Abstract
Background
There is growing evidence that adolescence is a key period for neuronal maturation. Despite the high prevalence of marijuana use among adolescents and young adults in the United States and internationally, very little is known about its impact on the developing brain. Based on neuroimaging literature on normal brain developmental during adolescence, we hypothesized that individuals with heavy cannabis use (HCU) would have brain structure abnormalities in similar brain regions that undergo development during late adolescence, particularly the fronto-temporal connection.
Method
Fourteen young adult males in residential treatment for cannabis dependence and 14 age-matched healthy male control subjects were recruited. Patients had a history of HCU throughout adolescence; 5 had concurrent alcohol abuse. Subjects underwent structural and diffusion tensor magnetic resonance imaging. White matter integrity was compared between subject groups using voxelwise and fiber tractography analysis.
Results
Voxelwise and tractography analyses revealed that adolescents with HCU had reduced fractional anisotropy, increased radial diffusivity, and increased trace in the homologous areas known to be involved in ongoing development during late adolescence, particularly in the fronto-temporal connection via arcuate fasciculus.
Conclusions
Our results support the hypothesis that heavy cannabis use during adolescence may affect the trajectory of normal brain maturation. Due to concurrent alcohol consumption in five HCU subjects, conclusions from this study should be considered preliminary, as the DTI findings reported here may be reflective of the combination of alcohol and marijuana use. Further research in larger samples, longitudinal in nature, and controlling for alcohol consumption is needed to better understand the pathophysiology of the effect of cannabis on the developing brain.
Keywords: Brain, Cannabis, Adolescent, Diffusion tensor imaging, Tractography, Magnetic resonance imaging
According to the Substance Abuse and Mental Health Services Administration (2006), one in two adolescents/early adults have tried cannabis at least once in their lifetime. A recent national survey also reported a history of cannabis (i.e., marijuana) use in approximately 45% of twelfth graders in the United States, with five percent reporting current daily use (Terry-McElrath, Johnston, O’Malley, & Yamaguchi, 2005). In addition, recent studies support the hypothesis that adolescent cannabis use is a gateway to illicit drug use in early adulthood (Fergusson, Boden, & Horwood, 2006).
In addition, there is increasing concern regarding the potential long-term effects of early cannabis use on neurodevelopment, as data from several epidemiological studies have linked early cannabis use (before age 15 years) with the subsequent development of psychiatric disturbances. Several lines of evidence suggest that long-term cannabis use may be more detrimental to the developing brain than for the mature brain in samples of preclinical individuals (Cha, White, Kuhn, Wilson, & Swartzwelder, 2006; Landfield, Cadwallader, & Vinsant, 1988; Stiglick & Kalant, 1985), adolescent cannabis users (Medina et al., 2007a), and adult cannabis users with previously documented neuropsychological deficits (Ehrenreich et al., 1999; Pope et al., 2003). Furthermore, morphometric studies involving adult marijuana users have documented, albeit inconsistently (Block et al., 2000), alterations in brain tissue composition among members of this cohort (Matochik, Eldreth, Cadet, & Bolla, 2005), particularly among adults with a history of early use (i.e., use prior to age 17 years) (Wilson et al., 2000). Previous studies have also reported early-age cannabis users to be more vulnerable to manifest psychosis in adulthood than adult users (Arseneault, Cannon, Witton, & Murray, 2004; Caspi et al., 2005).
Most recently, a structural brain imaging investigation examining white matter abnormalities among adolescent cannabis users yielded evidence that both marijuana use and white matter volume are each independent predictors of depressive symptomatology (Medina, Nagel, Park, McQueeny, & Tapert, 2007b). It has also been hypothesized that long-term exposure to cannabis may be associated with the downregulation of the CB1 receptors and suppress oligodendrocyte function over time (Molina-Holgado et al., 2002). In support of this hypothesis, long-term cannabinoid exposure has been found to be associated with decreased expression of myelin-related genes (Grigorenko et al., 2002). Together, these data provide support for a hypothesis that recurrent exposure to cannabis during adolescence may adversely affect white matter development.
Diffusion tensor imaging (DTI) is a powerful magnetic resonance imaging (MRI) technique suited to the study of white matter (WM) axonal structure because it can be used to quantify the magnitude and directionality of tissue water mobility in three dimensions. DTI has been utilized for the characterization of developmental changes in WM diffusion properties throughout the first two decades of life (Ashtari et al., 2007a; Barnea-Goraly et al., 2005; Guo et al., 2007; Schmithorst, Wilke, Dardzinski, & Holland, 2002). Results of DTI studies in typically developing children have shown prominent age-related increases in fractional anisotropy (FA) in numerous brain regions, including the left arcuate fasciculus (Ashtari et al., 2007a; Guo et al., 2007; Schmithorst et al., 2002), prefrontal cortex (Barnea-Goraly et al., 2005), internal capsule (Ashtari et al., 2007a; Barnea-Goraly et al., 2005; Guo et al., 2007; Schmithorst et al., 2002), corpus callosum (Ashtari et al., 2007a; Barnea-Goraly et al., 2005), right inferior longitudinal fasciculus (Guo et al., 2007; Schmithorst et al., 2002) and ventral visual stream WM (Barnea-Goraly et al., 2005), as well as areas extending from sensorimotor regions that appear to correspond to the corticothalamic and cortico-spinal tracts (Schmithorst et al., 2002).
To our knowledge, only a handful of studies have utilized DTI to examine the effect of chronic cannabis use on white matter integrity in adults. Gruber & Yurgelun-Todd (2005) used a region of interest (ROI) approach in 9 heavy cannabis users (HCUs), aged 18–47 years, and 9 healthy comparison subjects. The authors used four ROIs and placed them on a single slice covering only the frontal regions and reported no significant differences in FA, but did find a trend-level (p = 0.09) increase of the trace value parameter among HCUs. Similarly, Delisi and colleagues (2006) reported no evidence of WM integrity loss in young adults aged 18–27 years (n=10) who were frequent cannabis users during adolescence, relative to 10 age- and sex- matched controls, using whole brain voxelwise analysis. Arnone and colleagues (2006) compared 11 HCUs with demographically matched normal controls using a histogram approach and reported a median FA decrease among marijuana users (p = 0.03). In a more recent study by Arnone and colleagues (2008), the authors reported decreased FA and mean diffusivity in the corpus callusom in a group of adult HCUs. The lack of consistency in findings across these initial studies of adult cannabis users could be a reflection of small sample sizes, sample heterogeneity (e.g., duration and severity of marijuana use, variation in abstinence duration prior to DTI scanning, etc.), and varied methods of image acquisition and analysis.
In the current, preliminary study, we have employed a DTI technique with fifteen gradient directions and isotropic slices using a whole brain voxelwise analysis and diffusion tractography to examine potential relationships between white matter tissue organization (microstructure) and adolescent heavy cannabis use. In addition, we carried out analyses for all diffusion indices, including fractional anisotropy and radial, axial, and mean diffusivity, to study differences in brain structure between adolescent HCUs and healthy comparison subjects without a history of cannabis use. Based on our previous study of normative brain development (Ashtari et al., 2007a) and earlier investigations outlining the areas of brain maturation during adolescence, as well as brain areas where the endogenous cannabinoid system has been documented to influence the development of brain white matter, we hypothesized that subjects with a history of HCU would have diminished FA and increased mean and radial diffusivity in the following brain regions undergoing developmental change during adolescence, as documented by Ashtari and colleagues (2007a): the arcuate fasciculus, internal capsule/thalamic radiation, corpus callosum (documented at a more relaxed threshold in Ashtari, Cervellione, et al., 2007) and regions of the prefrontal cortex.
Materials and Methods
Subjects
Fourteen male adolescents and young adults (mean age = 19.3; SD = 0.8) with a history of heavy cannabis use throughout adolescence, and who were currently enrolled in a residential drug-treatment rehabilitation center to reduce cannabis consumption, were recruited. All individuals were court-ordered after being repeatedly prosecuted for crimes related to possession, sale, or purchase of marijuana. No patient had been court-ordered for other crimes, such as violent offenses. All individuals had been undergoing residential treatment for the duration of the drug-free period reported here (at least 3 months; mean duration = 6.7 months). In addition, all patients were required to have routine in-patient urine toxicology tests for the duration of their in-patient treatment, thus confirming their continued abstinence. All individuals met DSM-IV criteria for cannabis dependence, in remission (American-Psychiatric-Association, 1994). Patients reported using cannabis daily for at least one year prior to enrolling in treatment (mean = 5.8 joints per day). Patients were excluded if they had a lifetime history of more than 10 uses of any illicit substances besides cannabis. Additionally, patients were excluded if they had a history of any serious psychiatric illness, including any psychotic disorder, bipolar disorder, or major depressive disorder. Due to the very high incidence of cannabis abuse with comorbid heavy tobacco use and/or alcohol consumption, use of these substances were not considered exclusionary for this pilot study. Five patients met DSM-IV criteria for past alcohol abuse: the other 9 reported no more than regular use (i.e. one day per week). All patients were alcohol-free for the duration of inpatient rehabilitation.
Fourteen healthy male comparison subjects (mean age = 18.5; SD = 1.4) were recruited from an adolescent medical clinic at Schneider Children’s Hospital (New Hyde Park, NY, USA) via community fliers and word-of-mouth referral. These subjects were similar to cannabis users in terms of demographic and socioeconomic makeup (Table 1). Controls were excluded if they had any current or past DSM-IV diagnosis, history of psychological counseling, a self-reported history of more than 5 lifetime exposures to any illicit drug, or more than occasional use of alcohol (defined as more than 5 drinks per month) at any point in the past. Additional exclusion criteria for all subjects included mental retardation (as evidenced by intelligence test scores, DSM-IV diagnosis or documented history), known neurological illness, history of head injury with loss of consciousness for more than 30 seconds, any focal findings revealed by routine clinical scan at the time of research MRI and current use of psychotropic medications. All participants were interviewed by a research psychologist or trained psychometrician under the supervision of a board-certified child and adolescent psychiatrist (Kaufman et al., 1997).
Table 1.
Subject Demographics
| Patients with Cannabis Use (n=14) | Healthy Controls (n=14) | Statistic Test | p | |
|---|---|---|---|---|
| Mean Age (SD), years | 19.3 (0.8) | 18.5 (1.4) | t = −1.88 | .07 |
| Age Range | 18.00 – 21.20 | 17.30 – 21.50 | ||
| Sex (M/F) | 14/0 | 14/0 | ||
| Ethnicity (Caucasian/non-Caucasian)* | 0/14 | 3/11 | .22 | |
| Handedness (dextral/non-dextral)* | 10/3 | 12/1 | .59 | |
| Parental SES (high/low) 1 | 9/1 | 13/0 | .44 | |
| Mean WRAT3 score (SD) 2 | 84.3 (13.9) | 104.0 (17.6) | t = 3.29 | <.005 |
| Mean Age at Onset of Cannabis Use (SD), years | 13.1 (1.6) | |||
| Mean Duration of Use (SD) | 5.3 (2.1) years | |||
| Mean Amount Used (SD)joints per day** | 5.8 (2.6) | |||
| Mean Time since Last Use (SD), months | 6.7 (3.7) |
Note: Independent samples t – tests and Fisher’s Exact tests utilized as appropriate.
One missing data point for patients and one for controls.
Joints per day during the one year prior to current abstinence period.
Socioeconomic status (SES) (Hollingshead 1975), missing data for controls and patients
Wide Range Achievement Test (WRAT3) Reading subtest (Wilkinson 1993)
Study procedures were approved by the North Shore-Long Island Jewish Health System Institutional Review Board. Written informed consent, or parental consent with participant assent, were obtained as appropriate. Patients provided written approval for research staff to review in-patient rehabilitation charts to collect more detailed information about history of drug use and psychological functioning and to corroborate self-reports gathered during clinical interviews.
Magnetic Resonance Imaging
The imaging protocol and analysis methods have been described in detail elsewhere (Ashtari et al., 2005). MRI scans were conducted at the Long Island Jewish Medical Center on a 1.5T GE Neuro Vascular Interactive (NV/i) system. This unit was equipped with a high strength (50 mT/m) and high-speed gradient system (Slew Rate = 150 T/m/sec).
The diffusion tensor sequence used in this study utilized 15 gradient directions with b-factor of 1000 s/mm2, two b0, 50 isotropic slices (2.5×2.5×2.5 mm3) through the whole brain. Images were acquired parallel to the anterior-posterior commissures (AC-PC) using a dual spin-echo prepared sequence to reduce eddy current related image geometric distortions (Reese, Heid, Weisskoff, & Wedeen, 2003) and a ramp-sampled spin-echo, single shot echo-planar imaging (EPI) method with TR = 14 s, TE = 77 ms, matrix size = 88×88 (zero-filled to 256×256), FOV = 22×22 cm2, slice thickness = 2.5 mm with no gap, NEX = 2, and a total acquisition time of 8:24 minutes. All images were investigated to be free of motion or ghosting, high frequency and/or wrap around artifacts at the time of image acquisition.
In addition to the diffusion tensor images, a matching fast spin echo (FSE) double-echo sequence was acquired as well as a fluid-attenuated inversion recovery (FLAIR) sequence for clinical purposes. A 3D spoiled gradient-echo (SPGR) sequence with inversion preparation pulse (IR-Prep) was also obtained for registration purposes.
Image Processing: Spatial Normalization
Details of normalization methodology used here are presented in earlier reports (Ardekani et al., 2005; Ashtari et al., 2005; Kumra et al., 2005). Briefly, inter- and intra-subject registrations were carried out to ensure image alignment prior to vector and voxel averaging. We implemented an elastic registration algorithm for correction of the EPI image geometric distortions (Ardekani et al., 2005). In this algorithm, a subject image (b = 0 DTI image) is elastically deformed to match a target image (T2 FSE image). The algorithm is used for two purposes in the present paper: (a) for distortion correction of the DTI echo-planar images; and (b) for spatial normalization of the subjects’ SPGR images. Rigid-body registration was used (Ardekani, Nierenberg, Hoptman, Javitt, & Lim, 2003) for intra-subject registration to control for any subtle head motion or possible subject motion that may have occurred between or within sequence acquisitions. A single subject with the median brain volume among all participants was used as the template image. The whole-brain 3D SPGR of the template image was transformed into Talairach space using AFNI (http://afni.nimh.nih.gov/afni/). The diffusion-weighted images of each subject were spatially normalized to this template image. The transformations from all three registrations were combined mathematically into a single transformation to reduce multiple interpolations.
Eigenvalues and eigenvectors of the diffusion tensor matrix for each voxel were computed from the 17 DTI volumes (15 gradient directions and two b = 0 images) for each subject using methods described by Basser (1995) and Pierpaoli and Basser (1996). Using the computed eigenvalues (λ1, λ2, and λ3), the magnitude images for radial diffusivity λ⊥[(λ2 +λ3)/2], axial diffusivity λ|| (λ1), trace of the diffusion tensor (apparent diffusion coefficient or mean diffusivity) Dtr (λ1+ λ2 +λ3), and FA were calculated. Axial (λ||), radial (λ⊥), mean diffusivity (Dtr) and FA maps were transferred into Talairach space using the above registration procedures. It should be noted that the λ||, λ⊥, and FA maps are first computed in the subject’s native image space and then transformed into the common Talairach coordinates using the above mentioned three-step registration process. All images were smoothed with a 6 mm full-width-half-maximum (FWHM) Gaussian kernel in 3D. Using the above transformations, we obtained 28 1 mm isotropic FA maps (14 patients and 14 healthy volunteers) in common Talairach space.
Tractography Procedures and Extraction of Specific Fibers
Fiber tractography was performed using DTIStudio, which is based on the fiber assignment by continuous tracking (FACT) method (Mori et al., 2002; Xue, van Zijl, Crain, Solaiyappan, & Mori, 1999). Fibers are selected based on initiating a seed pixel in the anatomy of choice. From this seed point a line is propagated which follows the principal eigenvector in 3D contiguous space from voxel to voxel (Mori et al., 2002). A more detailed description of the DTIStudio and its functionalities is presented in a recent paper by Jiang and colleagues (2006). A threshold of 0.20 for FA value was used (Mori et al., 2002) and a turning angle of 41° (Okada et al., 2006; Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004) was used to perform tractography.
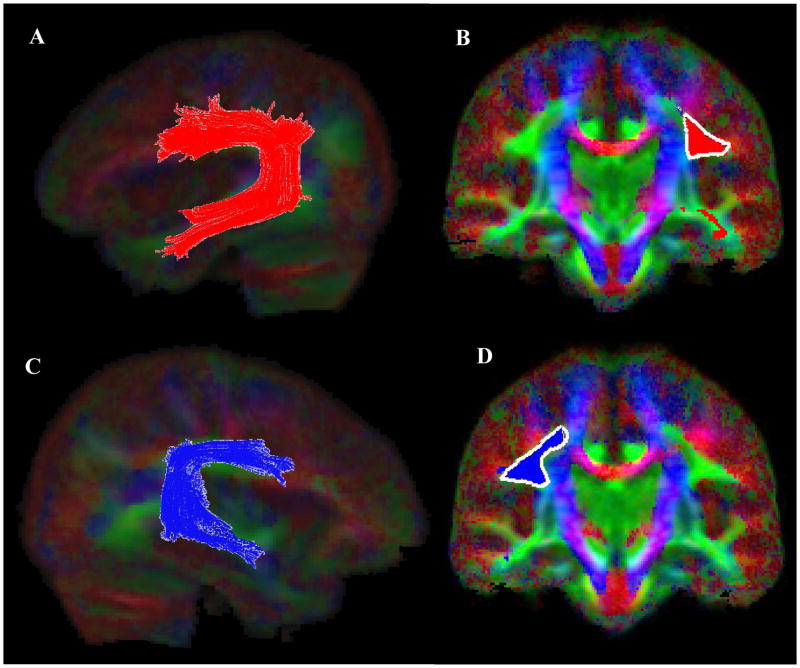
We used the results of our voxelwise analyses as a guide to place the seeds for tractography (Ashtari et al., 2007b). Since the posterior internal capsule/thalamic radiation is known to have complex projections to various parts of the brain (Behrens et al., 2003) we limited our tractography to the left and the right arcuate fasciculi. The left and the right motor tracts were extracted as control fibers. Similar to the study by Mori et al. (2002) the left and the right arcuate fibers were extracted by placing a single ROI in the densely concentrated fiber bundle along the superior segment of the arcuate fibers (between Broca’s and Wernicke’s areas). To clearly identify the arcuate fiber bundles, placement of ROI was performed on color-coded diffusion images (Figure 5; Images B &D). A subset of projections that were not part of arcuate tracts were excluded using the “NOT” operation of the DTIStudio. As is depicted in Figure 5 (Images A & C) arcuate fibers occupy extensive areas with branches penetrating the frontal, parietal, and temporal cortex.
Figure 5.
Extraction of the arcuate fasciculus (AF) fiber bundle (left AF; Image A, right AF; Image C) using the DTIStudio with a FA threshold of 0.2 and tract turning angle of 41 degrees. The seed ROI locations on the color coronal slices used in conjunction with the “OR” function of the DTIStudio are depicted for the left and the right AF in Images B and D respectfully.
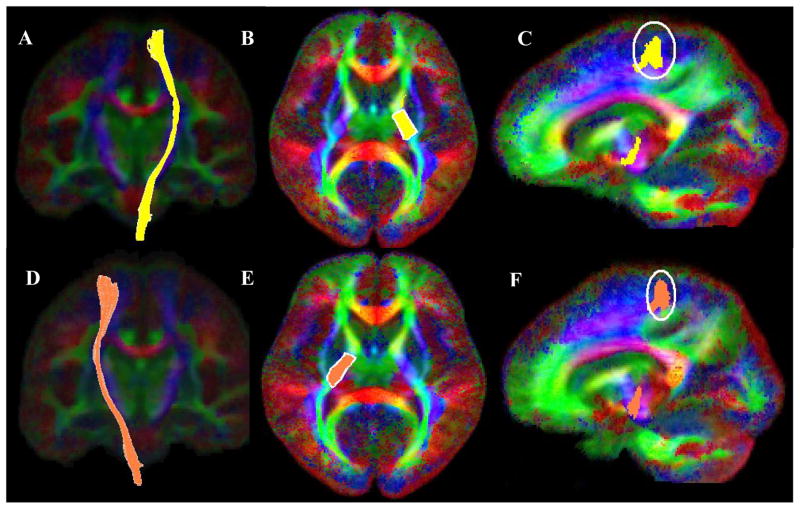
Motor tracts were extracted using two ROI’s (Lee et al., 2005; Mori et al., 2002; Okada et al., 2006; Wakana et al., 2004). Initial ROI was placed in the posterior segment of the internal capsule (Figure 6; Images B & E) using the “OR” function of the DTIStudio. This region is known to encompass both the pyramidal tract and somatosensory fibers. Since anatomically motor fiber must pass through the precentral gyrus, the second ROI was placed in the precentral gyrus using the “AND” function of the DTIStudio (Figure 6; Images C & F). Fiber branches to the cerebellum and pons or other non-motor brain areas were excluded using the “NOT” function. The resultant left and the right motor fibers are depicted in Figure 6; Images A & D respectively.
Figure 6.
Fiber bundle extraction for the left (Image A) and the right (Image D) motor fibers using the DTIStudio with a FA threshold of 0.2 and tract turning angle of 41 degrees. Motor fibers were extracted using two ROIs, first with the “OR” function a seed ROI was placed at the posterior segment of the internal capsule (left motor; Image B, right motor; Image E). The “AND” function was used in conjunction with a second ROI (left motor; Image C, right motor; Image F) encompassing the precentral gyrus projection of the fibers created with the first ROI.
Group Averaging of Diffusion Tensor Data for Tractography
Similar to single subject tractography in DTIStudio, group tractography needs an average FA and an ‘average’ principal eigenvector for all subjects. Using the above mentioned rigorous registration procedure, also reported in our earlier reports (Ashtari et al., 2005; Szeszko et al., 2005), a deformation field specific to each subject, that can be used for spatial normalization of the subject’s DTI images to a standardized space (i.e., Talairach space) is obtained. This deformation field is a vector-valued function of a vector variable that maps the coordinates of any given grid point in the standardized space to its corresponding point in the subject’s original DTI image space (Ardekani et al., 2005). Deformation field of each subject is then applied to the subject’s FA image and an arithmetic average of all spatially normalized group FA images is then calculated. The principal eigenvector field for the group was obtained by first computing the Jacobian matrix (Kaplan, 2003) of the deformation field at each grid point on the standardized space for each subject. The eigenvector in each subject’s original DTI image space that would be mapped to the particular grid point in the standardized space was then reoriented by pre-multiplying it with the computed Jacobian, as suggested by (Alexander, Pierpaoli, Basser, & Gee, 2001). Since DTIStudio only requires the principle eigenvector to perform tractography, we have not applied reorientation to the remaining two radial eigenvectors. Reorientation of the principle eigenvector was subsequently repeated for all subjects at all grid points in the standardized space. Thus, for each grid point, we obtained as many (re-oriented) eigenvectors as there were subjects in the study (N=28). Finally, at each grid point, a principal component analysis (PCA) (Kent, Bibby, & Marida, 1980) was performed on the n=28 eigenvectors. The principal eigenvector of this PCA was then taken to represent the whole group at the grid point in question.
Statistical Analyses
All analyses examining demographic and clinical characteristics of the study sample were conducted using SPSS (SPSS Inc., Chicago, Illinois). Demographic differences between cannabis users and comparison subjects were analyzed using independent samples t tests and Fisher’s exact tests, as appropriate. WRAT3 (Wilkinson, 1993) reading scores were used as to estimate for intelligence. Since WRAT3 reading score was significantly different between groups (t = 3.29, p < .01), statistical analyses were performed controlling for this variable.
Voxelwise Analyses
Voxelwise analysis of covariance (VANCOVA) was performed for the FA, axial, radial and mean diffusivity images between patients and controls covarying for subject’s estimates of premorbid intelligence (WRAT3 scores). To control for Type I errors (false positives) we used the false discovery rate (FDR) measure (Benjamini & Hochberg, 1995) provided by the ‘FDR’ program as part of the FSL software package (http://www.fmrib.ox.ac.uk/fsl/). FDR represents the expected proportion of rejected hypotheses that are false positives. In the present study, we used a 1% false discovery rate (FDR = 0.01) for a significance level of p = 0.001, unless no significant clusters were observed. Specifically, for the current study: FA analyses FDR = 0.01, p < .001; axial and mean diffusivity FDR = 0.015, p < .005; radial diffusivity FDR =0.04 and p < .005. As an additional safeguard against false positives, clusters greater than 100 voxels in size for FA and 200 for the other diffusion index maps were retained. Tract based analyses were performed using ANCOVA on all extracted fibers controlling for reading scores (WRAT3) between HCUs and healthy comparison subjects.
Results
Sample Characteristics
Sample characteristics are summarized in Table 1. HCUs with mean age of 19.3 years (range: 18.0–21.2 years) reported a mean age of first cannabis use of 13.1 years (range: 9.0–15.0 years) and were drug-free for a median duration of 6.7 months (range: 3–11 months) prior to MRI scan. In the one-year prior to the current period of abstinence, HCUs reported using an average of 5.8 joints per day. Five HCUs met DSM-IV criteria for past alcohol abuse; the remaining nine reported no more than regular use. Alcohol use was not quantified to any further extent. Two HCUs had a history of attention deficit hyperactive disorder (ADHD) and three others had a comorbid diagnosis of conduct disorder. Comparison subjects, with mean age of 18.5 years (range: 17.30–21.50 years), were similar to cannabis users in distributions of age, gender, parental socio-economic status (SES), ethnicity and handedness.
Voxelwise Analysis
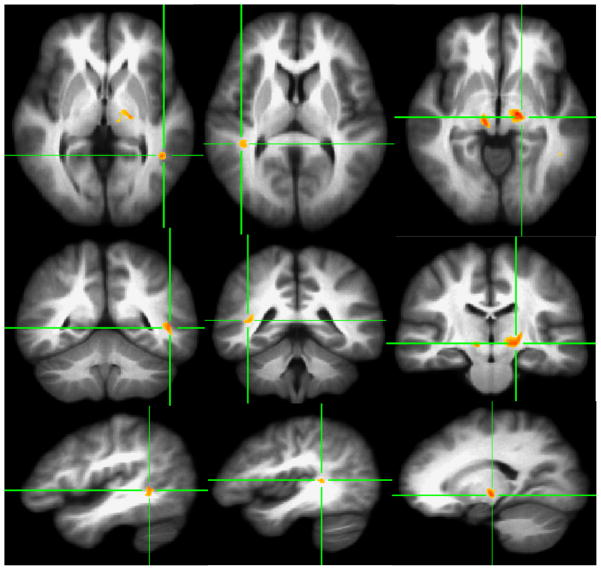
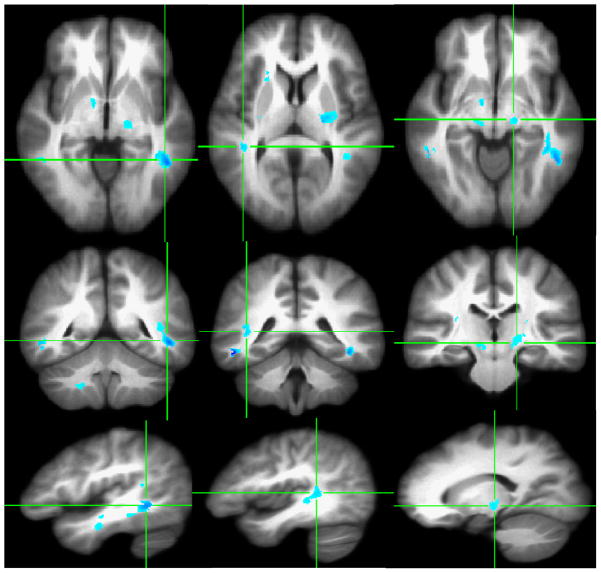
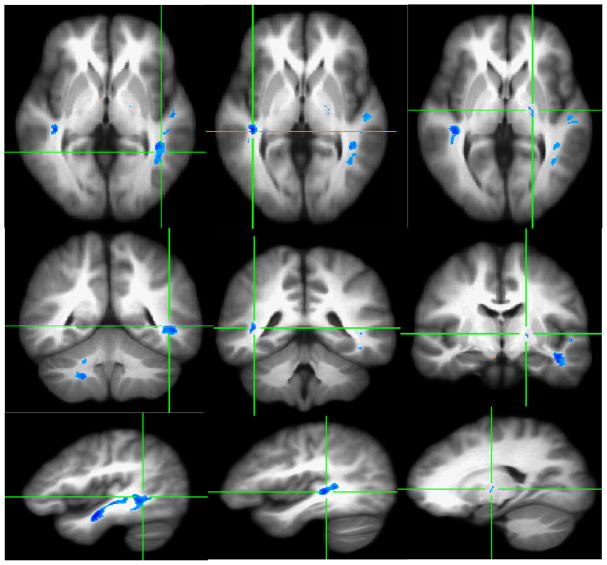
At a statistical significance level of p < .001 (i.e., corrected p < .01) and a cluster size extent threshold of ≥100 contiguous voxels, four clusters with decreased FA were identified in the left posterior internal capsule/thalamic radiation, left middle temporal gyrus, right posterior internal capsule and right superior temporal gyrus (Figure 1). Significant increase in radial (λ⊥) and trace values (Dtr) at p < .005 (corrected p < .015) and 200 contiguous voxels in HCUs, as compared to healthy comparison subjects, are shown in Figures 2 and 3 in the homologous brain areas that showed decreased FA (see Figure 2 and 3 and compare the locations with Figure 1).
Figure 1.
VANCOVA analysis shows decreased FA for ≥100 contiguous voxels at P<.001 superimposed on the averaged Talairach transferred images of all subjects. Reduced FA clusters are depicted along the left middle temporal lobe (First Column), the right superior temporal gyrus (Second Column), and the bilateral posterior internal capsules/thalamic radiations (Third Column).
Figure 2.
VANCOVA analysis shows increased radial diffusivity (λ⊥) in ≥200 contiguous voxels at P<.005 superimposed on the averaged Talairach transferred images of all subjects. Increased radial diffusivity is depicted along the left middle temporal gyrus (First Column), right superior temporal gyrus (Second Column) and the bilateral posterior internal capsule/thalamic radiations (Third Column).
Figure 3.
VANCOVA analysis shows increased trace (Dtr) in ≥200 contiguous voxels at P<.005 superimposed on the averaged Talairach transferred images of all subjects. Increased trace is depicted along the left middle temporal gyrus (First Column), right superior temporal gyrus (Second Column) and the left posterior internal capsule/thalamic radiations (Third Column).
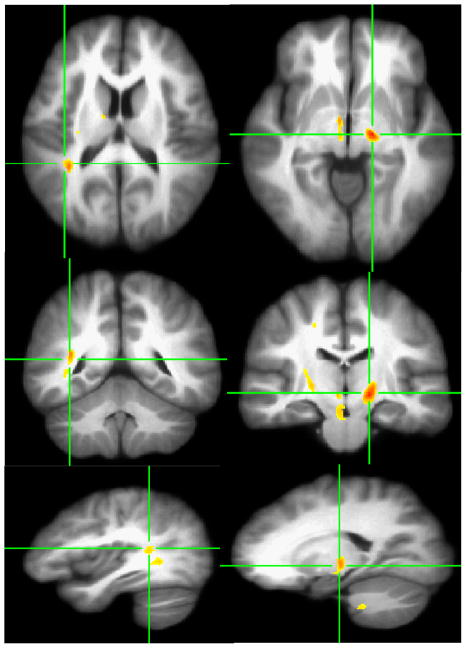
Decreased axial diffusivity for HCUs in some homologous areas with decreased FA and increased radial and mean diffusivity was observed at p < .005 (corrected p < .04) and extent threshold of ≥200 voxels (see Figure 4). A summary of these findings with their respective Talairach coordinates and cluster sizes are presented in Table 2.
Figure 4.
VANCOVA analysis shows decreased axial diffusivity (λ1) in ≥200 contiguous voxels at P<.005 superimposed on the averaged Talairach transferred images of all subjects. Decreased axial diffusivity is depicted the right superior temporal gyrus (First Column), the left posterior internal capsule/thalamic radiations (Second Column).
Table 2.
Talairach Coordinates and Voxelwise Analysis Results of Fractional Anisotropy, Radial Diffusivity (λ⊥), and Trace (Dtr) Parameters of Heavy Cannabis Users (n=14) Relative to Normal Controls (n=14)
| Changes in Diffusion Parameters | Anatomical Location | Cluster Size (# of Connected Voxels) | X | Y | Z |
|---|---|---|---|---|---|
| Fractional Anisotropy | |||||
| Right Posterior Internal Capsule | 302 | 10 | −18 | −4 | |
| Left Posterior Internal Capsule | 687 | −15 | −18 | −2 | |
| Left Middle Temporal Gyrus | 176 | −44 | −47 | 4 | |
| Right Superior Temporal Gyrus | 166 | 39 | −33 | 11 | |
| Radial Diffusivity | |||||
| Left Middle Temporal | 1714 | −43 | −40 | −1 | |
| Left Posterior Internal Capsule | 1207 | −23 | −14 | 6 | |
| Right Superior Temporal Gyrus | 362 | 41 | −32 | 6 | |
| Right posterior Internal Capsule | 774 | 28 | −10 | 14 | |
| Trace | |||||
| Left Middle Temporal | 1031 | −44 | −11 | −12 | |
| Left Middle Temporal | 843 | −42 | −44 | −2 | |
| Rt Superior Temporal | 431 | 40 | −30 | 4 | |
| Lt. posterior Internal capsule | 488 | −25 | −13 | 10 | |
| Axial Diffusivity | |||||
| Lt Posterior Internal capsule | 734 | −16 | −14 | −3 | |
| Rt posterior Internal Capsule | 358 | 25 | −17 | 6 | |
| Rt superior temporal gyrus | 588 | 36 | −41 | 10 |
Tractography
The left and the right arcuate fibers and the left and the right motor fibers were extracted ten times using DTIStudio. The tract-specific fibers were superimposed as an anatomical ROI onto the Talairach-transferred FA, λ11, λ⊥, and Dtr images for each subject to obtain the scalar values for their respective diffusion parameters. Results of the tractography analyses comparing these scalar values between HCUs and healthy comparison subjects (controlling for WRAT3 reading score) are presented in Table 3. HCUs exhibited decreased FA in the left arcuate tract at a trend level (p = .11) and at a significant level for the right arcuate (p = .02). HCUs showed a significant increase in λ⊥ for the left arcuate (p = .02) and the right arcuate (p = .01). HCUs showed a significant increase in Dtr for the left arcuate (p = .04) and the right arcuate (p = .03). HCUs showed decreased λ11 for the left (p = .01) and the right (p = .02) motor tracts.
Table 3.
Mean of Ten SLF and Motor Tracts Specific Measurements of Diffusion Indices and Statistical Analysis Between Healthy Cannabis Users and Normal Controls.
| Fiber Bundle | Healthy Controls (n=14) | Heavy Cannabis Users (n=14) | Test Statistic* | P |
|---|---|---|---|---|
| Diffusion Measures 10 Measurements (Arbitrary Units) |
Mean (SD)** | Mean (SD)** | ||
| Left SLF | ||||
| Fractional Anisotropy (FA) | 311.07 (15.97) | 294.73 (17.89) | 2.774 | .11 |
| Radial Diffusivity (λ⊥) | 788.58 (68.64) | 843.73 (54.22) | 5.92 | .02 |
| Axial Diffusivity (λ11) | 1376.79 (38.25) | 1351.98 (36.91) | 0.727 | .40 |
| Trace (Dtr) | 2956.53 (145.43) | 3042.27 (103.06) | 4.655 | .04 |
| Right SLF | ||||
| Fractional Anisotropy (FA) | 306.83 (12.73) | 292.57 (14.13) | 6.151 | .02 |
| Radial Diffusivity (λ⊥) | 774.09 (73.20) | 832.56 (53.82) | 7.331 | .01 |
| Axial Diffusivity (λ11) | 1356.74 (34.31) | 1331.24 (42.96) | 1.174 | .29 |
| Trace (Dtr) | 2904.98 (156.86) | 2997.69(114.90) | 5.162 | .03 |
| Left Motor | ||||
| Fractional Anisotropy (FA) | 377.81 (20.07) | 371.52 (19.88) | 1.293 | .27 |
| Radial Diffusivity (λ⊥) | 744.49 (98.12) | 717.14 (38.55) | .001 | .97 |
| Axial Diffusivity (λ11) | 1461.65 (118.26) | 1340.71 (66.25) | 7.739 | .01 |
| Trace (Dtr) | 2950.69 (281.76) | 2774.40 (93.25) | 1.777 | .20 |
| Right Motor | ||||
| Fractional Anisotropy (FA) | 367.41 (18.01) | 359.90 (19.88) | 1.146 | .30 |
| Radial Diffusivity (λ⊥) | 736.37 (79.90) | 722.86 (57.12) | .189 | .67 |
| Axial Diffusivity (λ11) | 1425.58 (110.27) | 1319.32 (65.19) | 6.519 | .02 |
| Trace (Dtr) | 2898.37 (242.93) | 2761.29 (139.12) | .948 | .34 |
All analyses were performed using analysis of covariance, controlling for WRAT3 (Wilkinson 1993) Reading scores.
All diffusion parameters were multiple by 1000.
Clinical Correlates
As a set of post-hoc exploratory analyses, we examined the relationship between neuroimaging measure results and clinical variables, specifically age at onset of cannabis use, duration of use, amount of use, and time abstinent. Among HCUs, Spearman correlations were performed between each of the clinical variables and values for each of the significant brain regions specified in Tables 2 and 3. Results of all analyses were non-significant at p < .05.
Discussion
In the current preliminary invetigation, voxelwise analysis and tractography were used to compare the WM fiber tract integrity of adolescents/young adults with HCU throughout adolescence to demographically-matched healthy comparison subjects. In the first set of analyses, VANCOVA at an FDR corrected statistical threshold (p < .01, 100 extent threshold) revealed four clusters of decreased FA among HCUs relative to healthy comparison subjects, including: bilateral posterior internal capsule/thalamic radiation, the left middle temporal gyrus and the right superior temporal gyrus. In addition, HCUs had increased radial diffusivity, increased trace values and decreased axial diffusivity in some homologous brain regions where decreased FA was observed. Decreased FA with a simultaneous increase in radial diffusivity and trace values within the same brain regions is suggestive of decreased myelination, which could reflect an arrest or delay in the myelination process. As such, the results of the current study indicate that heavy cannabis use during adolescence may lead to decreased myelination during adolescence.
The second set of analyses involved fiber tractography for the right and left arcuate fiber tracts, and was guided by the results of the voxelwise analysis. Tractography results, for most diffusion parameters, showed a similar pattern of abnormality as found in the voxelwise analysis, including decreased FA, increased λ⊥, and increased Dtr. Temporal lobe damage (i.e., damage to the arcuate regions) has been reported in previous functional brain studies of HCUs (Amen & Waugh, 1998; Mathew, Wilson, Humphreys, Lowe, & Wiethe, 1992).
In order to provide divergent validity and specificity to our findings, the left and the right motor fiber bundles were extracted as control tracts. Analysis of the control tracts showed no significant differences in FA, or in radial or mean diffusivity, but revealed significantly lower axial diffusivity in HCUs for both the left and right motor fibers. Though we did not expect to observe any changes in the control fibers, this finding may be an indication of early changes in these fibers in HCUs, as compared with healthy comparison subjects.
Evidence that Heavy Cannabis Use Disrupts Normal White Matter
Neurological effects of cannabis are largely mediated by the binding of its active ingredient, delta9-tetrahydrocannabinol (THC) to cannabinoid CB1 receptors in the brain and CB2 receptors the outside brain (Martin, Compton, Prescott, Barrett, & Razdan, 1995; Matsuda, Lolait, Brownstein, Young, & Bonner, 1990; Munro, Thomas, & Abu-Shaar, 1993). Although it has been recently shown that CB2 receptors are present in the brain (Onaivi et al., 2006) it appears that the abuse-related effects of cannabinoids are mostly, if not exclusively, mediated by CB1 receptors.
Cannabinoid receptors have been known to interact with brain WM in early and late gestation periods (Berrendero et al., 1998), thus supporting the potential influence of the endogenous cannabinoid system in brain WM development processes, such as neuronal migration, axonal elongation and myelin formation. Hence, it is possible that long-term exposure to cannabis may have profound effects on the number and/or function of oligodendrocytes (Molina-Holgado et al., 2002). Abnormalities of oligodendroglial cells could easily lead to abnormalities in myelin integrity, including myelin initiation, deposition, compaction and maintenance (Davis et al., 2003). Long term cannabis effects on the brain have also been shown in other animal (Kittler et al., 2000) and human studies (Arnone et al., 2006, 2008; Matochik et al., 2005) examining the influence of HCU on white matter density. Consistent with previous human and animal studies, the voxelwise analyses of the current report reveals patterns of altered water diffusion along the axons of several white matter fiber bundles in a group of HCUs, relative to healthy comparison subjects. Clusters of reduced FA along the posterior internal capsule/thalamic radiation areas and bilateral temporal regions were found in some of the same regions reported to undergo significant brain development during adolescence (Ashtari et al., 2007a; Barnea-Goraly et al., 2005; Guo et al., 2007; Schmithorst et al., 2002). These clusters of reduced FA were accompanied by increases in measures of trace and radial diffusivity, suggesting a pattern of myelination deficits within the axons (Ashtari et al., 2007a). Together, these results suggest that HCU during adolescence may interfere with the normal development of myelin, the insulating material around axons. Furthermore, our tractography findings for two critical fiber tracts involved in brain development during late adolescence (i.e., bilateral arcuate fiber tracts) (Ashtari et al., 2007a; Barnea-Goraly et al., 2005; Guo et al., 2007) showed a similar pattern of water diffusion abnormality along the entire fiber bundles (i.e., decreased FA accompanied by increased radial and mean diffusivity). If the diffusion abnormalities along the entire arcuate fiber bundles were limited to only those clusters reported in the voxelwise analyses, no significant effects would have been observed using the averaged diffusion parameters of the whole arcuate tracts. Therefore, we consider the tractography results an independent outcome of our data to further support the voxelwise findings in suggesting that HCU during adolescence may interfere with normal brain development.
To date, very few studies have been conducted using neuroimaging techniques to assess structural abnormalities in the brains of patients with HCU. One of the most recent studies on the effects of HCU on the brain was conducted by Yücel and colleagues (2008). Results of this study provide new evidence for the cannabis effect on brain structural abnormalities in the hippocampus and amygdala in long-term HCUs. In this report, Yücel and colleagues concluded that while modest use of cannabis may not lead to significant neurotoxic effects, heavy daily use of cannabis might indeed be toxic to human brain tissue (Yücel et al., 2008). It should be mentioned that the white matter changes of the arcuate fasciculi reported in the current study are in line with the volumetric changes of medial temporal structures reported by Yücel and colleagues. Although we did not find clinical correlates between measures of cannabis use and diffusion parameters, Yücel and colleagues reported the hippocampal volume to be inversely associated with cumulative exposure to cannabis. As there may be a link between these structures, future studies are needed to carefully examine the relationship between the fiber bundles of the arcuate fasciculus and medial temporal structures such as hippocampus and amygdala.
Clinical neuroimaging of marijuana users to assess changes in neurovasculature, blood flow, and presence of infarct or other ischemic processes, were presented in a review by Rojas (2005). Structural and functional effects of marijuana use on the brain were recently published in a review by Quickfall and Crockford (2006), who reported inconsistent structural, but robust functional, brain abnormalities in HCUs. This review, however, did not cover diffusion studies conducted with cannabis users. A comprehensive review of the diffusion imaging in the addiction literature was subsequently published by Arnone (2006), whereby only three DTI studies related to cannabis use were documented: some of which reported contradictory results to those presented here. There are a number of possible reasons why our results differed from previous DTI studies. Gruber and Yurgelun-Todd (2005) were the first to report on the neurotoxic effects of cannabis using DTI; however, DTI was performed as an adjunctive component to a functional paradigm in that initial investigation, and was limited to the frontal lobe regions of the brain. In addition they used ROI as the method of choice for their DTI analysis, reporting no diffusion changes in the frontal lobe for the cannabis users. Similar to Gruber and colleagues (2005), we did not observe white matter abnormalities in frontal regions for HCUs, as compared to healthy comparison subjects. However, since we utilized a whole brain analysis which revealed differences in regions other than the frontal regions, the areas reported on in the current study were not assessed by Gruber and Yurgelun-Todd (2005). A second DTI study (published in abstract form) by Arnone and colleagues (2006) also used a limited ROI approach, thus examining a limited number of brain regions, and similarly reported a general reduction of FA for cannabis users. Both of these studies had methodological DTI acquisition limitations, in that they did not use optimal gradient directions to increase signal-to-noise ratio; they used thick image slices (i.e. 5 mm as compared to 2.5 mm in the current report); and, they did not perform a whole brain analysis. In a more recent report by Arnone and colleagues (2008) the authors used a more sophisticated diffusion imaging methodology, but they limited their analyses to the corpus callosum and its projections connecting to various brain regions. Although similar to our findings Arnone and colleagues (2008) showed reduced FA and increased mean diffusivity in HCUs, the authors failed to match their HCUs with normal controls on measures of IQ and socioeconomic status (SES), and no abstinence period was reported.
In an investigation most similar to the current study, especially in terms of image analysis methodology, DeLisi and colleagues (2006) reported no evidence of WM integrity loss in young adults who were frequent cannabis users during adolescence. This investigation, however, differed from the current study in numerous ways, particularly in terms of sample demographics and image acquisition characteristics. First, in DeLisi and colleagues’ investigation, the minimum-use threshold for inclusion in the study (i.e., a minimum of 21 times during any single year, during adolescence) was much lower than that within the criteria set forth by Bolla and colleagues (2005). In addition, DeLisi and colleagues reported on a sample with a wide age range (17–37 years) and a small number of subjects (n=10), as well as a longer duration of elapsed time since last use. This time lapse may have increased the potential for a history of drug use recall bias. Furthermore, DeLisi and colleagues did not provide data on variables related to the chronology of drug use and the period of highest use (e.g., during early, middle or late adolescence), which potentially could have influenced the DTI results. In the current study, cannabis users began smoking marijuana approximately at age 13, and, on average, continued to do so until about ages 18–19: This reflects the potential neurotoxic exposure to cannabis during a critical period of brain development in adolescence. The amount of cannabis use was also substantially greater in our cohort, who reported consuming about 5.8 joints per day during the final year, prior to abstinence. Therefore, results reported by DeLisi may not be generalizable to samples other than those of recreational cannabis users. Aside from sample differences, our study also differed moderately from DeLisi and colleagues’ in terms of methods of diffusion image acquisition. In the current study, we employed a larger number of gradient directions (15 diffusion directions, and 2 b=0 images) covering the whole brain with fifty 2.5-mm isotropic slices. DeLisi and colleagues, however, used six gradient directions and one b=0 image with non-isotropic 5-mm slice thickness.
Clinical Correlates
Based on the results of the clinical-related analyses of the current study, the relationship between cannabis use patterns and WM structural abnormalities remains unclear. As with the few prior examinations that have utilized MRI technology to examine the long-term effects of cannabis use on the brain (Quickfall et al., 2006), there were no significant correlations between clinical and imaging measures. The most straightforward reason for these null findings is the small sample size of the current, and prior, studies. Hence, paired with the small range of variability in the clinical measures, the statistical power to detect an association was very low. Additionally, subjects were recruited from within a limited age range and were very similar in drug use characteristics. Furthermore, the clinical measures we used to characterize the sample were not well defined: e.g., “Amount used” was operationalized as ‘joints’ per day, which does not account for variability in the respondent’s definition of a ‘joint’; “length of use” was defined in our study as the time from initial use to time of current abstinence period, since individuals may have had different accelerations of use from time of first experimentation to heavy use, this may have affected the measure of clinical variables. Clinical measures were based on self-report; therefore the accuracy of the data and the reliability of the results of analyses including these measures is also questionable. It is also possible that early life trauma, stress, alcohol consumption and other background variables that co-vary with cannabis use could be significant predictors of WM abnormalities in HCUs (i.e., HCU is a non-specific factor associated with altered white matter development). Hence, further studies that are prospective in design should attempt to better classify and more accurately assess these clinical variables. Longitudinal studies assessing the same study cohort - before and after a carefully monitored abstinence period from marijuana use - are needed to determine whether brain changes are permanent or can be remediated by rehabilitation and intervention.
Strengths and Limitations
There are a number of limitations to report for the current study, many of which are also present in other investigations of this type. The sample size in this study was small, partially due to the difficulty in recruiting a sample that would meet all necessary inclusion/exclusion criteria. Due to the paucity of research that has been conducted in this area, we believe that keeping strict study criteria, and, therefore, a homogenous sample was important to this preliminary investigation. This sample included only clinically-referred male patients from mid-to-low socio-economic backgrounds, and with low IQ scores. As mentioned previously, although we have documented and quantified many aspects of marijuana use/abuse in our sample (e.g. the amount of use, initial age and length of use, and period of abstinence), these data are highly dependent on the veracity, validity and reliability of self-reports. Although we attempted to corroborate self-report information (e.g. by researching patients’ treatment charts), we were not able to do so in all cases. This limitation, however, is inherent to all studies using measures of self-report. We did not control for the effects of nicotine consumption in our sample. A recent paper by Arnone and colleagues (2008) elegantly explains that structural changes due to nicotine use in cannabis consumers is unlikely.
We also did not control for the amount of alcohol intake in HCUs. Arnone and colleagues (2006) showed that excessive alcohol consumption has been associated with structural changes using DTI. Although the DTI changes associated with alcohol abuse, documented by Arnone and colleagues, were primarily evident in the corpus callosum, conclusions from our report should be considered preliminary, as the DTI findings reported here maybe the combination of alcohol and marijuana effects.
With regards to the nicotine effect on human brain white matter, in a recent report by Durazzo and colleagues (2007), the authors studied three groups of patient to assess the effects of nicotine and alcohol on brain gray and white matter, and CSF contents. Regional volumetric comparisons were made among age-matched smoking heavy drinkers (n = 17), non-smoking heavy drinkers (n = 16), and non-smoking light drinkers (nsLD; n = 20). Nicotine effects in this study were only reported for gray matter and not white matter. In a separate study by Gazdzinski and colleagues (2005), the authors concluded that comorbid chronic cigarette smoking in heavy drinkers accounts for some of the variance associated with cortical gray matter loss. In the same paper, the authors reported chronic cigarette smoking to be associated with decreased parietal and temporal gray matter and increased temporal white matter. Based on the above preliminary studies, we have assumed that the breach of white matter integrity observed in the current study is likely not due to nicotine use.
In terms of sample acquisition and characteristics, this study is cross-sectional in nature, which makes it impossible to determine the causality of the findings (Di Forti, Morrison, Butt, & Murray, 2007). For instance, there are a number of plausible trajectories that our results could indicate: 1) WM myelination deficits in these brain areas may be evident before the start of cannabis use, and thus may represent a risk factor for drug dependence; 2) cannabis use may result in deficits in myelination that were observed in this study; 3) there may be a third variable that causes a predisposition to myelin development dysfunction and cannabis abuse (e.g., the presence of below-average IQ or environmental factors). Skranes and colleagues (2007) recently reported a positive relationship between FA in various brain areas (e.g. posterior internal capsule and arcuate fasciculus) and IQ. Therefore, low IQ among heavy cannabis users is a potential confound of the current study and needs to be addressed in future investigations.
There are numerous psychiatric disorders reported to manifest with alterations of brain diffusion parameters, such as ADHD (Ashtari et al., 2005), depression (Li et al., 2007), alcoholism (De Bellis et al., 2008), and heroin use (Lim et al., 2008; Moeller et al., 2007). In the current study, the brain areas reported to have WM deficiencies overlap with those brain regions reported to be still developing in normal healthy individuals, throughout adolescence (Ashtari et al., 2007a). We cannot rule out, however, that the presence of comorbid conditions (especially degree of past alcohol consumption) and low IQ in the group of HCUs may have influenced the neuroimaging results in our study (Ashtari et al., 2005; Lyoo, Lee, Jung, Noam, & Renshaw, 2002; Nagel, Schweinsburg, Phan, & Tapert, 2005).
As shown by Catani and colleagues (2007), there is great variability within the normal population regarding the anatomy of the arcuate tracts with an extreme leftward lateralization (especially in males) in direct connections between Broca’s and Wernicke’s language areas. Therefore, hypo-connectivity of the right arcuate fasciculus reported here may, to some extent, be due to such inherent differences among male controls. However, we believe since our entire pool of subjects (patients and controls) are composed of males, the hypo-connectivity of the right arcuate fasciculus in males (Catani et al, 2007) should be equally presented in both HCU and normal groups and, therefore, should not significantly influence the results we report here.
The current study has a number of advantages over previous DTI studies: 1) we acquired images from thin, 2.5-mm slices (as opposed to the 5-mm slices used in all previous studies, with the exception of the recent report by Arnone and colleagues (2008); 2) the present study implemented a tractography protocol; 3) our present study used a well-defined sample of HCUs with a very narrow age range (i.e., between 16–20 years old); and 4) we included a broader range of diffusion measures (i.e., axial diffusivity, radial diffusivity and trace) to characterize the WM abnormalities. In performing tractography, there may exist more than one fiber crossing the same pixel (Wiegell, Larsson, & Wedeen, 2000). We have assumed that in a major fiber bundle, such as arcuate fasciculus, there are fewer numbers of fiber crossings. This may not be an unreasonable assumption as the characteristic C-shaped trajectory of the arcuate fasciculus (see Figure 5 Images A and C) corresponds well with what has been postulated by neuroanatomists on the basis of postmortem results (Carpenter, 1976).
Conclusion
In summary, the current study may differ from previous DTI reports in terms of patient sample characteristics with respect to severity and duration of use, and length of abstinence. We also differed in terms of our image processing approaches with some of previous DTI reports that elected an ROI approach as opposed to a whole brain voxelwise analysis. Furthermore, in order to better understand brain water diffusion behavior in cannabis users, we employed other diffusion parameters (i.e., axial and radial diffusivity and trace) as well as FA. Most importantly, to further confirm the diffusion abnormalities found in our voxelwise analysis, we performed tractography to extract and analyze the entire fiber tracts. To our knowledge, the study we present here is the first to use a combination of DTI and fiber tractography to explore the associations between cannabis use and brain white matter deficits.
In conclusion, there is growing evidence suggesting that adolescence is a key period for neuronal maturation. The results of the current study support that heavy cannabis use during adolescence is related to brain damage in areas known to be involved in ongoing development during late adolescence, particularly in the fronto-temporal connection via arcuate fasciculus. These results suggest that early-onset substance use may affect the development of fronto-temporal white matter circuits, potentially resulting in disturbed memory, and deficits in executive and affective functioning (Lubman, Yücel, & Hall, 2007). Since five of the HCU subjects were alcohol abusers, conclusions from our report should be considered preliminary as the DTI findings reported here may be due to combination of alcohol and marijuana use. Adolescence, however, being marked as a critical time for brain maturation and development, may be a vulnerable period to partake in risky behaviors, such as marijuana or alcohol use, for both physiological and psychological reasons.
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIMH Grant MH – 070612; the NIMH (Dr. Ashtari), 1RO1-MH073150-01A2 (Dr. Kumra) and 7K23MH64556-06 (Dr. Kumra). NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The authors greatly appreciate the aid and cooperation of the residents and staff of Aurora Concepts during the course of this study, as well as the participation of the healthy control subjects. Special acknowledgements in particular to the following individuals for their help in making this project a successful one: Mr. David Roofeh, Dr. Joseph Rhinewine, Ms. Hana Kester, Mr. Britt Anderson, Ms. Emily Thaden, and Dr. Serge Sevy.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–9. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Amen DG, Waugh M. High resolution brain SPECT imaging of marijuana smokers with AD/HD. J Psychoactive Drugs. 1998;30:209–14. doi: 10.1080/02791072.1998.10399692. [DOI] [PubMed] [Google Scholar]
- American-Psychiatric-Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–9. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Arnone D, Abou-Saleh MT, Barrick TR. Diffusion tensor imaging of the corpus callosum in addiction. Neuropsychobiology. 2006;54:107–13. doi: 10.1159/000096992. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41:1067–74. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–7. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007a;35:501–10. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cottone J, Ardekani BA, Cervellione K, Szeszko PR, Wu J, Chen S, Kumra S. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry. 2007b;64:1270–80. doi: 10.1001/archpsyc.64.11.1270. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–55. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–44. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B, Methodological. 1995;57:289–300. [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–88. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11:491–6. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–92. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Carpenter MB. Anatomical organization of the corpus striatum and related nuclei. Res Publ Assoc Res Nerv Ment Dis. 1976;55:1–36. [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–27. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A. 2007;104:17163–8. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:448–55. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–56. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, Macfall J. Diffusion Tensor Measures of the Corpus Callosum in Adolescents With Adolescent Onset Alcohol Use Disorders. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cogitive disorders: the chicken or the egg? Curr Opin Psychiatry. 2007;20:228–34. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: Effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101:556–69. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: Preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Grigorenko E, Kittler J, Clayton C, Wallace D, Zhuang S, Bridges D, Bundey S, Boon A, Pagget C, Hayashizaki S, Lowe G, Hampson R, Deadwyler S. Assessment of cannabinoid induced gene changes: tolerance and neuroprotection. Chem Phys Lipids. 2002;121:257–66. doi: 10.1016/s0009-3084(02)00161-5. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Guo X, Chen C, Chen K, Jin Z, Peng D, Yao L. Brain development in Chinese children and adolescents: a structural MRI study. Neuroreport. 2007;18:875–80. doi: 10.1097/WNR.0b013e328152777e. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DTIStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–16. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kaplan W. Advanced calculus. 5. Boston, MA: Addison Wesley; 2003. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kent JT, Bibby JM, Marida KV. Multivariate analysis: Probability and mathematical statistics. San Diego, CA: Academic Press; 1980. [Google Scholar]
- Kittler JT, Grigorenko EV, Clayton C, Zhuang SY, Bundey SC, Trower MM, Wallace D, Hampson R, Deadwyler S. Large-scale analysis of gene expression changes during acute and chronic exposure to [Delta]9-THC in rats. Physiol Genomics. 2000;3:175–85. doi: 10.1152/physiolgenomics.2000.3.3.175. [DOI] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu J, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2005;44:934–41. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res. 1988;443:47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, Mori S. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005;25:53–65. doi: 10.1148/rg.251045085. discussion 66-8. [DOI] [PubMed] [Google Scholar]
- Li L, Ma N, Li Z, Tan L, Liu J, Gong G, Shu N, He Z, Jiang T, Xu L. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–8. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–72. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Hall WD. Substance use and the adolescent brain: a toxic combination? J Psychopharmacol. 2007;21:792–4. doi: 10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Compr Psychiatry. 2002;43:361–8. doi: 10.1053/comp.2002.34636. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Prescott WR, Barrett RL, Razdan RK. Pharmacological evaluation of dimethylheptyl analogs of delta 9-THC: reassessment of the putative three-point cannabinoid-receptor interaction. Drug Alcohol Depend. 1995;37:231–40. doi: 10.1016/0376-8716(94)01081-u. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Humphreys DF, Lowe JV, Wiethe KE. Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab. 1992;12:750–8. doi: 10.1038/jcbfm.1992.106. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007a;13:807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007b;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–8. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–53. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Miki Y, Fushimi Y, Hanakawa T, Kanagaki M, Yamamoto A, Urayama S, Fukuyama H, Hiraoka M, Togashi K. Diffusion-tensor fiber tractography: intraindividual comparison of 3.0-T and 1.5-T MR imaging. Radiology. 2006;238:668–78. doi: 10.1148/radiol.2382042192. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–36. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. J Neuropsychiatry Clin Neurosci. 2006;18:318–32. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rojas NL, Chan E. Old and new controversies in the alternative treatment of attention-deficit hyperactivity disorder. Ment Retard Dev Disabil Res Rev. 2005;11:116–30. doi: 10.1002/mrdd.20064. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–8. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology (Berl) 1985;85:436–9. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–5. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, Johnston LD, O’Malley PM, Yamaguchi R. Substance abuse counseling services in secondary schools: a national study of schools and students, 1999–2003. J Sch Health. 2005;75:334–41. doi: 10.1111/j.1746-1561.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wiegell MR, Larsson HB, Wedeen VJ. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology. 2000;217:897–903. doi: 10.1148/radiology.217.3.r00nv43897. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. WRAT3: Wide Range Achievement Test Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123–7. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]