Abstract
Inflammatory process is involved in the pathogenesis of diabetic nephropathy. In this article, we show that cholecystokinin (CCK) is expressed in the kidney and exerts renoprotective effects through its anti-inflammatory actions. DNA microarray showed that CCK was upregulated in the kidney of diabetic wild-type (WT) mice but not in diabetic intracellular adhesion molecule-1 knockout mice. We induced diabetes in CCK-1 receptor (CCK-1R) and CCK-2R double-knockout (CCK-1R−/−,-2R−/−) mice, and furthermore, we performed a bone marrow transplantation study using CCK-1R−/− mice to determine the role of CCK-1R on macrophages in the diabetic kidney. Diabetic CCK-1R−/−,-2R−/− mice revealed enhanced albuminuria and inflammation in the kidney compared with diabetic WT mice. In addition, diabetic WT mice with CCK-1R−/− bone marrow–derived cells developed more albuminuria than diabetic CCK-1R−/− mice with WT bone marrow–derived cells. Administration of sulfated cholecystokinin octapeptide (CCK-8S) ameliorated albuminuria, podocyte loss, expression of proinflammatory genes, and infiltration of macrophages in the kidneys of diabetic rats. Furthermore, CCK-8S inhibited both expression of tumor necrosis factor-α and chemotaxis in cultured THP-1 cells. These results suggest that CCK suppresses the activation of macrophage and expression of proinflammatory genes in diabetic kidney. Our findings may provide a novel strategy of therapy for the early stage of diabetic nephropathy.
As the incidence of diabetes continues to increase in almost all areas of developing and developed countries, diabetic nephropathy has become the most common cause of end-stage renal disease worldwide (1). In addition, accumulating evidence suggests that the development of diabetic nephropathy leads directly to increased cardiovascular mortality (2). Recent studies have suggested that the inflammatory process plays a crucial role in the pathogenesis of diabetic nephropathy (3).
We previously focused on the relationship between intracellular adhesion molecule-1 (ICAM-1) expression and macrophage infiltration in the diabetic kidney. We reported that ICAM-1 was overexpressed on endothelial cells and mediated macrophage infiltration in the diabetic kidney (4). Furthermore, we demonstrated that blockade of macrophage infiltration using anti–ICAM-1 antibody (4) or ICAM-1 knockout (ICAM-1−/−) mice (5) ameliorated diabetic renal injury, suggesting that the inflammatory axis of ICAM-1 activation to macrophage infiltration plays a pivotal role in the development of diabetic nephropathy.
In the current study, we performed a comprehensive, microarray-based analysis to clarify the genes responsible for the difference in urinary albumin excretion between diabetic ICAM-1−/− mice and diabetic wild-type (WT) mice. Unexpectedly, we found that cholecystokinin (CCK) mRNA expression was increased in the diabetic kidney of WT mice, whereas no significant increase was observed in nondiabetic WT mice.
CCK is a peptide hormone discovered in the small intestine (6,7) and is secreted from endocrine I cells of the duodenum and the jejunum into the bloodstream after a meal (8). CCK is well known as a regulator in the digestive tract and as a neurotransmitter in the nervous system (9,10). In addition to these well-known effects of CCK, anti-inflammatory effects of CCK have been reported (11–14).
To examine the role of CCK in the pathogenesis of diabetic nephropathy, diabetes was induced in CCK-1 receptor (CCK-1R) and CCK-2 receptor (CCK-2R) double-knockout (CCK-1R−/−,-2R−/−) mice. It is noteworthy that diabetic CCK-1R−/−,-2R−/− mice exhibited increased albuminuria and showed increased levels of proinflammatory genes in the kidney cortex. Therefore, we speculated CCK had renoprotective effects, and we further examined the effects of sulfated cholecystokinin octapeptide (CCK-8S) both in vivo and in vitro.
RESEARCH DESIGN AND METHODS
ICAM-1−/− mice studies.
Male ICAM-1−/− mice (C57BL/6J background) (15) were purchased from The Jackson Laboratory (Bar Harbor, ME). Male C57BL/6J (ICAM-1+/+) mice were used as controls. WT and ICAM-1−/− mice aged 8 weeks were divided into the following four groups (n = 5 each): 1) nondiabetic WT mice, 2) nondiabetic ICAM-1−/− mice, 3) streptozotocin (STZ)-induced diabetic WT mice, and 4) STZ-induced diabetic ICAM-1−/− mice. STZ was purchased from Sigma-Aldrich (St. Louis, MO). Mice in the diabetic groups received two intraperitoneal doses of STZ (each 100 mg/kg) given 7 days apart. Blood glucose levels were determined 7 days after STZ injection, and only mice with blood glucose concentrations >16 mmol/L were used in the study. Nondiabetic WT and ICAM-1−/− mice received citrate buffer injections only. All animal procedures were performed according to the guidelines as described previously (16). Three months after the induction of diabetes, all mice were killed, and the kidneys were harvested.
Oligonucleotide microarray.
Total RNA was extracted from each specimen of the renal cortex using the standard protocol included with the RNeasy Midi Kit (Qiagen, Valencia, CA) at 3 months. Preparation of biotin-labeled target cRNA and hybridization of probe arrays (CodeLink UniSet Mouse I Bioarray) were performed according to the manufacturer’s instructions (Amersham Biosciences, Uppsala, Sweden) (Gene Expression Omnibus accession numbers are available in the Supplementary Data).
Microarray data analysis.
The criteria for selecting genes that were induced or reduced by a diabetic state were as follows: 1) the gene flags were “true,” and 2) the ratio of the gene expression level in diabetic WT mice to that in diabetic ICAM-1−/− mice was >2 or <0.5. We then selected 193 genes for further analysis. All normalized data values were replaced to log base 2 and subjected to hierarchical clustering as described previously (16).
CCK receptor knockout mice studies.
CCK-1R−/−, CCK-2R−/−, and CCK-1R−/−,-2R−/− mice (C57BL/6J background) (17) were obtained from the Tokyo Metropolitan Institute of Gerontology. CCK-1R−/− mice and CCK-2R−/− mice were generated as described previously (18,19). C57BL/6J (CCK-1R+/+,-2R+/+) mice were used as controls. Male WT and CCK-1R−/−,-2R−/− mice aged 8 weeks were divided into four groups (n = 7 each): 1) nondiabetic WT mice, 2) nondiabetic CCK-1R−/−,-2R−/− mice, 3) STZ-induced diabetic WT mice, and 4) diabetic CCK-1R−/−,-2R−/− mice. Diabetes was induced as described above. Blood pressure, blood glucose, A1C, serum creatinine, urine creatinine, and urinary albumin were measured as described previously (16). Three months after the induction of diabetes, all mice were killed, and the kidneys were harvested as described previously (16). Male CCK-1R−/− and CCK-2R−/− mice aged 8 weeks (n = 7 each) were also used for comparison of albuminuria after induction of diabetes.
Bone marrow transplantation studies.
Bone marrow transplantation (BMT) was performed as described previously (20,21). Briefly, male WT and CCK-1R−/− mice aged 7–9 weeks received 9 Gy of total body irradiation. Postirradiated male CCK-1R−/− mice received a bone marrow transplant from WT mice (WT→1R−/−; n = 6). Postirradiated WT mice received a BMT from CCK-1R−/− mice (1R−/−→WT; n = 6) or WT mice (WT→WT; n = 4). Four weeks after BMT, diabetes was induced in all mice by STZ as described above. Four weeks after the induction of diabetes, all mice were killed. DNA was isolated from bone marrow extracts of all recipient mice using a DNeasy Blood & Tissue Kit (Qiagen). The chimerism was confirmed by PCR (Supplementary Fig. 1) at the termination of the study as described previously (22). The specific oligonucleotide primer sequences are shown in Supplementary Table 1.
Interventional animal studies.
Male Sprague-Dawley (SD) rats were purchased from CLEA Japan (Tokyo, Japan). SD rats aged 4 weeks were divided into three groups (n = 7 each): 1) nondiabetic control group (NDM), 2) STZ-induced diabetic group (DM), and 3) CCK-8S–treated diabetic group (DM-CCK). At the age of 5 weeks, rats chosen for the DM and DM-CCK groups were injected intravenously with STZ (65 mg/kg body wt) in citrate buffer (pH 4.5). Rats in the NDM group received citrate buffer injections only. At the age of 6 weeks, Alzet osmotic minipumps (Durect Corporation, Cupertino, CA) were implanted subcutaneously in the backs of all the rats. Rats in the DM-CCK group were continuously infused with CCK-8S (Bachem, Bubendorf, Switzerland) dissolved in 0.9% saline and given at a rate of 5 μg CCK-8S/kg · h−1. Animals in the NDM and DM groups were continuously infused with 0.9% saline only. Food intake was calculated as the average over 3 days. Serum CCK concentration in both diabetic groups was measured using the CCK Enzyme Immunoassay Kit (RayBiotech, Norcross, GA) according to the manufacturer’s instructions. Because the life expectancy of the osmotic pumps was 4 weeks, all pumps were replaced with new filled pumps when the rats reached the age of 10 weeks. Eight weeks after the induction of diabetes, all rats were killed, and the kidneys were harvested as described previously (16). Glomeruli were isolated from the left kidney by a previously developed sieving technique (23).
Histological analysis.
Periodic acid-methenamine silver (PAM)-stained sections were analyzed as described previously (24). To evaluate the glomerular size and mesangial matrix area, we examined 10 randomly selected glomeruli per mouse and 15 randomly selected glomeruli per rat under high magnification (×400). Quantitative analysis for all staining was performed in a blinded manner.
Immunoperoxidase staining.
Immunoperoxidase staining was performed as described previously (4). Primary antibodies were monoclonal antibody against rat monocytes/macrophages (ED1, 1:50; Serotec, Oxford, U.K.), polyclonal antibody against WT-1 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), or polyclonal antibody against cholecystokinin octapeptide (1:500; Phoenix Pharmaceuticals, Belmont, CA), all of which were applied for 12 h at 4°C. Secondary antibodies were biotin-labeled goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or biotin-labeled goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 60 min at room temperature. Intraglomerular ED1-positive cells or WT-1–positive cells were counted in 20 glomeruli per animal (n = 4/group).
Immunohistochemical staining.
Immunofluorescence staining was performed using the methods described previously (5). Rabbit antitype IV collagen Ab (1:200; LSL, Tokyo, Japan) was used for the primary reactions for 60 min at room temperature, followed by a second reaction with fluorescein isothicyanate-conjugated goat anti-rabbit IgG (H+L; Zymed Laboratories, San Francisco, CA) for 30 min at room temperature. The immunofluorescence intensity of type IV collagen was quantified as described previously (24). We evaluated 15 glomeruli per animal (n = 4/group).
RNA extraction and quantitative real-time PCR.
RNA extraction, real-time PCR, and visualization of gene expression were performed as described previously (24). The specific oligonucleotide primer sequences are shown in Supplementary Table 2.
Nuclear protein extract.
Nuclear proteins were extracted from kidney tissues with a nuclear extract kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions.
Nuclear factor-κB activity measurement.
Nuclear factor-κB (NF-κB) p65-dependent DNA-binding activity was determined by TransAM NFκB p65 (Active Motif) according to the manufacturer’s instructions.
Cell culture.
THP-1 cells were obtained from DS Pharma Biomedical (Osaka, Japan) and cultured according to the manufacturer’s instructions. PBS without calcium and magnesium [PBS (−)] was purchased from Invitrogen (Carlsbad, CA).
Tumor necrosis factor-α mRNA expression in THP-1 cells.
THP-1 cells were precultured in the RPMI 1640 (without glucose) medium supplemented with 10% FCS and 5.5 mmol/L d-glucose (Sigma-Aldrich) for 72 h. The cells were centrifuged, washed in PBS (−), centrifuged, and serum starved for 12 h in RPMI 1640 medium containing 5.5 mmol/L d-glucose. After starvation, the cells were adjusted to a cell density of 4 × 105 cells/mL in RPMI 1640 medium containing 5.5 mmol/L d-glucose and 1% FCS and placed in six-well plates (Falcon, Franklin Lakes, NJ). A control scrambled peptide (H-Gly-Asp-Tyr-Asp-Met-Trp-Met-Phe-NH2) and proglumide (a nonselective CCK receptor antagonist that interacts with both CCK receptors and can cross brain–blood barrier) were purchased from Sigma-Aldrich. The cells were exposed to the following stimuli (n = 5/group): 1) 5.5 mmol/L d-glucose (normal glucose [NG]); 2) 15 mmol/L d-glucose (high glucose [HG]); 3) 5.5 mmol/L d-glucose with 9.5 mmol/L mannitol (osmotic control [Mn]); 4) HG with scrambled peptide (10−6 M); 5) HG with CCK-8S (10−8 M); 6) HG with CCK-8S (10−7 M); 7) HG with CCK-8S (10−6 M); and 8) HG with CCK-8S (10−6 M) and proglumide (10−5 M).
CCK-8S and proglumide were added daily. After incubation for 72 h, total RNA was extracted, and quantitative real-time RT-PCR was performed as described above. Tumor necrosis factor-α (TNF-α) mRNA expression levels were normalized by β-actin in each sample. Values (means ± SEM) were expressed as the ratios of average values in HG.
Cell migration assays.
THP-1 cell migration was analyzed with a modified Boyden chamber assay using a 24-well transwell with 5.0-μm pores (Corning Life Sciences, Corning, NY) as described previously (25,26). THP-1 cells were preincubated for 24 h in serum-free RPMI 1640 supplemented with 0.1% bovine serum albumin (Sigma-Aldrich). After starvation, CCK-8S, scrambled peptide, or proglumide was added to THP-1 cells at different concentrations, and the cells were added to the top chamber. CCK-8S or scrambled peptide was incubated from 15 min before addition to the top chamber, and proglumide was added 15 min before addition of CCK-8S. The medium in the lower well contained 100 ng/mL of recombinant human CC chemokine ligand 2 (CCL2; R&D Systems, Minneapolis, MN). Cells that migrated to the bottom side of the membrane were quantitated by CyQuant DNA-binding fluorescence (Invitrogen) according to the manufacturer’s instructions (n = 6 each).
Statistical analysis.
All values are expressed as the means ± SEM. Differences between groups were examined for statistical significance using one-way ANOVA followed by Scheffe’s test. A P value <0.05 was considered statistically significant.
RESULTS
Enhanced CCK expression in the kidney tissues of diabetic WT mice.
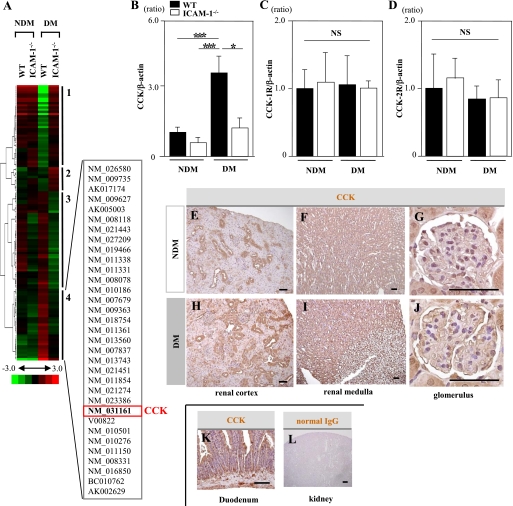
Hierarchical clustering identified 33 genes that were significantly upregulated only in diabetic WT mice but not remarkably changed in nondiabetic WT mice, nondiabetic ICAM-1−/− mice, or diabetic ICAM-1−/− mice (cluster 4; Fig. 1A). We focused on CCK because CCK is one of the most upregulated genes in cluster 4. Real-time RT-PCR revealed that the expression of CCK mRNA in the kidney cortices was significantly higher in diabetic WT mice than in diabetic ICAM-1−/− mice (Fig. 1B), whereas there was no difference in CCK-1R or CCK-2R mRNA expression (Fig. 1C and D). We confirmed the distribution of CCK in the kidney tissues by immunoperoxidase staining. CCK was widely distributed in kidney tissues of nondiabetic WT mouse (Fig. 1E–G). In diabetic WT mice, the distal tubules and glomeruli were stained more intensely than in nondiabetic WT mice (Fig. 1H–J).
FIG. 1.
The expression and distribution of CCK in kidney tissues of mice.A: Cluster analysis of differentially expressed genes comparing NDM WT and ICAM-1−/− mice and STZ-induced DM WT and ICAM-1−/− mice (n = 5/group). The dendrogram on the left of the cluster shows the relatedness of the change in gene expression. On the right of the cluster diagram, four groups of genes (1–4) are identified based on their gene expression changes. The list of constitutive genes involved in cluster 4 is given using the GenBank accession numbers. CCK was included in this cluster. B: CCK mRNA expression in the renal cortices was significantly increased in diabetic WT mice and significantly reduced to a nondiabetic level in diabetic ICAM-1−/− mice. C and D: The mRNA expressions of CCK-1R and CCK-2R were almost the same among the four groups. Values (means ± SEM) are presented as the ratio of nondiabetic WT. Data shown are representative of three separate experiments performed with five mice per group. E–G: Immunohistological staining of renal tissue specimens obtained from nondiabetic WT mice. The CCK-positive area was mainly observed in the distal tubules (E) and collecting ducts (F) and weakly in glomeruli (G). H–J: Immunohistological staining of kidney tissue specimens obtained from diabetic WT mice. The distal tubules (H) and glomeruli (J) were stained more intensely compared with those in the nondiabetic WT mice. A duodenal tissue specimen was used as a positive control (K). Normal IgG was also used as a negative control (L). Scale bars, 50 μm. *P < 0.05. ***P < 0.001. NS, P > 0.05. (A high-quality digital representation of this figure is available in the online issue.)
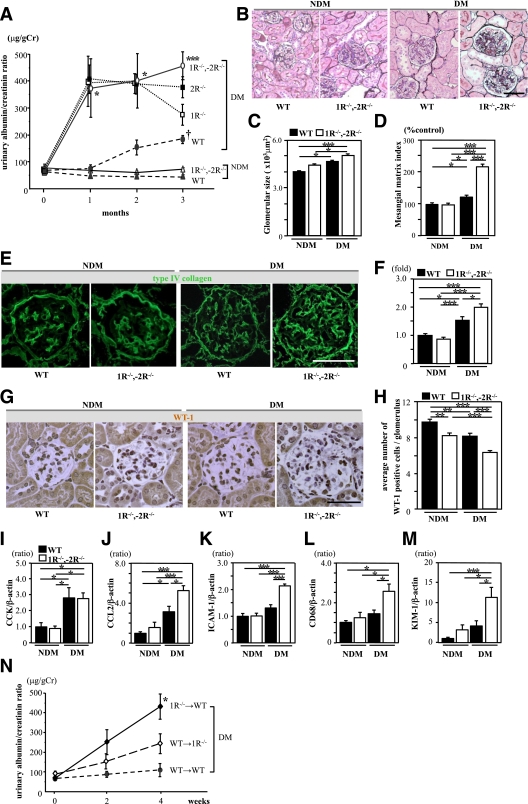
Diabetic CCK-1R−/−,-2R−/− mice exhibited increased albuminuria with upregulated proinflammatory genes in the kidney.
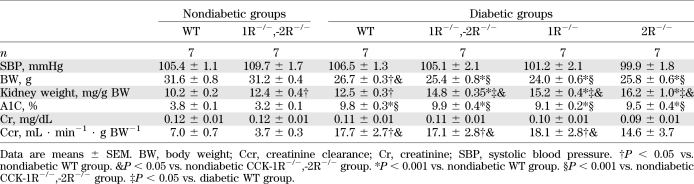
At 3 months after induction of diabetes, there was no significant difference in A1C, body weights, and creatinine clearance between the diabetic WT and diabetic CCK-1R−/−,-2R−/− groups (Table 1). Kidney weight per body weight was increased not only in the diabetic WT and diabetic CCK-1R−/−,-2R−/− groups but also in the nondiabetic CCK-1R−/−,-2R−/− group (Table 1). It was noteworthy that the urinary albumin/creatinine ratio was markedly increased in diabetic CCK-1R−/−,-2R−/− mice from 1 month to the end of the observation period compared with the diabetic WT mice (Fig. 2A). Furthermore, we compared levels of albuminuria among diabetic WT, CCK-1R−/−, CCK-2R−/−, and CCK-1R−/−,-2R−/− mice. Although there was no statistical significance, CCK-1R−/−,-2R−/− mice exhibited the most increased albuminuria at 3 months (Fig. 2A). Representative findings of the glomeruli in PAM-stained sections are shown in Fig. 2B. Glomerular hypertrophy was observed in both diabetic groups compared with the nondiabetic WT group at the end of the 3-month observation period (Fig. 2C). Mesangial matrix expansion was observed in both diabetic groups, but was more prominent in the diabetic CCK-1R−/−,-2R−/− group than in the diabetic WT group (Fig. 2D). Type IV collagen intensity was higher in both diabetic groups than nondiabetic groups, and the intensity in the diabetic CCK-1R−/−,-2R−/− group was further increased as compared with that in the diabetic WT group (Fig. 2E and F). Immunoperoxidase staining of WT-1, a normal podocyte marker, was performed to investigate the effect of CCK-8S in the progression of podocyte loss (Fig. 2G). The number of WT-1–positive cells per glomerulus was significantly decreased in the diabetic WT and both CCK-1R−/−,-2R−/− groups, whereas podocyte loss was more prominent in the diabetic CCK-1R−/−,-2R−/− group (Fig. 2H). CCK mRNA expression in the renal cortex was increased to the same extent in the diabetic WT group and diabetic CCK-1R−/−,-2R−/− group compared with the nondiabetic groups (Fig. 2I). In contrast, mRNA of CCL2, ICAM-1, cluster of differentiation (CD) 68, and kidney injury molecule-1 (KIM-1; a marker of tubular damage) were significantly upregulated in the diabetic CCK-1R−/−,-2R−/− group compared with the diabetic WT group (Fig. 2J–M). These findings suggest that diabetic renal injuries were exacerbated by deletion of both CCK-1R and CCK-2R via the inflammatory process. Furthermore, we performed BMT study to clarify whether deficiency of CCK-1R on infiltrating macrophages or resident renal cells is more important for the exacerbation of diabetic renal injury. BMT study showed that 1R−/−→WT mice exhibited significantly increased relative kidney weight (Supplementary Table 3) and albuminuria (Fig. 2N) compared with WT→1R−/− and WT→WT mice, suggesting the importance of CCK-1R on macrophages.
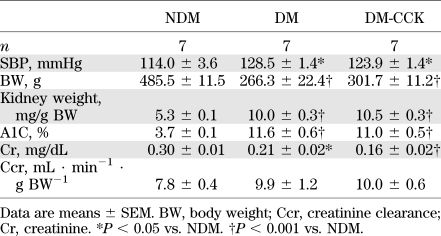
TABLE 1.
Metabolic characteristics of WT mice and CCK receptor knockout mice (3 months after induction of diabetes)
FIG. 2.
Diabetic CCK-1R−/−,-2R−/− mice exhibited increased albuminuria and enhanced proinflammatory genes in the kidney. A: Time course of urinary albumin/creatinine ratio (UACR). The UACR of diabetic CCK-1R−/−,-2R−/− mice (○) was markedly increased as compared with that of diabetic WT mice (●) (n = 7/group). The UACR of CCK-1R−/−,-2R−/− mice was higher than that of CCK-1R−/− mice (□) or CCK-2R−/− mice (■) at 3 months. *P < 0.05 vs. diabetic WT and nondiabetic groups; ***P < 0.001 vs. diabetic WT and nondiabetic groups; †P < 0.05 vs. nondiabetic groups. B: PAM staining of the kidney at 3 months. Scale bars, 50 μm. C: Glomerular hypertrophy was observed in both diabetic groups as compared with nondiabetic WT mice (n = 5/group). *P < 0.05; ***P < 0.001. D: The mesangial matrix index, calculated by the PAM-positive area in the tuft area, was significantly increased in diabetic CCK-1R−/−,-2R−/− mice as compared with the other three groups. Ten randomly selected glomeruli per mouse were examined (n = 5/group). *P < 0.05; ***P < 0.001. E: Expression of type IV collagen in kidney tissue. Scale bars, 50 μm. F: Collagen IV-positive area in glomeruli (folds versus the nondiabetic WT group). Type IV collagen was significantly increased in the diabetic CCK-1R−/−,-2R−/− group compared with the diabetic WT group. Fifteen randomly selected glomeruli per mouse were examined (n = 4/group). *P < 0.05; ***P < 0.001. G: Expression of WT-1 in glomeruli. H: The average number of WT-1–positive cells in glomeruli. Podocyte loss was significantly increased in diabetic CCK-1R−/−,-2R−/− mice as compared with the other three groups. Twenty randomly selected glomeruli per mouse were examined (n = 4/group). Values are the means ± SEM. **P < 0.01; ***P < 0.001. Scale bars, 50 μm. I–L: Expression of CCK and proinflammatory genes in the renal cortex. Expression of CCK was significantly increased to similar levels in both diabetic groups compared with the nondiabetic groups, whereas the expressions of CCL2, ICAM-1, and CD68 were significantly upregulated only in the diabetic CCK-1R−/−,-2R−/− group (n = 6/group). Values are presented as ratio of nondiabetic WT. Results (mean ± SEM) are representative of three independent experiments. M: Expression of KIM-1 gene in the kidney. Expression of KIM-1 was significantly increased in diabetic CCK-1R−/−,-2R−/− mice as compared with the other three groups (n = 6/group). Values are presented as the ratio of nondiabetic WT. Results (mean ± SEM) are representative of three independent experiments. N: Time course of UACR after induction of diabetes. The UACR of WT mice that received a bone marrow transplant from CCK-1 receptor-deficient mice (1R−/− →WT; ♦) was markedly increased after induction of diabetes as compared with that of other groups. WT→WT (●), WT mice that received a BMT from WT mice; WT→1R−/− (◇), CCK-1 receptor-deficient mice that received a BMT from WT mice. Values are the means ± SEM. *P < 0.05 vs. other groups. (A high-quality digital representation of this figure is available in the online issue.)
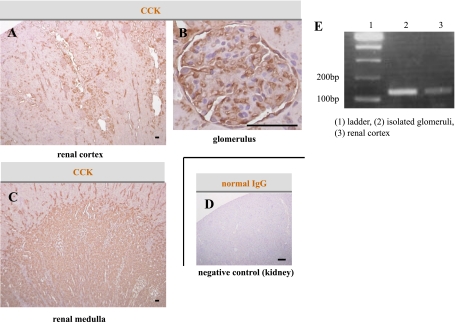
Distribution of CCK in rat renal tissues.
In the renal cortex of adult rats, the distribution of CCK was comparatively localized in distal tubules and glomeruli (Fig. 3A and B). In the renal medulla, the collecting ducts were stained intensely (Fig. 3C). We also identified CCK mRNA expression by real-time RT-PCR in the kidney cortex and isolated glomeruli obtained from normal adult rats (Fig. 3E).
FIG. 3.
Distribution of CCK in the rat renal tissues. A–D: Immunohistological staining of renal tissue specimens obtained from nondiabetic rats. The CCK-positive area was mainly observed in the distal tubules (A), glomeruli (B), and collecting ducts (C). Scale bars, 50 μm. Normal IgG was also used as a negative control (D). Scale bar, 500 μm. E: CCK mRNA expression was observed in isolated glomeruli and the renal cortex obtained from nondiabetic rats. (A high-quality digital representation of this figure is available in the online issue.)
CCK-8S ameliorates urinary albumin excretion and inhibits both macrophage infiltration and podocyte loss in glomeruli.
At 8 weeks after induction of diabetes, systolic blood pressure, A1C, and kidney weight per body weight were elevated to the same level in both diabetic groups. However, there was no significant difference between DM and DM-CCK (Table 2). The body weight and serum creatinine of both diabetic groups were lower than that of the NDM animals. However, there was no significant difference between the DM and DM-CCK groups (Table 2). Fasting serum CCK levels in the DM-CCK group was increased ∼3.9-fold than that of the DM group (514 ± 89 vs. 132 ± 33 pg/mL).
TABLE 2.
Metabolic characteristics of untreated rats and CCK-8S–treated rats (8 weeks after induction of diabetes)
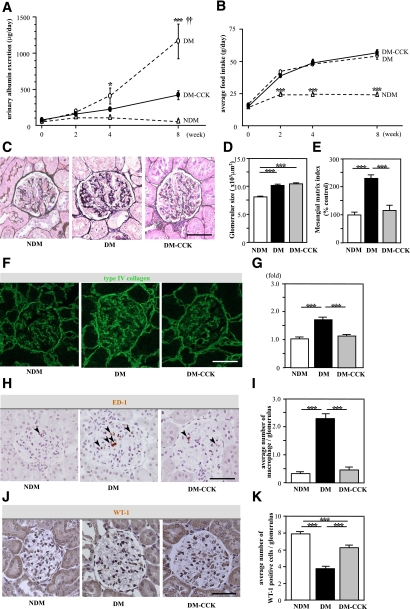
It is noteworthy that CCK-8S treatment significantly reduced urinary albumin excretion compared with the DM group at 8 weeks (Fig. 4A). Food intake was increased to the same extent in both diabetic groups compared with the NDM group after induction of diabetes (Fig. 4B). Glomerular hypertrophy was observed in both diabetic groups as compared with the NDM group. There was no significant difference in glomerular size between the DM and DM-CCK groups (Fig. 4C and D). Mesangial matrix expansion was observed in the DM group; however, CCK-8S treatment significantly reduced mesangial matrix expansion compared with DM (Fig. 4E). Type IV collagen intensity was higher in the DM than the NDM group. CCK-8S treatment markedly reduced type IV collagen intensity compared with the DM animals (Fig. 4F and G). The average number of macrophages (ED1-positive cells) per glomerulus was markedly increased in the DM compared with the NDM group, whereas macrophage infiltration was significantly inhibited by CCK-8S treatment (Fig. 4H and I). The number of WT-1–positive cells per glomerulus was significantly decreased in the DM, whereas podocyte loss was significantly inhibited by CCK-8S treatment (Fig. 4J and K).
FIG. 4.
CCK-8S ameliorates urinary albumin excretion and inhibits macrophage infiltration in glomeruli. A: Time course of urinary albumin excretion (UAE). The UAE of untreated diabetic rats (DM; ○) was increased progressively compared with that in nondiabetic rats (NDM; △), whereas it was suppressed in CCK-8S–treated diabetic rats (DM-CCK; ●) at week 8 (n = 7/group). Values are the means ± SEM. *P < 0.05 vs. NDM; ***P < 0.001 vs. NDM; ††P < 0.01 vs. DM-CCK. B: There was no difference in the amount of food intake between DM (○) and DM-CCK (●). ***P < 0.001 vs. DM and DM-CCK. Values are the means ± SEM. C: PAM staining of the kidney at 8 weeks. D: Glomerular hypertrophy was observed in both diabetic groups as compared with nondiabetic rats. Values are the means ± SEM. ***P < 0.001. E: Mesangial matrix index was ameliorated in CCK-8S–treated diabetic rats compared with untreated diabetic rats. Values are the means ± SEM. ***P < 0.001. Fifteen randomly selected glomeruli per rat were examined (n = 5/group). F: Expression of type IV collagen in kidney tissue. G: Type IV collagen-positive area in glomeruli (folds versus NDM). Type IV collagen was significantly increased in the DM compared with the DM-CCK group. Fifteen randomly selected glomeruli per rat were examined (n = 4/group). Values are the means ± SEM. ***P < 0.001. H: Macrophage infiltration into glomeruli at 8 weeks. Arrowheads indicate macrophages. I: The average number of intraglomerular macrophages. Macrophage infiltration into glomeruli was remarkable in the DM, whereas it was minimal in the DM-CCK group. Twenty randomly selected glomeruli per rat were examined (n = 4/group). Values are the means ± SEM. ***P < 0.001. J: Expression of WT-1 in glomeruli. K: The average number of WT-1–positive cells in glomeruli. Podocyte loss was inhibited in CCK-8S–treated diabetic rats compared with untreated diabetic rats. Twenty randomly selected glomeruli per rat were examined (n = 4/group). Values are the means ± SEM. ***P < 0.001. Scale bars, 50 μm. (A high-quality digital representation of this figure is available in the online issue.)
CCK-8S inhibits expression of proinflammatory genes and NF-κB activation in diabetic kidney.
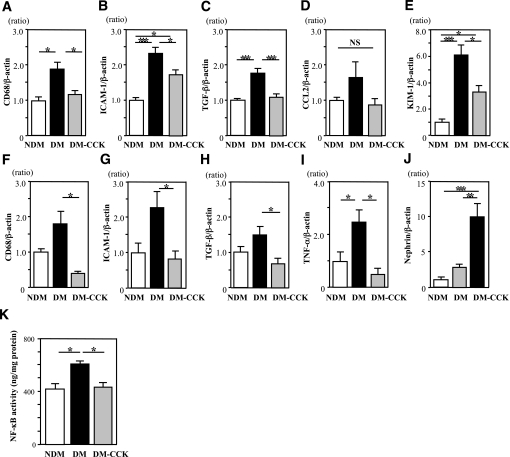
The mRNA expressions of CD68, ICAM-1, and TGF-β in the renal cortex were significantly upregulated in the DM group, and these increases were significantly suppressed by CCK-8S treatment (Fig. 5A–C). The increase of KIM-1 mRNA expression in the kidney of DM group was partially but significantly suppressed by CCK-8S treatment (Fig. 5E). In isolated glomeruli, CCK-8S treatment also decreased the mRNA expressions of CD68, ICAM-1, TGF-β, and TNF-α as compared with DM (Fig. 5F–I). Interestingly, CCK-8S treatment markedly increased the mRNA expression of nephrin in glomeruli as compared with DM (Fig. 5J). These findings suggest that CCK-8S inhibits the development of albuminuria via inhibition of proinflammatory genes in the diabetic kidney. NF-κB p65-dependent DNA-binding activity in the renal cortex was significantly increased in the DM compared with the NDM group. CCK-8S treatment significantly decreased the NF-κB p65-dependent DNA-binding activity (Fig. 5K).
FIG. 5.
CCK-8S inhibits expression of proinflammatory genes and NF-κB p65-dependent DNA-binding activity in the kidney of diabetic rats. A–D: Expression of proinflammatory genes in the renal cortex. CCK-8S treatment significantly decreased the mRNA levels of CD68 (A), ICAM-1 (B), and TGF-β (C) in the renal cortex as compared with those in untreated diabetic rats (n = 6/group). E: Expression of KIM-1 gene in the kidney was significantly inhibited by CCK-8S treatment. F–I: Expression of proinflammatory and nephrin genes in the isolated glomeruli (n = 4/group). CCK-8S treatment was significantly decreased the mRNA level of CD68 (F), ICAM-1 (G), TGF-β (H), and TNF-α (I) in isolated glomeruli as compared with untreated diabetic rats. In contrast, CCK-8S treatment was significantly increased the mRNA level of nephrin (J) in isolated glomeruli as compared with untreated diabetic rats. Values (means ± SEM) are presented as the ratio of NDM. *P < 0.05; **P < 0.01; ***P < 0.001; NS, P > 0.05. K: CCK-8S treatment ameliorates diabetes-induced NF-κB p65-dependent DNA-binding activity in the nuclear extracts of the rat kidney cortex (n = 6/group). *P < 0.05. Results (means ± SEM) are representative of two to three independent experiments.
CCK-8S suppresses TNF-α expression and chemotaxis in THP-1 cells.
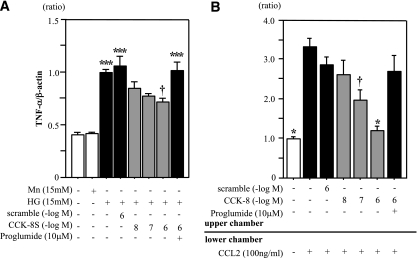
TNF-α mRNA expression was significantly increased in the untreated HG group (Fig. 6A). Although TNF-α mRNA expression was not suppressed in the scrambled peptide-treated HG group, it was suppressed in the CCK-8S–treated (10−6 M) HG group compared with the untreated HG group (Fig. 6A). In addition, the anti-inflammatory effect of CCK-8S was largely abrogated by proglumide (an antagonist for both CCK receptors) (Fig. 6A). The number of THP-1 cells migrated into the lower chamber of the transwell was significantly reduced by CCK-8S treatment in a dose-dependent manner and was not reduced by scrambled peptide. These antimigratory effects of CCK-8S were completely abolished by proglumide (Fig. 6B).
FIG. 6.
CCK-8S suppresses both expression of TNF-α and chemotaxis in THP-1 cells. A: THP-1 cells were cultured under different conditions for 72 h. CCK-8S inhibited HG-induced TNF-α expression in THP-1 cells (n = 5 each). Values are presented as the ratio of HG group. ***P < 0.001 vs. the NG and Mn groups; †P < 0.05 vs. the HG, scrambled peptide, and proglumide (a nonselective CCK receptor antagonist) groups. B: CCK-8S blocks the CCL2-induced chemotaxis in THP-1 cells (n = 5 per group). Values are presented as the ratio of untreated group. *P < 0.05 vs. the CCL2, CCL2 with scrambled peptide, and CCL2 with proglumide groups; †P < 0.05 vs. the CCL2 group. Results (means ± SEM) are representative of two to three independent experiments.
DISCUSSION
In present study, we found that CCK is one of the significantly upregulated genes in the diabetic WT kidney compared with the diabetic ICAM-1−/− kidney. We hypothesized that CCK might regulate inflammatory response in the diabetic kidney; however, little is known about the role of CCK and its receptors in the development of diabetic nephropathy.
Two types of CCK receptors have been identified (27,28). These receptors have been classified as CCK-1R and CCK-2R based on their highly distinctive ligand selectivities (18). The two types of CCK receptors are distributed in various cells or tissues, including the kidneys (29–33) and macrophages (34,35). CCK-1R−/−,-2R−/− mice are fertile and show no apparent developmental defects. It has been reported that the weights of the kidneys and liver are significantly increased in CCK-1R−/−,-2R−/− mice compared with WT mice, although no abnormality is visible in these organs (17). In this study, deletion of both CCK-1R and CCK-2R enhanced inflammatory reactions and exacerbated the development of albuminuria after induction of diabetes. Our results suggest that CCK is increased in the diabetic kidney of mice and may regulate macrophage-related proinflammatory genes via CCK receptors. Furthermore, BMT study revealed that CCK-1R on macrophages played a more important role in the early stage of diabetic nephropathy than CCK-1R on resident renal cells. In the BMT study, we used CCK-1R−/− mice, because CCK-1R on macrophages plays a more dominant role in the anti-inflammatory effect of CCK-8S than CCK-2R (12). In contrast, CCK-2R is expressed on renal cells including murine mesangial cells (32). And the systemic absence of CCK-2R also exacerbated the development of albuminuria after induction of diabetes almost same extent as in CCK-1R−/− mice. Therefore, although further BMT study is needed, endogenous CCK might act against not only infiltrating macrophages but also resident renal cells via CCK-2R.
Several transcription factors have been implicated in the glucose-mediated expression of genes involved in diabetic nephropathy (36). NF-κB is one of the key mediators in the inflammatory response and plays a pivotal role in the progression of diabetic nephropathy (37). Activation of NF-κB in both kidney tissues obtained by biopsies and human peripheral blood mononuclear cells has been shown to correlate with degree of diabetic nephropathy (38,39). And NF-κB is also involved in regulation of ICAM-1 expression in diabetic kidney (36). Li et al. (12) reported that CCK-8S inhibited lipopolysaccharide-induced cytokine production via suppression of NF-κB activity. We showed that CCK-8S significantly suppressed NF-κB activation in the diabetic kidney, suggesting that CCK-8S may inhibit ICAM-1 expression via inhibition of NF-κB activity and thus lead to suppression of macrophage infiltration in the diabetic kidney.
Guha et al. (40) reported that high glucose-induced TNF-α mRNA expression in THP-1 cells was mediated by NF-κB. Our results indicate that inhibition of both high glucose-induced TNF-α expression via NF-κB and CCL2-induced migration might be involved in the anti-inflammatory effects of CCK-8S in THP-1 cells. Because the nephrin gene expression in cultured podocyte is repressed by TNF-α at a transcriptional level (41), our results suggest that CCK-8S may prevent podocyte loss via inhibition of TNF-α mRNA expression in diabetic glomeruli.
Aunapuu et al. (42) recently reported that CCK overexpression was associated with renal morphological damage in transgenic mice without a significant difference in kidney weight or proteinuria, but with a thickened glomerular basement membrane. CCK is expressed from embryonic day (E) 8.5, whereas both CCK-1R and CCK-2R have been identified from E10.5; in addition, CCK is thought to affect tissue growth and differentiation (43). In another study, both CCK-1R and CCK-2R expression were confirmed from E14.5 in kidney tissues (33), and therefore, CCK might regulate renal microstructural growth. In nondiabetic CCK-1R−/−,-2R−/− mice, increased kidney and liver weight (17) and reduction in the number of glomerular podocytes also suggest that CCK may play a role in regulating the development or growth of these organs. In the current study, we examined the effect of CCK-8S using an STZ-induced diabetic model, but no nephrotoxicity was observed.
Because the half-time of CCK-8S in blood is short, it would be difficult to use a longer period for maintaining renoprotective effect in patients with diabetic nephropathy. Recently, León-Tamariz et al. (44) reported that PEGylated CCK-10, which did not cross the blood-brain barrier, maintained blood concentration longer than free CCK. Such drugs with a longer duration of action may be more suitable for clinical use.
In conclusion, we have shown that CCK is expressed in the kidney, and deficiency of both CCK-1R and CCK-2R accelerates development of albuminuria by enhancement of inflammation in the kidneys after induction of diabetes. Administration of CCK-8S confers protection against renal inflammation, leading to a reduction of albuminuria in diabetic rats. Furthermore, CCK-8S directly inhibits high glucose-induced TNF-α expression and migration in cultured THP-1 cells. Our findings may provide a novel strategy of therapy for the early stage of diabetic nephropathy and other inflammatory diseases.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports and Technology of Japan (Grant 21591031 to K.S.) and the Ministry of Health, Labour and Welfare of Japan.
No potential conflicts of interest relevant to this article were reported.
S.M. researched data, contributed to discussion, and wrote the manuscript. K.S. contributed to discussion and reviewed and edited the manuscript. K.M. researched data and reviewed and edited the manuscript. S.O., M.S., R.K., D.H., N.K., T.T., S.N., C.S., A.F., H.N., and H.A.U. researched data. H.U.K., D.O., and H.M. contributed to discussion. K.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0402/-/DC1.
REFERENCES
- 1.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999;34:795–808 [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–232 [DOI] [PubMed] [Google Scholar]
- 3.Nelson CL, Karschimkus CS, Dragicevic G, et al. Systemic and vascular inflammation is elevated in early IgA and type 1 diabetic nephropathies and relates to vascular disease risk factors and renal function. Nephrol Dial Transplant 2005;20:2420–2426 [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto H, Shikata K, Hirata K, et al. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes 1997;46:2075–2081 [DOI] [PubMed] [Google Scholar]
- 5.Okada S, Shikata K, Matsuda M, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 2003;52:2586–2593 [DOI] [PubMed] [Google Scholar]
- 6.Jorpes E, Mutt V. Cholecystokinin and pancreozymin, one single hormone? Acta Physiol Scand 1966;66:196–202 [DOI] [PubMed] [Google Scholar]
- 7.Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem 1968;6:156–162 [DOI] [PubMed] [Google Scholar]
- 8.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev 2006;86:805–847 [DOI] [PubMed] [Google Scholar]
- 9.Rehfeld JF. Clinical endocrinology and metabolism. Cholecystokinin. Best Pract Res Clin Endocrinol Metab 2004;18:569–586 [DOI] [PubMed] [Google Scholar]
- 10.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides 1994;15:731–755 [DOI] [PubMed] [Google Scholar]
- 11.Meng AH, Ling YL, Zhang XP, Zhang JL. Anti-inflammatory effect of cholecystokinin and its signal transduction mechanism in endotoxic shock rat. World J Gastroenterol 2002;8:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Ni Z, Cong B, et al. CCK-8 inhibits LPS-induced IL-1beta production in pulmonary interstitial macrophages by modulating PKA, p38, and NF-kappaB pathway. Shock 2007;27:678–686 [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt A, Cakir B, Ercan F, Yeğen BC. Anti-inflammatory effects of leptin and cholecystokinin on acetic acid-induced colitis in rats: role of capsaicin-sensitive vagal afferent fibers. Regul Pept 2003;116:109–118 [DOI] [PubMed] [Google Scholar]
- 14.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med 2005;202:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sligh JE, Jr, Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA 1993;90:8529–8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Usui HK, Shikata K, Sasaki M, et al. Macrophage scavenger receptor-a-deficient mice are resistant against diabetic nephropathy through amelioration of microinflammation. [Published erratum appears in Diabetes 2007;56:897.] Diabetes 2007;56:363–372 [DOI] [PubMed] [Google Scholar]
- 17.Miyasaka K, Ichikawa M, Ohta M, et al. Energy metabolism and turnover are increased in mice lacking the cholecystokinin-B receptor. J Nutr 2002;132:739–741 [DOI] [PubMed] [Google Scholar]
- 18.Takiguchi S, Suzuki S, Sato Y, et al. Role of CCK-A receptor for pancreatic function in mice: a study in CCK-A receptor knockout mice. Pancreas 2002;24:276–283 [DOI] [PubMed] [Google Scholar]
- 19.Nagata A, Ito M, Iwata N, et al. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci USA 1996;93:11825–11830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asakura S, Hashimoto D, Takashima S, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Invest 2010;120:2370–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimori H, Maeda Y, Teshima T, et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by downregulating Th1 and Th17. Blood 2012;119:285–295 [DOI] [PubMed] [Google Scholar]
- 22.Uchida HA, Poduri A, Subramanian V, Cassis LA, Daugherty A. Urokinase-type plasminogen activator deficiency in bone marrow-derived cells augments rupture of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2011;31:2845–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong JS, Drummond KN. Method for preparation of glomeruli for metabolic studies. J Lab Clin Med 1968;71:1034–1039 [PubMed] [Google Scholar]
- 24.Kodera R, Shikata K, Kataoka HU, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011;54:965–978 [DOI] [PubMed] [Google Scholar]
- 25.Wågsäter D, Zhu C, Björck HM, Eriksson P. Effects of PDGF-C and PDGF-D on monocyte migration and MMP-2 and MMP-9 expression. Atherosclerosis 2009;202:415–423 [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Garre D, Muñoz-Pacheco P, González-Rubio ML, Aragoncillo P, Granados R, Fernández-Cruz A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br J Pharmacol 2009;156:1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wank SA, Harkins R, Jensen RT, Shapira H, de Weerth A, Slattery T. Purification, molecular cloning, and functional expression of the cholecystokinin receptor from rat pancreas. Proc Natl Acad Sci USA 1992;89:3125–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopin AS, Lee YM, McBride EW, et al. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci USA 1992;89:3605–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacourse KA, Lay JM, Swanberg LJ, Jenkins C, Samuelson LC. Molecular structure of the mouse CCK-A receptor gene. Biochem Biophys Res Commun 1997;236:630–635 [DOI] [PubMed] [Google Scholar]
- 30.Galindo J, Jones N, Powell GL, Hollingsworth SJ, Shankley N. Advanced qRT-PCR technology allows detection of the cholecystokinin 1 receptor (CCK1R) expression in human pancreas. Pancreas 2005;31:325–331 [DOI] [PubMed] [Google Scholar]
- 31.von Schrenck T, Ahrens M, de Weerth A, et al. CCKB/gastrin receptors mediate changes in sodium and potassium absorption in the isolated perfused rat kidney. Kidney Int 2000;58:995–1003 [DOI] [PubMed] [Google Scholar]
- 32.de Weerth A, Jonas L, Schade R, et al. Gastrin/cholecystokinin type B receptors in the kidney: molecular, pharmacological, functional characterization, and localization. Eur J Clin Invest 1998;28:592–601 [DOI] [PubMed] [Google Scholar]
- 33.Lay JM, Jenkins C, Friis-Hansen L, Samuelson LC. Structure and developmental expression of the mouse CCK-B receptor gene. Biochem Biophys Res Commun 2000;272:837–842 [DOI] [PubMed] [Google Scholar]
- 34.Sacerdote P, Wiedermann CJ, Wahl LM, Pert CB, Ruff MR. Visualization of cholecystokinin receptors on a subset of human monocytes and in rat spleen. Peptides 1991;12:167–176 [DOI] [PubMed] [Google Scholar]
- 35.Xu SJ, Gao WJ, Cong B, Yao YX, Gu ZY. Effect of lipopolysaccharide on expression and characterization of cholecystokinin receptors in rat pulmonary interstitial macrophages. Acta Pharmacol Sin 2004;25:1347–1353 [PubMed] [Google Scholar]
- 36.Sanchez AP, Sharma K. Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev Mol Med 2009;11:e13. [DOI] [PubMed] [Google Scholar]
- 37.Lee FT, Cao Z, Long DM, et al. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol 2004;15:2139–2151 [DOI] [PubMed] [Google Scholar]
- 38.Nam JS, Cho MH, Lee GT, et al. The activation of NF-kappaB and AP-1 in peripheral blood mononuclear cells isolated from patients with diabetic nephropathy. Diabetes Res Clin Pract 2008;81:25–32 [DOI] [PubMed] [Google Scholar]
- 39.Hofmann MA, Schiekofer S, Isermann B, et al. Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia 1999;42:222–232 [DOI] [PubMed] [Google Scholar]
- 40.Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem 2000;275:17728–17739 [DOI] [PubMed] [Google Scholar]
- 41.Takano Y, Yamauchi K, Hayakawa K, et al. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett 2007;581:421–426 [DOI] [PubMed] [Google Scholar]
- 42.Aunapuu M, Roosaar P, Järveots T, et al. Altered renal morphology in transgenic mice with cholecystokinin overexpression. Transgenic Res 2008;17:1079–1089 [DOI] [PubMed] [Google Scholar]
- 43.Lay JM, Gillespie PJ, Samuelson LC. Murine prenatal expression of cholecystokinin in neural crest, enteric neurons, and enteroendocrine cells. Dev Dyn 1999;216:190–200 [DOI] [PubMed] [Google Scholar]
- 44.León-Tamariz F, Verbaeys I, Van Boven M, et al. Biodistribution and pharmacokinetics of PEG-10kDa-cholecystokinin-10 in rats after different routes of administration. Curr Drug Deliv 2010;7:137–143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.