Abstract
There is accumulating evidence that autoimmunity to insulin B chain peptide, amino acids 9–23 (insulin B:9–23), is central to development of autoimmune diabetes of the NOD mouse model. We hypothesized that enhanced susceptibility to autoimmune diabetes is the result of targeting of insulin by a T-cell receptor (TCR) sequence commonly encoded in the germline. In this study, we aimed to demonstrate that a particular Vα gene TRAV5D-4 with multiple junction sequences is sufficient to induce anti-islet autoimmunity by studying retrogenic mouse lines expressing α-chains with different Vα TRAV genes. Retrogenic NOD strains expressing Vα TRAV5D-4 α-chains with many different complementarity determining region (CDR) 3 sequences, even those derived from TCRs recognizing islet-irrelevant molecules, developed anti-insulin autoimmunity. Induction of insulin autoantibodies by TRAV5D-4 α-chains was abrogated by the mutation of insulin peptide B:9–23 or that of two amino acid residues in CDR1 and 2 of the TRAV5D-4. TRAV13–1, the human ortholog of murine TRAV5D-4, was also capable of inducing in vivo anti-insulin autoimmunity when combined with different murine CDR3 sequences. Targeting primary autoantigenic peptides by simple germline-encoded TCR motifs may underlie enhanced susceptibility to the development of autoimmune diabetes.
Type 1 diabetes of both humans and NOD mice is characterized by selective destruction of β-cells within pancreatic islets (1–4). For the NOD mouse, multiple studies demonstrate that insulin is a primary autoantigen for triggering anti-islet autoimmunity (5,6). To date, with the exception of preproinsulin (7), deletion of several characterized islet target antigens does not alter progression to diabetes in the NOD mouse (8–10). Even the prominent CD8 targeting of the molecule islet glucose-6-phosphatase catalytic subunit-related protein indicate that the insulin B chain peptide, amino acids 9–23 (insulin B:9–23) containing a native tyrosine at position 16, is essential for development of diabetes (13). NOD mice lacking native insulin but producing an insulin with a mutation of the B:9–23 sequence (B:16A) do not develop diabetes (7), and elimination of insulin-reactive T cells results in the dramatic prevention of diabetes (14,15).
Most T-cell receptor (TCR) interactions with peptide–major histocompatability complex (MHC) complexes occur through binding of six complementarity determining regions (CDR; three each for α- and β-chains). The CDR3 region is most often crucial for antigen recognition (16). In this region, which includes the N region for α-chains and nDn region for β-chains, highly variable amino acid sequences are generated from gene rearrangements of V and J segments (plus D segment for β-chains) (16). The CDR1 and CDR2 regions are germline-encoded by the V segments and, for many TCRs, predominantly interact with the α helices of the MHC molecule (17,18). In NOD mice, TCRs targeting the insulin B:9–23 peptide presented by the I-Ag7 MHC class II molecule frequently use the Vα gene segment TRAV5D-4*04 (formerly termed Vα 13S3) rearranged to the Jα gene segments TRAJ53 and 42 (19,20). Among these TCRs, the N region sequences of the α-chains were highly variable, and no consistent TCR β-chain usage was apparent. Two anti-insulin B:9–23 TCR α-chains (derived from T-cell clones 12–4.1 and 12–4.4) using the same Vα (TRAV5D-4*04) and Jα (TRAJ53) gene segments, but having unique N region sequences, were capable of inducing insulin autoimmunity in Cα knockout NOD mice (21). In this article, we show that the sequences underlying such induction of insulin autoimmunity are relatively simple. Namely, the germline-encoded sequences of Vα TRAV5D-4 CDR1 and CDR2 combined with many CDR3 sequences and diverse Jα elements are sufficient to induce anti-insulin autoimmunity.
RESEARCH DESIGN AND METHODS
Mice.
NOD.scid mice (NOD.CB17-Prkdcscid/J, 001303) and Cα knockout NOD mice (NOD.129P2(C)-Tcratm1Mjo/DoiJ, 004444) were purchased from The Jackson Laboratory (Bar Harbor, ME). B16:A double insulin-knockout NOD.scid mice were generated in the Eisenbarth laboratory (13). All three strains, NOD/Bdc mice, and retrogenic mice were maintained in a pathogen-free animal colony at the Barbara Davis Center satellite animal facility and the Center for Comparative Medicine. All animal experiments were approved by the Animal Care and Use Committee of the University of Colorado Denver.
Generation of α-chain retrogenic mice.
Retrogenic mice were generated using the modified version of the protocol described previously (22,23). TCR α-chain constructs were either generated by PCR using cDNA from original T-cell clones (12–4.4, 12–4.1, BDC-6.9, BDC-10.1, BDC-2.5, 14H4, 5F2, and 6C5) or were assembled based on sequences. For the NY4.1 α-chain, a sequence published in the National Center for Biotechnology Information was used (accession number U80816). The 2H6 sequence was kindly provided from Dr. Li Wen (Yale University, New Haven, CT). TCR α-chain constructs encoding all α-chains detected by the 454 high-throughput sequencing and chimeric human Vα TRAV13–1 α-chains were also assembled by PCR with overlapping primers. TCR α-chain constructs were cloned into murine stem cell virus (MSCV)-based retroviral vectors carrying green fluorescent protein (GFP) (pMIGII) (22). Phoenix cells were cotransfected with the pMIGII plasmids and the pCL-Eco packaging vector using Lipofectamine 2000 (Life Technologies/Invitrogen) to produce replication-incompetent retroviruses encoding TCR α-chain sequences. Bone marrow cells were prepared from Cα knockout NOD mice treated with 5-fluorouracil (Sigma-Aldrich) and spin-infected with the retroviral supernatant daily for 4 consecutive days. The bone marrow cells were cultured in complete DMEM containing 20% heat-inactivated fetal bovine serum, 20 ng/mL IL-3, 50 ng/mL IL-6, and 50 ng/mL stem cell factor (Life Technologies/Invitrogen). NOD.scid mice or B16:A double insulin-knockout NOD.scid mice received 210 rad of radiation from the IBL 437C 137Cs irradiator (CIS Bio International) or 225 rad from the RS2000 X-ray irradiator (Rad Source Technologies, Suwanee, GA) and were injected with the bone marrow cells (2 × 106 cells) intravenously. For all experiments, cultured bone marrow cells were assessed for GFP expression by flow cytometry prior to the injection; ∼50–70% of bone marrow cells were positive for GFP. Peripheral blood mononuclear cells (PBMC) from all recipient mice were assessed for T-cell generation 5 weeks post-bone marrow transplantation; generally, 5–20% of PBMC expressed GFP and CD4. PBMC were stained with anti-CD4, anti-CD8, and anti-TCRβ antibodies (eBioscience) and analyzed on a FACSCalibur (BD Biosciences, San Jose, CA).
Measurement of micro-insulin autoantibody assay.
All retrogenic mice were bled at 4, 8, 12, 16, and 20 weeks after bone marrow transfer. Female NOD mice were bled at 4, 8, 10, 12, 16, and 20 weeks of age for comparison. Insulin autoantibody (IAA) levels were measured with the 96-well filtration plate micro-IAA assay previously described and expressed as an index (24). A value ≥0.01 is defined as positive.
Assessment of diabetes incidence.
The blood glucose levels were measured weekly. Mice were considered diabetic after two consecutive blood glucose values >250 mg/dL.
Assessment of insulitis.
The pancreata were obtained when killed (8–20 weeks after bone marrow transplant) and were fixed in 10% formalin. Paraffin-embedded tissue sections were stained with hematoxylin and eosin, and sections from islet grafts were also stained with polyclonal guinea pig anti-insulin antibodies (Linco Research Immunology, St. Charles, MO), followed by incubation with a peroxidase-labeled anti-guinea pig IgG antibody (Kirkegaard and Perry Laboratories). More than 10 pancreatic islets from an individual mouse were randomly selected and evaluated for lymphocytic infiltration (no insulitis, peri-islet insulitis, intraislet insulitis) by the same reader (M.N.) blinded to the group of mice.
IFN-γ enzyme-linked immunospot assay.
IFN-γ enzyme-linked immunospot (ELISPOT) assay was performed according to the manufacturer’s instructions (BD Biosciences). Spleen cells, harvested from retrogenic mice (7 × 105 cells/well), were incubated in the presence or absence of 100 μg/mL of antigens (insulin B:9–23: SHLVEALYLVCGERG; B16:A B:9–23: SHLVEALALVCGERG; tetanus toxin 830–843 peptide: QYIKANSKFIGITE; Genemed Synthesis, San Antonio, TX) for 3 days. Plates were developed using the AEC substrate system (BD Biosciences) and analyzed with the CTL Immunospot SpotMap 4.0 software (Cellular Technology, Shaker Heights, OH). A Stimulation Index >3 (the number of spots in cultures with peptide/without peptide) is considered positive.
TCR β-chain sequencing and reconstitution.
Pancreatic islets were isolated from four 12–4.1 and five 8–1.1 α-chain retrogenic mice by collagenase digestion (Sigma-Aldrich) of the pancreas and purification by Histopaque (Sigma-Aldrich) as described previously (25). Total RNA was directly extracted from islets using the RNeasy Mini kit (Qiagen), and single-strand cDNA was synthesized using the Clontech SMARTer RACE cDNA Amplification Kit (Clontech, Mountain View, CA) with oligo-dT primers according to the manufacturer’s instructions. The cDNA products connected to the SMARTer IIA Oligonucleotide were subject to touchdown PCR with the Universal Primer A Mix (Clontech) and the reverse primer annealing to the TRBC1/2 regions (5′-AGCCCATGGAACTGCACTTGGCAGCG-3′) to amplify TCR β-chains. The PCR products purified by gel extraction (Qiagen) were then cloned into pCR4-TOPO TA cloning vectors (Life Technologies/Invitrogen) and sequenced bidirectionally on the 3130xl Genetic Analyzer (Life Technologies/Applied Biosystems). For TCR reconstitution, TCR β-chain constructs obtained from the TA cloning and TCR α-chain constructs (8–1.1 or 12–4.1) were separately subcloned in pMIGII, pMSCVhygro (Clontech), or pMSCVpuro (Clontech) retroviral vectors, and the plasmids containing individual α- and β-chain genes were cotransfected to Phoenix cells to produce retroviruses. The 5KCα-β- hybridoma line lacking TCR α- and β-chains (23) were spin-infected with retroviral supernatants and cultured with hygromycin or puromycin followed by cell sorting of GFP-positive cells on the MoFlo cell sorter (DakoCytomation, Carpinteria, CA). TCR expression was also confirmed by staining with anti-mouse TCRβ antibody (clone H57–597; BD Biosciences).
TCR α-chain sequencing.
Pancreatic islets were isolated from two 20-week-old NOD/Bdc mice, and total RNA was extracted as described above. Single-strand cDNA was synthesized from 2 μg of RNA with random hexadeoxynucleotide primers and Moloney murine leukemia virus reverse transcriptase (GE Healthcare). To amplify α-chains containing TRAV5D-4, TRAV6, and TRAV13–1 segments, two-step PCR was performed. The first round of PCR included eight cycles of amplification with fusion primers that included TCR-specific primers and 454 adapters. The TCR-specific primers were designed to target the FR2 region of each TRAV segment (TRAV5D-4: 5′-GGAGAGAATCCTAAGCTCATCATTGAC-3′, TRAV6: 5′-CTGGAGAAGGTCCACAGCTCC-3′, and TRAV13–1: 5′-CTGGGGGAAGACTCGTCAGAC-3′) and the TRAC region (5′-GTGCTGTCCTGAGACCGAGG-3′). The first PCR products were then further amplified with the 454 adaptor primers, agarose gel-purified, subject to emulsion PCR with the GS-FLX-LR standard chemistry, and sequenced on the 454 GS-FLX instrument (Roche, Branford, CT).
Generation of hybridomas expressing a TCR with an alanine mutation.
TCR α-chain constructs were assembled by PCR with overlapping primers containing sequences for alanine substitution. TCR β-chain constructs were generated by PCR using cDNA from original T-cell clones and combined with paired α-chain constructs linked by the PTV1.2A sequence by PCR. TCR α-chain–PTV1.2A–β-chain constructs were then cloned in the pMIGII plasmids. 5KCα-β-hybridoma cells were spin-infected with replication-incompetent retroviruses generated as described above and sorted by GFP expression on the MoFlo cell sorter (DakoCytomation).
IL-2 enzyme-linked immunosorbent assay.
TCR-expressing 5KCα-β- hybridomas (1 × 105 cells) were incubated in the presence or absence of 200 μg/mL of peptides (insulin B:9–23, hen egg lysozyme [HEL]:11–25: AMKRHGLDNYRGYSL; Genemed Synthesis) or 100 pancreatic islets harvested from NOD.scid mice along with spleen cells from young NOD mice (1 × 105 cells/well) overnight. IL-2 secretion in supernatants was measured by enzyme-linked immunosorbent assay according to manufacturer’s instructions (BD Biosciences).
Statistics.
The incidence of insulin autoantibodies and positive ELISPOT responses were analyzed with the χ2 test. Peak values of insulin autoantibodies were analyzed with the unpaired two-tailed Student t test. Survival curves were analyzed using a log-rank test. The percentages of CD4 and CD8 T cells were analyzed with one-way ANOVA. All statistical tests were performed using Prism software (GraphPad, La Jolla, CA).
RESULTS
Retrogenic NOD mice expressing Vα TRAV5D-4 α-chains with multiple CDR3α sequences develop anti-insulin autoimmunity.
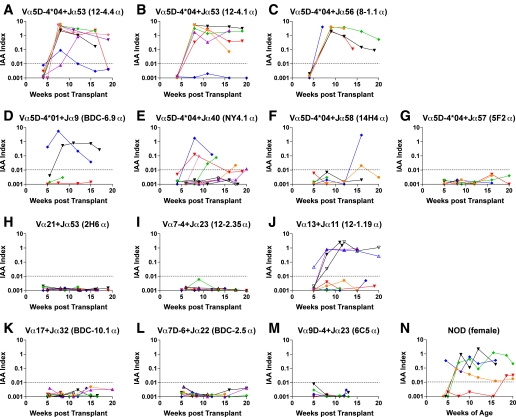
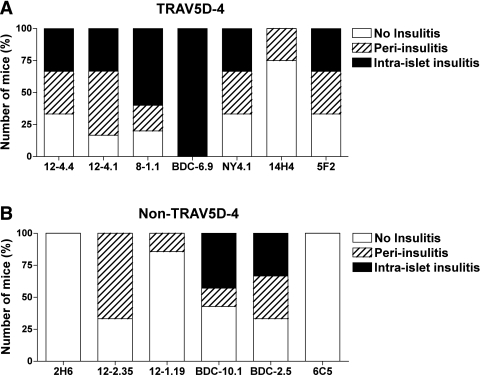
To investigate which α-chain TCR sequences are sufficient to induce insulin autoantibodies, we first created a series of retrogenic mice expressing TCR α-chain sequences derived from NOD CD4 T-cell clones into TCR Cα knockout NOD mice (Supplementary Table 1). In these mice, individual T cells express the introduced TCR receptor α-chain that associates with endogenously selected β-chain sequences produced in vivo. The α-chain TCR sequences used are derived from clones that react to the insulin B:9–23 peptide, unknown islet antigens, or completely unrelated antigens. Despite different N regions and Jα sequences, six out of seven α-chain retrogenic strains expressing the TRAV5D-4 sequence developed high levels of insulin autoantibodies (Fig. 1A–G and Supplementary Table 1). Of note, α-chains originally derived from diabetogenic T-cell clones that react with unknown islet antigens [i.e., NY4.1 described by Santamaria et al. (26) and BDC-6.9 established by Haskins et al. (27)], or even a nondiabetogenic clone reactive with an HEL peptide [14H4 established by Vignali and colleagues (28)], could induce insulin autoantibodies. Three strains expressing α-chains originally reacting with the insulin B:9–23 peptide developed relatively the higher level of insulin autoantibodies compared with female wild-type NOD mice (Fig. 1A–C and N; P = 0.03 [12–4.4], 0.04 [12–4.1], and <0.01 [8–1.1] versus wild-type NOD). In contrast, only one of the retrogenic strains expressing TCR α-chains other than Vα TRAV5D-4 developed insulin autoantibodies (Fig. 1H–M; P < 0.02 versus strains that express α-chains with TRAV5D-4). Retrogenic mice expressing TCR α-chains derived from clones known to be highly diabetogenic, but that do not use Vα TRAV5D-4 [BDC-2.5 and BDC-10.1 established by Haskins et al. (27)], and those expressing TCR α-chains from clones that respond to the insulin B:9–23 peptide [2H6 established by Wen and colleagues (29) and 12–2.35 established by Daniel and Wegmann (30)] did not lead to the production of insulin autoantibodies. The only non–TRAV5D-4 α-chain that induced insulin autoantibodies is derived from a diabetogenic insulin B:9–23-reactive T-cell clone [12–1.19 established by Daniel and Wegmann (30)]. It is notable that none of the three additional retrogenic mouse strains expressing α-chains containing this Vα segment (TRAV13–1) used by this 12–1.19 α-chain developed insulin autoantibodies (see below; Fig. 4D). Although TRAV5D-4 sequences induced insulin autoantibodies, only a subset of the Vα TRAV5D-4 retrogenic mice developed overt diabetes (Supplementary Fig. 1), and the severity of lymphocytic infiltration in the islets was consistent with the diabetes incidence (Fig. 2). Of note, although α-chain retrogenic mice are lymphopenic compared with wild-type mice in general, and peripheral CD4 and CD8 T-cell populations have broad variations even in the same strain (Supplementary Fig. 2), the number of T cells in the periphery did not correlate with the development of insulin autoantibodies, insulitis, and diabetes, and the percentages of CD4 and CD8 T cells do not statistically differ by strains by one-way ANOVA.
FIG. 1.
Cα knockout NOD mice retrogenic for α-chains containing TRAV5D-4 but not non–TRAV5D-4 develop anti-insulin autoimmunity. Levels of IAA in mice retrogenic for TCR α-chains with TRAV5D-4 (A–G) or with non–TRAV5D-4 (H–M) and in female NOD/Bdc mice (N). The α-chain sequences are derived from NOD CD4 T-cell clones (A: 12–4.4 [n = 8]; B: 12–4.1 [n = 6]; C: 8–1.1 [n = 5]; D: BDC-6.9 [n = 4]; E: NY4.1 [n = 9]; F: 14H4 [n = 5]; G: 5F2 [n = 5]; H: 2H6 [n = 7]; I: 12–2.35 [n = 7]; J: 12–1.19 [n = 8]; K: BDC-10.1 [n = 7]; L: BDC-2.5 [n = 7]; M: 6C5 [n = 4]). Symbols represent individual mice, and each panel represents a different retrogenic strain with a unique N and TRAJ region. IAA index ≥0.01 is defined as positive.
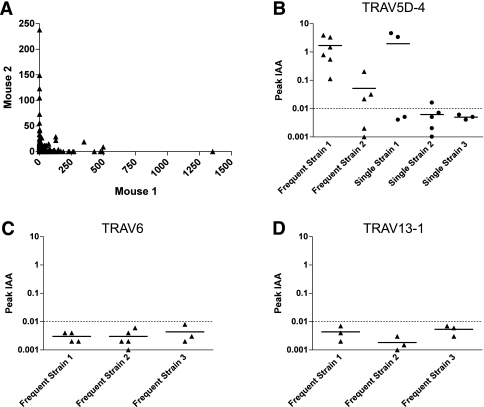
FIG. 4.
Retrogenic mice expressing α-chains detected by the 454 high-throughput sequencing. A: TRAV5D-4 α-chains infiltrating islets of two different 20-week-old NOD mice (Mouse 1 and Mouse 2) were sequenced. Individual symbols represent the number of unique CDR3 amino acid sequences that were detected in Mouse 1 (x-axis) and Mouse 2 (y-axis). B, C, and D: The peak value of insulin autoantibodies of retrogenic mice expressing α-chains that were detected by 454 sequencing (B: TRAV5D-4; C: TRAV6; D: TRAV13–1). Equal to or greater than three mice per individual strains were bled to measure insulin autoantibodies every 4 weeks between 4 and 16 weeks after bone marrow transplantation. Symbols represent individual mice. IAA index ≥0.01 is defined as positive.
FIG. 2.
Lymphocytic infiltration in the islets of TRAV5D-4 and non–TRAV5D-4 α-chain retrogenic mice. The pancreata from TRAV5D-4 (A: 12–4.4 [n = 6]; 12–4.1 [n = 6]; 8–1.1 [n = 5]; BDC-6.9 [n = 3]; NY4.1 [n = 4]; 14H4 [n = 5]; 5F2 [n = 3]) and non–TRAV5D-4 (B: 2H6 [n = 4]; 12–2.35 [n = 3]; 12–1.19 [n = 7]; BDC-10.1 [n = 7]; BDC-2.5 [n = 6]; 6C5 [n = 3]) α-chain retrogenic mice were evaluated for the development of insulitis. The number of mice with or without peri-islet insulitis and intraislet insulitis is shown.
Expression of the insulin B:9–23 sequence is required for the development of anti-insulin autoimmunity.
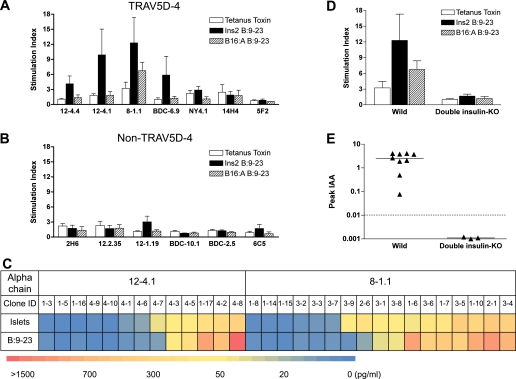
We next asked whether development of insulin autoimmunity in TRAV5D-4 α-chain retrogenic mice is associated with the recognition of the insulin B:9–23 peptide. Spleen cells from strains expressing TRAV5D-4 α-chains that produced high levels of insulin autoantibodies (from clones 12–4.1, 12–4.4, 8–1.1, and BDC-6.9) responded to insulin B:9–23, but did not respond to either the insulin B:9–23 peptide with B16:A mutation or a control tetanus toxin 830–843 peptide (Fig. 3A). Responses to the insulin B:9–23 peptide by spleen cells from 14H4, 5F2, and NY4.1 α-chain retrogenic mice were undetectable. The levels of insulin autoantibodies in these strains are not as high as those of other TRAV5D-4 α-chain strains showing reactivity to the insulin B:9–23 peptide, which may suggest the low probability of B:9–23-reactive T cells in the strains without the response to B:9–23. In the retrogenic mice that express an α-chain other than TRAV5D-4, spleen cells from only the 12–1.19 strain, which developed insulin autoantibodies, responded to insulin B:9–23 (Fig. 3B; P < 0.05 versus strains that express α-chains with TRAV5D-4).
FIG. 3.
TRAV5D-4 α-chain retrogenic mice develop anti-insulin autoimmunity in response to the native insulin B:9–23 peptide. A, B, and D: Response to insulin B:9–23 peptides. Spleen cells from α-chain retrogenic mice (A: mice expressing an α-chain with TRAV5D-4; B: mice expressing an α-chain with non–TRAV5D-4; D: B16:A double insulin-knockout mice expressing an α-chain with 8–1.1 TRAV5D-4) were tested for response to tetanus toxin peptide 830–843 (open bar), insulin B:9–23 peptide (closed bar), and B16:A B:9–23 peptide (hatched bar) by IFN-γ ELISPOT assay. Data are mean ± SEM and cumulative from equal to or greater than three independent experiments. Stimulation Index >3 (the number of spots in cultures with peptide/without peptide) is considered positive. C: The heat map of IL-2 secretion in response to islets and the insulin B:9–23 peptide by TCR-null CD4+ 5KC cell lines expressing β-chain sequences derived from islets of TRAV5D-4 α-chain retrogenic mice along with the original α-chain. Data are mean and are cumulative from two independent experiments. E: The peak value of insulin autoantibodies of 8–1.1 TRAV5D-4 α-chain retrogenic mice with/without native insulin B:9–23 expression. Insulin autoantibodies were measured every 4 weeks between 4 and 16 weeks after bone marrow transplantation. Symbols represent individual mice (wild-type retrogenic recipient mice, n = 9; insulin B:9–23-negative (double insulin-knockout) retrogenic recipient mice, n = 3). IAA index ≥0.01 is defined as positive.
To further examine how frequently T cells infiltrating pancreatic islets of TRAV5D-4 α-chain retrogenic mice recognize the insulin B:9–23 peptide, we reconstituted β-chains detected in the islets along with the original α-chain on the TCR-null 5KC CD4+ T cells (a T-cell hybridoma without T-cell receptor α- and β-chain genes) (31). We analyzed 146 β-chain sequences amplified by 5′ rapid amplification of cDNA ends PCR from the islets of retrogenic mice expressing either the 12–4.1 or 8–1.1 α-chain and found 90 unique junction sequences (Supplementary Table 2). We randomly chose 30 unique β-chain sequences and tested 5KC cell lines expressing those β-chain sequences for response to islets and the insulin B:9–23 peptide. As shown in Fig. 3C, 17 out of 30 5KC CD4+ T-cell lines responded to islets isolated from NOD.scid mice, and 14 of the 17 islet-responding lines (82%) were insulin B:9–23-reactive. Thus, the majority of TCRs of CD4 T cells infiltrating and responding to pancreatic islets of TRAV5D-4 α-chain retrogenic mice reacted with the insulin B:9–23 peptide.
We then asked whether the expression of native insulin B:9–23 sequence is required for TRAV5D-4 α-chain retrogenic mice to develop anti-insulin autoimmunity in vivo. To test this, we used B16:A double insulin-knockout NOD.scid mice (both native Ins1 and Ins2 genes knocked out) as bone marrow recipients (13). With the knockout of native insulin genes (Ins1 and Ins2) and a preproinsulin transgene producing insulin in islets with alanine rather than tyrosine at position B16, retrogenic mice do not express native insulin in their islets and lymphoid epithelial cells. The lack of native insulin B:9–23 expression in the recipient mice abrogated development of insulin autoantibodies (Fig. 3E; P < 0.01), diabetes, and lymphocyte reactivity to the insulin B:9–23 (Fig. 3D; P < 0.02). Thus, development of anti-insulin autoimmunity of TRAV5D-4 α-chain retrogenic mice is dependent upon the expression of the native insulin B:9–23 sequence.
TRAV5D-4 α-chains with variable CDR3 sequences induce anti-insulin autoimmunity.
To characterize the utilization of the Vα TRAV5D-4 in NOD pancreatic islets, we PCR-amplified TCRs from cDNA of 20-week-old NOD islets and sequenced these amplicons using high-throughput sequencing. Within ∼100 islets extracted from two mice, we found >1,000 unique in-frame TRAV5D-4 sequences with relatively few expanded identical CDR3 sequences. Individual mice had notably different TRAV5D-4 repertoires and TCR variant frequencies in islets (Fig. 4A); for example, we detected approximately 1,400 counts of the same TRAV5D-4 sequence in one mouse (x-axis), whereas that sequence was not detected at all in the second mouse (y-axis). To assess whether multiple TRAV5D-4 α-chain sequences observed in these islets would induce insulin autoantibodies in retrogenic mice, we produced five TRAV5D-4 α-chain retrogenic strains using sequences obtained from the 454 sequencing (Supplementary Table 3), and four out of five strains developed insulin autoantibodies (Fig. 4B). Of the sequences chosen, two were observed at high frequency and three were singletons (observed once) in the high-throughput sequencing experiments. Thus, multiple intraislet α-chains containing Vα TRAV5D-4 with different CDR3 sequences are capable of inducing anti-insulin autoimmunity. As controls, we created retrogenics with TRAV6 or TRAV13–1 α-chain sequences that were also observed at high frequency in islets based on 454 sequencing (Supplementary Table 3). None of the strains with these control Vα segments developed insulin autoantibodies (Fig. 4C and D; P < 0.01 versus strains that express α-chains with TRAV5D-4).
Essential amino acid sequences in the TRAV5D-4 CDR1 and CDR2 for the recognition of insulin B:9–23.
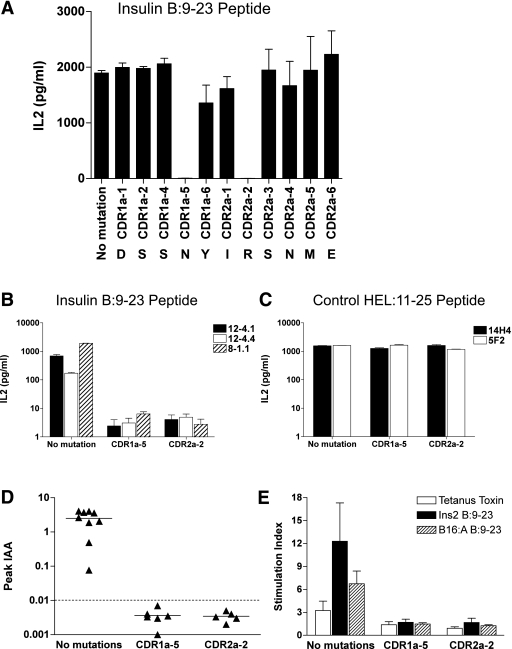
Evidence that TRAV5D-4 α-chains with diverse CDR3α sequences are capable of inducing anti-insulin autoimmunity (in response to the insulin B:9–23 peptide) led to the hypothesis that the TRAV5D-4 CDR1 and/or CDR2 sequences might be crucial to the recognition of the insulin B:9–23/I-Ag7 complex. To assess which specific amino acids in CDR1 and CDR2 are important for response to the insulin B:9–23, we performed an in vitro alanine scan of the α-chain CDR1 and CDR2 regions in the 8–1.1 TRAV5D-4 αβ anti-insulin B:9–23 TCR. For this experiment, we used 5KC CD4+ T cells (31) as recipients of TCR reconstitution, and the complete set of alanine mutations at each position of CDR1α and CDR2α were tested for response to insulin B:9–23 peptides in 5KC T-cell lines. As shown in Fig. 5A, mutations of asparagine in CDR1α (position 5) and arginine in CDR2α (position 2) abrogated the response to the insulin B:9–23 peptide. Notably, reactivity of other insulin B:9–23-reactive TCRs (12–4.1 and 12–4.4) also depended on these two amino acid sequences at positions CDR1α-5 and CDR2α-2 (Fig. 5B). In contrast, the two anti-HEL:11–25-reactive TCRs that also contain Vα TRAV5D-4 (14H4 and 5F2) responded to their peptide target presented by I-Ag7, despite replacing either CDR1α-5 or CDR2α-2 with alanine (Fig. 5C). Importantly, retrogenic mice with these TRAV5D-4 chains containing alanine mutations at CDR1α-5 and CDR2α-2 positions did not develop any anti-insulin antibodies (Fig. 5D; for each position, P < 0.01 versus no mutated TCR), and T-cell responsiveness to insulin B:9–23 in these mice, as measured by IFN-γ ELISPOT, was completely abrogated (Fig. 5E; for each position, P < 0.02 versus no mutated TCR). This lack of anti-insulin autoimmunity was not due to a lack of T cells in the periphery as both CD4 and CD8 T cells were present at levels comparable to the nonmutated 8–1.1 α-chain retrogenic mice.
FIG. 5.
Two amino acid residues at CDR1 and CDR2 of Vα TRAV5D-4 are essential for the recognition of insulin B:9–23 and anti-insulin autoimmunity induced by Vα TRAV5D-4. A: Alanine scan of the 8–1.1 insulin B:9–23-reactive TCR. 5KC cells expressing the 8–1.1 TCR with alanine mutations at individual CDRα positions were tested for the IL2 secretion in response to insulin B:9–23 peptide. Reactivity was lost only when CDR1α-5 and CDR2α-2 were mutated to alanine. Data are mean ± SEM and cumulative from two independent experiments. 5KC cells expressing TCRs (B: insulin B:9–23-reactive TCRs; C: HEL:11–25-reactive TCRs) with or without an alanine mutation at position CDR1α-5 or CDR2α-2 were tested for the IL-2 secretion in response to insulin B:9–23 (B) or HEL:11–25 (C). All three insulin B:9–23-reactive TCRs lost reactivity with these mutations, whereas anti-HEL peptide response by two HEL:11–25-reactive TCRs was unaffected. Data are mean ± SEM and cumulative from equal to or greater than three independent experiments. D: Insulin autoantibodies of mice retrogenic for 8–1.1 α-chain with or without alanine mutation at CDR1α-5 or CDR2α-2. Equal to or greater than five mice per individual strains were bled to measure insulin autoantibodies every 4 weeks between 4 and 16 weeks after bone marrow transplantation. Symbols represent individual mice. IAA index ≥0.01 is defined as positive. E: Spleen cells from mice retrogenic for 8–1.1 insulin B:9–23-reactive α-chain with or without an alanine mutation at CDR1α-5 or CDR2α-2 were tested for IFN-γ response to tetanus toxin peptide 830–843 (open bar), insulin B:9–23 peptide (closed bar), and B16:A B:9–23 peptide (hatched bar) by IFN-γ ELISPOT assay. Data are mean ± SEM and are cumulative from equal to or greater than three independent experiments. Stimulation Index >3 is considered positive.
Chimeric α-chains with the human ortholog of murine TRAV5D-4 are capable of inducing anti-insulin autoimmunity.
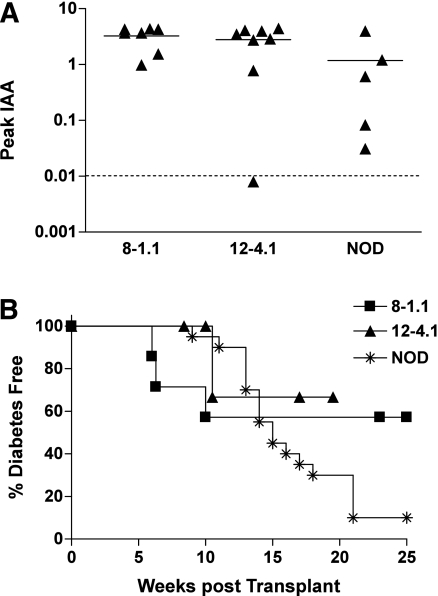
To explore the potential relevance of our findings to human type 1 diabetes, we produced retrogenic NOD mice with a chimeric human Vα TRAV13–1 α-chain. TRAV13–1 is the human ortholog of murine TRAV5D-4 (70% amino acid identity) with identical amino acids at the key positions of CDR1α-5 and CDR2α-2 (asparagine and arginine) as the murine TRAV5D-4 (Supplementary Fig. 3). In these retrogenic experiments, we engineered mice to express chimeric α-chains composed of the Vα sequences from human TRAV13–1, but with N, Jα, and Cα regions from mice, specifically 8–1.1 or 12–4.1 α-chain CDR3 sequence. These chimeric α-chain retrogenic mice developed insulin autoantibodies (Fig. 6A) and diabetes (Fig. 6B).
FIG. 6.
Retrogenic mice expressing chimeric human TRAV13–1 α-chains. Development of insulin autoantibodies (A) and diabetes incidence (B) of retrogenic mice expressing chimeric 12–4.1 and 8–1.1 α-chains for which Vα sequences were replaced with human TRAV13–1. Data of female wild-type NOD/Bdc mice are included for comparison. A: Chimeric retrogenic mice expressing human TRAV13–1 α-chains developed insulin autoantibodies. Symbols represent the peak values of insulin autoantibodies of individual mice (8–1.1: n = 8; 12–4.1: n = 8; NOD/Bdc: n = 5). IAA index ≥0.01 is defined as positive. B: Chimeric retrogenic mice (8–1.1: squares [n = 4]; 12–4.1 [n = 4]: triangles) developed diabetes.
DISCUSSION
It is generally assumed that for typical immune responses, CDR3 sequences of both α- and β-chains dominate the specificity to peptide recognition. In our experiments, different levels of insulin autoantibodies were induced in retrogenic mice by TRAV5D-4 α-chains with diverse CDR3 sequences (Fig. 1). The quantitative differences between retrogenic strains expressing TRAV5D-4 α-chains are likely determined by influence of varying CDR3 and Jα sequences contributing to recognition of the insulin B:9–23-I-Ag7 complex. Our study provides evidence that amino acids asparagine and arginine in CDR1 and CDR2 (respectively) in the TRAV5D-4 gene segment are essential for anti-insulin reactivity. Mutating a single amino acid of CDR1 and CDR2 in vitro and in vivo abrogated targeting of insulin B:9–23. This suggests that the CDR1 and CDR2 regions of the specific germline-encoded Vα sequence predispose TCRs to target the insulin B:9–23 peptide presented by I-Ag7 and are usually sufficient for the induction of anti-insulin autoimmunity in NOD mice. Such a simple TCR motif may relate to the ease and frequency of activating islet autoimmunity when other diabetes-promoting genetic and/or environmental factors exist.
It was notable that retrogenic strains expressing TRAV5D-4 α-chains developed peri-islet insulitis or even intraislet insulitis, which indicates that even T cells expressing islet-unrelated TRAV5D-4 α-chains are capable of recognizing islet antigens. TRAV5D-4 α-chain retrogenic mice had greater lymphocytic infiltration than non–TRAV5D-4 α-chain strains. TRAV5D-4 α-chains, even ones derived from islet-unrelated antigens, paired with appropriate β-chains may have a higher chance to recognize islet antigens. The evidence that TRAV5D-4 α-chains induce insulin autoantibodies may suggest that such TCRs recognize the insulin B:9–23 peptide and initiate islet infiltration. Indeed, NOD mice transgenic or retrogenic for the 12–4.1 and 12–4.4 TRAV5D-4 α-chains have the greater number of T cells reacting with the insulin B:9–23 peptide (32).
There are other examples of dominant V gene segment usage in autoimmune diseases. Tisch and colleagues (33) have found a preponderance of a specific TCR Vβ usage among T cells responding to the anti-islet BDC-2.5 mimotope. In the BB rat model, a specific Vβ sequence (Tcrb-V13) that is polymorphic among rat strains contributes to disease (34). The dominance of specific germline-encoded TCR gene sequences may be a general phenomenon in other autoimmune diseases. For example, polymorphisms of the TCR α-chain locus are associated with narcolepsy, which is strongly associated with DQB1*0602 (35). Crystal structures of the autoimmune TCRs isolated from patients having multiple sclerosis suggest unusual binding modes (36–38), which may relate to potential dominance of selected Vα or Vβ targeting.
Unanue and colleagues (39) have recently provided evidence that the recognized insulin B:9–23 peptides are produced only within islets of NOD mice. In addition, Stadinski et al. (40) have reported that the insulin B:9–23 peptide is recognized in a specific low-affinity register (register 3 with arginine in pocket 9) by autoreactive NOD CD4 T cells. Both of these phenomena might favor escape of autoreactive T cells from negative thymic selection as well as T-cell recognition at islets and draining pancreatic lymph nodes. Furthermore, a recent study by von Boehmer’s group (41) shows that the poor recognition of insulin B:9–23 results in the inefficient induction of regulatory T cells. Such poor recognition events involving the trimolecular complex consisting of a TRAV5D-4 α-chain, insulin B:9–23 peptide, and particular MHC molecule may be critical to the development of type 1 diabetes in NOD mice and, by analogy, possibly in humans. The identical insulin B:9–23 peptide sequence is present in human and mouse (Ins2), and an ortholog of mouse TRAV5D-4 (human TRAV13–1) is able to substitute for TRAV5D-4 in α-chain retrogenic mice. Of note, the structure of I-Ag7 is similar to that of the diabetes-susceptible DQ8 MHC class II molecule. Both molecules lack an aspartic acid residue at position 57 of the β-chains (42) and select common peptides that are naturally processed (43). We propose the concept that an accident of nature with the insulin B:9–23 peptide bound by the susceptible MHC class II and recognition of such a peptide–MHC complex by relatively large number of TCRs containing germline-encoded TRAV5D-4 (compared with TCRs with specific CDR3 sequences) may underlie the heightened risk of autoimmune diabetes for a species.
The current study indicates that despite dramatic diversity in N region and Jα sequences, producing retrogenic mice having a common Vα sequence, TRAV5D-4, results in insulin autoimmunity. In the presence of the appropriate class II MHC alleles, such a Vα may set the stage for generation of insulin-specific autoimmunity. Even the related human sequence (TRAV13–1) as a chimeric retrogenic with murine CDR3 and Cα region induces insulin autoantibodies. In conclusion, by use of an α-chain retrogenic mouse model, we identified a pathogenic α-chain sequence that elicits insulin autoimmunity via the recognition of insulin B:9–23 peptides. It is notable that the Vα TRAV5D-4 sequence that is encoded in the germline with fixed CDR1 and CDR2, but a diversity of CDR3 sequences, recognizes one essential peptide to provoke autoimmunity. Targeting of the interaction of pivotal germline Vα or Vβ sequences with cognate peptide–MHC complexes may provide a disease-specific immunotherapeutic strategy for prevention of autoimmunity.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-055969 and P30-DK-057516), the National Institutes of Health Autoimmune Prevention Center (U19-AI-050864), the Immune Tolerance Network (N01-AI-015416), JDRF (4-2007-1056), the Brehm Coalition, and the Children’s Diabetes Foundation (to G.S.E.). M.N. was supported by a K99 career development grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K99-DK-080885) and JDRF (44-2008-913). T.C. was supported by a National Institutes of Health/National Library of Medicine postdoctoral training grant (LM009451). D.A.A.V. was supported by the National Institutes of Health, JDRF, a National Cancer Institute Comprehensive Cancer Center Support CORE grant, and the American Lebanese Syrian Associated Charities. L.G. was supported by JDRF (1-2008-588). D.P. was supported by the National Institutes of Health (Grant R01-GM-083127).
M.N. is on a University provisional patent for treating islet autoimmunity targeting MHC–peptide complex with small molecules. G.S.E. is on two University provisional patents for treating islet autoimmunity targeting MHC-peptide complex with small molecules or antibodies. G.S.E. has a research grant from Novartis in the same area. No other potential conflicts of interest relevant to this article were reported.
M.N., T.C., L.G., D.P., and G.S.E. designed the study. M.N., T.C., T.S., X.H., and K.J. performed experiments. M.N., T.S., L.G., and G.S.E. analyzed data. T.C. and D.P. conducted biocomputational analyses. M.N., T.C., T.S., K.H., D.A.A.V., L.G., D.P., and G.S.E. wrote and edited the manuscript. D.A.A.V. provided training in and advice on the generation of retrogenic mice. K.H. and D.A.A.V. provided essential reagents and T-cell clones. M.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Li Wen (Yale University) and Dr. Garrison C. Fathman (Stanford University) for providing T-cell clone sequences; Amanda Burton and Erica Vincent (St. Jude Children’s Research Hospital) for training and advice in the generation of retrogenic mice and providing TCR reagents; Randall Wong, Carrie Toews, Leslie Rook, and Philip Pratt (University of Colorado School of Medicine) for technical assistance; and Drs. Jean M. Jasinski and Edwin Liu (University of Colorado School of Medicine) for discussions and critical reading of the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1113/-/DC1.
REFERENCES
- 1.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol 2007;148:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson MA, Gianani R. The pancreas in human type 1 diabetes: providing new answers to age-old questions. Curr Opin Endocrinol Diabetes Obes 2009;16:279–285 [DOI] [PubMed] [Google Scholar]
- 3.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity 2010;32:437–445 [DOI] [PubMed] [Google Scholar]
- 4.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010;464:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michels AW, Nakayama M. The anti-insulin trimolecular complex in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17:329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol 2008;20:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 2005;435:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubosaki A, Gross S, Miura J, et al. Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes 2004;53:1684–1691 [DOI] [PubMed] [Google Scholar]
- 9.Kubosaki A, Miura J, Notkins AL. IA-2 is not required for the development of diabetes in NOD mice. Diabetologia 2004;47:149–150 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Yamato E, Tashiro F, et al. Development of autoimmune diabetes in glutamic acid decarboxylase 65 (GAD65) knockout NOD mice. Diabetologia 2004;47:221–224 [DOI] [PubMed] [Google Scholar]
- 11.Lieberman SM, Evans AM, Han B, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA 2003;100:8384–8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthy B, Dudek NL, McKenzie MD, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 2006;116:3258–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama M, Beilke JN, Jasinski JM, et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J Clin Invest 2007;117:1835–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French MB, Allison J, Cram DS, et al. Transgenic expression of mouse proinsulin II prevents diabetes in nonobese diabetic mice. Diabetes 1996;46:34–39 [DOI] [PubMed] [Google Scholar]
- 15.Jaeckel E, Lipes MA, von Boehmer H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat Immunol 2004;5:1028–1035 [DOI] [PubMed] [Google Scholar]
- 16.Gras S, Kjer-Nielsen L, Burrows SR, McCluskey J, Rossjohn J. T-cell receptor bias and immunity. Curr Opin Immunol 2008;20:119–125 [DOI] [PubMed] [Google Scholar]
- 17.Marrack P, Scott-Browne JP, Dai S, Gapin L, Kappler JW. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu Rev Immunol 2008;26:171–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat Immunol 2009;10:143–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simone E, Daniel D, Schloot N, et al. T cell receptor restriction of diabetogenic autoimmune NOD T cells. Proc Natl Acad Sci USA 1997;94:2518–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abiru N, Wegmann D, Kawasaki E, Gottlieb P, Simone E, Eisenbarth GS. Dual overlapping peptides recognized by insulin peptide B:9-23 T cell receptor AV13S3 T cell clones of the NOD mouse. J Autoimmun 2000;14:231–237 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M, Jasinski J, Liu E, et al. Conserved T cell receptor alpha-chain induces insulin autoantibodies. Proc Natl Acad Sci USA 2008;105:10090–10094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA. Generation of T-cell receptor retrogenic mice. Nat Protoc 2006;1:406–417 [DOI] [PubMed] [Google Scholar]
- 23.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature 2009;458:1043–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolls MR, Coulombe M, Beilke J, Gelhaus HC, Gill RG. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J Immunol 2002;169:4831–4839 [DOI] [PubMed] [Google Scholar]
- 26.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 1997;186:1663–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci USA 1989;86:8000–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton AR, Vincent E, Arnold PY, et al. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes 2008;57:1321–1330 [DOI] [PubMed] [Google Scholar]
- 29.Du W, Wong FS, Li MO, et al. TGF-beta signaling is required for the function of insulin-reactive T regulatory cells. J Clin Invest 2006;116:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniel D, Wegmann DR. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23). Proc Natl Acad Sci USA 1996;93:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White J, Pullen A, Choi K, Marrack P, Kappler JW. Antigen recognition properties of mutant V beta 3+ T cell receptors are consistent with an immunoglobulin-like structure for the receptor. J Exp Med 1993;177:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Jasinski JM, Kobayashi M, et al. Analysis of T cell receptor beta chains that combine with dominant conserved TRAV5D-4*04 anti-insulin B:9-23 alpha chains. J Autoimmun 2009;33:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, He Q, Garland A, et al. beta cell-specific CD4+ T cell clonotypes in peripheral blood and the pancreatic islets are distinct. J Immunol 2009;183:7585–7591 [DOI] [PubMed] [Google Scholar]
- 34.Mordes JP, Cort L, Norowski E, et al. Analysis of the rat Iddm14 diabetes susceptibility locus in multiple rat strains: identification of a susceptibility haplotype in the Tcrb-V locus. Mamm Genome 2009;20:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. [Published erratum appears in Nat Genet 2009;41:859.] Nat Genet 2009;41:708–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn M, Nicholson MJ, Pyrdol J, Wucherpfennig KW. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat Immunol 2005;6:490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Huang Y, Lue J, Quandt JA, Martin R, Mariuzza RA. Structure of a human autoimmune TCR bound to a myelin basic protein self-peptide and a multiple sclerosis-associated MHC class II molecule. EMBO J 2005;24:2968–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wucherpfennig KW, Call MJ, Deng L, Mariuzza R. Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr Opin Immunol 2009;21:590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol 2010;11:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA 2010;107:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J Exp Med 2011;208:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 1987;329:599–604 [DOI] [PubMed] [Google Scholar]
- 43.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest 2005;115:2268–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.