Abstract
Objective
Cetuximab (Erbitux®) was approved for the treatment of metastatic colorectal cancer in Japan in 2008. To verify information on the safety in practical use of cetuximab, we conducted post-marketing surveillance in accordance with the conditions for approval.
Methods
All patients to be treated with cetuximab were enrolled by the central enrolment method. Data on treatment status, and incidence and severity of adverse drug reactions were collected. The target number of patients was 1800.
Results
A total of 2126 patients were enrolled from 637 institutions. Among 2006 patients analysed, 93.2% received cetuximab as third-line or later treatment. The median duration of treatment was 15.3 weeks, and 11.1% of patients received treatment for >48 weeks. The incidence of adverse drug reactions was 89.6%, of which ≥grade 3 was 21.5%. The incidence of infusion reactions was 5.7% (any grade), with 83.3% of them occurring at the first administration. The incidence of skin disorders was 83.7% (any grade), and the time to event varied for each skin disorder. The incidence of interstitial lung diseases was 1.2% (any grade). Diarrhoea and haematotoxicity scarcely occurred with cetuximab alone.
Conclusions
In this surveillance, the incidence and categories of adverse drug reactions are not distinct from previous reports. Although most patients received cetuximab as third-line or later treatment, treatment was maintained with a median duration of 15 weeks. Cetuximab treatment in practical use is considered to be well tolerated and clinically useful in Japanese patients with metastatic colorectal cancer.
Keywords: colorectal cancer, cetuximab, post-marketing surveillance
INTRODUCTION
Cetuximab (Erbitux®) is a human/mouse chimeric monoclonal immunoglobulin G1 antibody that targets the epidermal growth factor receptor (EGFR) to inhibit its signalling and shows anti-tumour effects by binding to EGFR competitively with ligands (1,2). Cetuximab, administered alone or in combination with irinotecan, showed efficacy in the treatment of patients with EGFR-positive metastatic colorectal cancer (CRC) who are refractory to irinotecan (3). Subsequent studies confirmed the efficacy and safety of cetuximab alone or in combination with chemotherapy (4,5). Moreover, it was reported that KRAS status is a predictive marker of response to cetuximab (6–8).
Based on these studies and a Japanese phase II study (9) in which cetuximab was administered in combination with irinotecan in 39 patients with EGFR-positive metastatic CRC refractory to irinotecan, cetuximab was approved in Japan as second-line and later treatment for EGFR-positive metastatic CRC in July 2008.
In Japan, post-marketing surveillance (PMS) has been introduced to verify the safety and the clinical efficacy of medicines in practical use, and practice standards of PMS have been established under a ministerial order. As a condition of its approval, PMS of all patients receiving cetuximab during a certain period was requested by the Ministry of Health, Labour and Welfare.
In this report, treatment status and safety in the clinical use of cetuximab are examined based on prospectively aggregated PMS data.
PATIENTS AND METHODS
Enrolment
Following the launch of cetuximab on 19 September 2008, all patients to be treated with cetuximab were enrolled in advance using the central enrolment method. Patient information, including gender, age and treatment line, was collected from a company (Merck Serono Co., Ltd. and Bristol-Myers K.K.) prepared enrolment sheet. The company checked whether the patients met the following conditions for proper use upon approval: positive EGFR, no history of hypersensitivity to the components of the product, performance status (PS) 0–1, no interstitial lung diseases (ILDs) and refractoriness or intolerance to previous chemotherapy.
To detect adverse drug reactions (ADRs) with an incidence of 0.2% and a probability of at least 95%, and to complete the enrolment within 1 year after launch, the target number of patients was determined to be 1800.
Treatment
In accordance with the statement on the package insert, the initial dose of cetuximab was administered at 400 mg/m2 over 2 h followed by weekly infusions of 250 mg/m2 over 1 h. As there were no data available on the efficacy and safety of cetuximab in combination with oxaliplatin-based regimens at the beginning of the surveillance in Japan, it was recommended to use irinotecan or FOLFIRI (folinic acid, fluorouracil and irinotecan) as a combination chemotherapy.
To reduce the risk of infusion reactions (IRs), pre-medication with antihistamines is recommended in the ‘precautions for use’ section on the package insert for cetuximab. Likewise, concomitant use of corticosteroid is also suggested to reduce the risk of IRs.
Monitoring
The observation period was defined as the time between the first administration and the last administration of cetuximab. The case report forms including information of treatment status and ADRs filled out by physicians were collected three times (at Week 4, Week 8 and final administration).
Safety Evaluation
Severities of adverse events (AEs) were assessed mainly according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0). As priority survey items, the incidence and severity of IRs, skin disorders, ILDs, electrolyte abnormalities including hypomagnesaemia, cardiotoxicity, gastrointestinal disorders, thrombosis/embolism, delayed wound healing and eye disorders (e.g. keratitis) were surveyed. AEs that the physicians and the company defined as being related to cetuximab treatment were analysed as ADRs.
Statistical Analysis
All analyses were performed using SAS (version 9.2; SAS Institute, Inc., Cary, NC, USA). The incidences of ADRs, the number of treatment and duration of treatment were compared among patients characteristics and therapeutic factors using the χ2 test, Fisher's exact test, the Wilcoxon rank sum test or the non-paired t-test. P< 0.05 was considered to represent a statistically significant difference. The Kaplan–Meier method was used to calculate the cumulative incidence of ADRs.
RESULTS
Patient Characteristics
Between 19 September 2008 and 5 January 2009, 2126 patients were enrolled from 637 institutions. Excluding 154 patients who did not receive cetuximab treatment, but including 34 who were treated with cetuximab outside of the enrolment condition, 2006 patients were included in the analysis. The cut-off date was set at 5 January 2010 (including patients continuing treatment).
The patient characteristics are shown in Table 1. The median age was 64 years (range, 18–87), the male/female ratio was 1:0.6 and 93.2% of patients received cetuximab as third-line or later treatment (Table 1). In this surveillance, KRAS testing was performed in 15%.
Table 1.
Patient characteristics and clinical use
| n | % | |
|---|---|---|
| (A) Patient characteristics | ||
| Sex | ||
| Male | 1234 | 61.5 |
| Female | 772 | 38.5 |
| Age | ||
| <65 years | 1032 | 51.4 |
| ≥65 years | 971 | 48.4 |
| Unknown | 3 | 0.2 |
| Tumour site (including overlapping sites) | ||
| Colon | 1235 | 61.6 |
| Rectum | 775 | 38.6 |
| PS | ||
| 0 | 1370 | 68.3 |
| 1 | 630 | 31.4 |
| 2 | 2 | 0.1 |
| Unknown | 4 | 0.2 |
| Treatment line | ||
| Second line | 133 | 6.6 |
| Third line and later treatment | 1869 | 93.2 |
| Others | 4 | 0.2 |
| Previous chemotherapy | ||
| (−) | 3 | 0.2 |
| (+) | 2003 | 99.9 |
| FOLFIRI | 1510 | 75.3 |
| FOLFOX | 1854 | 92.4 |
| 5-FU/LV | 370 | 18.4 |
| Irinotecan | 261 | 13.0 |
| UFT/LV | 337 | 16.8 |
| Bevacizumab | 923 | 46.0 |
| Previous surgery | ||
| (−) | 81 | 4.0 |
| (+) | 1925 | 96.0 |
| Previous radiation therapy | ||
| (−) | 1650 | 82.3 |
| (+) | 355 | 17.7 |
| Unknown | 1 | 0.0 |
| EGFR-IHC | ||
| Positive | 1974 | 98.4 |
| Negative | 29 | 1.4 |
| Not tested | 3 | 0.2 |
| KRAS status | ||
| Wild | 249 | 12.4 |
| Mutant | 53 | 2.6 |
| Not tested | 1691 | 84.3 |
| Unknown | 13 | 0.7 |
| Comorbidity | ||
| (−) | 1019 | 50.8 |
| (+) | 974 | 48.6 |
| Unknown | 13 | 0.6 |
| (B) Clinical use | ||
| No. of treatments | ||
| <4 | 253 | 12.6 |
| 4 to <16 | 900 | 44.9 |
| 16 to <32 | 524 | 26.1 |
| 32 to <48 | 255 | 12.7 |
| ≥48 | 74 | 3.7 |
| Duration of treatment | ||
| <4 weeks | 276 | 13.8 |
| 4 to < 16 weeks | 739 | 36.8 |
| 16 to <32 weeks | 489 | 24.4 |
| 32 to <48 weeks | 280 | 14.0 |
| ≥48 weeks | 222 | 11.1 |
| Cumulative dose | ||
| <1500 mg/m2 | 435 | 21.7 |
| 1500 to <3000 mg/m2 | 493 | 24.6 |
| 3000 to < 9000 mg/m2 | 832 | 41.5 |
| ≥9000 mg/m2 | 236 | 11.8 |
| Unknown | 10 | 0.5 |
| Combination chemotherapy | ||
| (−) | 460 | 22.9 |
| (+) | 1546 | 77.1 |
| Irinotecan | 1255 | 62.6 |
| FOLFIRI | 256 | 12.8 |
| Others | 35 | 1.7 |
| Combination radiation therapy | ||
| (−) | 1943 | 96.9 |
| (+) | 59 | 2.9 |
| Unknown | 4 | 0.2 |
| Pre-medicationa | ||
| No | 14 | 0.7 |
| Antihistamine alone | 185 | 9.2 |
| Corticosteroid alone | 23 | 1.2 |
| Antihistamine + corticosteroid | 1783 | 88.9 |
PS, performance status; 5-FU, 5-fluorouracil; LV, leucovorin; UFT, tegafur-uracil; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
aExcluded unknown (one patient).
State of Clinical Use
In total, 77.1% of patients received cetuximab in combination with chemotherapy, and the rest received cetuximab alone. Almost all patients (99.3%) received pre-medication, and most of these (88.9%) were treated with both antihistamines and corticosteroids (Table 1).
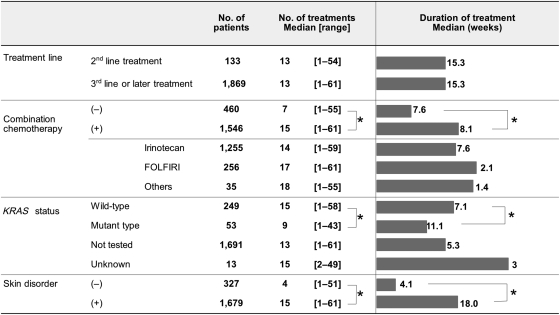
The median duration of treatment was 15.3 weeks (range, 1–73.9), the median numbers of treatment was 13 (range, 1–61) and the median cumulative dose was 3255 mg/m2 (range, 50.0–17 680.9). A total of 11.1% of patients continued cetuximab treatment for >48 weeks (Table 1). The number of treatment and the duration of treatment by patient characteristics are shown in Fig. 1. Treatment duration did not differ among treatment line. The number of treatment was significantly higher, and the duration of treatment was significantly longer in the patients with combination chemotherapy, wild KRAS tumours and skin disorders (P< 0.0001).
Figure 1.
Number and duration of treatment by patient characteristics. The bars show the median duration of the cetuximab treatment for each subgroup of patients. *P< 0.0001 (the non-paired t-test).
Safety
The overall incidence of ADRs (any grade) was 89.6% (n = 1797), and that of ADRs of ≥grade 3 was 21.5% (n= 432) (Table 2). Among patient characteristics affecting the incidence of ≥grade 3 ADRs, significant differences were observed in the comorbidity (presence, 25.5% vs. none, 18.1%; P< 0.0001) and combination chemotherapy (presence, 23.2% vs. none, 15.9%; P= 0.0006). There were no significant differences by age (<65 years, 21.4% vs. ≥ 65 years, 21.6%; P= 0.91).
Table 2.
Incidence of adverse drug reactions (ADRs)
| Any grade |
≥Grade 3 |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Overall incidence of ADRs | 1797 | 89.6 | 432 | 21.5 |
| Incidence of priority survey items | ||||
| Infusion reactions | 114 | 5.7 | 22 | 1.1 |
| Skin disorders | 1679 | 83.7 | 212 | 10.6 |
| Interstitial lung diseases | 24 | 1.2 | 15 | 0.7 |
| Electrolyte abnormalities including hypomagnesaemia | 231 | 11.5 | 24 | 1.2 |
| Cardiotoxicity | 17 | 0.9 | 5 | 0.2 |
| Gastrointestinal disorders | 464 | 23.1 | 77 | 3.8 |
| Thrombosis/embolism | 11 | 0.6 | 8 | 0.4 |
| Delayed wound healing | 5 | 0.3 | 1 | 0.05 |
| Eye disorders | 53 | 2.6 | 1 | 0.05 |
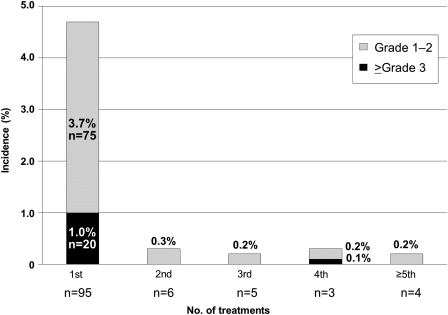
Incidences of the priority survey items are shown in Table 2. IRs were observed in 5.7% of patients (n= 114), whereas IRs ≥grade 3 were observed in 1.1% (n= 22). Most IRs (83.3%; n= 95), especially those of ≥grade 3 (20 out of 22) occurred at the first administration of cetuximab (Fig. 2). The median time to the onset of ≥grade 3 IRs was 10 min (range, 2 min to 8 h). Among the 22 patients with IRs of ≥grade 3, 21 developed IRs within 60 min after the start of administration. There were no differences in the incidence of IRs among pre-medication groups (data not shown).
Figure 2.
Timing of infusion reactions (IRs). The bars show the incidence of IRs for each number of cetuximab treatments received. One patient with a grade 3 IR was excluded because the time to the onset of IR was unknown.
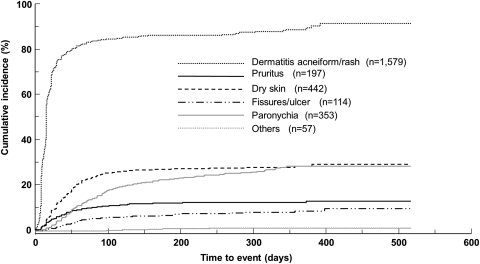
Skin disorders were the most frequently observed ADRs, accounting for 83.7% of patients (n= 1679, any grade). In the seven categories grouped by symptom, dermatitis acneiform/rash (n= 1579, 78.7%) was observed most frequently, followed by dry skin (n= 442, 22.0%) and paronychia (n= 353, 17.6%). The cumulative incidence of each disorder over time after the start of administration of cetuximab is shown in Fig. 3. The median time to the onset of each skin disorder was 15 days for dermatitis acneiform/rash, 30 days for dry skin and 52 days for paronychia.
Figure 3.
Cumulative incidence of skin disorders. The lines show the cumulative incidence of each skin disorder over time from the start of administration of cetuximab using the Kaplan–Meier methods. Skin disorders were divided into seven categories as grouped by symptoms, which is different from the counting based on the system organ class (SOC) classification of Medical Dictionary for Regulatory Activities Terminology (MedDRA).
ILDs were observed in 1.2% of patients (n= 24), and ILDs ≥grade 3 were reported in 0.7% (n= 15). The outcomes included recovery in 2 patients, lower severity in 6 patients, non-recovery in 5 patients, death in 10 patients and unknown in 1 patient. The median time to onset of ILDs was 101 days (range, 17–431), indicating no certain tendency in time to onset of ILDs.
Electrolyte abnormalities (any grade, 11.5%; ≥grade 3, 1.2%) included hypomagnesaemia (10.8%), hypocalcaemia (0.5%), hyperkalaemia (0.4%), hypokalaemia (0.3%), hyponatraemia (0.2%), hypochloraemia (0.1%) and hypophosphataemia (0.1%). The median time to onset of hypomagnesaemia was 57 days (range, 3–418 days), and a higher rate of hypomagnesaemia was observed in patients undergoing long-term administration.
Cardiotoxicity was observed in 17 patients (0.9%) and cardiotoxicity ≥grade 3 was reported in 0.2% (n= 5). Cardiotoxicity included myocardial infarction, cardiac failure, right cardiac failure and coronary spastic angina. All five patients with cardiotoxicity ≥grade 3 were treated with cetuximab plus irinotecan or FOLFIRI, and four patients died.
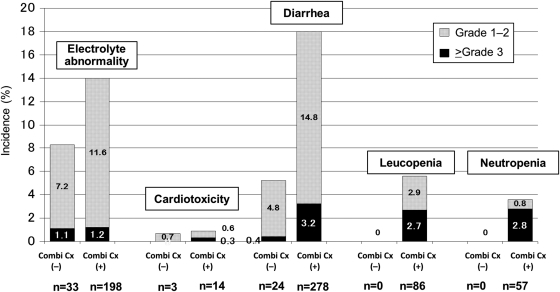
Other ADRs developed including diarrhoea (any grade, 15.1%; ≥grade 3 2.5%), leukopenia (4.3%; 2.0%) and neutropenia (2.8%; 2.2%). Leukopenia and neutropenia did not occur in the cetuximab alone group, and the incidence of diarrhoea in this group was lower than that in the combination chemotherapy group (5.2 vs. 18.0%, P< 0.001) (Fig. 4).
Figure 4.
The incidence of adverse drug reactions (ADRs) with/without combination chemotherapy. The bars show the incidence of ADRs in patients with/without combination chemotherapy. Combi Cx (−): patients receiving cetuximab alone. Combi Cx (+): patients receiving cetuximab in combination with chemotherapy.
A total of 780 patients died during the surveillance period. Of these, a causal relationship with cetuximab could not be ruled out in 22 patients (1.1%). Of the 66 patients (3.3%) who died within 30 days, a causal relationship with cetuximab could not be ruled out in seven patients (0.4%).
DISCUSSION
This report shows the results of a large-scale surveillance in which all patients treated with cetuximab were collected prospectively within a certain period after launch.
The incidence of ADRs in this surveillance (89.6%) was similar to that (93.0%) reported in the MABEL study, in which the efficacy and safety of the combination with irinotecan were studied in 1147 patients with EGFR-positive metastatic CRC refractory or intolerant to irinotecan (10), and that (100%) reported in a Japanese phase II study (9).
Because IRs occurred frequently at the first administration of cetuximab and most of the severe reactions occurred within 1 h after administration, careful observation during the first 1 h is therefore important in cetuximab treatment. It was reported that the incidence of IRs was decreased by administering antihistamine and corticosteroid as pre-medication (11). Accordingly, the Japanese package insert for cetuximab recommends pre-medication with an antihistamine (and corticosteroid), and 88.9% of patients received both pre-medications in this surveillance. So, the incidence of IRs in this report (5.7%) might be lower than that in the MABEL study (15.4%).
Skin disorders were the most frequent ADRs, and the time to event was different for each disorder similar to previous reports (12). The occurrence of skin disorders is considered a predictive factor for the efficacy of EGFR inhibitors (4,13), and it is important to manage skin disorders by considering the occurrence time of each symptom to continue the cetuximab treatment.
The incidence of ILDs in this surveillance (1.2%) was higher than that in the MABEL study (0.3%) (14). With other drugs, it has been suggested that the incidence of ILDs in Japanese patients is higher than that in Caucasian patients (15,16). ILDs are often followed by serious outcomes including death, while no certain tendency in time of onset of ILDs was observed in this surveillance. Urgent consultation with a respiratory specialist and the introduction of corticosteroid pulse therapy should be considered when observing suspicious symptoms and/or images of ILDs.
The incidence of cardiotoxicity was low in this surveillance (0.9%), and all patients with cardiotoxicity ≥grade 3 were treated with irinotecan as combination chemotherapy. Cardiotoxicity has also been reported following fluorouracil treatment (17) and it is important to pay close attention when administering cetuximab in combination with these drugs.
Diarrhoea, leukopenia and neutropenia are frequently observed ADRs caused by cytotoxic anti-cancer drugs, but in this surveillance, they were rarely observed with administration of cetuximab alone. Based on this finding, cetuximab can be used safely in elderly patients and those receiving the drug as later-line treatment.
Although 93.2% of 2006 patients in this surveillance were treated in third-line or later treatment, the median duration of treatment was 15.3 weeks (range, 1–73.9), and 11.1% received long-term treatment >48 weeks, while that in the CO.17 study, in which cetuximab alone was administered to patients refractory or intolerant to fluorouracil, oxaliplatin and irinotecan, was 8.1 weeks (range, 1–60) (4). Based on these observations, cetuximab treatment is considered controllable and clinically effective in practical use in a later-line setting in Japan.
In addition, higher number of treatments and longer duration of treatment were observed in the patients with wild KRAS tumours and those who developed skin disorders. Treatment duration might be considered as a surrogate of the efficacy of treatment including cetuximab. Thus, it might indicate that these patients had obtained the clinical benefit from cetuximab treatment.
KRAS status has been reported to be a predictive factor of response to cetuximab since 2006 (6), and the indication for the use of cetuximab was changed to KRAS wild-type metastatic CRC in July 2008 by the European Medicines Agency, and in September 2009 by the US Food and Drug Administration. At the beginning of this surveillance, there was no description regarding KRAS status on the Japanese package insert for cetuximab, and KRAS testing was not covered by insurance. Therefore, only 15% (n= 263) of the patients included in this surveillance had undergone KRAS testing. It is now recommended that KRAS testing, which has been covered by insurance since April 2010 in Japan, should be conducted before the administration of cetuximab.
CONCLUSIONS
We conducted a prospective PMS in Japanese patients receiving cetuximab for the treatment of metastatic CRC. There was no notable difference in the profiles and incidence of ADRs compared with previous reports from other countries. Although >90% of the patients received cetuximab as third-line or later treatment, the treatment was maintained with a median duration of 15 weeks. Thus, cetuximab is considered well tolerated and clinically useful for Japanese patients.
Funding
This PMS was funded and undertaken by Merck Serono Co., Ltd. and Bristol-Myers Squibb Corp. in accordance with the conditions for approval. Merck Serono Co., Ltd. was responsible for development of the protocol and for the analysis of the data.
Conflict of interest statement
Megumi Ishiguro received a consulting fee from Taiho Pharmaceutical Co., Ltd, and lecture honoraria from Chugai Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd and Yakult Honsha Co., Ltd; Toshiaki Watanabe received lecture honoraria from Chugai Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd, Yakult Honsha Co., Ltd, Takeda Pharmaceutical Company, Merck Serono Co., Ltd and Bristol-Myers K.K.; Taroh Satoh received lecture honoraria from Chugai Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd, Yakult Honsha Co., Ltd, Takeda Pharmaceutical Company, Daiichi Sankyo Company, Merck Serono Co., Ltd and Bristol-Myers K.K.; Yuh Sakata received lecture honoraria from Taiho Pharmaceutical Co., Ltd and Yakult Honsha Co., Ltd and a royalty from International Inc. Synergy; Kenichi Sugihara received lecture honoraria from Chugai Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd, Merck Serono Co., Ltd, Takeda Pharmaceutical Company, Bristol-Myers K.K. and Yakult Honsha Co., Ltd; Hideyuki Ito is an employee of Merck Serono Co., Ltd; Taku Seriu is an employee of Bristol-Myers K.K.
Acknowledgements
We are grateful to the physicians and patients for their cooperation in this surveillance, Merck Serono Co., Ltd., Japan and Bristol-Myers K.K., Japan. All authors are members of the cetuximab advisory board.
References
- 1.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 2.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–70. [PubMed] [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 4.Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 5.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–19. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 6.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chien CRC, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 8.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. KRAS mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 9.Tahara M, Shirao K, Boku N, Yamaguchi K, Komatsu Y, Inaba Y, et al. Multicenter phase II study of cetuximab plus irinotecan in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin and fluoropyrimidines. Jpn J Clin Oncol. 2008;38:762–9. doi: 10.1093/jjco/hyn102. [DOI] [PubMed] [Google Scholar]
- 10.Wilke H, Glynne-Jones R, Thaler J, Adenis A, Preusser P, Aguiler EA, et al. Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL Study. J Clin Oncol. 2008;26:5335–43. doi: 10.1200/JCO.2008.16.3758. [DOI] [PubMed] [Google Scholar]
- 11.Siena S, Glynne-Jones R, Adenis A, Thaler J, Preusser P, Aguiler EA, et al. Reduced incidence of infusion-related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined steroid and antihistamine premedication. Cancer. 2010;116:1827–37. doi: 10.1002/cncr.24945. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E. Challenges in the use of epidermal growth factor receptor inhibitors in colorectal cancer. Oncologist. 2006;11:1010–7. doi: 10.1634/theoncologist.11-9-1010. [DOI] [PubMed] [Google Scholar]
- 13.Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–21. doi: 10.1158/1078-0432.CCR-06-2610. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi K, Satoh T, Watanabe T, Ishiguro M, Maruyama K, Seriu T, et al. The final report of post-marketing surveillance for cetuximab in colorectal cancer in Japan. J Clin Oncol. 2011;29(suppl 4):5–90. doi: 10.1093/jjco/hys005. (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuboi M, Le Chevalier T. Interstitial lung disease in patients with non-small-cell lung cancer treated with epidermal growth factor receptor inhibitors. Med Oncol. 2006;23:161–70. doi: 10.1385/MO:23:2:161. [DOI] [PubMed] [Google Scholar]
- 16.Azuma A, Kudoh S. High prevalence of drug-induced pneumonia in Japan. Jpn Med Assoc J. 2007;50:405–11. [Google Scholar]
- 17.Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8:191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]