Abstract
Hepatocellular carcinoma (HCC), one of the most prevalent and lethal cancers, has shown an alarming rise in the USA. Without effective therapy for HCC, novel chemopreventive strategies may effectively circumvent the current morbidity and mortality. Oxidative stress predisposes to hepatocarcinogenesis and is the major driving force of HCC. Pomegranate, an ancient fruit, is gaining tremendous attention due to its powerful antioxidant properties. Here, we examined mechanism-based chemopreventive potential of a pomegranate emulsion (PE) against dietary carcinogen diethylnitrosamine (DENA)-induced rat hepatocarcinogenesis that mimics human HCC. PE treatment (1 or 10 g/kg), started 4 weeks prior to the DENA challenge and continued for 18 weeks thereafter, showed striking chemopreventive activity demonstrated by reduced incidence, number, multiplicity, size and volume of hepatic nodules, precursors of HCC. Both doses of PE significantly attenuated the number and area of γ-glutamyl transpeptidase-positive hepatic foci compared with the DENA control. PE also attenuated DENA-induced hepatic lipid peroxidation and protein oxidation. Mechanistic studies revealed that PE elevated gene expression of an array of hepatic antioxidant and carcinogen detoxifying enzymes in DENA-exposed animals. PE elevated protein and messenger RNA expression of the hepatic nuclear factor E2-related factor 2 (Nrf2). Our results provide substantial evidence, for the first time, that pomegranate constituents afford chemoprevention of hepatocarcinogenesis possibly through potent antioxidant activity achieved by upregulation of several housekeeping genes under the control of Nrf2 without toxicity. The outcome of this study strongly supports the development of pomegranate-derived products in the prevention and treatment of human HCC, which remains a devastating disease.
Introduction
Hepatocellular carcinoma (HCC), one of the most lethal cancers, results in >1 million deaths worldwide per year. Most detected HCC cases are found in the developing countries of Asia and Africa, but recent trends show a rapid surge in the occurrence of this disease in Japan, Western Europe as well as North America. The incidence of HCC has dramatically increased in the USA by >70% in the past 25 years (1), with over 24 000 new cases and nearly 19 000 deaths expected in 2010 (2). HCC develops most frequently in a milieu of chronic oxidative stress and hepatic inflammation primarily due to viral infections (hepatitis B and C), alcohol abuse, metabolic diseases and exposure to dietary carcinogens such as aflatoxins and nitrosamines (3–5). Currently available treatment options, such as surgical resection and liver transplantation have their own limitations, and the only drug (sorafenib), approved by the United States Food and Drug Administration for the treatment of advanced HCC, causes serious adverse effects (6). Considering the limited treatment options and dismal prognosis for HCC, chemoprevention has been considered the best strategy to reduce its current morbidity and mortality.
The early stages of human as well as animal hepatocellular carcinogenesis are characterized by the appearance of preneoplastic lesions, including enzyme-altered liver cell foci and nodules, which could be used as early tumor biomarkers for monitoring liver cancer progression as well as chemopreventive efficacies of candidate agents. The initiation–promotion or two-stage hepatocarcinogenesis model in rodents resembles the early events of human HCC. The initiation stage of rat liver cancer can be achieved by the administration of diethylnitrosamine (DENA), a potent hepatotoxic carcinogen and mutagen, which is metabolized to reactive electrophilic species known to form genetic lesions as well as chromosomal aberrations. Administration of promoting agents, for example, phenobarbital (PB), results in selective enhancement of the proliferation and clonal expansion of the initiated hepatocytes (7). The DENA/PB rodent model of hepatocarcinogenesis has proved useful in the elucidation of the potential chemopreventive properties of novel agents of dietary origin (8–11).
The pomegranate (Punica granatum, Punicaceae) fruit is extensively cultivated today throughout the Mediterranean and in Afghanistan, India, China, Russia, Japan, Mexico as well as the USA (mainly in Arizona, California and Texas). Pomegranate’s importance to Buddhism, Christianity, Islam, Judaism and Zoroastrianism has been noted (12). ‘A pharmacy unto itself’ pomegranate has been extensively utilized in the Unani and Ayurvedic systems of medicine for the treatment of various ailments. The ‘superfruit’ pomegranate is gaining tremendous importance because of its potent antioxidant properties (13,14) attributed primarily to polyphenolic constituents, like anthocyanins, hydrolyzable tannins (ellagitannins and gallotannins) and condensed tannins (proanthocyanidins) (13,15,16). Some of these antioxidant constituents are bioavailable and safe (17). The antioxidant activity of pomegranate extracts surpasses that of red wine, green tea, tomatoes and vitamin E (13). Pomegranate extracts and constituents exert numerous beneficial activities, e.g. protection against and/or treatment of cancer, neurological damages, inflammation, ulcers, diabetes, dental disorders, high cholesterol, cardiovascular disease, obesity, bacterial infections, erectile dysfunction and male infertility (18). Accordingly, consumption of pomegranate juice has skyrocketed (17).
Pomegranate suppresses the proliferation of human prostate, breast, lung and colon cancer cells in vitro (16,19). In vivo and ex vivo studies have also shown that pomegranate products prevent and/or reduce chemically induced tumors in skin, breast, lung and colon (20–25). Pomegranate-derived constituents suppress the growth and metastatic potential of lung and prostate tumor cells implanted in rodents (26–31). Finally, a phase II clinical trial linked oral consumption of pomegranate juice with significant prolongation of prostate-specific antigen doubling time for men with rising prostate-specific antigen after surgery or radiotherapy for prostate carcinoma with no serious adverse effects (32).
Nevertheless, the chemopreventive potential of this superfruit has not been yet explored against experimentally induced liver tumorigenesis. Recent studies showed pomegranate fruit and flower extracts to exhibit free-radical scavenging properties with simultaneous potent hepatoprotection against chemically induced liver damage in rodents (33–35). Since oxidative stress is a key modulator of multistage hepatocarcinogenesis (36,37), pomegranate-derived antioxidant constituents may protect against neoplastic hepatocellular transformation. Accordingly, the current study was initiated to investigate the chemopreventive action of pomegranate against environmental and dietary carcinogen DENA-initiated early events of rat hepatocarcinogenesis that closely mimics the human disease. The antioxidant effects of pomegranate and related molecular events during hepatocarcinogenesis were also monitored further to illuminate mechanisms of pomegranate action. This is the first study demonstrating the chemopreventive efficacy of pomegranate against chemically induced hepatic neoplasia, possibly through antioxidant signaling mechanisms.
Materials and methods
Materials
Pomegranate emulsion (PE) was purchased from Rimonest Ltd (Haifa, Israel). The emulsion is a proprietary combination of pomegranate aqueous phase extract and pomegranate seed oil. The aqueous phase was fermented with Rimonest strain 018 Saccharomyces cerevisiae with extracts of pomegranate juice, peels, leaves and flowers, organically grown at Kibbutz Sde Eliahu (near Beit Shan, Israel) in 2007 and 2008, and pomegranate seed oil was obtained by mechanical extrusion of the clean, dried seeds without the addition of heat (cold press). Chemical profiling has thus far revealed a preponderance of mixed octadecatrienoic acids (38), sterols (Wiesman, Z, Lansky, E.P, unpublished data) and steroids, especially 17-alpha-estradiol (39) and the tocol, gamma tocopherol in the lipid phase and gallic acid, 5-hydroxymethylfurfural, ferulic acid, punicalagins A and B, caffeic acid, corilagin, protocatechuic acid, trans-p-coumaric acid and ellagic acid in the aqueous phase (40). The sham emulsion (obtained from Rimonest Ltd) was prepared by emulsifying refined sunflower seed oil with water using a proprietary emulsifying complex. DENA and PB were purchased from Sigma–Aldrich (St Louis, MO), rabbit polyclonal nuclear factor E2-related factor 2 (Nrf2) antibody and ABC staining systems from Santa Cruz Biotechnology (Santa Cruz, CA) and OxyBlot Protein Oxidation Detection kit from Chemicon (Temecula, CA).
Animals and treatment schedule
Male Sprague–Dawley rats, 50–74 g each, were purchased from Harlan Laboratories (Indianapolis, IN). Animal housing, care and experiments were in conformity with the guidelines of the Institutional Animal Care and Use Committee of Northeastern Ohio Universities Colleges of Medicine and Pharmacy. The animals received well defined, Constant Nutrition® formula pulverized diet (Formulab 5008 from LabDiet, St Louis, MO) and drinking water ad libitum. The potential chemopreventive role of PE was investigated using our well established and previously published DENA-initiated and PB-promoted two-stage hepatocarcinogenesis rat model (10), with slight modification (Figure 1A). Following 1 week acclimatization, the rats were randomly divided into five groups of 6–12 animals each. Group A was maintained as untreated normal group. Group B animals were fed a sham emulsion (10 g/kg body wt) through oral gavage (per os) using a feeding needle (Popper & Sons, New Hyde Park, NY) three times a week (Monday, Wednesday and Friday). The remaining three groups were similarly administered (per os) PE at 1 g/kg (group C) or 10 g/kg (groups D and E). These doses were based on a preliminary study that showed inhibition of growth of xenografted tumor in rodents (Wiesman, Z, Lansky, E.P, unpublished data). Following 4 weeks of this regimen, hepatocarcinogenesis was initiated in all animals of groups B, C and D by a single intraperitoneal injection of DENA (200 mg/kg body wt) in peanut oil. Animals of groups A (normal group) and E (PE control) were injected with an equal volume of peanut oil. Following a 2 week recovery, the promoter PB was added into the drinking water of DENA-injected groups (i.e. groups B, C and D) at a concentration of 0.05% wt/vol for 16 consecutive weeks. Feeding of rats with PE (in groups C, D and E) and sham emulsion (in group B) continued throughout the entire experimental period. Food and water intake as well as behavioral changes were monitored twice weekly, and animal body weights recorded every 2 weeks. All animals were killed 18 weeks following DENA or vehicle injection, i.e. at 22 weeks following commencement of the study.
Fig. 1.
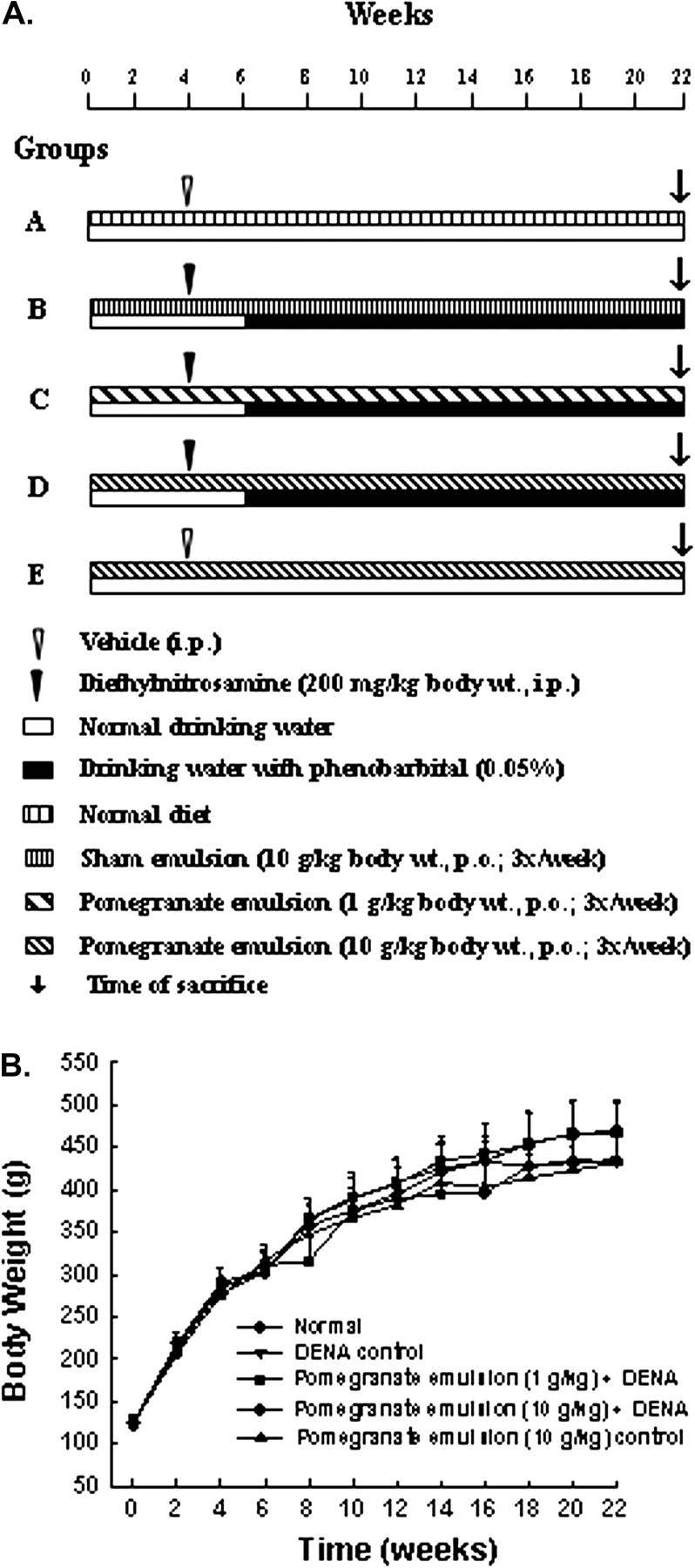
Experimental design and animal growth during the entire term of the study. (A) Schematic representation of the experimental protocol involving the two-stage rat hepatocarcinogenesis initiated with DENA and promoted by PB. (B) Effect of PE on body weight gain during DENA-induced hepatocarcinogenesis in rats. Each data point indicates mean ± standard error of the mean. There were 6–12 animals in various groups. No significant difference in body weights was observed among various rat groups at any time point.
Hepatic nodulogenesis
Under anesthesia, livers were perfused with heparinized saline and subsequently excised. Each liver was examined macroscopically on the surface and in 3 mm cross-sections for gross visible hepatocyte nodules. The nodules (approximated spheres) were measured in two perpendicular planes with a vernier caliper to the nearest millimeter to obtain an average diameter of each nodule. Estimates of nodular volume were determined as described recently (10).
Histopathology
Representative liver specimens (∼5 mm thick) were collected from several lobes, immediately immersed in 4% paraformaldehyde and stored at 4°C. The liver samples were embedded in embedding medium, and freeze-cut serial sections (∼10 μm thick) were prepared using a cryostat. Serial sections were stained with hematoxylin and eosin and specific hepatocellular lesions were recognized by light microscopy following established criteria (8).
Histochemical analyses of γ-glutamyl transpeptidase-positive foci
Histochemical determination of γ-glutamyl transpeptidase (GGT)-positive foci was performed by the modified method of Rutenberg et al. (41). In brief, hepatic sections were incubated at room temperature in dark for 45 min with 0.1 M Tris solution (pH 7.4) containing γ-glutamyl-4-methoxy-2-naphthylamide (125 μg/ml), glycylglycine (500 μg/ml) and fast blue BB (500 μg/ml). The slides were then rinsed with normal saline and incubated for 5 min with 0.1 M cupric sulfate solution. Finally, the slides were rinsed with saline, counterstained with hematoxylin, mounted and finally observed under a light microscope. Single GGT-positive hepatocytes were ignored, whereas the numbers of GGT-positive lesions >0.2 mm in diameter were counted. Four sections from each liver were examined for quantitative evaluation of the GGT-positive foci. The slides were coded so that the particular dietary treatment was unknown to the individual performing the histochemical determinations.
Hepatic oxidative stress
Liver specimens were immediately flash frozen. Hepatic lipid peroxidation was determined by estimation of thiobarbituric acid-reactive substances (TBARS) and protein oxidation measured according to protein carbonyl levels (42).
Reverse transcription–polymerase chain reaction
Several genes related to oxidative stress were evaluated by reverse transcription–polymerase chain reaction (PCR). Total RNA was extracted from 20 mg of flash frozen liver using Qiagen RNeasy plus mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Complementary DNA was synthesized from 0.5 μg of total RNA using Superscript II complementary DNA synthesis kit (Invitrogen, Carlsbad, CA). The primer sequences were designed as per supplementary Table S1, available at Carcinogenesis Online. The PCR products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining under ultraviolet. The amount of transcripts of the target genes was normalized to endogenous reference gene GAPDH.
Western blot
For Nrf2 western blotting analysis, whole-cell lysates were prepared from frozen liver tissues. Samples containing 40 μg protein were run on 8–16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred electrophoretically to a nitrocellulose membrane. Following blotting, the membrane was incubated overnight at 4°C in the presence of 1:200 dilution of anti-Nrf2 antibody (Santa Cruz Biotechnology) followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody at a dilution of 1:3000 at room temperature for 1 h. The transferred proteins were visualized by using an enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL). Normalization of western blot was ensured by β-actin.
Immunohistochemistry
Immunohistochemical detection of Nrf2 in hepatic sections was performed by standard immunohistochemical techniques (42).
Statistical analysis
Results are expressed as means ± standard errors of the mean. One-way analysis of variance was used to estimate the overall significance followed by post-hoc analysis using Student–Neuman–Keuls test. A P value of <0.05 was considered significant. All statistical analyses were performed using commercial software (SigmaStat 3.1; Systat Software, San Jose, CA).
Results
General observations
During the entire study period, no differences in food or water intake were noted among the various experimental groups. There was no treatment-related mortality of rats from any group before the termination of the study, i.e. 22 weeks.
Body, liver and relative liver weights
No statistical differences were noticed in the average body weights of any of the treatment and normal groups at various time points (Figure 1B) or at the end of the study (supplementary Table S2 is available at Carcinogenesis Online), suggesting that PE did not retard the animals’ growth. Average liver weight of DENA control (group B) was significantly (P < 0.001) higher than that of normal group (group A) (supplementary Table S2 is available at Carcinogenesis Online). Though PE at 1 g/kg (in group C) did not alter liver weights compared with group B, PE at 10 g/kg (in group D) significantly reduced liver weights (P < 0.05) compared with group B. The relative liver weights of group B were significantly (P < 0.001) higher than in group A (supplementary Table S2 is available at Carcinogenesis Online). There was no significant difference in relative liver weights of all DENA-treated animals in the presence or absence of PE, and PE at a dose of 10 g/kg body wt (in group E) did not influence liver weights relative to group A (supplementary Table S2 is available at Carcinogenesis Online).
PE inhibits DENA-induced nodulogenesis
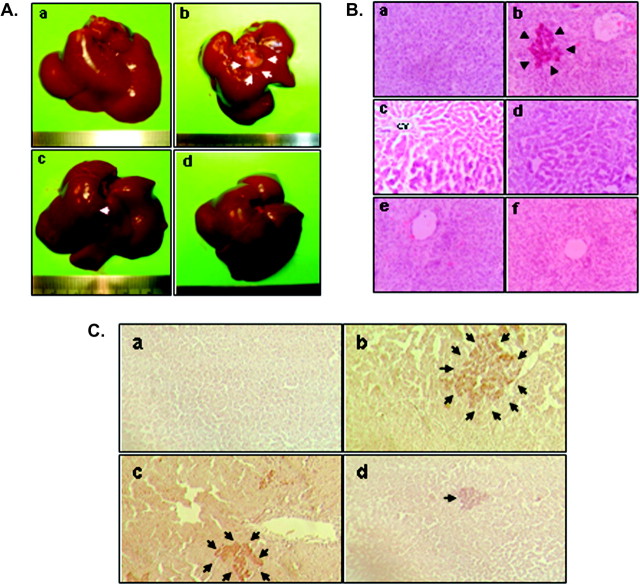
Though no visible hepatocyte nodules were found in the livers of normal group (group A) or PE control (group E), macroscopic nodules did arise from the livers of various DENA-treated groups (Figure 2A). Table I summarizes nodule incidence, total number of nodules and average number of nodules/nodule-bearing liver (nodule multiplicity) of DENA-initiated groups with or without PE treatment. At 1 g/kg, PE treatment reduced nodule incidence in group C compared with DENA control (group B), but the result did not reach significance. Significantly (P < 0.05) decreased nodule incidence occurred with PE at a dose of 10 g/kg (group D) compared with group B. The total number of nodules was less in the two PE-treated groups (C and D) than in group B. Both doses of PE significantly (P < 0.01 or 0.001) attenuated nodule multiplicity relative to group B. Table I also highlights the size distribution of visible nodules, mean nodular volume and nodular volume as a percentage of liver volume of various experimental groups. PE treatment at 1 or 10 g/kg significantly (P < 0.05 or 0.001) reduced appearance of nodules ≥3 mm compared with group B. Mean nodular volume was significantly (P < 0.05 or 0.01) less in PE-treated groups (C and D) relative to group B. Decreased nodular volume as a percentage of liver volume, though not significant, was observed in all PE-fed groups (C and D) compared with group B.
Fig. 2.
Pomegranate chemoprevention of DENA-initiated hepatocarcinogenesis in rats. (A) Morphological examination of rat liver tissue at the termination of the study. Macroscopically visible hepatic nodules are depicted by arrows. Representative livers were excised from several groups 22 weeks following the initiation of the study: (a) normal (group A) showing absence of nodules; (b) DENA control (group B) showing a large nodule; (c) PE at 1 g/kg body wt + DENA (group C) showing a small nodule and (d) PE at 10 g/kg body wt + DENA (group D) with no visible nodules. The animals fed PE at 10 g/kg only (group E) also showed no visible nodules (figure not shown). (B) Histopathological analysis of representative liver tissue from various experimental animals as observed by H&E staining (magnification: ×250). (a) Normal untreated rat liver (group A) showing normal cellular architecture; (b) DENA control (group B) showing eosinophilic foci and (c) areas of aberrant hepatocellular phenotype with irregular sinusoids and variation in nuclear size, shape and chromatin condensation; (d) liver section from PE (1 g/kg body wt) + DENA (group C) showing moderate improvement of hepatic histopathological indices over group B; (e) section from PE (10 g/kg body wt) + DENA (group D) showing hepatocytes exhibiting near-normal architecture and (f) section from PE (10 g/kg body wt) control group (group E) demonstrating characteristics of normal liver. CV, central vein. (C) Histochemical detection of hepatic GGT-positive foci in various rat groups (magnification: ×100). (a) Absence of foci in normal group; (b) a large GGT-positive focus in DENA control liver; (c) a medium size focus in low dose (1 g/kg) PE plus DENA-treated liver and (d) a small focus in high dose (10 g/kg) PE plus DENA-treated liver. GGT-positive foci of various sizes are indicated by arrows.
Table I.
Effects of pomegranate treatment on the development and growth of macroscopic hepatocyte nodules induced by DENA-PB in rats
| Groups | Rats with nodules/total rats | Nodule incidence (%) | Total no. of nodules | Average no. of nodules/nodule-bearing liver (nodule multiplicity) | Nodules relative to size (% of total no.) |
Mean nodular volumea (cm3) | Nodular volume/liver volumeb (%) | ||
| ≥3 mm | <3 to >1 mm | ≤1 mm | |||||||
| B—Sham + DENA | 11/12 | 92 | 199 | 18.1 ± 1.4c | 46 ± 2 | 27 ± 2 | 27 ± 2 | 0.10 ± 0.01 | 0.52 ± 0.04 |
| C—Pomegranate (1 g/kg) + DENA | 8/12 | 66 | 112 | 14.0 ± 0.7e | 37 ± 4g | 31 ± 3 | 32 ± 3 | 0.08 ± 0.00g | 0.39 ± 0.04 |
| D—Pomegranate (10 g/kg) + DENA | 5/12 | 42d | 56 | 11.2 ± 0.7f | 30 ± 3e | 35 ± 2 | 35 ± 4 | 0.06 ± 0.00e | 0.40 ± 0.05 |
Animals from normal (Group A) and pomegranate (10 g/kg) control group (Group E) did not show any visible hepatocyte nodule.
Individual nodule volume was calculated from two perpendicular diameters on each nodule. For details, please see Material and Methods.
One gram of liver was assumed to occupy 1 cm3 for this calculation.
Values are presented as means ± SEMs.
dP < 0.05 compared with Group B by Fisher’s exact probability test. eP < 0.01, fP < 0.001 and gP < 0.05 compared with Group B.
PE improves hepatocellular architecture in DENA hepatocarcinogenesis
While hepatic sections from normal animals (group A) revealed normal liver parenchymal architecture (Figure 2B, a), numerous hepatic lesions, predominantly liver cell foci and nodules, were found throughout the liver parenchyma in DENA-treated rats (group B). The altered hepatic foci were of eosinophilic, basophilic, clear and mixed cell types. A preponderance of eosinophilic foci (Figure 2B, b), rather than mixed or clear cell foci, was prominently observed in the hepatic sections from DENA control animals (group B). Liver sections from this group were enlarged and oval-shaped hepatocytes with granular cytoplasm, extensive vacuolation and multinucleate (Figure 2B, c) were observed. Numerous abnormal hepatocytes were enlarged with hyperchromatic nuclei and prominent, centrally located nucleoli. Treatment with PE at a dose of 1 g/kg (group C) improved hepatocellular characteristics, i.e. more regular and less altered hepatocytes compared with group B (Figure 2B, d). A predominant presence of clear cell foci rather than eosinophilic or mixed cell foci was observed in this group. The cellular architecture of liver sections from rats receiving PE at 10 g/kg (group D) was almost comparable with that of normal animals. The hepatocytes from this group exhibited a compact cytoplasm and only limited clear cell foci. Furthermore, the size of the nuclei resembled those of normal hepatocytes with few binucleated cells (Figure 2B, e). Normal rats fed with PE at 10 g/kg (group E) showed normal hepatocellular architecture (Figure 2B, f), suggesting no hepatotoxicity from chronic PE treatment (22 weeks).
PE suppresses the induction of GGT-positive foci
While no GGT-positive hepatic foci were detected in normal group (group A) (Figure 2C, a) as well as PE control (group E) (figure not shown), liver sections from all DENA-exposed groups exhibited various sizes of foci with positive GGT expression (Figure 2C, b–d). As presented in Table II, a significant (P < 0.01 or 0.001) decrease in the number of DENA-induced GGT-positive foci was observed in groups receiving both doses of PE (groups C and D). Coadministration of PE in DENA-treated groups also attenuated the GGT-positive focal area as compared with DENA control. Interestingly, a significant (P < 0.01 or 0.001) result was achieved with PE at a dose of 1 or 10 g/kg.
Table II.
Effects of pomegranate on the induction of GGT-positive foci during DENA/PB hepatocarcinogenesis in rats
| Groups | No. of foci/cm2 | Focal area (mm2/cm2) |
| B—Sham + DENA | 34.4 ± 2.9a | 4.32 ± 0.36 |
| C—Pomegranate (1 g/kg) + DENA | 20.2 ± 2.5b | 2.53 ± 0.32b |
| D—Pomegranate (10 g/kg) + DENA | 12.3 ± 1.1c | 1.55 ± 0.14c |
Animals from normal (Group A) and pomegranate (10 g/kg) control group (Group E) did not show any GGT-positive foci.
Values are presented as means ± SEMs of four animals.
bP < 0.01 and cP < 0.001 compared with Group B.
PE exerts antioxidant effects during hepatocarcinogenesis
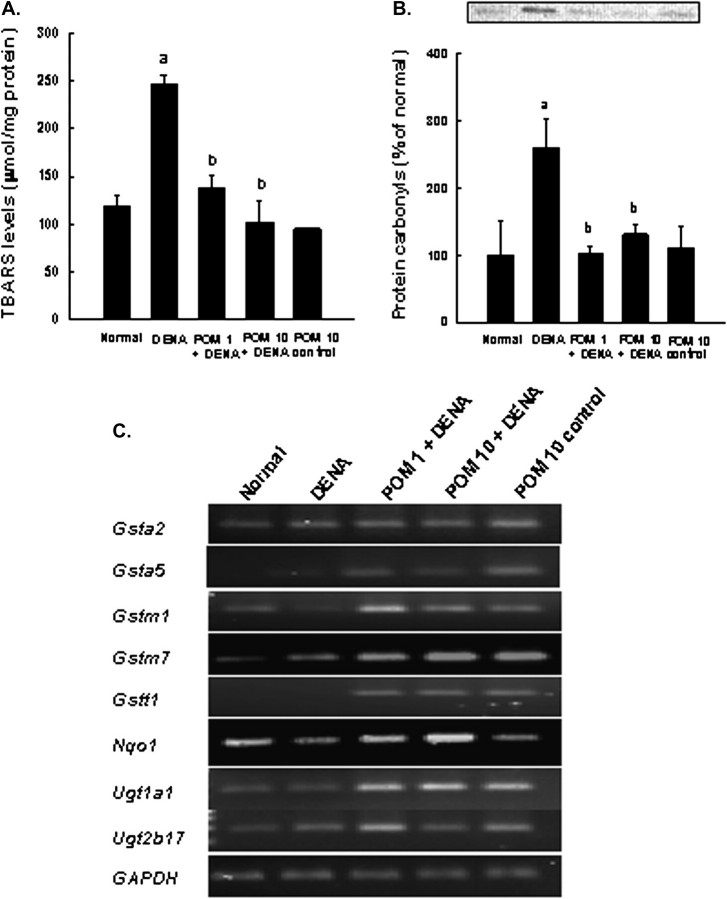
Hepatic TBARS levels reflect the extent of lipid peroxidation and index magnitude of oxidative stress in the liver. DENA treatment significantly (P < 0.001) increased the generation of TBARS in rat liver (Figure 3A). PE dose dependently inhibited oxidative damage, specifically DENA-induced hepatic lipid peroxidation. At 10 g/kg, PE not only abrogated DENA-induced lipid peroxidation but also returned the TBARS levels to normal. To explore the effects of PE feeding on oxidative modifications of hepatic proteins during DENA hepatocarcinogenesis, the carbonyl content of proteins in various experimental groups was measured. DENA administration significantly (P < 0.05) increased the protein carbonyl levels as measured by the immunoblotting technique, compared with normal animals (Figure 3B). Both doses of PE limited DENA-induced protein carbonyl content. No difference between protein carbonyl levels in the livers of rats fed the highest dose of PE and normal animals occurred.
Fig. 3.
Antioxidant effects of PE during DENA-induced hepatocarcinogenesis in rats. Rats were killed 22 weeks following the commencement of the study. (A) Effects of PE on hepatic lipid peroxidation measured by TBARS in DENA-challenged animals. Each bar represents the mean ± SEM (n = 4–6 livers). aP < 0.001 as compared with normal group. bP < 0.001 as compared with DENA control. (B) Effects of PE on hepatic protein carbonyl formation in rats subjected to DENA hepatocarcinogenesis. Each bar represents the mean ± SEM (n = 4–6 livers). aP < 0.05 as compared with normal group. bP < 0.05 as compared with DENA control. (C) PE-mediated upregulation of the genes of phase 2 xenobiotic-metabolizing enzymes related to oxidative stress in DENA-initiated hepatocarcinogenesis in rats. Total RNA was extracted from the livers of several groups. The resultant complementary DNA following reverse transcription was subjected to PCR using specific primer sequences for each gene. Representative reverse transcriptase–PCR gel pictures are shown with GAPDH as the housekeeping gene.
PE upregulates antioxidant genes during hepatocarcinogenesis
Consistent with the crucial role of oxidative stress hepatocarcinogenesis (36,37), PE suppressed lipid and protein oxidation as mentioned above. Reverse transcriptase–PCR analysis of several phase 2 xenobiotic-metabolizing genes involved in oxidative stress response showed messenger RNA (mRNA) levels of glutathione S-transferase (GST) isozymes alpha2, alpha5, mu1, mu7 and theta1 (Gsta2, Gsta5, Gstm1, Gstm7 and Gstt1, respectively) either marginally induced or unaltered in the livers of DENA-exposed rats compared with untreated normal rats (Figure 3C). PE treatment at 1 or 10 mg/kg in DENA-exposed animals substantially increased mRNA levels of these genes compared with DENA controls, though mRNA expression of the aforementioned genes remained significantly elevated in livers of rats treated only with PE compared with their normal counterparts. Similarly, the mRNA levels of genes of other phase 2 enzymes, i.e. NAD(P)H:quinone oxidoreductase 1 (Nqo1), uridine diphosphate-glucuronosyltransferase isoforms alpha1 and beta17 (Ugt1a1 and Ugt2b17, respectively) did not differ significantly from DENA control or normal animals. On the other hand, clear upregulation of these genes was noticed following chronic treatment with PE in DENA-challenged animals. Transcription levels of these genes were significantly elevated in the livers of PE control compared with normal animals.
PE induces Nrf2 expression during DENA hepatocarcinogenesis
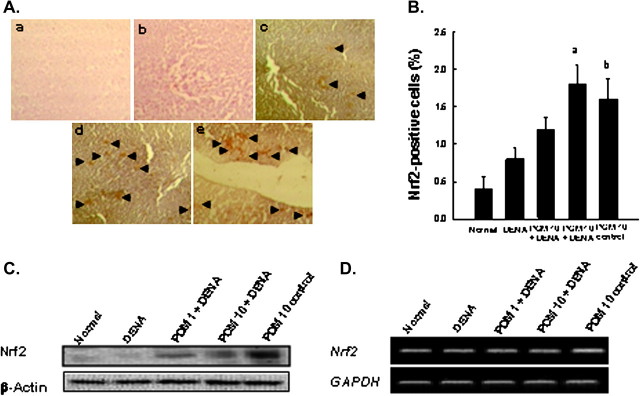
Since Nrf2 signaling is known to play a crucial role in the suppression of oxidative stress (43), we next investigated the role of Nrf2 in the observed antioxidant responses of PE during DENA hepatocarcinogenesis. Very limited expression of Nrf2 in liver sections of normal (Figure 4A, a) or only DENA-treated animals (Figure 4A, b) contrasted with numerous Nrf2 expression in the two DENA plus PE-treated groups (Figure 4A, c and d) or PE control group (Figure 4A, e). Most of the immunopositivity for Nrf2 in these groups was observed in the nucleus, indicating activation of Nrf2 and its subsequent nuclear translocation. Quantitative analysis of Nrf2 immunopositive cells showed limited number of hepatic Nrf2-positive cells in normal or DENA control group (Figure 4B). PE dose dependently increased Nrf2-positive cells in DENA-treated animals and significantly (P < 0.01) with the highest dose of PE compared with DENA control. These results are supported by Nrf2 western blot analysis, which indicate either lack of expression or very weak expression of hepatic Nrf2 protein in normal or DENA control animals (Figure 4C). PE treatment dose dependently elevated the level of Nrf2 in DENA-initiated animals. A drastic elevation of this protein level was also noticed in the group that received only the highest dose of PE. We have also determined the Nrf2 gene expression by measuring mRNA levels of Nrf2 in hepatic tissues of various experimental animals. DENA treatment alone did not influence hepatic Nrf2 mRNA expression compared with normal animals, but both doses of PE elicited augmented Nrf2 mRNA expression in the livers of DENA-challenged animals compared with DENA control (Figure 4D). Transcription of Nrf2 in PE control animals was significantly upregulated relative to normal counterparts. All these results highlight the involvement of Nrf2 in the chemopreventive effects of PE against DENA-initiated hepatocarcinogenesis in rats.
Fig. 4.
Effects of PE on hepatic Nrf2 expression during DENA-induced hepatocellular carcinogenesis in rats. Rats were killed and estimations were performed 22 weeks following the commencement of the study. (A) Immunohistochemical staining of Nrf2 (magnification: ×100). Arrowheads indicate immunohistochemical staining of Nrf2. Representative observation of Nrf2 immunoreactivity in different groups: very limited expression in (a) normal and (b) DENA control liver; (c) moderate induction in PE (1 g/kg) plus DENA; (d) substantial induction in PE (10 g/kg) plus DENA and (e) PE (10 g/kg) control group. (B) Immunochemical quantification of Nrf2-positive cells in livers of various groups. One thousand hepatocytes were counted per animal and the results were based on four animals per group. Each bar represents the mean ± standard error of the mean (n = 4 livers). aP < 0.01 as compared with DENA control and bP < 0.01 as compared with normal group. (C) Representative western blot analysis of hepatic Nrf2 protein expression. Total cellular protein was separated and blotted with anti-Nrf2 antibody. (D) The mRNA levels Nrf2 in several rat groups. Total RNA was isolated from liver and subjected to reverse transcription. The resulting complementary DNA was subjected to PCR using specific primer sequences for Nrf2. Representative reverse transcriptase–PCR gel pictures are shown with GAPDH as the housekeeping gene.
Discussion
Experimental liver cancer in rodents induced by DENA, an environmental and dietary hepatocarcinogen, has been considered as one of the best-characterized experimental models of HCC, allowing for the screening of potential anticancer compounds at various stages of neoplastic transformation and development (8,9). DENA-induced preneoplastic foci and preneoplastic and neoplastic nodule formation in rodents closely mimics HCC development in humans (44). Cross-species comparison of gene expression showed DENA-induced liver tumors in rodents to closely mimic a subclass of human HCC (45), allowing for the extrapolation of potential clinical chemopreventive effects of candidate agents.
Here, chemopreventive action of a complex (aqueous and lipid phase) pomegranate product on development and growth of early hepatic preneoplastic lesions was established in a two-stage model of rat hepatocarcinogenesis initiated with DENA and promoted by PB. The results demonstrate a potent chemopreventive effect of PE against chemically induced rat liver tumorigenesis. Pomegranate constituents have been previously reported to inhibit chemically induced tumors in skin, breast, lung and colon (20–25), and here, we present evidence, for the first time, of pomegranate-mediated chemopreventive action against experimental hepatocarcinogenesis. Feeding of DENA-exposed rats with PE resulted in fewer animals with visible hepatocyte nodules and smaller nodule multiplicity (the principal endpoint for evaluating the chemopreventive potential of a candidate agent) compared with DENA-treated animals fed a sham emulsion. Furthermore, PE dose dependently mediated reduction of the number of nodules >3 mm and a parallel attenuation of nodular volume. Though not all hepatocyte nodules become malignant during the lifespan of animals, several lines of evidence indicate that the nodules are precursors of hepatic cancer (46). Moreover, experience in both experimental and human disease correlates both frequency and size of nodular hyperplasia to hepatic carcinoma (47). Thus, PE-mediated inhibition of hepatic nodule formation and suppression of nodule growth as observed here are important steps for liver cancer chemoprevention. Our histopathology findings further lend credence to the chemopreventive potential of pomegranate-derived constituents against hepatic cancer.
The membrane-bound enzyme GGT has been widely accepted as a marker of biochemical alteration in hepatocellular foci, nodules and tumors in rats. It is well known that induction of GGT in preneoplastic foci represents an early event in hepatocarcinogenesis, and GGT-positive foci appear to be the first discernible evidence for the occurrence of tumor initiation (48). Elevated metabolism of extracellular glutathione by GGT results in a radical-rich microenvironment in close proximity to the foci, leading to oxidative damage that further fuels the carcinogenic process. Immunohistochemical detection and morphometric analysis directed at GGT-positive foci has become established in quantitative assessment of the process of hepatocarcinogenesis and is widely considered a useful tool for evaluation of modulation of hepatocarcinogenesis by dietary constituents. The results of the present study demonstrated that PE not only exerted a striking inhibitory effect on the formation of GGT-positive foci (number) but also caused a significant attenuation of focal area. According to the well-accepted hypothesis of Pitot et al. (49), the number and size of altered hepatocyte foci signify initiating and promoting activities, respectively. In view of these observations, our results suggest that PE not only affects the initiation phase of DENA hepatocarcinogenesis but also suppress the promotional stage by restricting the growth of preneoplastic foci.
Oxidative stress, an imbalance between generation of reactive oxygen species and antioxidant defense mechanisms, predisposes to hepatocarcinogenesis and drives HCC in chronic liver ailments. DENA initiates hepatocarcinogenesis through production of reactive oxygen species, including DNA-binding ethylcarbonium ions, resulting in adducts and superoxide radicals via lipid peroxidation of phospholipid membrane fatty acids (50) and continuous administration of PB effects release of free radicals. Here, DENA initiation followed by PB promotion induced dramatic hepatic lipid peroxidation in rats as evidenced by a drastic increase in TBARS levels, indicating severe oxidative stress. Reactive oxygen species could modify the chemical structure of proteins with formation of protein carbonyls due to oxidative cleavage of the main peptide backbone or by oxidation of amino acids, including arginine, lysine, proline and threonine. Protein carbonyls, the most ubiquitously used markers of protein oxidation, are elevated in the plasma of HCC patients due to oxidative stress (51). In addition to lipid peroxidation, we also observed elevated hepatic protein carbonyl formation in DENA-treated animals, indicating oxidative protein damage. Our data also show that PE reversed DENA-induced lipid peroxidation, suggesting the ability of pomegranate constituents to scavenge free radicals produced by the hepatocarcinogen DENA. PE completely abrogated DENA-induced enhanced protein carbonyl formation, implicating the ability of pomegranate-derived agents to suppress hepatic oxidative stress. These results support numerous studies showing that inhibition of lipid peroxidation and oxidative damage figures prominently in the hepatoprotective effects of pomegranate against chemically induced hepatic damage in rodents (33–35). Nevertheless, our present results provide the first experimental evidence that antioxidant properties of pomegranate products play a valuable role in the inhibition of chemically induced hepatocarcinogenesis in rats.
Antioxidant and detoxifying enzymes, e.g. NQO1, GST and uridine diphosphate-glucuronosyltransferase, protect mammalian cells from oxidative stress, and consequently, reduce the propensity of tissues to develop malignancy (52). To delineate the underlying mechanism of the antioxidant effect of PE during DENA hepatocarcinogenesis, reverse transcriptase–PCR analysis was performed to monitor the mRNA expression of the aforementioned genes associated with oxidative stress. The mRNA levels of Nqo1, Gsta2, Gsta5, Gstm1, Gstm7, Ugt1a1 and Ugt2b17 were elevated in parallel with suppression of lipid and protein oxidation by coadministration of PE with DENA. The observation that oral feeding of rats with PE alone upregulated mRNA levels of these genes, suggests that pomegranate constituents exert antioxidant properties through transcriptional regulation of antioxidant and phase 2 enzymes.
It is well known that DENA undergoes metabolic activation conferred by phase 1 cytochrome P-450 (CYP) enzymes to yield electrophilic reactive products that mediate hepatocarcinogenesis. Moreover, PB induces hepatic CYP activity linked to promotion of DENA-initiated hepatocellular transformation. CYP1A2 and CYP2E1, isoenzymes constitutively expressed in the liver, have been shown to activate both DENA and PB (50,53). Although the effects of PE on phase 1 hepatic enzymes have not yet been elucidated, PE-derived constituents probably attenuate DENA activation through inhibition of CYP isoenzymes and thereby reduce the formation of active metabolites. Similarly, pomegranate juice consumption decreased total hepatic CYP content as well as CYP1A2 expression in rodents (54).
As the transcription factor Nrf2, a member of the basic leucine zipper family, plays an essential role in the antioxidant-response element-mediated expression of many antioxidant and phase 2 detoxifying enzymes, including NQO1, GST-alpha2, GST-alpha5 and GST-m1 (55), possible involvement of Nrf2 in the observed upregulation of the genes of these enzymes by PE was investigated. Nrf2 is normally sequestered in the cytosol by the Kelch-like erythroid Cap `n' collar homolog-associated protein 1 (Keap1). Upon oxidative or electrophilic stress or stimulation by compounds with ability to oxidize or covalently modify thiol groups of Keap1, Nrf2 dissociates from Keap1 and undergoes nuclear translocation, binding to the antioxidant-response element in the promoter regions of target genes (56), effecting synthesis of the aforementioned antioxidant and detoxifying enzymes. Recent studies with Nrf2-deficient mice highlighted the role of Nrf2 in protecting liver from xenobiotic-initiated hepatocarcinogenesis (57). Thus, Nrf2 could be a key target of chemoprevention of HCC. In the present study, we have observed for the first time an increased hepatic Nrf2 protein with enhanced nuclear translocation in rats pretreated with PE and subsequently exposed to the potent hepatocarcinogen DENA. A parallel increase in the transcription of Nrf2 gene and protein by PE during rat liver carcinogenesis further supported the immunohistochemical findings. In view of these observations, the inhibitory effects of PE against DENA-induced lipid peroxidation and protein oxidation as observed here could be achieved by the induction of antioxidant and phase 2 conjugating enzymes via upregulation of Nrf2. Pomegranate bioactive constituents may act by induction of Nrf2-regulated cytoprotective enzymes, putatively resulting in enhanced excretion of electrophilic carcinogen and reduced formation of free radicals that initiate hepatocarcinogenesis.
Identification of the active component(s) of PE responsible for the results presented remains to be elucidated, but studies on pomegranate clearly showed synergistic interactions of phytochemicals present in three anatomically discrete sections of pomegranate fruit (peel, juice and seeds) in inhibiting the growth of cancer cells of human origin. Therapeutically, beneficial pomegranate constituents include gallic acid and ellagic acid, ellagitanins including punicalagins, octadecatrienoic fatty acids including punicic acid and flavonoids, including anthocyanidins, anthocyanins and estrogenic flavonols and flavones. Specific combinations of phytochemicals may be more effective against cancer than isolated compounds (reviewed in ref. 58). As single plants evolve complex means for fighting their own enemies, cancer clones also evolve complex mechanisms for attaining advantage over their hosts. The chemical complexity used by plants for their own purposes may also be, in part, transferable to the drug designer for resolving physiological ailments, including cancer (39), leading to a complex, synergistic drug for the prevention of cancer. The PE used in the present study has been developed by combining the extract of the whole fruit (including peel) with seed oil to maximize the product’s inherent bioactive synergy.
In conclusion, the results here clearly demonstrate that pomegranate bioactive constituents exert a striking chemopreventive effect against DENA-induced rat liver carcinogenesis by suppressing hepatic oxidative insult without any toxic manifestation. The present study also demonstrates that attenuation of oxidative stress could be mediated through induction of antioxidant and phase 2 xenobiotic-metabolizing enzymes. The PE-mediated upregulation of Nrf2 at the transcriptional as well as the translational level could be attributed to elevation of housekeeping enzymes. The steady-state induction of these cytoprotective enzymes may lead to a move and shift of the metabolic profile and reduction of intercellular levels of carcinogen-derived reactive intermediates and consequent initiation of hepatocarcinogenesis. Since oxidative stress begets both the development and progression of human liver cancer, our findings underscore the tremendous potential of alleviating oxidative injury through modulation of Nrf2 signaling as a putative strategy for liver cancer chemoprevention and treatment by pomegranate-derived products.
Supplemental material
Supplementary Tables S1 and S2 can be found at http://carcin.oxfordjournals.org/.
Funding
This work was supported by a start-up research grant of Northeastern Ohio Universities Colleges of Medicine and Pharmacy to A.B.
Supplementary Material
Acknowledgments
We thank Rajiv Lotey and Thomas Mbimba for assistance with animal feeding and tissue harvesting, Kendra F. Barnes and Animesh Mandal for analytical work, and Danielle M. Petit for immunohistochemical studies.
Conflict of Interest Statement: E.P.L. has stock and ownership interests in Punisyn Pharmaceuticals, Ltd. The other authors have no conflict of interest.
Glossary
Abbreviations
- CYP
cytochrome P-450
- DENA
diethylnitrosamine
- GGT
γ-glutamyl transpeptidase
- GST
glutathione S-transferase
- HCC
hepatocellular carcinoma
- Keap1
Kelch-like ECH-associated protein 1
- mRNA
messenger RNA
- NQO1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
nuclear factor E2-related factor 2
- PB
phenobarbital
- PCR
polymerase chain reaction
- PE
pomegranate emulsion
- TBARS
thiobarbituric acid-reactive substance
References
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, et al. Cancer statistics. CA Cancer J. Clin. 2010;60:1–24. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Schütte K, et al. Hepatocellular carcinoma – epidemiological trends and risk factors. Dig. Dis. 2009;27:80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 4.Kensler TW, et al. Chemoprevention of hepatocellular carcinoma in aflatoxin endemic areas. Gastroenterology. 2004;127:S310–S318. doi: 10.1053/j.gastro.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Bartsch H, et al. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984;5:1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- 6.Je Y, et al. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and mata-analysis of clinical trials. Lancet Oncol. 2009;10:967–974. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 7.Newell P, et al. Experimental models of hepatocellular carcinoma. J. Hepatol. 2008;48:858–879. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishayee A, et al. Inhibitory effect of vanadium on rat liver carcinogenesis initiated with diethylnitrosamine and promoted with phenobarbital. Br. J. Cancer. 1995;71:1214–1220. doi: 10.1038/bjc.1995.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishayee A, et al. Further evidence for chemopreventive potential of beta-carotene against experimental carcinogenesis: diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis is prevented more effectively by beta-carotene than retinoic acid. Nutr. Cancer. 2000;37:89–98. doi: 10.1207/S15327914NC3701_12. [DOI] [PubMed] [Google Scholar]
- 10.Bishayee A, et al. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chem. Biol. Interact. 2009;179:131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Bishayee A, et al. Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. doi: 10.1016/j.jnutbio.2010.09.001. in press doi:10.1016/j.jnutbio.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Langley P. Why a pomegranate? Br. Med. J. 2000;321:1153–1154. doi: 10.1136/bmj.321.7269.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil MI, et al. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 14.Faria A, et al. Effect of pomegranate (Punica granatum) juice intake on hepatic oxidative stress. Eur. J. Nutr. 2007;46:271–278. doi: 10.1007/s00394-007-0661-z. [DOI] [PubMed] [Google Scholar]
- 15.Seeram NP, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Lansky EP, et al. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Mertens-Talcott SU, et al. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy volunteers. J. Agric. Food Chem. 2006;54:8956–8961. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- 18.Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): a review. Alter. Med. Rev. 2008;13:128–144. [PubMed] [Google Scholar]
- 19.Adhami VM, et al. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr. Cancer. 2009;61:811–815. doi: 10.1080/01635580903285064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hora JJ, et al. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J. Med. Food. 2003;6:157–161. doi: 10.1089/10966200360716553. [DOI] [PubMed] [Google Scholar]
- 21.Afaq F, et al. Anthocyanin- and hydrolysable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 22.Kim ND, et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002;71:203–217. doi: 10.1023/a:1014405730585. [DOI] [PubMed] [Google Scholar]
- 23.Khan N, et al. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 24.Kohno H, et al. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004;95:481–486. doi: 10.1111/j.1349-7006.2004.tb03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boateng J, et al. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007;45:725–732. doi: 10.1016/j.fct.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Khan N, et al. Pomegranate fruit extract inhibits prosturvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- 27.Albrecht M, et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J. Med. Food. 2004;7:274–283. doi: 10.1089/jmf.2004.7.274. [DOI] [PubMed] [Google Scholar]
- 28.Malik A, et al. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl Acad. Sci. USA. 2005;102:14813–14818. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeram NP, et al. Pomegranate ellagitinnin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 30.Rettig MB, et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-κB-dependent mechanism. Mol. Cancer Ther. 2008;7:2262–2271. doi: 10.1158/1535-7163.MCT-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartippour MR, et al. Ellagitanin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int. J. Oncol. 2008;31:475–480. [PubMed] [Google Scholar]
- 32.Pantuck AJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 33.Chidambara Murthy KN, et al. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J. Agric. Food Chem. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 34.Kaur G, et al. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem. Toxicol. 2006;44:984–993. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Celik I, et al. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem. Toxicol. 2009;47:145–149. doi: 10.1016/j.fct.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Perez Y, et al. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Lett. 2005;217:25–32. doi: 10.1016/j.canlet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Kawanishi S, et al. Oxidative stress and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 38.Tran HN, et al. Pomegranate (Punica granatum) seed linolenic acid isomers: concentration-dependent modulation of estrogen receptor activity. Endocr. Res. 2010;35:1–16. doi: 10.3109/07435800903524161. [DOI] [PubMed] [Google Scholar]
- 39.Lansky EP, et al. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Invest. New Drugs. 2005;23:11–20. doi: 10.1023/B:DRUG.0000047101.02178.07. [DOI] [PubMed] [Google Scholar]
- 40.Khan GN, et al. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integr. Cancer Ther. 2009;8:242–253. doi: 10.1177/1534735409341405. [DOI] [PubMed] [Google Scholar]
- 41.Rutenburg AM, et al. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J. Histochem. Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- 42.Bishayee A, et al. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev. Res. 2010;3:753–763. doi: 10.1158/1940-6207.CAPR-09-0171. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, et al. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Natl Acad. Sci. USA. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verna L, et al. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee JS, et al. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat. Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 46.Farber E, et al. Hepatocarcinogenesis: a dynamic cellular perspective. Lab. Invest. 1987;56:4–22. [PubMed] [Google Scholar]
- 47.Farber E. Clonal adaptation during carcinogenesis. Biochem. Pharmacol. 1990;39:1837–1846. doi: 10.1016/0006-2952(90)90599-g. [DOI] [PubMed] [Google Scholar]
- 48.Pitot HC, et al. The stages of initiation and promotion in hepatocarcinogenesis. Biochim. Biophys. Acta. 1980;605:191–215. doi: 10.1016/0304-419x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- 49.Pitot HC, et al. Critical parameters in the quantitation of the stages of initiation, promotion and progression in one model of hepatocarcinogenesis in the rat. Toxicol. Pathol. 1989;17:594–605. doi: 10.1177/0192623389017004105. [DOI] [PubMed] [Google Scholar]
- 50.Kang J, et al. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis. Cancer Res. 2007;67:11141–11146. doi: 10.1158/0008-5472.CAN-07-1369. [DOI] [PubMed] [Google Scholar]
- 51.Liu ZM, et al. Hepatitis B virus infection contributes to oxidative stress in a population exposed to aflatoxin B1 and high-risk for hepatocellular carcinoma. Cancer Lett. 2008;263:212–222. doi: 10.1016/j.canlet.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talaley P, et al. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 53.Lin HL, et al. N-Nitrosodimethylamine-mediated formation of oxidized and methylated DNA bases in a cytochrome P450 2E1 expressing cell line. Chem. Res. Toxicol. 2001;14:562–566. doi: 10.1021/tx0001979. [DOI] [PubMed] [Google Scholar]
- 54.Faria A, et al. Pomegranate juice effects on cytochrome P450s expression: in vivo studies. J. Med. Food. 2007;10:643–649. doi: 10.1089/jmf.2007.403. [DOI] [PubMed] [Google Scholar]
- 55.Hayes JD, et al. Cancer chemoprevention mechanisms mediated through the Keap1–Nrf2 pathway. Antioxid. Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi M, et al. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene expression. Antioxid. Redox Signal. 2005;7:382–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 57.Kitamura Y, et al. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazo[4,5- f]quinoline. Cancer Sci. 2007;98:19–24. doi: 10.1111/j.1349-7006.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Kok TM, et al. Mechanisms of combined action of different chemopreventive dietary compounds. Eur. J. Nutr. 2008;47:51–59. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.