Abstract
We present the clinical case of a patient with a spheno-orbital meningioma. Literature review of the treatment options, including the application of piezoelectric or ultrasound surgery and orbital reconstruction after meningioma resection, is also presented. Complete resection was performed by means of a frontotemporal craniotomy and an orbitozygomatic approach. Piezoelectric osteotomy was used around the optic nerve canal and the superior orbital fissure to minimize the damage to soft tissues. Orbital wall reconstruction was done using a titanium mesh previously premolded using a skull model. The superior orbital rim was reconstructed with calvarial bone grafts, and the sphenotemporal bone defect was covered with a titanium mesh cranioplasty. Ultrasonic vibrations to perform osteotomies in craniofacial surgery provide an interesting tool to reduce damage to surrounding soft tissues. Reconstruction of the roof and lateral orbital wall with premolded titanium meshes with a skull model is a safe and easy method to achieve a good orbital reconstruction and to avoid secondary sequelae.
Keywords: Orbital meningioma, piezosurgery, premolded titanium mesh, orbital reconstruction
Meningiomas are the most common benign intracranial lesions and the second most common intracranial tumors after gliomas, representing ~18% of all intracranial neoplasms.1 Spheno-orbital meningiomas represent up to 9% of all intracranial meningiomas.2 Meningiomas extending into the orbital region may be classified as primary, if they come from the optic nerve sheath, or as secondary meningiomas (this is the most common situation), if their origin is the inner and outer aspects of the sphenoid wing. Bone is involved in ~30% of these tumors; 12% may be primarily intraosseous and their origin is from within the orbital bones.3 Meningioma en plaque is a subgroup of meningiomas defined by a sheetlike appearance that infiltrates dura mater and eventually bone.4 The amount of bone infiltrated (hyperostosis) is often disproportionate compared with the relative small amount of intracranial tumor.5
Nowadays, most authors advocate for an early and aggressive surgical resection of spheno-orbital meningiomas to prevent recurrences.6,7 Due to their proximity to the cavernous sinus, carotid artery, optic nerve, and other important structures, these lesions are often unresectable when they recur. Actually, one reason for recurrence is the fear to produce iatrogenic morbidity or even mortality associated with a too-radical tumor resection in this area.8 Ultrasound piezosurgery, or ultrasound bone cutting, was initially presented by Vercelloti et al in 20019 as a new technique to cut the bone without damaging the soft tissue in the maxillary sinus augmentation procedure. Although piezosurgery has been mainly used in oral surgery, in recent years, applications in neurosurgery and craniofacial surgery have been proposed.10,11,12
Radical surgical resection of these tumors must be followed by reconstruction of the cranio-orbital skeleton to prevent enophthalmos and postoperative cosmetic sequelae.3,6
We present the clinical case of a patient with a spheno-orbital meningioma en plaque. We discuss the treatment, emphasizing both the application of the piezoelectric osteotomy in skull base surgery and the fronto-orbital reconstruction.
CASE REPORT
A 52-year-old woman was referred to our outpatient clinic presenting a left, slowly progressive, non-pulsating, irreducible proptosis. Campimetry showed a slight inferonasal defect in the left eye, but no other ophthalmologic or neurological clinical signs were observed. Neuroimaging with computed tomography (CT) and magnetic resonance imaging (MRI) revealed a hyperostotic lesion in the left sphenoid wings, roof, and lateral wall of the orbit and the squama of the temporal bone (Fig. 1). Initial suspected diagnosis was fibrous dysplasia. However, fat-suppression and postcontrast MRI sequences demonstrated a dural enhancement nearby the sphenoid wing, and an open biopsy by means of a blepharoplasty approach confirmed the diagnosis of meningioma.
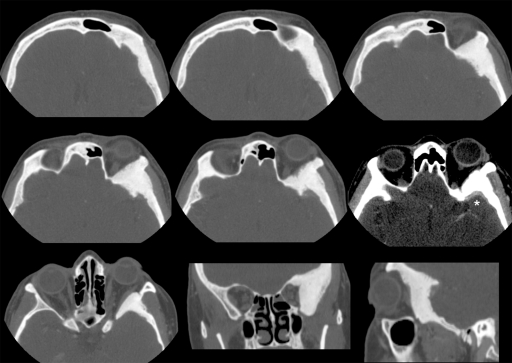
Figure 1.
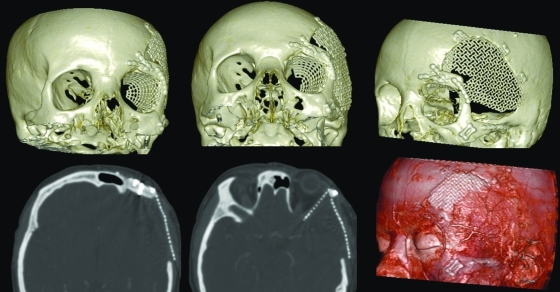
Axial, coronal, and sagittal views of the preoperative computed tomography scan showing the hyperostotic lesion located in the left spheno-orbital region. The window of one of the axial sections has been softened to show the typical meningeal contrast enhancement adjacent to the sphenoid wing (*).
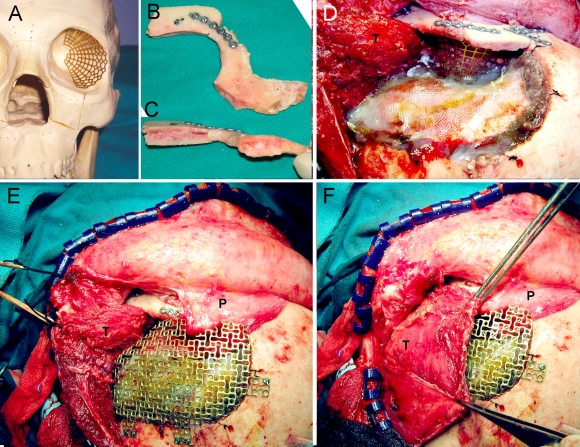
The patient underwent surgery. Using a bicoronal approach, an inferiorly based pericranial flap was elevated and subfascial dissection was done, protecting the frontal branch of the facial nerve, to expose the superior and lateral orbital rims and the zygomatic arch. The temporal muscle was detached and a frontotemporal/pterional craniotomy and an orbitozygomatic approach were performed (Fig. 2). All the hyperostotic bone was resected, including excision of the orbital roof to the anterior clinoid process and the lateral wall to the inferior orbital fissure. A partial anterior clinoidectomy was performed, and the optic canal and superior orbital fissure were decompressed using the piezoelectric device (Fig. 3). The dura of the temporal fossa was excised as far as possible, up to the superior orbital fissure, cavernous sinus, and infraorbital nerve, and the defect was reconstructed using a hermetic pericranial graft (Fig. 4). The roof and lateral wall of the orbit were reconstructed using a titanium mesh previously premolded in a standard skull model. The superior orbital rim was reconstructed with split cranial bone grafts, which were cut to size. The orbitozygomatic bone was reattached with titanium miniplates and screws. A pericranial flap was brought over the orbit, and the cranial bone defect was reconstructed using a titanium mesh cranioplasty. The temporal muscle was reattached with sutures to the titanium mesh, the temporal fascia was sutured, and the skin was closed in two layers (Fig. 5).
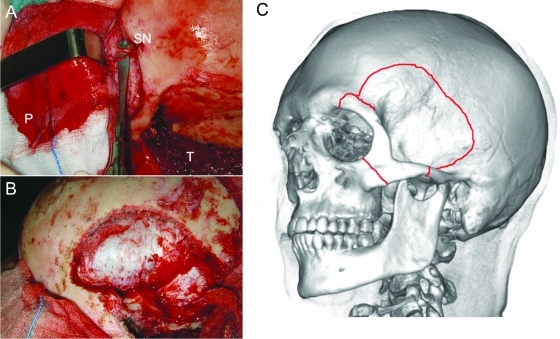
Figure 2.
(A) Intraoperative view after elevating the pericranial flap and detaching the temporalis muscle. (B) Frontotemporal craniotomy. (C) Schematic representation of the craniotomy and the orbitozygomatic osteotomy. P, pericranial flap; T, temporalis muscle; SN, supraorbital nerve.
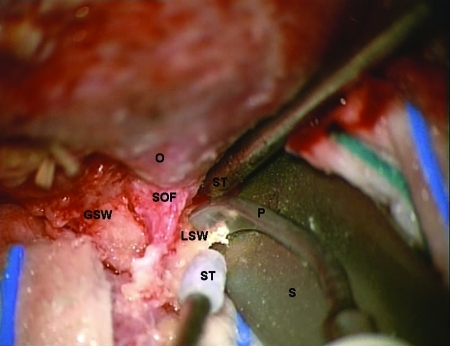
Figure 3.
Intraoperative view under microscopic magnification showing the decompression of the superior orbital fissure with the piezoelectric device. The orbital roof has already been removed. The spatula is protecting the frontal lobe dura. O, orbit; GSW, greater sphenoid wing; SOF, superior orbital fissure; LSW, lesser sphenoid wing; P, piezoelectric device; ST, suction tube; S, spatula.
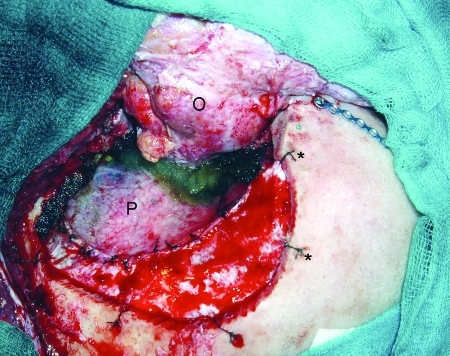
Figure 4.
Operative view after tumor resection. O, orbit; P, pericranial graft. *Dural tenting sutures.
Figure 5.
(A) Titanium mesh blended using a standard skull model. (B and C) Superior orbital rim reconstructed with two calvarial split grafts and fixed to the orbitozygomatic bone. (D) Operative view after placing the titanium mesh and reattaching the orbitozygomatic complex. (E) Titanium mesh for the cranial defect. (F) Temporalis muscle sutured back in place. P, pericranial flap; T, temporalis muscle.
Histological evaluation of the specimen showed infiltration, in both the dura matter and the resected bone, by a grade I meningothelial meningioma (according to the 2007 World Health Organization classification13).
No postoperative complications were observed, and good functional and cosmetic results were achieved correcting proptosis (Fig. 6). The patient had no recurrence after 1-year follow-up (Fig. 7), and the left eye visual field defect was corrected in the campimetry (Fig. 8).
Figure 6.
Clinical pictures of the patient before (A) and 1 year after surgery (B, C, and D).
Figure 7.
Postoperative computed tomography scan after 1-year follow-up.
Figure 8.
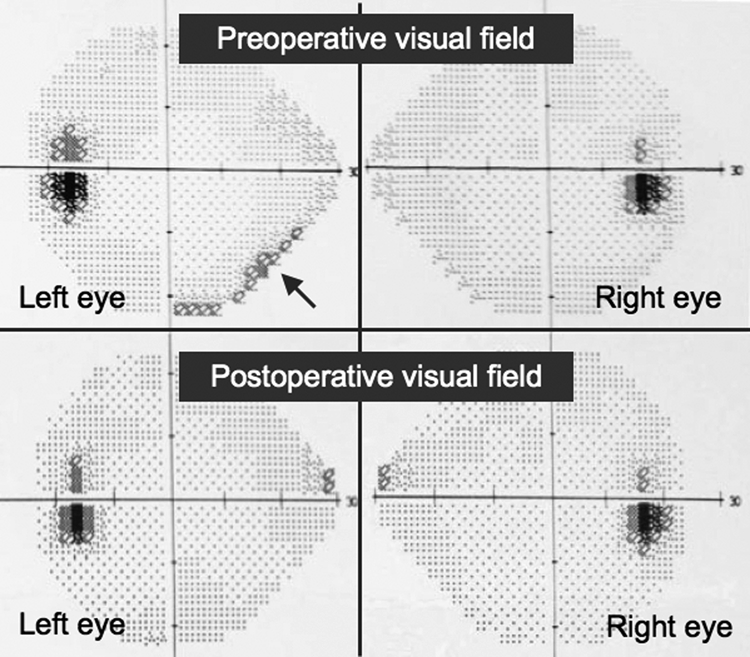
Pre- and postoperative campimetry showing correction of the left eye inferonasal defect.
DISCUSSION
Clinical presentation of orbital meningiomas was initially described by Cushing and Eisenhardt.4 It is mainly characterized by painless progressive proptosis and unilateral visual loss. Meningiomas of the orbit can compress or infiltrate the optic nerve, the intraorbital contents, the cavernous sinus, and the frontotemporal lobes. Other clinical features include optic disc changes, diplopia, headaches, nausea, and vomiting.
Radiological imaging is needed to delimitate the extension of the tumor within the orbit and the intracranial region. Radiological findings in orbital meningiomas may include hyperostosis, thickening of the optic nerve or the “tram track sign” (two strips of lucency around the enlarged optic nerve), and calcification, which can be seen in CT scanning. This may also show additional features including intracranial changes as intracranial mass, cerebral edema, and subdural ossification. MRI may identify smaller tumors and postcontrast, fat-suppressed, T1-weighted MRI can also show meningeal enhancement, helping to delineate the dural and orbital involvement.6,8
Differential diagnosis of orbital meningiomas must be done with lesions involving the optic nerve and adjacent structures3: tumors arising from the optic nerve (meningioma, glioma, neuroma/neurofibroma, metastatic tumors, arachnoid cysts, juvenile xanthogranulomas), inflammatory diseases of the optic nerve (multiple sclerosis optic neuritis, infectious optic neuritis, sarcoidosis), central retinal vein occlusion, tumors arising from adjacent regions (meningioma, glioma, cavernous hemangioma, hemangioblastoma, hemangiopericytoma, bone tumor, malignant sinonasal tumor, metastasis, dermoid tumor), fibrous dysplasia, Paget disease, cholesteatoma, benign mixed lacrimal gland/mucocele, venous varix, arteriovenous malformation, and nonspecific orbital inflammation. Of all these entities, fibrous dysplasia is one of the most common misdiagnosis preoperatively. Maroon et al8 found in the literature that despite complete excision attempts, a 35 to 50% probability of recurrence existed, and that one reason for failure to diagnose this tumor is that it can be confused with other entities such as fibrous dysplasia. Thus, in those cases of spheno-orbital hyperostotic lesions without significant intracranial involvement, which may be confused with fibrous dysplasia, it is especially recommended to perform a gadolinium-enhanced MRI. This resonance usually shows the intracranial dural involvement that is not observed with CT scanning. However, an open biopsy of the hyperostotic bone may also be useful, as in the present case.
Hyperostosis associated with meningiomas is likely caused by tumor invasion, and this is why a complete resection of the hyperostotic bone must be done when possible, as most of the recurrences seem to be due to residual tumor. Bikmaz et al reported 14 patients with hyperostotic sphenoid wing meningiomas completely removed, and only one of them recurred over a mean follow-up of 36 months.6 Because aggressive resection, although difficult, can be achieved with low mortality and morbidity rates, these authors advocate for a complete resection with extensive excision of the involved bone and dura to minimize the chance of recurrence.
To perform a more aggressive, but also safer resection, ultrasound piezosurgery can be used. This is a minimally invasive technique that decreases the damage of surrounding soft tissues. The piezoelectric effect was first described by Jacques and Pierre Curie in 1880, who discovered an unusual characteristic of certain minerals: under a mechanical force, the minerals become electrically polarized. A year later, Gabriel Lippmann discovered the converse piezoelectric effect: when one of those crystalline minerals is exposed to an electric field, it is lengthened and shortened according to the polarity of the field and in proportion to its strength. Devices designed for piezosurgery operate by this inverse effect. Frequencies of 25 to 29 kHz cut only mineralized tissue, and soft tissues are cut at frequencies higher than 50 kHz.12 Piezoelectric or ultrasonic surgery has been applied in periodontology and endodontic surgery, implantology (sinus floor elevation, splitting of crestal bone), orthognathic surgery, rhinoplasty, otologic, cranial base, spine, orthopedic, and hand surgeries.12,14 Authors like Gleizal et al have proposed the use of piezoelectric devices in craniofacial surgery.10,11 They reported the use of piezosurgery to remove the superior orbital roof in 30 cases of craniofaciostenosis, to perform a Le Fort III osteotomy for the Crouzon syndrome in two patients, and to cut the parietal and frontal bone in 30 cases of craniofaciostenosis, decreasing the rate of soft tissues injuries compared with the use of the mechanical saw.11 In the present case, we have found the piezoelectric device very useful to perform a safe osteotomy around the optic nerve canal and the superior orbital fissure. We have also found it useful in frontal, supraorbital rim, and subcranial osteotomies. However, piezosurgery has some limitations to be taken into account and adds an extra cost to the surgery. Nerves or other delicate tissues may be damaged when direct mechanical pressure is applied unintentionally with the tip of the tool. Also, there is a risk of thermal injury if not enough irrigation is used. Between the piezosurgery tips at hand, we chose a short, angled bone harvesting saw for the decompression of the optic nerve and superior orbital fissure (Fig. 3), but longer tips with a more obtuse angle might perform better in this particular situation. Another consideration of available piezosurgery devices is the increase in surgical time in comparison with other techniques, but as noted by Gleizal et al,11 this may be influenced by a learning curve; they have reduced the surgical time to 20% after the use of piezosurgery for over 2 years. The piezoelectric device also needs to be connected to a console with an irrigation unit and a foot pedal, which limits the space in the operating room. As faster and more efficient systems are being developed, we believe piezoelectric surgery will be an essential tool in skull base surgery.
Reconstruction of the roof and/or lateral orbit wall has been considered unnecessary by some authors: Maroon et al8 argue that they had no pulsating exophthalmos in over 200 patients treated with orbital decompression; Schick et al5 reconstruct the lateral orbit wall, but they consider further reconstruction of the orbital roof unnecessary. However, radical resection of the involved bone without proper reconstruction may lead to postoperative aesthetic and functional complications such as meningocele formation, diplopia from extraocular muscle fibrosis, orbital pain, pulsating enophthalmos, and restrictive ptosis.15 As proposed by Gaillard et al,16 an adequate reconstruction of the bony landmarks that have been abolished during the tumor resection may be useful to prevent dissemination of recurrence in multiple directions and to make dissection easier in future surgical procedures.
Different materials have been used to reconstruct the orbit, such as autogenous bone grafts, chondrocostal grafts, titanium meshes, and other alloplastic materials.2,7,16 In comparison with autologous reconstruction, titanium meshes are easier to contour and to adapt to the shape of the orbit, and they have no donor site morbidity. However, titanium meshes may be expensive and sometimes difficult to remove when needed. Reconstruction with autogenous grafts is better indicated in the growing orbit.
Volume symmetry in orbital reconstruction may be difficult to achieve, although it has been shown that a variation of around 10 to 20% of the volume in cases of posttraumatic enophthalmos is clinically imperceptible.17 Regarding orbital wall reconstruction using titanium meshes, Bikmaz et al6 recommend placing the titanium mesh over the bone before removing the tumor and molding it into the original configuration of the patient's skull base for later reconstruction of the orbit. However, the tumor may be modifying the original shape of the orbital walls, making this intraoperative molding difficult. An alternative method is the use of a standard skull model preoperatively to shape and cut the mesh, as described by Andrades et al18 for the treatment of floor and medial orbital wall fractures. They found that the most important factor influencing postoperative volume correction in these cases was the use of this prefabricated mesh. In the present case, the titanium mesh for the orbital roof and lateral wall was premolded in a similar way using a standard skull model, achieving excellent results.
Regarding cranial and orbital reconstruction, computer-assisted surgery provides excellent tools to achieve optimal results. CT digital data allow biomedical modeling with stereolithography and computer-assisted design (CAD) and computer-assisted manufacture (CAM) to fabricate customized implants. CAD-CAM approaches are difficult when the resection and reconstruction are done at the same time because the craniofacial defect is not present in the preoperative CT scan. The use of rapid prototyping to build anatomic orbital models of the patient mirroring the healthy orbit, which was applied in reconstruction after orbital floor fractures, has been described.19 Pritz and Burgett15 recently described their technique using a mirror-image implant from a computer-generated model to reconstruct the spheno-orbital area after meningioma resection. When craniofacial resection and reconstruction are done at the same surgery, we prefer CAD-CAM to draw the area of resection in the preoperative CT scan using the navigation software and then to generate a digital implant mirroring the healthy orbit. We send this digital information to the manufacturer to obtain the patient-specific implant. However, all these CAD-CAM approaches are more expensive than standard titanium meshes, and a longer planning time is needed before surgery.
CONCLUSIONS
A complete resection of spheno-orbital meningiomas requires removing all the hyperostotic bone. New technical procedures, such as piezosurgery, allow performing bone cutting with no or minimal damage to adjacent soft tissues, and it is especially useful to perform the osteotomy around the optic nerve and the superior orbital fissure.
Adequate reconstruction of the craniofacial skeleton after spheno-orbital meningioma excision is recommended to prevent postoperative complications. When the tumor is disturbing the original anatomic shape of the orbit, the use of CAD-CAM implants and computer-assisted surgery are helpful. However, premolding the orbital mesh using a standard skull model is an easy and not expensive alternative to achieve good results and can be done by any surgeon.
References
- Rachlin J, Rosenblum M. Etiology and biology of meningiomas. In: Al-Mefty O, editor. Meningiomas. New York: Raven Press; 1991. pp. 27–36. [Google Scholar]
- Leake D, Gunnlaugsson C, Urban J, Marentette L. Reconstruction after resection of sphenoid wing meningiomas. Arch Facial Plast Surg. 2005;7:99–103. doi: 10.1001/archfaci.7.2.99. [DOI] [PubMed] [Google Scholar]
- Boulos P T, Dumont A S, Mandell J W, Jane J A., Sr Meningiomas of the orbit: contemporary considerations. Neurosurg Focus. 2001;10:E5. doi: 10.3171/foc.2001.10.5.6. [DOI] [PubMed] [Google Scholar]
- Cushing H, Eisenhardt L. Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical End Results. Springfield, IL: Charles C. Thomas; 1938. [Google Scholar]
- Schick U, Bleyen J, Bani A, Hassler W. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104:208–214. doi: 10.3171/jns.2006.104.2.208. [DOI] [PubMed] [Google Scholar]
- Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107:905–912. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- Shrivastava R K, Sen C, Costantino P D, Della Rocca R. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103:491–497. doi: 10.3171/jns.2005.103.3.0491. [DOI] [PubMed] [Google Scholar]
- Maroon J C, Kennerdell J S, Vidovich D V, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994;80:202–208. doi: 10.3171/jns.1994.80.2.0202. [DOI] [PubMed] [Google Scholar]
- Vercellotti T, De Paoli S, Nevins M. The piezoelectric bony window osteotomy and sinus membrane elevation: introduction of a new technique for simplification of the sinus augmentation procedure. Int J Periodontics Restorative Dent. 2001;21:561–567. [PubMed] [Google Scholar]
- Gleizal A, Béra J C, Lavandier B, Béziat J L. [Craniofacial approach for orbital tumors and ultrasonic bone cutting] J Fr Ophtalmol. 2007;30:882–891. doi: 10.1016/s0181-5512(07)74023-8. [DOI] [PubMed] [Google Scholar]
- Gleizal A, Bera J C, Lavandier B, Beziat J L. Piezoelectric osteotomy: a new technique for bone surgery—advantages in craniofacial surgery. Childs Nerv Syst. 2007;23:509–513. doi: 10.1007/s00381-006-0250-0. [DOI] [PubMed] [Google Scholar]
- Labanca M, Azzola F, Vinci R, Rodella L F. Piezoelectric surgery: twenty years of use. Br J Oral Maxillofac Surg. 2008;46:265–269. doi: 10.1016/j.bjoms.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Perry A, Luis D, Scheithauer B, et al. Meningiomas. World Health Organization Classification of Tumors of the Central Nervous System. 4th ed. Lyon, France: IARC; 2007. [Google Scholar]
- Landes C A, Stübinger S, Ballon A, Sader R. Piezoosteotomy in orthognathic surgery versus conventional saw and chisel osteotomy. Oral Maxillofac Surg. 2008;12:139–147. doi: 10.1007/s10006-008-0123-7. [DOI] [PubMed] [Google Scholar]
- Pritz M B, Burgett R A. Spheno-orbital reconstruction after meningioma resection. Skull Base. 2009;19:163–170. doi: 10.1055/s-0028-1096199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard S, Pellerin P, Dhellemmes P, Pertuzon B, Lejeune J P, Christiaens J L. Strategy of craniofacial reconstruction after resection of spheno-orbital “en plaque” meningiomas. Plast Reconstr Surg. 1997;100:1113–1120. doi: 10.1097/00006534-199710000-00004. [DOI] [PubMed] [Google Scholar]
- Schuknecht B, Carls F, Valavanis A, Sailer H F. CT assessment of orbital volume in late post-traumatic enophthalmos. Neuroradiology. 1996;38:470–475. doi: 10.1007/BF00607281. [DOI] [PubMed] [Google Scholar]
- Andrades P, Hernandez D, Falguera M I, et al. Degrees of tolerance in post-traumatic orbital volume correction: the role of prefabricated mesh. J Oral Maxillofac Surg. 2009;67:2404–2411. doi: 10.1016/j.joms.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Kozakiewicz M, Elgalal M, Loba P, et al. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J Craniomaxillofac Surg. 2009;37:229–234. doi: 10.1016/j.jcms.2008.11.009. [DOI] [PubMed] [Google Scholar]