Abstract
Although loss of functional β-cell mass is a hallmark of diabetes, no treatment approaches that halt this process are currently available. We recently identified thioredoxin-interacting protein (TXNIP) as an attractive target in this regard. Glucose and diabetes upregulate β-cell TXNIP expression, and TXNIP overexpression induces β-cell apoptosis. In contrast, genetic ablation of TXNIP promotes endogenous β-cell survival and prevents streptozotocin (STZ)- and obesity-induced diabetes. Finding an oral medication that could inhibit β-cell TXNIP expression would therefore represent a major breakthrough. We were surprised to discover that calcium channel blockers inhibited TXNIP expression in INS-1 cells and human islets and that orally administered verapamil reduced TXNIP expression and β-cell apoptosis, enhanced endogenous insulin levels, and rescued mice from STZ-induced diabetes. Verapamil also promoted β-cell survival and improved glucose homeostasis and insulin sensitivity in BTBR ob/ob mice. Our data further suggest that this verapamil-mediated TXNIP repression is conferred by reduction of intracellular calcium, inhibition of calcineurin signaling, and nuclear exclusion and decreased binding of carbohydrate response element–binding protein to the E-box repeat in the TXNIP promoter. Thus, for the first time, we have identified an oral medication that can inhibit proapoptotic β-cell TXNIP expression, enhance β-cell survival and function, and prevent and even improve overt diabetes.
Loss of pancreatic β-cell mass is a key factor in the pathogenesis of type 1 and type 2 diabetes, but therapies that interfere with this process are currently lacking. We recently discovered thioredoxin-interacting protein (TXNIP), a ubiquitously expressed cellular redox regulator, as an attractive novel target in this regard. We originally identified TXNIP as the top glucose-induced gene in a human islet microarray study and found that β-cell expression of TXNIP is increased in diabetes (1–3). Moreover, TXNIP overexpression induces β-cell apoptosis and is essential for glucotoxicity-induced β-cell death (3), whereas lack of TXNIP promotes endogenous β-cell survival and prevents type 1 and type 2 diabetes (2,4,5). In addition, TXNIP has recently been discovered to induce inflammasome activation and interleukin-1β production, a cytokine involved in type 1 diabetes (6). While together these findings have established TXNIP as an attractive therapeutic target, effective TXNIP inhibitors are currently missing. Of interest, we found that calcium channel blockers such as verapamil (commonly used as antihypertensive drugs) can reduce TXNIP expression as well as apoptosis in the heart (7).
The present studies were therefore aimed at determining whether calcium channel blockers may also decrease β-cell TXNIP expression, enhance β-cell survival, and protect against diabetes. Using INS-1 cells, primary human islets, and models of type 1 and type 2 diabetes, our results demonstrate that oral verapamil can indeed rescue from β-cell death and diabetes and shed light on the mechanisms involved.
RESEARCH DESIGN AND METHODS
Cell culture.
INS-1 cells were grown in RPMI 1640 (Invitrogen) with 11.1 mmol/L glucose, 10% FBS, 1% penicillin/streptomycin, 1 mmol/L sodium pyruvate, 2 mmol/L l-glutamine, 10 mmol/L HEPES, and 0.05 mmol/L 2-mercaptoethanol and incubated with verapamil, diltiazem, NiCl2, EGTA, or cyclosporine A (CyA; Sigma). Mouse pancreatic islets were isolated by collagenase digestion (8,9) and used for RNA or protein extraction or were embedded for immunohistochemistry and transferase-mediated dUTP nick-end labeling (TUNEL) analysis.
Human islets were obtained from the Islet Resource Facility of the University of Alabama at Birmingham Comprehensive Diabetes Center. After overnight incubation at 5 mmol/L glucose, human islets were incubated at 11.1 mmol/L glucose in the presence or absence of 50, 100, or 150 μmol/L verapamil for 24 h. Islets from the same donor were used as control.
Animal studies.
All mouse studies were approved by the University of Alabama at Birmingham Animal Care and Use Committee.
Wild-type, 8-week-old, male C57BL/6 mice (The Jackson Laboratory) were rendered diabetic by multiple low-dose injection of streptozotocin (STZ; 40 mg/kg body wt per day for 5 days, prepared freshly in 0.1 mmol/L sodium citrate at pH 4.5).
As a model of type 2 diabetes, leptin-deficient, obese, insulin-resistant, and diabetic BTBRlepob/ob (ob/ob) mice (The Jackson Laboratory) were used. BTBRlepob/ob mice develop severe diabetes at 4–5 weeks of age and suffer from β-cell apoptosis (2).
Mice received verapamil in their drinking water (1 mg/mL), resulting in an average dose of 100 mg/kg/day, which is the commonly used dose for mouse studies of this kind (7,10). (A subset of mice received a lower dose of 0.5 mg/mL in their drinking water, resulting in an average dose of 50 mg/kg/day.) Control mice were housed under identical conditions without verapamil. Blood glucose was measured with a FreeStyle Lite glucometer; serum insulin was assessed using a mouse insulin ELISA kit (Alpco Diagnostics, Salem, NH).
Insulin tolerance tests (ITTs) were performed after a 4-h fast using insulin 1 IU/kg i.p. (2). At killing, mouse pancreata were collected for immunohistochemistry or for islet isolation (2,3).
Plasmid construction and transient transfection assays.
Construction of the TXNIP promoter reporter and control LacZ plasmids were described previously (4), as was the carbohydrate response element–binding protein (ChREBP) overexpression plasmid (11).
To determine the TXNIP promoter region responsible for the verapamil effect, INS-1 cells were grown in 12-well plates and transfected with different TXNIP promoter deletion reporter plasmids (0.4 μg/well) together with pRL-TK control plasmid (20 ng/well; Promega) using DharmaFECT Duo transfection reagent (1 μL/well; Dharmacon/Thermo Scientific, Chicago, IL). Verapamil was added to the medium at a final concentration of 100 μmol/L. At 24 h after transfection, cells were harvested and luciferase activity was determined by Dual Luciferase assay kit (Promega).
To determine the role of ChREBP in the verapamil effects, INS-1 cells were grown in 6-well plates and transfected with ChREBP or control LacZ plasmids (2 μg/well) using DharmaFECT Duo transfection reagent (3 μL/well). At 24 h after transfection, verapamil was added to the medium with a final concentration of 50 μmol/L; 48 h after transfection, cells were harvested for RNA extraction.
Quantitative real-time RT-PCR.
Total RNA was extracted using RNeasy kit (QIAGEN) according to the manufacturer’s instructions. RNA (1 μg) was reverse transcribed to cDNA using the first strand cDNA synthesis kit (Roche).
Quantitative real-time PCR was performed on a Prism 7000 Sequence Detection System using SYBR Green (Applied Biosystems, Foster City, CA). Rat TXNIP primers have been described previously (4). All samples were corrected for the 18S ribosomal subunit (Applied Biosystems) run as an internal standard.
Western blotting.
Whole cell and nuclear protein extracts from INS-1 cells and mouse islets were prepared as described previously (3,12). The following antibodies were used: mouse anti-TXNIP IgG (1:400; JY2; MBL International), rabbit anti-ChREBP IgG (1:200; sc-33764), goat anti-mouse IgG (1:5,000; sc-2005), and goat anti-rabbit IgG (1:5,000; sc-2004) (Santa Cruz Biotechnology). Bands were visualized by ECL Plus (Amersham GE Health) and quantified by ImageQuant.
Immunohistochemistry and TUNEL assay.
Insulin staining and TUNEL were performed as previously described (2).
Chromatin immunoprecipitation.
Chromatin immunoprecipitation (ChIP) assays were performed as detailed previously (11).
Statistical analysis.
Student t tests were used to calculate the significance of a difference between two groups. For datasets of more than two groups and to analyze changes overtime, we performed one-way ANOVA calculations.
RESULTS
Calcium channel blockers reduce β-cell expression of proapoptotic TXNIP.
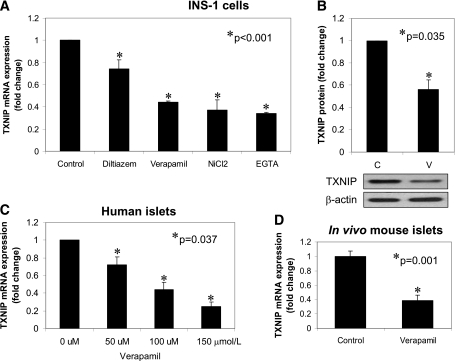
We previously identified TXNIP as a promising target to halt β-cell death and promote endogenous β-cell mass. Of interest, we recently discovered that calcium channel blockers are capable of reducing cardiac TXNIP expression (7), raising the question whether this class of antihypertensive drugs might be able to inhibit β-cell TXNIP expression. All first-generation calcium channel blockers inhibit l-type calcium channels, which are abundant in not only the heart but also pancreatic β-cells. To test the effects of calcium channel blockers on β-cell TXNIP expression, we therefore initially incubated rat INS-1 β-cells with diltiazem or verapamil (both first-generation calcium channel blockers) and measured changes in endogenous TXNIP mRNA expression by real-time PCR assay. Both decreased TXNIP mRNA significantly, with verapamil resulting in an ∼50% reduction. NiCl2, a T-type calcium channel inhibitor, also reduced TXNIP expression significantly, demonstrating that the observed effects were not dependent on the type of calcium channel affected. Furthermore, this inhibition of TXNIP expression could be mimicked by the calcium chelator EGTA, suggesting that the effects were primarily due to reduction in intracellular calcium (Fig. 1A). In contrast, another antihypertensive agent, propranolol (a β-blocker), had no effect on β-cell TXNIP expression (Supplementary Fig. 1A). Of interest, while the above studies were performed at 11.1 mmol/L glucose, verapamil failed to reduce TXNIP expression under low normal (5 mmol/L glucose) conditions (Supplementary Fig. 1B). Given the strong effect verapamil had and the fact that it is already an approved oral medication, we focused our subsequent studies on this calcium channel blocker and confirmed that verapamil also was able to significantly reduce TXNIP protein levels (Fig. 1B). In addition, we tested the effects of verapamil on human islets and found that it reduced TXNIP expression in a dose-dependent manner (Fig. 1C). To further test whether verapamil could reduce TXNIP levels in vivo in mouse islets, C57BL/6 mice received verapamil in their drinking water as described previously (7), and TXNIP expression was analyzed in their islets. The results demonstrated that TXNIP levels of verapamil-treated mice were significantly decreased compared with control mice (Fig. 1D).
FIG. 1.
Effects of calcium channel blockers on β-cell TXNIP expression. A: INS-1 cells were incubated for 24 h with diltiazem (50 μmol/L), verapamil (50 μmol/L), NiCl2 (500 μmol/L), or the calcium chelator EGTA (500 μmol/L), and TXNIP expression was assessed by quantitative RT-PCR. B: INS-1 cells were incubated for 48 h in the presence (V) or absence (C) of verapamil (50 μmol/L), and effects on TXNIP protein levels were assessed by Western blotting and corrected for β-actin. C: Isolated human islets were incubated for 24 h at the designated concentrations of verapamil, and TXNIP expression was assessed by quantitative RT-PCR. D: C57BL/6 mice received verapamil (100 mg/kg/day) in their drinking water for 3 weeks, and their islet TXNIP expression was assessed by quantitative RT-PCR. Bars represent means ± SEM of at least three independent experiments.
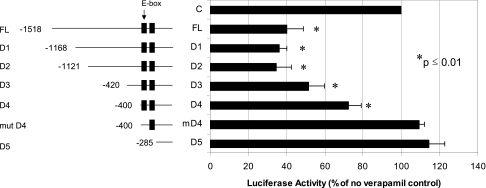
A specific E-box repeat in the TXNIP promoter is critical for verapamil-mediated inhibition of TXNIP transcription.
To further investigate how verapamil might regulate TXNIP expression and identify the cis-acting factors responsible, we used promoter deletion studies of the human TXNIP promoter and found that a conserved E-box repeat of two identical CACGAG elements was necessary for the observed repression of TXNIP by verapamil as demonstrated by the loss of verapamil-induced inhibition with mutation of the first E-box (mut D4) (Fig. 2). Of interest, we previously discovered that this E-box motif acts as a carbohydrate response element and serves as the binding site of the ChREBP (11).
FIG. 2.
Verapamil effects on TXNIP promoter activity. INS-1 cells were grown in 12-well plates and transfected with different TXNIP promoter reporter plasmids (0.4 μg/well) together with pRL-TK control plasmid (20 ng/well) using DharmaFECT Duo transfection reagent (1 μL/well). Verapamil was added to the medium with a final concentration of 100 μmol/L. At 24 h after transfection, cells were harvested and luciferase activity was determined by Dual Luciferase assay kit. Bars represent mean luciferase activity ± SEM of at least three independent experiments as compared with no verapamil control.
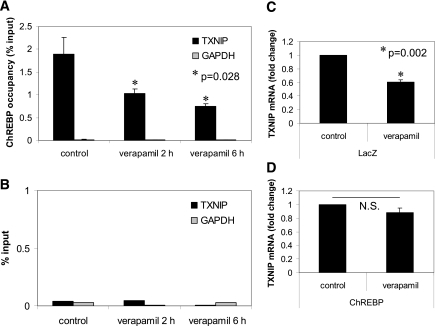
Verapamil inhibits ChREBP binding to the TXNIP promoter.
These findings raised the possibility that verapamil was acting through inhibition of ChREBP binding to the TXNIP promoter, resulting in inhibition of TXNIP transcription. In fact, using ChIP assays and PCR primers flanking the E-box repeat, we found that verapamil significantly reduced in vivo binding of ChREBP to the TXNIP promoter in a time-dependent manner (Fig. 3A), while IgG and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) negative controls showed no enrichment (Fig. 3B). Analysis of the liver-type pyruvate kinase (LPK) promoter containing a known ChREBP binding site revealed that verapamil was able to significantly reduce ChREBP occupancy of the LPK promoter in a time-dependent manner, demonstrating that its effects are not restricted to TXNIP and may include other ChREBP targets (Supplementary Fig. 2). The role of ChREBP was further confirmed by the fact that ChREBP overexpression blunted the verapamil effect (Fig. 3C and D).
FIG. 3.
ChIP analysis of ChREBP binding to the TXNIP promoter in response to verapamil. INS-1 cells were treated with verapamil for the designated times, and ChIP assays were performed as described previously (11) using 4 μg ChREBP antibody (A) or normal rabbit IgG (B), and real-time PCR was performed using primers flanking the E-boxes. Effects of ChREBP overexpression on verapamil-mediated inhibition of TXNIP expression. INS-1 cells were grown in 6-well plates and transfected with control LacZ (C) or ChREBP plasmids (2 μg/well) (D). At 24 h after transfection, verapamil was added to the medium with a final concentration of 50 μmol/L; 48 h after transfection, cells were harvested for RNA extraction and analyzed by quantitative RT-PCR. Bars represent means ± SEM of at least three independent experiments.
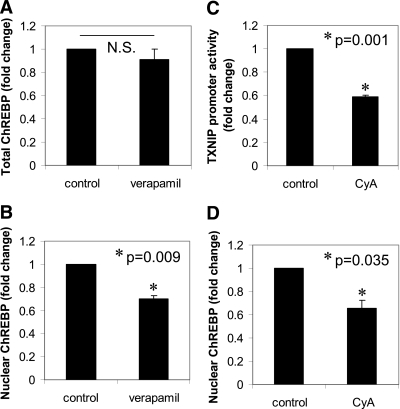
We further found that while ChREBP mRNA expression and total ChREBP protein levels remained unchanged (some data not shown) (Fig. 4A), nuclear ChREBP was significantly reduced in response to verapamil (Fig. 4B), which may explain the reduced ChREBP binding observed in our ChIP experiments. Furthermore, decreased intracellular calcium levels seem to be the key mediator of the verapamil effects because downregulation of TXNIP expression by calcium channel blockers was not class specific and was observed with both phenylalkylamines (i.e., verapamil) and benzothiazepines (i.e., diltiazem) as well as with the calcium chelator EGTA. Calcium can regulate gene expression by two major pathways, the calcium-dependent Ser-Thr protein phosphatase 2B (PP2B/calcineurin) and the calcium/calmodulin-dependent protein kinase (CaMK). In our case, we found that the calcineurin inhibitor, CyA, significantly reduced TXNIP mRNA levels (P = 0.011) (data not shown) and TXNIP promoter activity (Fig. 4C). In addition, CyA led to decreased nuclear ChREBP levels (Fig. 4D), thereby mimicking the various verapamil effects. In contrast, KN-93 (a CaMK inhibitor) did not affect TXNIP expression (data not shown). Since nuclear ChREBP localization is dependent on its dephosphorylation, we also investigated the possibility that verapamil, by inhibiting the calcium-dependent PP2B/calcineurin, would increase ChREBP phosphorylation and decrease its nuclear localization. To obtain direct evidence of endogenous ChREBP phosphorylation, we used a specific phospho-ChREBP antibody detecting only ChREBP phosphorylated at Ser196, the site critical for controlling nuclear translocation (13) (gift of Dr. C. Postic, Paris, France). Indeed, these studies revealed a more than twofold increase in the level of ChREBP phosphorylation in response to verapamil (Supplementary Fig. 3). Together, these findings suggest that verapamil-mediated inhibition of TXNIP expression is conferred by reduction of intracellular calcium, inhibition of calcium-regulated PP2B/calcineurin signaling, and increased Ser196 phosphorylation and nuclear exclusion of ChREBP.
FIG. 4.
Changes in nuclear ChREBP in response to verapamil and CyA. INS-1 cells were incubated in the presence or absence of verapamil (50 μmol/L) for 24 h, and ChREBP levels were analyzed by Western blotting in whole cell extracts (A) or nuclear fractions (B) as detailed in Research Design and Methods. C: INS-1 cells were transfected with the FL-TXNIP promoter reporter plasmid (0.4 μg/well) together with pRL-TK control plasmid (20 ng/well) using DharmaFECT Duo transfection reagent (1 μL/well) and incubated in the presence or absence of the calcineurin inhibitor CyA (5 μmol/L) for 24 h. Bars represent luciferase activity (mean ± SEM) of three independent experiments. D: INS-1 cells were incubated in the presence or absence of CyA (5 μmol/L) for 24 h, and ChREBP levels were assessed in the nuclear fractions (n = 3).
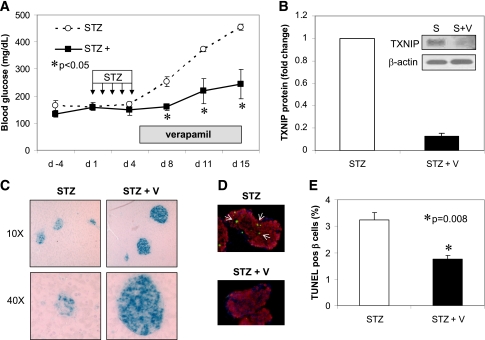
Oral verapamil administration prevents β-cell apoptosis and STZ-induced diabetes.
On the basis of our previous findings that TXNIP-deficient mice are protected against diabetes (2) and our current discovery that verapamil lowers β-cell TXNIP expression, we hypothesized that verapamil might protect against diabetes by promoting β-cell survival and preserving functional β-cell mass through inhibiting TXNIP expression. To test this hypothesis, we first used the diabetes model of multiple low-dose STZ injections. After completing the 5-day course of STZ, C57BL/6 mice were given oral verapamil in their drinking water as described previously (7,10) and blood glucose was followed for another 10 days. It is interesting that while control mice became overtly diabetic, reaching blood glucose levels >400 mg/dL, verapamil-treated mice maintained normal blood glucose levels (<250 mg/dL) (Fig. 5A). This protection was accompanied by a dramatic 80% reduction in TXNIP levels in isolated islets of verapamil-treated animals as assessed by Western blotting (Fig. 5B). In addition, immunohistochemistry of pancreas cross-sections showed severely disrupted islets in pancreata of STZ-treated mice, whereas normal insulin-containing islets were seen in the verapamil-treated mice (Fig. 5C). Moreover, β-cell apoptosis was significantly reduced in response to verapamil treatment as measured by TUNEL (Fig. 5D and E), suggesting that verapamil lowers proapoptotic TXNIP, promotes β-cell survival, and thereby prevents diabetes. These findings also are consistent with our results in genetic forms of TXNIP deficiency, including HcB-19 mice harboring a natural mutation in the TXNIP gene (whole body deficiency) and our β-cell specific TXNIP knockout mice (bTKO), both of which demonstrated decreased β-cell apoptosis and protection against STZ-induced diabetes. Of note, verapamil was well tolerated and did not lead to any weight loss or to any cardiovascular side effects, such as hypotension (Supplementary Figure 4A and data not shown, respectively).
FIG. 5.
Verapamil effects on STZ-induced diabetes and β-cell apoptosis. Male C57BL/6 mice received multiple low-dose STZ injections (40 mg/kg/day) for 5 days and then half the mice received verapamil (V) in their drinking water (100 mg/kg/day). A: Blood glucose (mean ± SEM) in STZ- and STZ + verapamil–treated mice (n = 3 mice per group). B: Verapamil effects on islet TXNIP protein levels in STZ-induced diabetic mice. S, STZ. C: Comparison of pancreas cross-section of STZ- and STZ + verapamil–treated mice (blue = insulin staining). D and E: β-Cell apoptosis as assessed by TUNEL in response to verapamil treatment in STZ-induced diabetic mice. pos, positive. White arrows point at apoptotic nuclei. In total, >2,000 β-cell nuclei were analyzed per group; bars represent means ± SEM. (A high-quality digital representation of this figure is available in the online issue.)
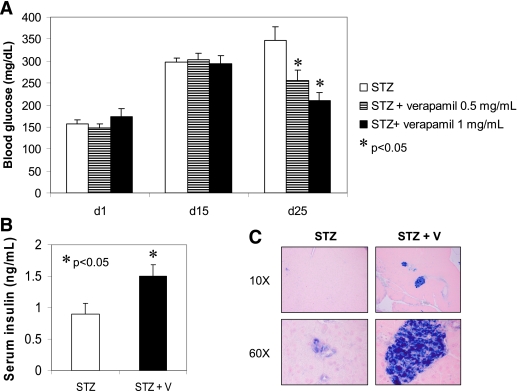
Oral verapamil improves STZ-induced diabetes and enhances β-cell survival and function.
To further address the question whether verapamil could reverse diabetes, not just prevent it, we performed an additional set of experiments in which we again induced diabetes in mice with multiple low-dose STZ injections but selected only the mice that actually had become overtly diabetic after 15 days (blood glucose >250 mg/dL) and only then started oral verapamil administration and followed mice for an additional 10 days. In addition, we also included an even lower dose of verapamil (0.5 mg/mL drinking water resulting in a dose of 50 mg/kg/day) to see whether the effect might be dose dependent. As shown in Fig. 6A, all three experimental groups were severely diabetic with blood glucose levels ∼300 mg/dL at day 15, and there was no difference between the groups; in addition, no significant difference in body weight was observed in response to verapamil or over the course of the experiment (Supplementary Fig. 4B). However, while diabetes progressed in the STZ control group, reaching blood glucose levels >350 mg/dL 25 days after STZ injection, low-dose verapamil significantly reduced blood glucose levels to 255 mg/dL, and high-dose verapamil even normalized blood glucose levels to ∼200 mg/dL, demonstrating that verapamil can improve overt diabetes and suggesting that the reduction in blood glucose levels is indeed dose dependent. Moreover, verapamil also led to a significant increase in serum insulin levels (Fig. 6B), and while healthy, insulin-containing islets were seen in the pancreas cross-sections of verapamil-treated mice, severe islet destruction was observed in STZ control mice (Fig. 6C). Together, this suggests that verapamil can enhance β-cell survival and function even in the face of severe diabetes.
FIG. 6.
Verapamil (V) effects on glucose homeostasis and β-cell function and survival in overtly diabetic mice. Male C57BL/6 mice received multiple low-dose STZ injections (40 mg/kg/day) for 5 days. At day 15, overtly diabetic mice (blood glucose >250 mg/dL) were selected to receive verapamil in their drinking water at a regular dose of 1 mg/mL or at a 50% lower dose of 0.5 mg/mL or to serve as controls. A: Blood glucose (mean ± SEM) in the three experimental groups. P values designate level of significance compared with the STZ control group (n = 4–8 mice per group). B: Serum insulin levels (mean ± SEM) in STZ-induced diabetic mice and STZ + verapamil–treated (0.5 mg/mL) mice (n = 6–7). C: Comparison of pancreas cross-section of STZ- and STZ + verapamil–treated mice (blue = insulin staining). (A high-quality digital representation of this figure is available in the online issue.)
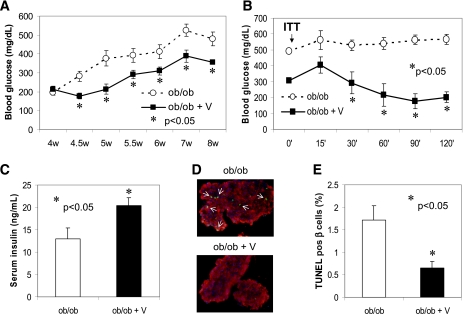
Oral verapamil administration promotes β-cell survival and improves glucose homeostasis in BTBR ob/ob mice.
To further evaluate whether verapamil also may have protective effects in the context of type 2 diabetes, we treated 4-week-old BTBR ob/ob mice with verapamil. Unlike on the C57BL/6 background, the leptin ob/ob mutation on the BTBR background leads to not only severe obesity and insulin resistance but also severe diabetes. It is interesting that while control ob/ob mice developed severe diabetes with blood glucose levels >500 mg/dL, oral verapamil significantly reduced blood glucose levels within a few days, and this difference was maintained throughout the experiment, even though verapamil was not able to completely protect against this very severe form of diabetes (Fig. 7A). This might be the result of some ongoing decrease in β-cell function in this diabetes model. Verapamil also led to a dramatic improvement in insulin sensitivity as assessed by ITTs (Fig. 7B)—and resulted in slightly reduced body weight (Supplementary Fig 5A)—which may contribute to the improved glucose homeostasis. However, verapamil also significantly increased serum insulin levels (Fig. 7C) and reduced β-cell apoptosis (Fig. 7D and E) even in the face of chronic hyperglycemia, suggesting again that enhanced β-cell survival and function was playing a major role in the observed effects. Similar to our findings in the STZ model, we found that our verapamil regimen reduced islet TXNIP levels by >70% in this model of obesity, insulin resistance, and type 2 diabetes (Supplementary Fig. 5B). Of note, our double mutant congenic BTBRlepob/obtxniphcb/hcb, representing a genetic model of TXNIP deficiency (HcB-19) combined with the BTBR ob/ob model of diabetes, also revealed decreased β-cell apoptosis and improved ITT and glucose homeostasis (2). Together with our current results, these findings strongly suggest that verapamil acts through TXNIP inhibition to promote β-cell survival and improve glucose homeostasis in this severe form of obesity-induced diabetes.
FIG. 7.
Verapamil (V) effects on obesity-induced diabetes and β-cell survival. BTBRlepob/ob mice, as a model of severe obesity, insulin resistance, and diabetes, received verapamil (1 mg/mL) in their drinking water starting at age 4 weeks. A: Blood glucose (mean ± SEM) in verapamil-treated and control ob/ob mice (n = 8–10 mice per group). W, weeks. B: ITTs were performed as described in research design and methods. Blood glucose (mean ± SEM) in verapamil-treated and control ob/ob mice is shown (n = 4). ■, verapamil-treated mice; ○, control ob/ob mice. C: Serum insulin levels (mean ± SEM) in 8-week-old ob/ob (white bar) and verapamil-treated ob/ob (black bar) mice (n = 4–5). D and E: β-Cell apoptosis as assessed by TUNEL in response to verapamil treatment in obesity-induced BTBR ob/ob mice. pos, positive. White arrows point at apoptotic nuclei. In total, >3,000 β-cell nuclei were analyzed per group; bars represent means ± SEM. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
The results of the current study identify for the first time an effective pharmacological means (i.e., calcium channel blockers) to inhibit pancreatic β-cell expression of proapoptotic TXNIP, enhance β-cell survival and function, and thereby prevent and even improve overt diabetes and shed new light on the mechanisms involved.
We initially identified TXNIP as a strongly glucose-induced gene in a human islet microarray study (1) and went on to show that β-cell TXNIP expression is upregulated in islets of diabetic mice and induces β-cell apoptosis (2,4). In fact, we found that TXNIP was critical for glucotoxicity-induced β-cell death (3). In addition, we discovered that genetic TXNIP deficiency (whole body/HcB-19 or β-cell-specific/bTKO) promotes endogenous β-cell mass and protects mice against STZ-induced as well as obesity-induced diabetes, suggesting that TXNIP inhibition represents an attractive target for the treatment of type 1 and type 2 diabetes (2). However, until now, effective TXNIP inhibitors were lacking. Our current studies reveal that calcium channel blockers such as verapamil provide an effective tool to reduce β-cell TXNIP expression, inhibit β-cell apoptosis, enhance β-cell survival and function, and rescue mice from diabetes. They thereby provide a proof of principle that pharmacological downregulation of TXNIP can protect against diabetes, which is consistent with the proapoptotic properties of TXNIP as well as with our findings in genetic models of TXNIP deficiency, and further establish TXNIP as a therapeutic target amenable to pharmacological intervention. Of interest, verapamil was effective in reducing TXNIP expression only when glucose levels were elevated, which is a very attractive feature that would allow normalization of TXNIP levels in diabetes but eliminate unwanted effects due to excessive reduction of TXNIP below physiological levels. Moreover, our results indicate that verapamil administration was able to not only prevent diabetes progression (Fig. 5) but also halt/delay diabetes development and significantly improve glucose homeostasis and β-cell survival and function in already diabetic mice (Fig. 6). This result is obviously very promising in terms of the possibilities of translating these findings into patients with diabetes.
Verapamil is a first-generation l-type calcium channel blocker and as such, reduces cellular calcium influx. Since β-cell insulin release is dependent on calcium influx, inhibition of calcium entry would, from a purely functional point of view, be expected to block β-cell insulin release. The significant increase in serum insulin levels we observed in verapamil-treated animals (Figs. 6 and 7) is therefore particularly impressive, and in the context of reduced β-cell apoptosis, this finding underlines the importance and the extent of functional β-cell preservation achieved with verapamil. On the other hand, in the severely insulin-resistant ob/ob mice, verapamil also improved insulin sensitivity, and this may contribute to the overall improved glucose homeostasis in this model and indirectly promote β-cell survival and insulin secretion (Fig. 7). Of note, this finding is consistent with our previous results in whole body TXNIP-deficient HcB-19 mice (2) and demonstrates that similar to genetic TXNIP deficiency, pharmacological downregulation of TXNIP seems to have additional extrapancreatic effects that are beneficial for improving glucose homeostasis. On the basis of reported effects of TXNIP in extrapancreatic tissues, these are likely to include increased muscle and adipose glucose uptake (14) and decreased hepatic glucose production (15).
Using promoter deletion studies, we discovered that verapamil mediates its TXNIP-lowering effects via a specific cis-acting element consisting of an E-box repeat in the TXNIP promoter (Fig. 2). We previously reported that this E-box motif serves as the binding site for ChREBP, which acts as a key transcription factor controlling β-cell TXNIP transcription (11). We now found in our ChIP studies that verapamil decreases in vivo binding of ChREBP to this TXNIP promoter region (Fig. 3A). We further observed that while total ChREBP levels remained unchanged by verapamil (Fig. 4A), nuclear ChREBP was significantly reduced (Fig. 4B). This is consistent with the notion that nuclear localization of ChREBP plays a key role in the regulation of gene transcription by this transcription factor (16,17). Nevertheless, constitutive overexpression of ChREBP was able to blunt the verapamil effects and thereby further confirmed the critical role this pathway plays for the observed TXNIP-lowering verapamil effects (Fig. 3C and D). Of note, this novel pathway seems to have implication beyond TXNIP because verapamil also reduced ChREBP binding to LPK, another known ChREBP target (Supplementary Fig. 2). In addition, our findings indicate that the calcium channel blocker effects on TXNIP expression are primarily mediated by a decrease in intracellular calcium because we found that they were not agent or class specific (diltiazem and verapamil), were not restricted to l-type calcium channel blockers (NiCl2), and could be mimicked by calcium chelators (EGTA) (Fig. 1A). Moreover, we observed that the calcineurin inhibitor CyA was able to effectively mimic the verapamil effects on TXNIP transcription and ChREBP nuclear localization. It is interesting that calcineurin signaling recently has also been implicated in β-cell survival (18). Taken together, we propose a novel signaling pathway by which verapamil reduces intracellular calcium, which in turn controls calcineurin signaling and results in increased phosphorylation and nuclear exclusion of ChREBP, decreased ChREBP binding to the TXNIP promoter, and the observed reduction in TXNIP expression and β-cell apoptosis.
In the present studies, we have combined mechanistic molecular studies, in vitro INS-1 experiments, and in vivo studies using two different mouse models of type 1 and type 2 diabetes as well as human islets and different doses of verapamil to elucidate the potential of pharmacological downregulation of proapoptotic TXNIP for the treatment of diabetes. Our genetic models of whole body and β-cell TXNIP deficiency reported previously (2) strongly support the role of TXNIP inhibition in the observed antidiabetic effects. Additional attractive features of this approach in regard to potential translation into clinical studies include the possibility for oral administration of verapamil or other calcium channel blockers and the fact that this class of medications has been widely used for many years, including by patients with diabetes and cardiovascular disease. Moreover, concerns about off-target effects and cardiac complications are dramatically reduced by the fact that calcium channel blockers are heart medications and, more important, we and others have demonstrated their beneficial effect specifically in diabetic cardiomyopathy (7,19). In addition, while the strongest effects are expected in tissues with high expression levels of l-type calcium channels, such as heart and β-cells, downregulation of TXNIP in other tissues seems also to be beneficial (14,15) and might be responsible for the improved insulin sensitivity we observed in BTBR ob/ob mice (Fig. 7B) as well as our previously reported double mutant congenic BTBRlepob/obtxniphcb/hcb (2).
On the other hand, we obviously also wondered why such potentially beneficial effects of calcium channel blockers in terms of diabetes control and progression have not been recognized in humans. In this regard, we have to remember that calcium channel blockers are antihypertensive drugs and most of the larger clinical studies focus on mortality and cardiovascular events as primary and secondary outcomes, rather than metabolic control. Indeed, in this cohort, verapamil has been shown to have beneficial effects in diabetic cardiomyopathy (19). In addition, while our data indicate that verapamil is capable of reversing diabetes progression in overtly diabetic mice, the effect is more pronounced when the treatment is initiated earlier in the disease progress. However, unlike ACE inhibitors, calcium channel blockers are not typically used as the first-line antihypertensive drug in patients with diabetes, resulting in a diabetic population taking verapamil that is skewed toward those with long-term disease where the intervention may be less effective. In future clinical studies, it therefore will be interesting to evaluate whether early initiation of a calcium channel blocker such as verapamil might be beneficial in preserving endogenous insulin secretion and preventing or halting disease progression especially in the hypertensive patient. In fact, two spin-off studies of the International Verapamil SR/Trandolapril Study (INVEST) reveal that newly diagnosed diabetes was less frequent in verapamil SR–treated patients as compared with atenolol-treated patients (P < 0.01) and that verapamil SR reduced the risk of new-onset diabetes especially in Hispanics (20,21). Obviously, these clinical findings strongly support our more mechanistic studies and suggest that verapamil-derived approaches may indeed provide clinically relevant outcomes in terms of diabetes prevention.
In summary, the results of the current studies provide the basis for future clinical trials using calcium channel blockers (or other TXNIP inhibitors) that promise to enhance β-cell survival and function and lead to better therapies for patients with type 1 and type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Juvenile Diabetes Research Foundation and JNJSI (40-2011-1), as well as by the American Diabetes Association (7-07-CD-22) and the National Institutes of Health (R01-DK-078752). No other potential conflicts of interest relevant to this article were reported.
G.X. performed in vitro studies, analyzed data, and helped write the manuscript. J.C. performed and analyzed in vivo and primary islet studies. G.J. performed some of the protein studies. A.S. designed the study, performed analyses, and wrote the manuscript. A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0955/-/DC1.
REFERENCES
- 1.Shalev A, Pise-Masison CA, Radonovich M, et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 2002;143:3695–3698 [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Hui ST, Couto FM, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J 2008;22:3581–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes 2008;57:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 2005;146:2397–2405 [DOI] [PubMed] [Google Scholar]
- 5.Minn AH, Pise-Masison CA, Radonovich M, et al. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem Biophys Res Commun 2005;336:770–778 [DOI] [PubMed] [Google Scholar]
- 6.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010;11:136–140 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Cha-Molstad H, Szabo A, Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab 2009;296:E1133–E1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minn AH, Lan H, Rabaglia ME, et al. Increased insulin translation from an insulin splice-variant overexpressed in diabetes, obesity, and insulin resistance. Mol Endocrinol 2005;19:794–803 [DOI] [PubMed] [Google Scholar]
- 9.Lan H, Rabaglia ME, Stoehr JP, et al. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 2003;52:688–700 [DOI] [PubMed] [Google Scholar]
- 10.Cohn RD, Durbeej M, Moore SA, Coral-Vazquez R, Prouty S, Campbell KP. Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex. J Clin Invest 2001;107:R1–R7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem 2009;284:16898–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem 2010;285:3997–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denechaud PD, Bossard P, Lobaccaro JM, et al. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J Clin Invest 2008;118:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chutkow WA, Patwari P, Yoshioka J, Lee RT. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J Biol Chem 2008;283:2397–2406 [DOI] [PubMed]
- 16.Davies MN, O’Callaghan BL, Towle HC. Glucose activates ChREBP by increasing its rate of nuclear entry and relieving repression of its transcriptional activity. J Biol Chem 2008;283:24029–24038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A 2001;98:9116–9121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soleimanpour SA, Crutchlow MF, Ferrari AM, et al. Calcineurin signaling regulates human islet beta-cell survival. J Biol Chem 2010;285:40050–40059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzal N, Ganguly PK, Dhalla KS, Pierce GN, Singal PK, Dhalla NS. Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes 1988;37:936–942 [DOI] [PubMed] [Google Scholar]
- 20.Cooper-Dehoff R, Cohen JD, Bakris GL, et al. ; INVEST Investigators Predictors of development of diabetes mellitus in patients with coronary artery disease taking antihypertensive medications (findings from the INternational VErapamil SR-Trandolapril STudy [INVEST]). Am J Cardiol 2006;98:890–894 [DOI] [PubMed] [Google Scholar]
- 21.Cooper-DeHoff RM, Aranda JM, Jr, Gaxiola E, et al. ; INVEST Investigators Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients—findings from the International Verapamil SR/Trandolapril Study (INVEST). Am Heart J 2006;151:1072–1079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.