Abstract
Objectives
Therapeutic HIV vaccinations may alter the size of the resting memory CD4+ T-cell latent HIV reservoir as HIV establishes latency when memory responses are formed, including those toward HIV. Alternatively, latently infected CD4+ T cells maybe killed, while exiting the reservoir upon activation.
Methods
The effect of therapeutic immunization with modified vaccinia Ankara and Fowlpox-based HIV vaccines on the latent reservoir was examined in 19 young adults who were receiving effective antiretroviral therapy. Correlations between size of the reservoir [measured in infectious units per million (IUPM)] resting CD4+ T cells and HIV-specific immune responses, including immune activation were examined. Decay of the reservoir was assessed using random-effects model.
Results
A modest transient decrease in the size of the reservoir was observed at week 40 [mean −0.31 log10 IUPM (95% confidence interval: −0.60 to −0.03; P =0.03] following HIV vaccinations. The estimated half-life (T1/2) of the reservoir during the 40 weeks following vaccination was 9.8 months and statistically different from zero (P =0.02), but 35.3 months and not different from zero (P =0.21) over 72 weeks of study. Latent reservoir size at baseline was not correlated with HIV-specific CD4+, CD8+ responses or immune activation, but became correlated with CD4+ IFNγ (r =0.54, P =0.02) and IL-2 responses at 6 weeks after immunization (r =0.48, P =0.04).
Conclusion
Therapeutic HIV vaccinations led to a transient increase in decay of latently infected CD4+ T cells. Further studies of therapeutic HIV vaccines may provide important insights into facilitating decay of the latent reservoir.
Keywords: resting memory CD4+ T-cell latent reservoir, therapeutic HIV vaccines
Introduction
There is intense interest in developing strategies to target reservoirs of HIV that create barriers to cure [1,2]. One reservoir for HIV resides in resting memory CD4+ T cells [3–5], in which HIV establishes latency during formation of CD4+ T-cell memory responses [6], including those directed toward HIV [7,8]. The long life span of memory CD4+ T cells [6], and their capacity to undergo homeostatic proliferation, promotes life-long persistence of HIV [9]. Clearance of HIV from resting memory CD4+ T cells by immune mechanisms or antiretroviral drugs is further hindered by viral quiescence promoted by the resting state of the cells [1,2]. However, viral quiescence in resting memory CD4+ T cells may be reversed under specific or nonspecific immune activation stimuli allowing for targeting of latently infected CD4+ T cells by antiretroviral drugs or immune surveillance [1,2].
HIV vaccination of infected individuals is considered as one potential strategy to allow for eventual antiretroviral therapy discontinuation through augmentation of HIV-specific immune responses [10,11]. Therapeutic immunization may induce HIV expression in latently infected CD4+ T cells [1,2,12], which could in turn render those harboring latent proviral genomes susceptible to HIV-specific immune responses or to antiretroviral drugs that block steps in the virus life cycle occurring postintegration [1,2]. Additionally, augmentation of HIV-specific immunity [11], which wanes in HAART-treated individuals [13–15], may facilitate clearance of latently infected cells if they become activated. Alternatively, HIV vaccinations may serve to replenish the latent reservoir if by increasing target cell availability low-level virus replication is enhanced or with infection of HIV-specific memory CD4+ T cells.
Indeed, plasma viral loads were shown to increase transiently during routine immunization of HIV-infected individuals, even in those on effective HAART [16,17]. Furthermore, administering therapeutic HIV-pox-based vaccines to infected persons was shown to result in a shorter time to viral rebound and higher levels of viremia when antiretroviral therapy was discontinued [18]. In other studies, however, recipients of therapeutic HIV vaccines, who were on effective HAART, had a trend toward delay in rebound viremia [19] and lower levels of viremia (rAd5-HIV gag vaccine) [20] during treatment interruption. Even in untreated HIV-infected individuals, HIV-vaccinations were found to decrease plasma viral loads for up to 1 year after vaccination [21,22,23]. To our knowledge, there is no study reporting on the effect of therapeutic HIV vaccinations on the resting CD4+ T-cell reservoir in patients receiving HAART.
We examined, in a phase 1 clinical trial of recombinant modified vaccinia Ankara (MVA) and Fowlpox-based HIV-vaccines [Pediatric AIDS Clinical Trials Group (PACTG) P1059] [24] in young adults on effective HAART, the effects of immunization on size and decay of the resting CD4+ T-cell latent reservoir, and their correlations with immune activation, and HIV-specific T-cell immune responses.
Participants and methods
The study was approved by the Institutional Review Board at Johns Hopkins University School of Medicine. Written informed consent was obtained for each participant at the clinical sites participating in the trial (see below under participants). Blood samples were collected at each study site, and deindentified prior to shipment to the laboratory for analyses of the latent reservoir.
Participants
HIV-infected young adults who were receiving effective antiretroviral therapy (plasma HIV RNA <50 copies/ml) were enrolled between October 2005 and June 2006 in a phase 1 trial [Pediatric AIDS Clinical Trials Group (PACTG) P1059] of MVA and Fowlpox-based HIV vaccines with follow-up ending November 2007 [24]. The vaccines contained HIV env, gag, tat, rev, nef and reverse transcriptase genes [24]. As previously reported, the study participants were to receive two vaccinations with MVA-based vectors at study entry and week 4 and two additional vaccines with the Fowlpox-based vectors at weeks 8 and 24. Most participants received both MVA-based vaccines (N =19) and one dose of the Fowlpox-vaccine (N =18), but only 11 received the fourth Fowlpox-booster dose due to interrupted vaccine supply [24]. Two participants received only one and two vaccine doses, respectively, due to possible vaccine-related toxicities [24].
Study design
The frequencies of latently infected CD4+ T cells were quantified at two time points (screen and entry) before, and seven time points following HIV-vaccinations (weeks 2, 4, 6, 24, 26, 40 and 72).
Laboratory methods
Assessment of size of the resting CD4+ T-cell reservoir
We used a modification of previously published methods for measuring resting CD4+ T cells infected with replication-competent virus [25] and previously used to assess treatment intensification on the size and decay of the latent reservoir in adults [26]. Meaurements of the size of the latent reservoir were performed in real-time on freshly collected blood, therefore, issues surrounding assay performance per given time point that may occur with batching of samples are not likely to influence the results reported here. Briefly, cultured cells were derived from peripheral blood mononuclear cells that were enriched for resting CD4+ T cells [CD4+ T cells lacking expression of the activation marker human leukocyte antigen-DR (HLA-DR)] by removal of cells expressing CD69, CD25, CD8, CD16, CD14, and HLA-DR using magnetic bead depletion [25]. Enriched, resting, CD4+ T cells were activated in vitro to promote virus expression, and released virus was then expanded in CD4+ T lymphoblasts from HIV-seronegative donors. Infected cell frequencies were measured in infectious units per million (IUPM) resting CD4+ T cells based on maximum likelihood methods [25,27]. As previously reported, for cultures in which no viral isolates were recovered, an upper bound on the frequency of infected CD4+ T cells was assigned. The confidence interval (CI) for individual determinations is estimated at ±0.7 log10 IUPM [25].
HIV-specific immune responses
In the parent trial, HIV-specific immune responses were measured at screen, entry, and weeks 6 on all participants and week 26 on 11 participants receiving all four vaccinations [24]. HIV-specific immune studies were carried out with carboxyfluorescein succinimidyl ester-based assays to measure CD4+ T-cell lymphoproliferation following stimulation with either recombinant HIV p24 gag protein (Protein Sciences, Meriden, Connecticut, USA) or Aldrithiol-2-inactivated (AT2) HIVMN viral particles (kindly provided by Dr Jeffrey Lifson; AIDS Vaccine Program, National Cancer Institute, Frederick, MD); assessment of frequencies of cytokine-secreting (γ-interferon or IL-2) CD4+ and CD8+ T cells following stimulation with HIV gag p55, nef, or AT2- HIVMN and γ-IFN ELISPOT assays to measure the frequencies of HIV-specific CD8-T cells with pools of overlapping Clade B consensus peptides [24].
Statistical analysis
The demographic summaries include all 20 participants enrolled in the study. However, analyses of the effects of therapeutic HIV-vaccines on the latent reservoir were limited to 19 study participants in whom prevaccine measurements were obtained. In addition, measurements obtained during rebound viremia were excluded because the goal of the study was to examine the effect of HIV vaccines on infected resting CD4+ T cells with integrated HIV. These included: measurements collected after week 6 in two study participants who self-discontinued HAART and developed rebound viremia by week 24 (study participants #5, and #15), and after week 40 in a third participant (#11) who developed rebound viremia by week 72. Study participants who received two or fewer vaccine doses (#8 and #1, respectively) were excluded after weeks 8 and 4, respectively. The dataset at week 26 was, therefore, restricted to the nine participants who received all four vaccinations and maintained control of virus replication.
The frequencies of CD4+ T cells carrying replication-competent HIV were summarized using geometric means and 95% CI. The changes in log10 IUPM relative to baseline (defined as the average of the two prevaccine measurements) were summarized using means and 95% CI. These were estimated using a random effects model, in which indicator variables were created for each week and then compared with baseline. P values from the Student’s t-test against zero were reported. The 95% CIs around the average change in IUPM at various time points reflect the ranges within which the true population values are estimated to fall, based on the results of our sample. In cases in which these CIs do not include zero, there is a 95% certainty that the direction of the change in IUPM will be reproducible. The frequencies of latently infected CD4+ T cells (expressed as log10 IUPM) were also correlated with CD4+ and CD8+ T cells, HIV-specific CD4+ and CD8+ T cell responses and CD8+ T cell immune activation (HLA-DR+) before and following immunization; Spearman’s rank correlation coefficients were computed. Correlations between IUPM and HIV-specific CD4+ and CD8+ T-cell immune responses at week 26 were not examined due to small sample size. No corrections for multiple comparisons were performed. The estimated decay rate and 95% CI in log 10 IUPM values were obtained from a random-effects model of log-transformed latent reservoir measurement, described in detail elsewhere [28]. Half-life estimates were calculated assuming first-order decay kinetics [28].
Results
Baseline characteristics
Table 1 [29] summarizes the participants’ age, mode of infection, antiretroviral treatment histories, number of vaccines received and the frequencies of latently infected resting CD4+ T cells in IUPM measured at screen and study entry prior to HIV vaccinations. As previously reported [24], the median age of the cohort at time of HIV vaccination was 23 years (range; 19–24 years) and the median duration of control of virus replication with HAART was 3.3 years (range; 0.6–6.4). All study participants had clinically undetectable viral loads (plasma HIV RNA <50 copies/ml) at the time of enrollment and the median percentage CD4+ T cells was 38% (range: 18–51%). Five (25%) of the study participants were infected through perinatal transmission (Table 1). Fifty-five percent (11 of 20) of the study participants were receiving therapy with nonnucleoside reverse transcriptase inhibitor-based HAART and the remainder were on protease inhibitor-based regimens (Table 1). Most study participants received three vaccine doses (N =18) and 11 received the full vaccine series of four doses (N =11; Table 1).
Table 1.
Patient characteristics and frequencies of latently infected CD4+ T cells prior to HIV vaccinations.
| Participant no. [29] | Age (years) | Mode of infection | Pre-HAART regimen | HAART regimen | No. of vaccines received | Virus recovered (prevaccine) | Mean IUPMa (prevaccine) |
|---|---|---|---|---|---|---|---|
| 2 | 24 | Risk behavior | ZDV 3TC EFV | ZDV 3TC NFV | 4 | + | 0.37 |
| 3 | 24 | Risk behavior | None | ZDV 3TC EFV | 4 | + | 0.1 |
| 4 | 23 | Risk behavior | None | ZDV 3TC NVP | 4 | − | 0.1 |
| 5b | 23 | Risk behavior | None | ZDV 3TC EFV | 4 | + | 0.16 |
| 6 | 23 | Risk behavior | None | ZDV 3TC EFV | 4 | + | 0.1 |
| 7 | 20 | Risk behavior | None | ZDV 3TC NFV | 4 | + | 0.31 |
| 9 | 24 | Risk behavior | None | ZDV 3TC EFV | 4 | + | 0.1 |
| 10 | 21 | Risk behavior | None | DDI 3TC EFV | 4 | + | 0.96 |
| 11 | 23 | Perinatal | ZDV 3TC DDI ABC IDV EFV | D4T TDF LPV/r | 4 | + | 1.72 |
| 13 | 22 | Perinatal | ZDV DDI D4T ADF 3TC IDV APV NFV SQV DDC hydroxyurea | ZDV TDF FTC ATV/r | 4 | + | 0.78 |
| 12 | 20 | Risk behavior | ZDV 3TC ABC LPV/r | ABC 3TC ATV/r | 3 | + | 0.81 |
| 15b | 24 | Risk behavior | None | DDI FTC EFV | 3 | − | 0.1 |
| 16 | 21 | Risk behavior | None | DDI FTC EFV | 3 | + | 0.46 |
| 17 | 23 | Perinatal | ZDV DDI 3TC D4T DDC NVP ABC RTV APV LPV/r | TDF EFV ATV/r | 3 | + | 0.37 |
| 18 | 23 | Risk behavior | ZDV 3TC NFV | TDF FTC ATV/r | 3 | + | 5.66 |
| 19 | 19 | Perinatal | None | DDI NFV EFV | 3 | + | 2.18 |
| 20 | 22 | Risk behavior | None | DDI FTC EFV | 3 | + | 0.51 |
| 8 | 24 | Risk behavior | ZDV | ZDV 3TC NVP | 2 | + | 0.85 |
| 1 | 21 | Risk behavior | DDI 3TC TDF EFV | TDF FTC LPV/r | 1 | + | 0.22 |
3TC, lamivudine; ABC, abacavir; ADF, adefovir; APV, amprenavir; ATV, atazanavir; DDC, zalcitabine; DDI, didanosine; D4T, stavudine; EFV, efavirenz; FTC, emtricitabine; IDV, indinavir; LPV, lopinavir; NFV, nelfinavir; NVP, nevirapine;/r, boosted with ritonavir; RTV, ritonavir; SQV, saquinavir; TDF, tenofovir; ZDV, zidovudine.
IUPM, infectious units per million resting CD4+ T cells; average of IUPM at screen and entry.
Self-discontinued HAART.
Analysis of replication-competent HIV CD4+ T-cell reservoirs
The frequencies of latently infected resting CD4+ T cells were measured at two time points before and seven times following HIV vaccinations. One hundred and sixty-three viral cultures (35 before and 128 following HIV vaccinations) were performed on the cohort over a 2-year period; only measurements on the 19 of 20 study participants who had blood samples analyzed prevaccination were included in the analysis: 16 were studied at both prevaccination visits, and three at one time point prevaccination (Table 1). Patients in the analyzed cohort had a median of eight latent reservoir measurements performed over the course of the study (115 enhanced cultures).
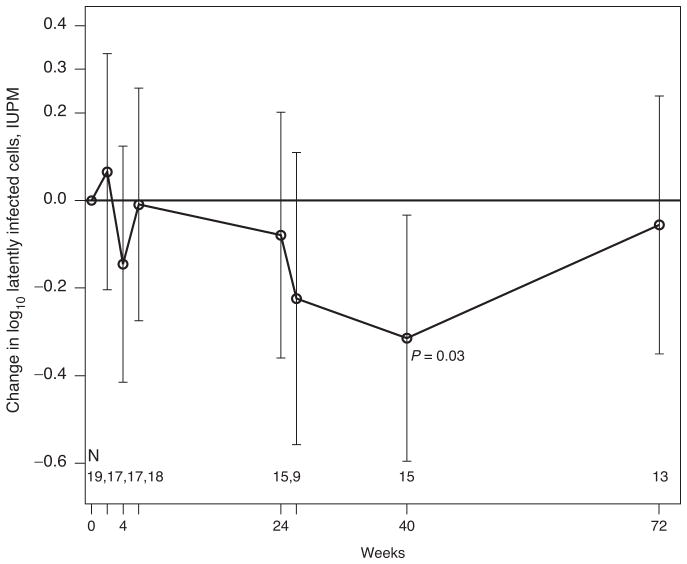
Replication-competent virus was recovered from 89% of the 19 participants studied prior to HIV vaccinations. The geometric mean frequency of latently infected cells (average of measurements at screen and entry) at baseline was 0.41 IUPM (95% CI: 0.23, 0.72), which is somewhat lower than mean levels (0.82 IUPM) reported for chronically HIV-infected adults on HAART [28]. The mean change in the frequencies of latently infected cells between the screen and study entry visits, which were separated by a median of 3 weeks was −0.25 log10 IUPM (95% CI; −0.72, 0.21), and not statistically different (P =0.27). Individuals who were infected through perinatal transmission had higher frequencies of latently infected CD4+ T cells at baseline compared with those infected through high-risk behavior [geometric mean frequency 1.02 vs. 0.32; 95% CI: (0.28, 3.69) vs. (0.17, 0.61) P =0.08]; both groups showed the same pattern of change over time (data not shown). The geometric mean frequencies of latently infected CD4+ T cells at weeks 2, 4 and 6 were 0.48 (95% CI: 0.24, 0.94), 0.29 (95% CI: 0.16, 0.52) and 0.40 (95% CI: 0.23, 0.68), respectively, and not statistically different from baseline values (P =0.14, 0.94 and 0.50, respectively). By week 24, the geometric mean frequency was slightly lower than baseline levels 0.37 (95% CI: 0.21, 0.65) in the 15 study participants who received the first three vaccinations and remained suppressed on HAART but not statistically different from baseline (P =0.46). At week 40, the geometric mean frequency in latently infected CD4+ T cells was lower than prevaccine levels [0.21 (95% CI: 0.14, 0.34); P =0.09], but by week 72, the last study time point, the geometric mean frequency in latently infected CD4+ T cells was nearly similar to prevaccine levels [0.38, (95% CI: 0.19, 0.74); P =0.53]. Figure 1 shows the mean change from prevaccine levels in the frequencies of latently infected cells over the course of the study as estimated using a random effects model of the log-transformed IUPM. A 0.07−log10 (95% CI; −0.20 to 0.34) increase in the mean frequency of latently infected CD4+ T cells was detected at two weeks following the first vaccine dose; this was not statistically significant (P =0.63). At all other time points, the mean change in log10 IUPM was negative, with a significant modest decrease at week 40 [mean −0.31 log IUPM (95% CI: −0.60 to −0.03; P =0.03)]. This effect was, however, transient and by week 72, the mean change in log10 IUPM was almost zero at −0.06 log10 IUPM (95% CI; −0.35 to 0.24; P =0.71). The decrease in IUPM at week 40 also corresponded with decrease in recovery of replication-competent virus. Prior to vaccination, 77% (27 of 35) of the viral cultures yielded replication-competent virus. Following vaccination, the recovery of replication competent virus ranged from 69 to 84% for all study visits except at week 40, at which virus recovery was lower at 40% (six of 15 cultures performed).
Fig. 1. Change in frequencies of resting CD4+ T cells containing replication-competent HIV in log 10 infectious units per million relative to baseline.
The bold line represents the mean change in log10 infectious units per million (IUPM) per time point analyzed. The 95% confidence interval for each time point analyzed is included. The P value for the change relative to baseline at the week 40 visit is denoted.
Using a random effects model with first order decay kinetics of the log-transformed IUPM measurements, the decay rate of the reservoir from baseline to week 40 was estimated to be −0.008 log10 IUPM per week; 95% CI; −0.01 to −0.002, and was statistically different from zero (P =0.02). The estimated mean half-life was 9.8 months (95% CI; 5.4–48.9 months) and closer to estimates (4.6–6 months) reported for patients initiating HAART during acute infection. However, this effect was transient and the estimated decay rate of the latent reservoir over 72 weeks of the trial was −0.002 log10 IUPM per week (95% CI: −0.005 to 0.001), and not statistically different from zero (P =0.21). The estimated mean half-life was 35.3 months (95% CI for half-life, 13.7 months to infinity) and similar to previously reported estimates in HIV-infected adults on stable HAART initiated during chronic infection (T1/2 = 44 months).
HIV-specific immune responses and latent reservoir size
Because HIV-specific CD4+ T cells contribute to this reservoir, correlations between the size of the latent reservoir (measured by IUPM) and HIV-specific CD4+ T-cell responses before and following HIV vaccinations were examined, as were the relationship between latent reservoir size and CD4+ and CD8+ T-cell counts, immune activation markers and HIV-specific CD8+ T-cell responses. At baseline, IUPM did not correlate with absolute CD4+ (r =−0.17; P =0.48), CD8+ T-cell counts (r =0.20; P =0.41), nor immune activation as assessed by frequencies of CD8+ T cells that coexpressed HLA-DR and CD38 (r =0.37; P =0.14). HIV-specific CD4+ T-cell lymphoproliferative responses as assessed by IL-2 production in response to whole inactivated virus (AT2 HIVMN) or p24 gag at baseline also did not correlate with latent reservoir size. At 6 weeks, the first time point at which HIV-specific immune responses were determined after vaccination, latent reservoir size became correlated with frequencies of HIV-specific IFNγ (r =0.54, P =0.02) and IL-2 producing CD4+ T cells (r =0.48, P =0.04). At baseline, latent reservoir size correlated with CD8+ T-cell responses directed toward HIV pol (r =0.57, P =0.01), and tat (r =0.57, P =0.01) and remained correlated at 6 weeks after vaccination. Even after removing outliers from baseline measurements, the correlation between reservoir size and HIV-specific CD8+ T-cell responses to HIV pol and tat remained (r =0.50, P =0.03 and r =0.49, P =0.04, respectively). In addition, change in IUPM at week 6 was negatively correlated with change from baseline in IL-2 producing HIV-specific CD8+ T cells (r =−0.62, P =0.01).
Discussion
This study is the first to our knowledge to examine whether therapeutic HIV vaccinations would lead to either decay or increase the size of the resting CD4+ T-cell latent HIV reservoir. There are several limitations to our analyses including the lack of a placebo group and the small sample size, and that nonsignificant results observed in the study may be due to the lack of statistical power. Furthermore, the exploratory nature of the study required analyses addressing various hypotheses and creating the possibility of chance findings. Despite these limitations, we found HIV-pox-based vaccinations to infected young adults who were receiving durable effective HAART resulted in a modest, but measurable and statistically significant transient decrease (an average of 0.31 log10 IUPM lower) in the frequencies of latently infected CD4+ T cells detected by 40 weeks following the first vaccine dose in recipients receiving three or four vaccinations. This decrease was associated with a faster rate of decay of the reservoir between study entry and week 40, in which the half-life of the reservoir was statistically different from zero, and estimated at 9.8 months and closer to those measured in adults treated during acute HIV infection (T1/2 = 4.6 to 6 months) [30,31]. However, over the 72 weeks of study the estimated half-life of the reservoir was 35.3 months and similar to that measured in chronically-infected adults on HAART alone (T1/2 = 44 months) [28]. Our results suggest, a measurable transient decline, although small, in the resting CD4+ T-cell reservoir occurred with HIV vaccinations. This effect may result from enhanced targeting of latently infected CD4+ T cells by vaccine-induced HIV-specific immune responses upon exiting the latent reservoir during activation; or by direct reactivation of HIV-specific memory CD4+ T cells harboring latent HIV with therapeutic immunizations, and warrants evaluation in other studies. It is, however, unclear what specific factors led to the transient decrease and subsequent rebound in the latent reservoir size by weeks 40 and 72, respectively, but may be consistent with transient increase followed by subsequent loss of HIV-specific immune responses, although the durability of the HIV-specific responses through 72 weeks of study were not evaluated in the parent trial. Alternatively, it is possible that over the time course of the study recirculation of infected cells to the peripheral blood compartment occurred. Importantly, HIV vaccinations were not observed to increase the size of the reservoir despite induction of HIV-specific CD4+ T-cell proliferative responses and cytokine secreting cells [24], which would be one concern for this treatment strategy [32].
In this proof-of-concept study, prevaccine frequencies of latently infected CD4+ T-cells in patients on standard HAART did not correlate with HIV-specific CD4+ T-cell responses but were correlated with HIV-specific CD8+ T-cell responses to HIV pol and tat. This finding may be explained by the possibility that patients with a larger reservoir size may experience more frequent episodes of HIV gene expression with reactivation of HIV from latency; virus production may then serve to boost HIV-specific CD8+ T-cell immune responses. Indeed, anti-tat cytotoxic T lymphocytes are frequently detected in HIV infected individuals [33], and anti-tat vaccine is under investigation [34]. However, a direct correlation between levels of CD8+ T-cell immune activation and reservoir size at baseline was not observed in this study, as has been reported by others [35,8,26]. A measureable transient, although likely clinically insignificant, decrease in the size of the reservoir seen with therapeutic immunization is suggestive of an interaction between HIV-specific immune responses and latent reservoir size, although these findings are limited by lack of a placebo group. In other words, subpopulations of latently infected CD4+ T cells that become activated during the course of infection may in turn become susceptible to vaccine-induced HIV-specific cytotoxic T-cell responses, that would block reversion HIV-infected CD4+ T cells to the resting state [6]. That interaction between persistently infected cells in HAART-treated patients and HIV-specific CD8+ T-cell responses may occur is suggested by our previous finding of sequence evolution in low-levels of plasma virus within relevant CD8+ T-cell epitopes, also detected transiently at week 40, in HLA-A2-positive individuals enrolled in the trial [29]. Together, these findings are suggestive of short-lived CD8+ T-cell-mediated effects induced with HIV-pox-based vaccinations in HAART-treated individuals. Understanding the pathogenesis of a transient decrease in the size of the reservoir in the context of HIV vaccinations is important for informing HIV therapeutic vaccine and eradication strategies, and merits confirmation in other vaccine trials. The long-term stability of the reservoir in the setting of immune activation and augmentation of HIV-specific immune responses suggests that although vaccine-induced immunity may have a transient, albeit modest effect on latently infected CD4+ T cells, alternate strategies will also be required for clearing HIV from this reservoir.
Acknowledgments
The authors thank the patients and their families for participating in this study, and the research personnel for their assistance at the study sites. Additional members of the P1059 Protocol Team include: Barbara E. Heckman, BS, Frontier Science and Technology Research Foundation, Amherst, NY; Bill G. Kapogiannis, MD, Medical Officer, NICHD, Bethesda MD; Lawrence Fox MD, PhD, Medical Officer, NIAID, Bethesda, MD; Elizabeth Hawkins, Clinical Manager, IMPAACT Operations Center, Silver Spring, MD.
Institutions and site staff who enrolled participants to the study IMPAACT P1059, conducted by the International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) group: Site 5048-USC LA NICHD CRS (James Homans, MD; Andrea Kovacs, MD; Michael Neely, MD; LaShonda Spencer, MD); Site 6501-St. Jude/UTHSC CRS (Lennie Lott, RN; Sally Discenza, FNP; Jill Utech, RN, MSN; Nehali Patel, MD); Site 4101-Columbia IMPAACT CRS (Alice Higgins, RN; Marc Foca, MD; Andrea Jurgrau, CNP; Gena Silva, RN); Site 5090-Children’s Hospital of Los Angeles NICHD CRS (Diane Tucker MSN; Marvin Belzer MD; This work was supported in part by Grant Number 1UL1RR031986, Children’s Hospital Los Angeles Clinical Translational Science Institute, with funds provided by the National Center for Research Resources (NCRR), NIH.); Site 4701-DUMC Ped. CRS (Piers Barker, MD; Joan Wilson, RN; Mary Jo Hassett, RN; Carole Mathison); Site 5052-Univ. of Colorado Denver NICHD CRS (Emily Barr, PNP-C, CNM; Jason Child, PharmD; Elizabeth J. McFarland, MD; Carol Salbenblatt, RN, MSN).
The study on latent reservoirs was supported by NIAID grants R01 A155312 and R01 A1062446, awarded to D. Persaud, and an IMPAACT Subspecialty grant. This is the main funding source for the study.
Footnotes
There are no conflicts of interest.
Name of Trial Registry:ClinicalTrials.gov. Clinical-Trials.gov Registry Number: NCT00107549
Conflict of interest
Grant support: This clinical trial (ClinicalTrials.gov identifier NCT00107549) was supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network of the National Institute of Allergy and Infectious Diseases (NIAID). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). Immunology studies were supported byan IMPAACT Immunology Laboratory grant and NIAID grant RO1 32391 to K. Luzuriaga.
References
- 1.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 2.Trono D, Van LC, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329:174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 5.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 6.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 7.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, Hatano H, Sinclair E, Lee TH, Busch MP, Martin JN, et al. HIV-specific CD4+ T cells may contribute to viral persistence in HIV controllers. Clin Infect Dis. 2011;52:681–687. doi: 10.1093/cid/ciq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305:205–208. doi: 10.1126/science.1100600. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi RT, Altfeld M. The quest for an HIV-1 therapeutic vaccine. J Infect Dis. 2005;192:556–559. doi: 10.1086/432014. [DOI] [PubMed] [Google Scholar]
- 12.Grossman Z, Feinberg MB, Paul WE. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc Natl Acad Sci U S A. 1998;95:6314–6319. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, et al. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxenius A, Price DA, Dawson SJ, Gunthard HF, Fischer M, Perrin L, et al. Residual HIV-specific CD4 and CD8 T cell frequencies after prolonged antiretroviral therapy reflect pre-treatment plasma virus load. AIDS. 2002;16:2317–2322. doi: 10.1097/00002030-200211220-00012. [DOI] [PubMed] [Google Scholar]
- 16.Castro P, Plana M, Gonzalez R, Lopez A, Vilella A, Argelich R, et al. Influence of a vaccination schedule on viral load rebound and immune responses in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses. 2009;25:1249–1259. doi: 10.1089/aid.2009.0015. [DOI] [PubMed] [Google Scholar]
- 17.Gunthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, Christopherson C, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 18.Autran B, Murphy RL, Costagliola D, Tubiana R, Clotet B, Gatell J, et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452) AIDS. 2008;22:1313–1322. doi: 10.1097/QAD.0b013e3282fdce94. [DOI] [PubMed] [Google Scholar]
- 19.Angel JB, Routy JP, Tremblay C, Ayers D, Woods R, Singer J, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. AIDS. 2011;25:731–739. doi: 10.1097/QAD.0b013e328344cea5. [DOI] [PubMed] [Google Scholar]
- 20.Li JZ, Brumme ZL, Brumme CJ, Wang H, Spritzler J, Robertson MN, et al. Factors associated with viral rebound in HIV-1-infected individuals enrolled in a therapeutic HIV-1 gag vaccine trial. J Infect Dis. 2011;203:976–983. doi: 10.1093/infdis/jiq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy Y, Durier C, Lascaux AS, Meiffredy V, Gahery-Segard H, Goujard C, et al. Sustained control of viremia following therapeutic immunization in chronically HIV-1-infected individuals. AIDS. 2006;20:405–413. doi: 10.1097/01.aids.0000206504.09159.d3. [DOI] [PubMed] [Google Scholar]
- 22.Garcia F, Climent N, Assoumou L, Gil C, Gonzalez N, Alcami J, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011;203:473–478. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10 :1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 24.Greenough TC, Cunningham CK, Muresan P, McManus M, Persaud D, Fenton T, et al. Safety and immunogenicity of recombinant poxvirus HIV-1 vaccines in young adults on highly active antiretroviral therapy. Vaccine. 2008;26:6883–6893. doi: 10.1016/j.vaccine.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi RT, Bosch RJ, Aga E, Albrecht M, Demeter LM, Dykes C, et al. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;201:293–296. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies III: validity tests for the single-hit Poisson model. J Immunol Methods. 1984;72:29–40. doi: 10.1016/0022-1759(84)90430-7. [DOI] [PubMed] [Google Scholar]
- 28.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 29.Shiu C, Cunningham CK, Greenough T, Muresan P, Sanchez-Merino V, Carey V, et al. Identification of ongoing human immunodeficiency virus type 1 (HIV-1) replication in residual viremia during recombinant HIV-1 poxvirus immunizations in patients with clinically undetectable viral loads on durable suppressive highly active antiretroviral therapy. J Virol. 2009;83 :9731–9742. doi: 10.1128/JVI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TW, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–1764. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 31.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 32.Papagno L, Alter G, Assoumou L, Murphy RL, Garcia F, Clotet B, et al. Comprehensive analysis of virus-specific T-cells provides clues for the failure of therapeutic immunization with ALVAC-HIV vaccine. AIDS. 2011;25:27–36. doi: 10.1097/QAD.0b013e328340fe55. [DOI] [PubMed] [Google Scholar]
- 33.Addo MM, Altfeld M, Rosenberg ES, Eldridge RL, Philips MN, Habeeb K, et al. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc Natl Acad Sci U S A. 2001;98 :1781–1786. doi: 10.1073/pnas.98.4.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ensoli B, Fiorelli V, Ensoli F, Cafaro A, Titti F, Butto S, et al. Candidate HIV-1 Tat vaccine development: from basic science to clinical trials. AIDS. 2006;20:2245–2261. doi: 10.1097/QAD.0b013e3280112cd1. [DOI] [PubMed] [Google Scholar]
- 35.Chun TW, Murray D, Justement JS, Hallahan CW, Moir S, Kovacs C, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–138. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]