neurons and are closely associated with synaptic markers in the healthy brain (Datwani et al., 2009; Goddard et al., 2007). MHCI immunostaining, using an antibody known to recognize both Kb and Db (McConnell et al., 2009; Needleman et al., 2010), is primarily associated with neurons in brain sections taken from the cortical penumbra 7d post MCAO or from unmanipulated cortex, as assessed by co-localization with the neuronal marker neuron specific enolase (NSE). Staining is not detected in astrocytes or microglia (Figure 2D, E; Figure S2). As expected, there is evidence of both astrocytic and microglial activation post MCAO (Figure 2E). Together these observations demonstrate that Kb and Db are upregulated following MCAO, and that within the cortical penumbra this upregulation is associated with increased protein expression in neurons and at or near synapses.
Improved outcomes in mice lacking PirB receptor
To explore further how absence of Kb and Db in the brain might lead to neuroprotection, we next examined mice lacking the MHCI receptor, PirB (Shatz, 2009; Syken et al., 2006; Takai, 2005). PirB is expressed in CNS neurons, including pyramidal cells throughout cerebral cortex. Seven days post MCAO, PirB KO mice had smaller infarcts than WT (KO: 18% vs WT: 35%; p=0.0001) even though infarct area was the same at 24hr post MCAO (Figure 3A). Between 1 to 7d post MCAO, infarct area in PirB KO mice decreased significantly (by 51%), as assessed by Cresyl violet staining. Since Cresyl violet stains acidic cellular components, particularly polyribosomes (Tureyen et al., 2004), the decrease in infarct area in KO mice may reflect recovery of protein synthesis in stressed cells within the penumbra. In KbDb KO mice at 7d post MCAO, infarct area is also reduced compared to WT (KbDb KO: 32% vs WT: 44%; p=0.03), but to a lesser degree than in PirB KO. Together, these data suggest that knock out of PirB has a similar or even greater effect on infarct size than when Kb and Db are deleted.
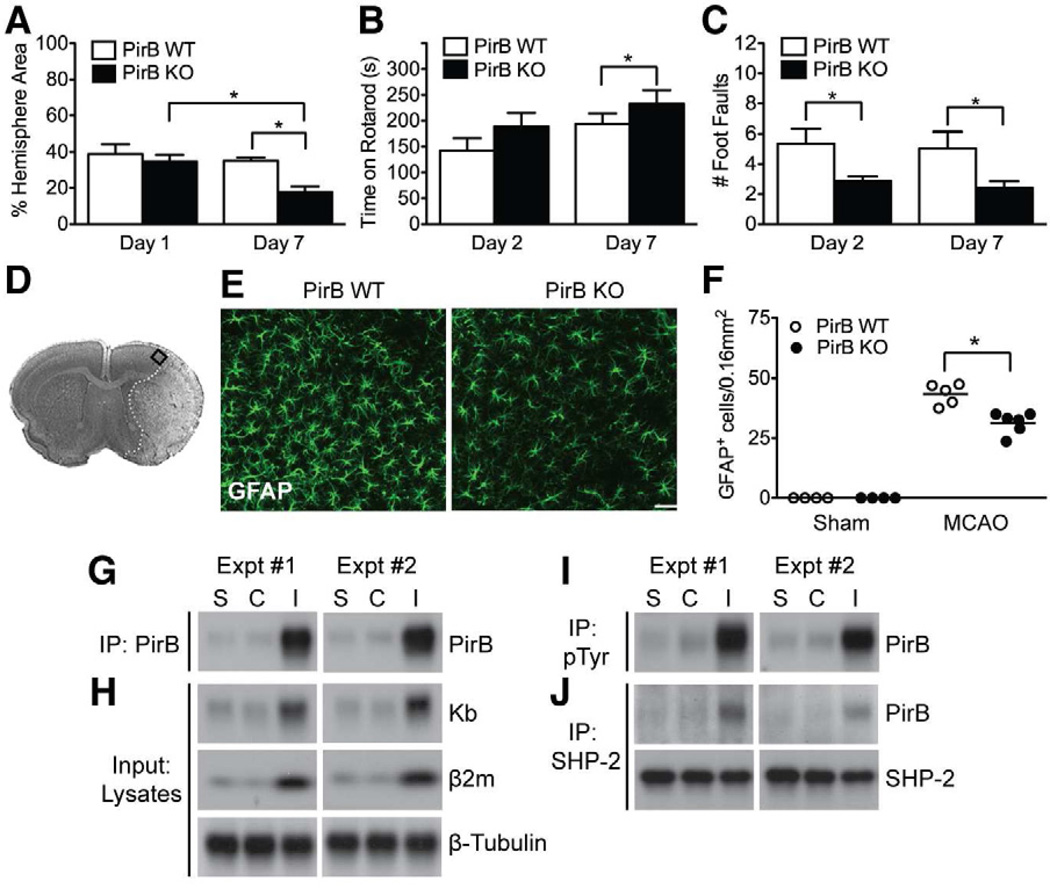
Figure 3.
PirB KO enhances recovery and dampens astrocytic response; MCAO increases PirB proximal signaling. (A) KO animals have smaller infarct areas 7d post MCAO (n=10 per genotype), but not 24hr (n=7 for WT, n=8 for KO). Infarct area decreased 51% in KO mice at 7d vs 1d post MCAO (p=0.003). (B, C) KO animals outperform WT cohorts on motor tasks after MCAO. Average Rotarod (B) and Foot Fault (C) performances at 2d and 7d post MCAO. N=10–20 animals per condition. Rotarod performance of PirB WT was not significantly different at 7d compared to 2d, p=0.61. Data represented as mean +/− SEM. (D– F) Astroglial activation is diminished in cortical penumbra 7d post MCAO in PirB KO mice. (D) Cresyl violet stained section; rectangle indicates cortical area used for cell counting. (E) Representative images of astrocytes immunostained for GFAP at a distance of 400μm from the ischemic core. (F) Quantitation of GFAP+ cells in WT vs KO. *P<0.05. N=5 animals per experimental condition. Scale bars, 50μm. (G– J) Enhanced PirB expression and PirB proximal signaling following MCAO. (G) Protein extracts from 7d post MCAO (M) or sham (S) brains were immunoprecipitated with anti-PirB antibody followed by Western blotting for PirB. In each experiment (n=2), 4 hemispheres from sham (S1–4; S5–8) or MCAO brains (M1–4; M5–8) were pooled for protein extraction. C=contralateral to injury; I=ipsilateral to injury. (H) Expression of Kb, β2m and β-tubulin in brain lysates from (G). (I, J) Aliquots of protein extracts from (G) were subjected to immunoprecipitation for phospho-tyrosine (I) or SHP-2 (J) to assess levels of tyrosine phosphorylation of PirB, and SHP-2 recruitment to PirB. See also Figures S1, S3.
To determine if protection in PirB KO mice is also associated with improved motor performance, animals were assessed on Rotarod and Foot Fault. Prior to MCAO, KO mice learned faster and remained on the rod longer than WT over the course of the pre-training period (p< 0.0001; Figure S1E; no genotype differences were observed with training on the Foot Fault task: p=0.45; Figure S1F). Following MCAO, PirB KO mice performed better than WT on Rotarod (p=0.001) and Foot Fault (p=0.02) by 7d post treatment; even at 2d post MCAO, KO mice performed better than WT on Foot Fault (p=0.01, Figures 3B, C). Together, these observations in PirB KO mice are strikingly similar to those for KbDb KO mice, suggesting that knocking out a receptor for these 2 MHCI molecules results in strong neuroprotection most apparent 7d post MCAO.
Astrocyte activation is reduced following MCAO in PirB KO mice
Experimentally and clinically, stroke is followed by an inflammatory response characterized by production of inflammatory cytokines, infiltration of leukocytes and monocytes, and activation of resident glial cells (Choe et al., 2011; Offner et al., 2006; Nedergaard and Dirnagl, 2005). While activated astrocytes and microglia can exert beneficial effects, inflammation can also compromise neuronal survival and worsen ischemic damage. To determine if PirB deletion alters glial activation 7d post MCAO, brain sections were immunolabeled for astrocyte and microglia/macrophage markers and the number of activated cells in the cortical penumbra (Figure 3D) were counted. The number of reactive astrocytes decreased in PirB KO vs WT (GFAP+: 26% decrease, p=0.001; Figure 3E, F; Vimentin+: 32% decrease, p=0.03; Figure S3A, C). In contrast, the number of microglia did not differ from WT (Figure S3B, D). Thus, the neuroprotection afforded by PirB deletion appears to be accompanied by diminished numbers of activated astrocytes but not microglia in the penumbra area. A decrease in Vimentin+ and GFAP+ reactive astrocytes has been associated with better regenerative capacity following spinal cord or traumatic brain injury (Menet et al., 2003; Wilhelmsson et al., 2004). The diminished astrocytic, but not microglial activation might reflect the contribution of astrocytes to synaptic plasticity and their close association to synapses (Beattie et al., 2002; reviewed in Giaume et al., 2010), where PirB and MHCI are thought to be located (Needleman et al., 2010; Shatz, 2009). Together, these observations suggest that the astrocytic response following MCAO in part relies upon PirB signaling.
PirB expression and immediate downstream signaling are increased following MCAO
Because outcome is improved in PirB KO mice, we assessed if PirB is upregulated in WT mice following MCAO. PirB protein levels are markedly increased in the damaged hemisphere post MCAO, compared to the undamaged contralateral side or to sham controls (Figure 3G). Western blot analysis (input) verified the increase in Kb protein level in the damaged hemisphere (Figure 3H), similar to that observed in synaptosomes (Figure 2). In the damaged hemisphere, there is also a significant increase in β2m (Figure 3H). Since β2m is necessary for stable cell surface expression of the majority of MHCI proteins (Huh et al., 2000), it is likely that the increase in levels of Kb and Db is accompanied by increased cell surface expression following MCAO.
MHCI binding to PirB facilitates tyrosine phosphorylation of PirB on cytoplasmic ITIM motifs, which in turn recruits SHP-1 and SHP-2 phosphatases to PirB and modulates downstream signal transduction pathways (Nakamura et al., 2004; Takai, 2005). Therefore, we examined if upregulation of Kb, Db, β2m and PirB following MCAO is associated with known PirB signaling components in brain (Syken et al., 2006). Both PirB phosphorylation and SHP-2 recruitment to PirB increase significantly following MCAO (Figure 3I, J). Thus, a notable consequence of MCAO is to engage the first key steps in PirB downstream signal transduction.
Diminished cell death in PirB and KbDb KO in an in vitro model of ischemia
PirB and KbDb KO mice have smaller infarcts and better motor recovery, suggesting that these molecules exert their deleterious effects in WT both by causing more cell death within the infarct and by limiting compensation via synaptic plasticity in surviving circuits. Because Kb, Db and PirB also function in the immune system, the smaller infarcts seen in KO mice might arise from a dysregulated immune response (Maenaka and Jones, 1999; Takai, 2005), rather than from absence of expression in the CNS. To examine this possibility, an in vitro model of ischemia was employed: 15 min of oxygen glucose deprivation (OGD) of hippocampal organotypic slice cultures. The slices contain resident astrocytes and microglia but few if any peripheral immune cells. Circulating neutrophils, which might be present initially within these slices, have life spans of only 8–20 hr and so are gone prior to experiments, which start after 2 weeks in vitro; no new peripheral immune cells can infiltrate in response to injury. The extent of neuronal cell death was assessed directly in CA1 using propidium iodide (PI) immunofluorescence 24hr after OGD insult (Ouyang et al., 2007; Figure 4A). Despite the absence of peripheral immune system infiltration, cultures from KbDb WT mice sustained significant damage, while cell death was significantly reduced in cultures from KbDb KO mice, as indicated by a 55% decrease in average PI fluorescence intensity (KbDb KO: 38±1.9 median pixel intensity vs WT: 89±3.4; p<0.0001, Figure 4A). Cultures from PirB KO mice also had less cell death than WT, visualized as a 54% decrease in average PI fluorescence intensity compared to WT (PirB KO: 64±3.7 median pixel intensity vs WT: 141±7.3; p<0.0001, Figure 4B). These observations demonstrate that in vitro as well as in vivo PirB, Kb and Db contribute to damage following ischemia. In addition, results suggest that in vivo the absence of these molecules in brain cells (neurons and/or resident glia), rather than just in the peripheral immune system, is neuroprotective.
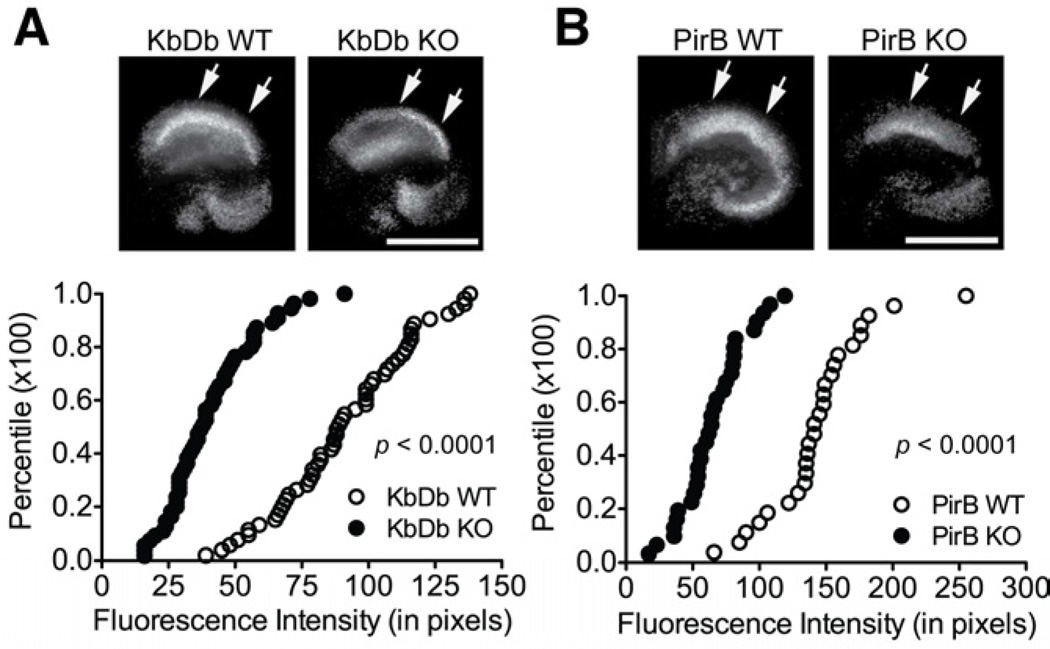
Figure 4.
Diminished neuronal death in CA1 region of hippocampal organotypic slice cultures from KO mice following in vitro ischemia. PI-immunofluorescence (bright white) in CA1 region (top) and cumulative histograms of neuronal damage as measured by fluorescence intensity at 24hr after OGD (bottom) show that deletion of Kb and Db (A), or PirB (B), reduces cell death compared to WT. For cumulative histograms, each point is one slice (KbDb KO: n=55 vs WT n=52 slices from 5 animals; PirB KO: n=27 vs WT n=30 slices from 4 animals). Arrows indicate measured CA1 area. Scale bars, 800μm.
Increased crossed projections of CST axons following MCAO in PirB KO mice
Functional recovery following stroke is associated with axonal plasticity as well as with altered gene expression profiles (Lee et al., 2004; Li et al., 2010; Netz et al., 1997; Stinear et al., 2006). Cortical injury increases axonal projections from Layer 5 (L5) pyramidal neurons in motor cortex opposite the lesioned hemisphere into denervated ipsilesional subcortical targets, including red nucleus and spinal cord (Lee et al., 2004; Naus et al., 1985; Rouiller et al., 1991). L5 pyramidal neurons express PirB, and protein can be detected in descending corticofugal axon tracts during development, as well as in cortical neuron growth cones in vitro (Syken et al., 2006). Deletion of PirB increases axon outgrowth on myelin inhibitory substrates in vitro (Atwal et al., 2008). Consequently it is possible that enhanced recovery from MCAO in PirB KO mice arises in part from an enhanced capacity of L5 pyramidal axons descending from the intact hemisphere to cross the midline into denervated territory.
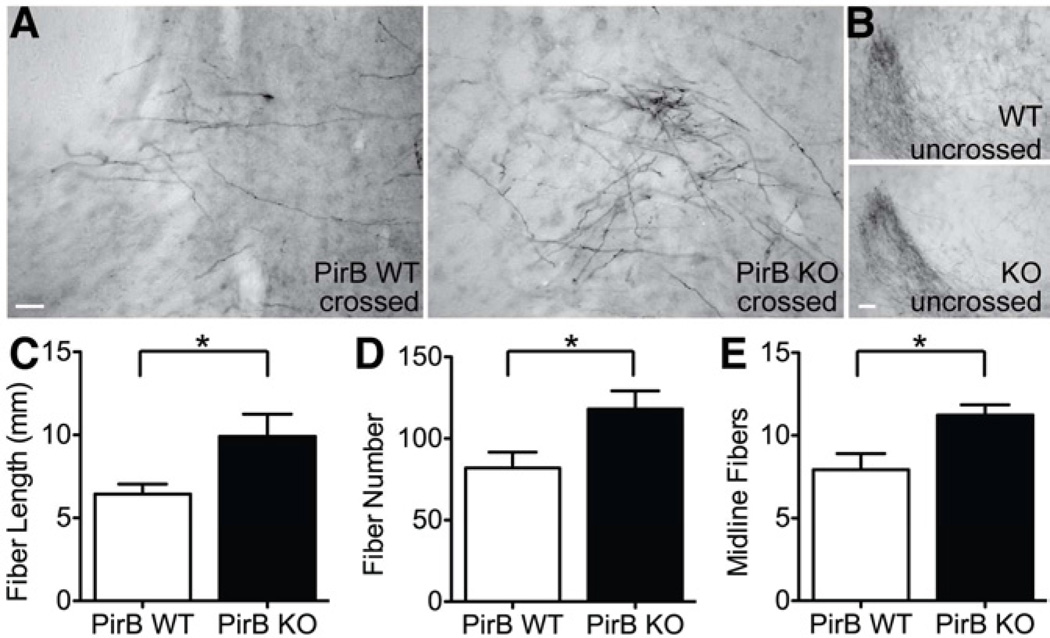
To determine if there are a greater number of crossed corticospinal tract (CST) fibers, the anterograde tracer BDA was injected into contralateral (undamaged hemisphere) motor cortex 14d post MCAO in PirB KO and WT to label the descending axons from L5 pyramidal neurons in the intact hemisphere. BDA-positive fibers were examined in the red nucleus ipsilateral (Figure 5A) or contralateral (Figure 5B) to the injury. In the ipsilateral red nucleus of PirB KO mice, there was an increase in all measured parameters of crossed axons: fiber length (Figure 5C, 52.3% increase in KO, p=0.032), fiber number (Figure 5D, 44.2% increase in KO, p=0.036) and fibers crossing the midline (Figure 5E, 41.8% increase in KO, p=0.024) were greater than in lesioned WT controls. To exclude the possibility that the increase in BDA-positive fibers was due to better labeling in KO than in WT mice, mean pixel intensity of BDA labeling in contralateral red nucleus was calculated. No difference was seen between KO and WT (WT=181.4±3.1, KO=175.8±4.1; p=0.30). The increase in labeled fibers in PirB KO mice is also unlikely to be due to a difference in infarct size, because average infarct index between WT and KO was not different at the conclusion of the tract tracing experiment (WT index=14.5±6.6, KO index=12.4±5.3, p=0.813). The increase in crossed CST fibers from intact motor cortex terminating within the denervated red nucleus in PirB KO mice could account for their improved behavioral outcome post MCAO and suggests that L5 pyramidal neurons in these mice have greater axonal plasticity in response to stroke.
Figure 5.
Increased corticospinal tract fibers in PirB KO vs WT mice 28d post MCAO. (A) Representative images of BDA-labeled crossed CST fibers originating in undamaged motor cortex and terminating in the red nucleus on the damaged side. (Midline is to right of each image.) Note more extensive labeling in KO. (B) The uncrossed CST projection to the red nucleus terminating on the undamaged side. (Midline is to left of each image.) Note similarity of labeling in KO vs WT. (C–E) Quantification of crossed fibers from undamaged CST by (C) total fiber length (WT=6.5±6.0mm, KO=9.9±1.3mm; p=0.032), (D) fiber number (WT=81.9±9.7, KO=118.1±11.1; p=0.036), or (E) number of fibers crossing the midline (WT=7.9±1.0, KO=11.2±0.6; p=0.024). N=5 KO, n=6 WT, 2–3 sections per animal analyzed. Scale bars, 100μm. Data are represented as mean +/− SEM.
Discussion
Here we show significant neuroprotection in the absence of either the innate immune receptor PirB or its two MHCI ligands Kb and Db using in vivo and in vitro ischemia models. Motor performance in KO mice recovered to a greater degree than in WT and infarct area was smaller in KO but only after 7d and not 24hr post MCAO. This delay is consistent with the idea that mechanisms of synaptic plasticity and functional recovery take time and may be more fully engaged in KO mice.
RT qPCR and Western blotting revealed dramatically increased expression levels of Kb and Db in the damaged hemisphere of WT mice. β2m protein, the light chain that is co-expressed with MHCI molecules (Zijlstra et al., 1990), is also elevated following MCAO, implying that there is an increase in stable cell surface expression of MHCI protein. Because PirB expression, phosphorylation and its interaction with SHP-2 are also increased, these observations argue mechanistically for an increase in signaling cascades downstream of the PirB receptor. Together, these experiments identify an entirely new set of molecules that, when present, exacerbate damage caused by stroke and when removed, permit more extensive recovery.
The greater recovery in PirB vs KbDb KO mice fits well with a model in which PirB binds not only Kb and Db, but also other ligands. In addition to classical MHCIs, PirB is also thought to bind Nogo in collaboration with the Nogo receptor (NgR) (Atwal et al., 2008), which itself cannot signal (Fournier et al., 2002). Mice lacking Nogo or NgR, like PirB mice, have enhanced synaptic plasticity (McGee et al., 2005), and blocking Nogo function also enhances recover following MCAO (Lee et al., 2004). Thus, deletion of PirB would be expected to have a larger effect than deleting only a subset of ligands. It will be worthwhile to explore PirB interaction with other ligands as well as receptors in the context of neuroprotection from stroke. An important implication of the findings reported here is that new avenues of therapy following stroke may be available because PirB has only a limited number of homologues in humans, members of the LILRB family (Takai, 2005). As a key step, it will be necessary to explore if acute blockade of PirB or LILRBs can also lead to neuroprotection.
Increased CST projection in PirB KO can account for enhanced functional recovery
Following stroke, neurons in undamaged cortical regions extend their axons into damaged regions and become responsive to motor or sensory functions perturbed by injury (Lee et al., 2004; Netz et al., 1997). In PirB KO mice, an increased number of midline crossing fibers from the undamaged corticospinal tract were seen extending into the denervated red nucleus 28d post MCAO. These observations support previous studies showing that PirB and MHCI ligands limit axonal outgrowth in development and regeneration following injury in vitro and in vivo (Atwal et al., 2008; Fujita et al., 2011; Washburn et al., 2011; Wu et al., 2011). In vivo, PirB downstream signaling inhibits Trk receptors that function to promote axonal outgrowth; KO of PirB increases TrkB signaling and neurite outgrowth following optic nerve injury (Fujita et al., 2011). However, our results contrast with recent studies reporting no difference in PirB KO CST axonal projections using a traumatic brain or spinal cord injury model (Nakamura et al., 2011; Omoto et al., 2010). Note that these studies used entirely different injury paradigms as well as a different PirB KO mouse. Our study suggests that the increased number and length of CST fibers from undamaged motor cortex extending into the denervated red nucleus can serve as a structural substrate for the improved recovery observed in PirB KO mice following MCAO.
Is protection due to changes in immune response, in neural plasticity or both?
In the healthy brain, neurons express both MHCI and PirB, with MHCI protein detected at synapses [(Datwani et al., 2009; Needleman et al., 2010); Figure 2]. Genetic deletion of either Kb and Db or PirB results in enhanced synaptic plasticity in visual cortex, hippocampus and cerebellum, in development and in adulthood (Datwani et al., 2009; Huh et al., 2000; McConnell et al., 2009; Syken et al., 2006), consistent with the proposal that MHCI and PirB receptor signaling limit synaptic plasticity in healthy brain (Shatz, 2009). Thus, the significant elevation of MHCI and PirB expression, as well as PirB proximal signaling components following MCAO (Figure 2, 3), could reduce synaptic plasticity of surviving neurons and circuits, thereby limiting functional recovery. Indeed, cellular correlates of synaptic plasticity such as LTP are blunted or absent after MCAO (Sopala et al., 2000; Wang et al., 2005). Following MCAO, neurons are the chief cell type in the brain in which MHCI expression is upregulated as identified by colocalization of the neuronal marker NSE with the OX18 antibody, known to recognize MHCIs in neurons and at synapses in rat and mouse [(Datwani et al., 2009; Needleman et al., 2010; Neumann et al., 1995); Figure 2]. An increase in Kb protein in synaptosomal preparations was also observed, consistent with the possibility that synaptic plasticity may be diminished after MCAO in WT mice. These biochemical preparations include not only pre- and postsynaptic membranes, but could contain glial processes that enwrap synapses, so it is possible that upregulation also reflects a glial contribution. However, EM studies of MHCI protein in healthy brain sections show localization primarily at synaptic and subsynaptic neuronal membranes (Needleman et al., 2010), implying that neuronal MHCI can be upregulated.
MHCIs and PirB are also normally expressed in the peripheral immune system (Takai, 2005). KbDb KO mice have compromised adaptive immune systems due to dampened CD8 T-cell responses (Schott et al., 2002). In contrast, PirB KO mice have intact, even hyperactive adaptive immune systems (Takai, 2005). These diametrically opposed peripheral immune responses are not easily reconciled with the observations here that ablation of either PirB or MHCI lead to neuroprotection. The fact that these molecules are expressed and signal in neurons suggests that neuroprotection is at least in part brain-specific. This conclusion is consistent with the OGD experiments using hippocampal slice cultures prepared from healthy brain, which lack functioning vasculature and in which peripheral immune cells cannot participate. Neurons in this model are thought to die as a consequence of apoptosis and necrosis (Meloni et al., 2011). The KO slice cultures suffered less neuronal cell death than WT cultures subjected to the same duration of OGD, consistent with the idea that the function of these molecules in the brain—in neurons or possibly other resident glial or microglia—normally contributes to damage following stroke. Future experiments involving cell type-specific knock out mice will help dissect the relative contribution of peripheral immune cells, neurons and brain glia to damage and impaired recovery.
Experimental Procedures
Animals
KbDb KO mice, offspring of breeding pairs on a C57BL/6 background generously provided by H. Ploegh (Cambridge, MA) (Vugmeyster et al., 1998; Ziskin et al., 2007). C57BL/6 (i.e., KbDb WT) controls were purchased (Charles River). PirB KO and PirB WT controls were previously generated in C. Shatz’s lab (Syken et al., 2006). Mice were maintained in a pathogen-free environment. All experiments using animals were performed blind to genotype and in accordance with a protocol approved by the Stanford University animal care and use committee and in keeping with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Focal Cerebral Ischemia (MCAO)
Transient ischemia was induced using the suture occlusion technique, as previously described (Han et al., 2009), with slight modifications, in male mice (P60–90). This age was chosen as it is beyond the developmental critical periods but the animals are still relatively young adults.
See Supplemental Information for complete details of Methods
Supplementary Material
Acknowledgements
We thank Nora Sotelo and Peggy Kemper for help with lab logistics and mice breeding, Dr. Hanmi Lee and Yoon Kim for additional troubleshooting for qPCR reactions, and Sarah Cheng for genotyping assistance. The PirB mutant mice were generated by Dr. J. Syken in the Shatz lab. Thanks also to Dr. Hidde Ploegh, MIT, for KbDb−/− mice. This work was supported by NIH Grants MH071666, EY02858, the Mathers Charitible Foundation and the Ellison Foundation to CJS, a National Defense Science & Engineering Graduate Fellowship (NDSEG) and National Science Foundation Graduate Research Fellowship (GRFP) to JA and NIH grants RO1 GM49831 and NS 053898 to RGG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol. Dis. 2010;37:259–266. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol. Dis. 2001;8:910–922. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- Choe CU, Lardong K, Gelderblom M, Ludewig P, Leypoldt F, Koch-Nolte F, Gerloff C, Magnus T. CD38 exacerbates focal cytokine production, postischemic inflammation and brain injury after focal cerebral ischemia. PLoS ONE. 2011;6:e19046. doi: 10.1371/journal.pone.0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Gould G, Wang X, Strittmatter SM. Nogo and the Nogo-66 receptor. Prog. Brain Res. 2002;137:361–369. doi: 10.1016/s0079-6123(02)37027-4. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Endo S, Takai T, Yamashita T. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO. 2011:1–13. doi: 10.1038/emboj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl. Acad. Sci. USA. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han RQ, Ouyang YB, Xu L, Agrawal R, Patterson AJ, Giffard RG. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth. Analg. 2009;108:280–287. doi: 10.1213/ane.0b013e318187ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J. Cereb. Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Chotiner JK, Watson JB. Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J. Neurosci. Methods. 1997;77:151–156. doi: 10.1016/s0165-0270(97)00120-9. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST. An age-related sprouting transcriptom provides molecular control of axonal sprouting after stroke. Nat. Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenaka K, Jones EY. MHC superfamily structure and the immune system. Curr. Opin. Struct. Biol. 1999;9:745–753. doi: 10.1016/s0959-440x(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Matsushita H, Endo S, Kobayashi E, Sakamoto Y, Kobayashi K, Kitaguchi K, Kuroki K, Söderhäll, Maenaka K, Nakamura A, Strittmatter SM, Takai T. Differential but competitive binding of Nogo protein and class i major histocompatibility complex (MHCI) to the PIR-B ectodomain provides an inhibition of cells. J. Biol. Chem. 2011;286:25739–25747. doi: 10.1074/jbc.M110.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc. Natl. Acad. Sci. USA. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni BP, Meade AJ, Kitikomolsuk D, Knuckey NW. Characterisation of neuronal cell death in acute and delayed in vitro ischemia (oxygen-glucose deprivation) models. J. Neurosci. Methods. 2011;195:67–74. doi: 10.1016/j.jneumeth.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Menet V, Prieto M, Privat A, Ribotta G. Axonal plasticity and functional recovery after spinal cord injuury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl. Acad. Sci. USA. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Kobayashi E, Takai T. Exacerbated graft-versus-host disease in Pirb−/− mice. Nat. Immunol. 2004;5:623–629. doi: 10.1038/ni1074. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T. Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J. Biol. Chem. 2011;286:1876–1883. doi: 10.1074/jbc.M110.163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus C, Flumerfelt BA, Hrycyshyn AW. An anterograde HRP-WGA study of aberrant corticorubral projections following neonatal lesions of the rat sensorimotor cortex. Exp. Brain Res. 1985;59:365–371. doi: 10.1007/BF00230916. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc. Natl. Acad. Sci. USA. 2010;107:16999–17004. doi: 10.1073/pnas.1006087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Neumann H, Cavalie A, Jenne DE, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J. Cereb. Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Omoto S, Ueno M, Mochio S, Takai T, Yamashita T. Genetic deletion of paired immunoglobulin-like receptor B does not promote axonal plasticity or functional recovery after traumatic brain injury. J. Neurosci. 2010;30:13045–13052. doi: 10.1523/JNEUROSCI.3228-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J. Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagina G, Eysel UT, Jancke D. Strengthening of lateral activation in adult rat visual cortex after retinal lesions captured with voltage-sensitive dye imaging in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:8743–8747. doi: 10.1073/pnas.0900068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl F, Lidman O. Neuroinflammation in the rat--CNS cells and their role in the regulation of immune reactions. Immunol. Rev. 2001;184:212–225. doi: 10.1034/j.1600-065x.2001.1840119.x. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Liang FY, Moret V, Wisendanger M. Trajectory of redirected corticospinal axons after unilateral lesion of the sensorimotor cortex in neonatal rat; a phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Exp. Neurol. 1991;114:53–65. doi: 10.1016/0014-4886(91)90084-p. [DOI] [PubMed] [Google Scholar]
- Schott E, Bertho N, Ge Q, Maurice MM, Ploegh HL. Class I negative CD8 T cells reveal the confounding role of peptide-transfer onto CD8 T cells stimulated with soluble H2-Kb molecules. Proc. Natl. Acad. Sci. USA. 2002;99:13735–13740. doi: 10.1073/pnas.212515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopala M, Frankiewicz T, Parsons C, Danysz W. Middle cerebral artery occlusion produces secondary, remote impairment in hippocampal plasticity of rats - involvement of N-methyl-D-aspartate receptors? Neurosci. Lett. 2000;281:2–3. doi: 10.1016/s0304-3940(00)00829-6. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2006;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuru Y, Fukumoto D, Yoshitomo M, Nemoto T, Tsukada H, Nabekura J. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J. Neurosci. 2009;29:10081–10086. doi: 10.1523/JNEUROSCI.1638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thams S, Oliveira A, Cullheim S. MHC class I expression and synaptic plasticity after nerve lesion. Brain Res. Rev. 2008;57:265–269. doi: 10.1016/j.brainresrev.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Sailor KA, Dempsey RJ. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci. Methods. 2004;139:203–207. doi: 10.1016/j.jneumeth.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H. Major histocompatibility complex (MHC) class I KbDb −/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc. Natl. Acad. Sci. USA. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kee N, Preston E, Wojtowicz JM. Electrophysiological correlates of neural plasticity compensating for ischemia-induced damage in the hippocampus. Exp. Brain Res. 2005;165:250–260. doi: 10.1007/s00221-005-2296-8. [DOI] [PubMed] [Google Scholar]
- Washburn LR, Zekzer D, Eitan S, Lu Y, Dang H, Middleton B, Evans CJ, Tian J, Kaufman DL. A potential role for shed soluble major histocompatability class I molecules as modulators of neurite outgrowth. PLoS ONE. 2011;6:e18439. doi: 10.1371/journal.pone.0018439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Bilousova T, Escande-Beillard N, Dang H, Hsieh T, Tian J, Kaufman DL. Major histocompatability complex class I-mediated inhibition of neurite outgrowth from peripheral nerves. Immunol. Lett. 2011;135:118–123. doi: 10.1016/j.imlet.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahachi H, Marik SA, McManus JN, Denk W, Gilbert CD. Rapid axonal sprouting and pruning accompany functional reorganization in primary visual cortex. Neuron. 2009;64:719–729. doi: 10.1016/j.neuron.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.