Abstract
We have shown previously that stimulation of the angiotensin II type 2 receptor (AT2R) results in nerve facilitation. In this study, we determined the capacity of candesartan to correct expression patterns characteristic of neuropathy and AT2R-mediated neurite outgrowth in the fructose-induced insulin-resistant rat, which is one of the human hyperinsulinemia models. Wistar rats received a 15% (w/v) fructose solution in their drinking water for 4 weeks (fructose-drinking rats [FDRs]), with or without candesartan (5 mg/kg/day). We evaluated physiological and behavioral parameters and performed immunohistochemical studies. We found that the FDR developed insulin resistance and downregulated both AT2R neuronal function and phosphorylated Akt expression in dorsal root ganglia (DRG) neurons. Candesartan improved neurite outgrowth in the FDR, which was associated with the restoration of AT2R and phosphorylated Akt expression. Furthermore, downregulation of phosphoinositide 3-kinase (PI3K) inhibited AT2R-mediated neurite outgrowth in control DRG cells. PI3K activation increased AT2R-mediated neurite outgrowth and phosphorylated Akt expression in FDR DRG cells. These results suggest that the decrease of AT2R-mediated neurite outgrowth in FDRs is likely to be the result of decreased PI3K-dependent Akt activation. Candesartan improved AT2R neuronal function and Akt phosphorylation, which were associated with sensory nerve defects and insulin sensitivity in the FDR.
Angiotensin II (Ang II) has been shown to be involved in the development of insulin resistance. Current evidence indicates that Ang II can negatively modulate the muscle insulin signaling pathway, leading to reduced insulin-stimulated glucose uptake (1,2), GLUT4 translocation (3), and inhibition of the insulin signaling pathway through Ang II type 1 receptors (AT1Rs) (4). Ang II activates two major types of seven-transmembrane domain G protein–coupled receptors: the AT1R and Ang II type 2 receptors (AT2Rs). AT1Rs function as regulators of the cardiovascular system, oxidative stress, and cell proliferation, whereas AT2Rs mediate the opposite effect of AT1Rs such as vasodilation. AT2R mRNA is highly expressed in neonates but decreases after birth (5). However, in pathological conditions such as vascular injury or atherosclerosis, the expression of AT2Rs is significantly increased (6). The AT2R is known to mediate the effects of nerve regeneration. Many reports have demonstrated that AT2Rs are involved in neuronal differentiation of PC12 cells (7).
Diabetic neuropathy is known to be associated with hyperglycemia. We demonstrated previously that fructose-drinking rats (FDR) mimic human metabolic syndrome, develop hyperinsulinemia but not hyperglycemia, and suppress the function and innervation of calcitonin gene–related peptide (CGRP), which is a major neurotransmitter in sensory nerves and is produced in the dorsal root ganglia (DRG) (8,9). Moreover, previous studies in our laboratory have shown that activation of AT2Rs facilitates reinnervation of mesenteric perivascular CGRP-containing nerves in nerve-injured rats after phenol treatment (10). Given the neurite outgrowth function of AT2Rs, we hypothesized that in insulin-resistant rats, which have impaired peripheral nerve function, neurite outgrowth may be affected via the AT2R. The purpose of the current study was to ascertain whether depressed CGRP nerves after fructose-induced insulin resistance was related to AT2R inactivation and to determine whether treatment with the AT1R antagonist candesartan cilexetil improves this depressed response. In addition, we focused on the phosphoinositide 3-kinase (PI3K)-Akt signaling pathway. A study has shown that Akt phosphorylation has consistently shown to have a positive influence on neurite elongation (11). Therefore, we investigated whether insulin resistance affected AT2R-mediated neurite outgrowth, particularly focusing on the PI3K-Akt pathway.
RESEARCH DESIGN AND METHODS
All animal procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the Japanese association for laboratory animal science. All experiments were approved by the Animal Care and Use Committee of the Okayama University of Science. According to these guidelines, efforts were made to minimize the number of animals used and their discomfort.
Fructose-drinking rats.
According to a method described previously (8), 25 6-week-old Wistar rats (purchased from Shimizu Experimental Animals, Shizuoka, Japan) were given normal drinking water (control group), and 50 were given 15% (w/v) fructose solution (fructose group). The 15% (w/v) fructose solution was given as drinking water from 6 weeks of age to 10 weeks of age. For drug treatment, animals received a daily oral administration of candesartan cilexetil (5 mg/kg/day; a gift of Takeda Pharmaceutical, Okayama, Japan) with 15% (w/v) fructose solution.
Oral glucose tolerance test.
At 10 weeks of age, rats were fasted overnight and then given an oral administration of D-glucose (2 g/kg body weight dissolved in 0.9% [w/v] saline). At 0, 30, 120, and 180 min after glucose administration, blood samples were taken from the caudate vein. The plasma level of glucose and insulin were measured using a glucose analyzer (ACCU-CHEK Advantage glucose testing system; Roche Diagnostics, Indianapolis, IN) and an ELISA insulin kit (Morinaga Biochemistry, Kanagawa, Japan), respectively. This ultrasensitive rat sandwich ELISA kit is designed for detection of insulin (0.1–6.4 ng/mL) in plasma, serum, and cell culture supernatant.
Hot-plate test.
An animal was placed on an aluminum plate (45 × 30 cm) maintained at 55 ± 0.5°C. Rats were placed on the hot plate, and the time taken to either lick the fore or hind-paws or jump off the hot plate was recorded as the latency time. The latency to respond was measured using a stopwatch. A cutoff time (30 s) was set to avoid tissue damage.
Tail immersion test.
An animal was gently restrained, and the distal half of the tail was immersed into a water bath at 50°C and the withdrawal latency for tail flicking was recorded using a stopwatch. A cutoff time (15 s) was set to avoid tissue damage.
Immunohistochemistry.
Immunohistochemical studies were evaluated as reported previously (9,10). In brief, animals were treated with a large dose of sodium pentobarbital (50 mg/kg i.p.). The superior mesenteric artery was removed and immersion-fixed in Zamboni solution for 48 h. After fixation, the artery was repeatedly washed in 10 mmol/L PBS (pH = 7.4), immersed in PBS containing 0.5% (v/v) Triton X-100 overnight, and incubated with PBS containing normal goat serum (1:100) for 60 min. The tissue was then incubated with rabbit polyclonal anti-CGRP (Enzo Life Sciences, Farmingdale, NY) antiserum at a dilution of 1:300 for 72 h at 4°C. After incubation, the artery was washed in PBS and the sites of antigen-antibody reaction were detected by incubation with fluorescein-5-isothiocyanate (FITC)-labeled goat anti–rabbit IgG (diluted 1:100) (ICN Pharmaceuticals, Aurora, OH) for 60 min. Thereafter, the artery was observed under a confocal laser scanning microscope (CLSM510; Carl Zeiss, Tokyo, Japan) in Okayama University Medical School Central Research Laboratory.
Immunohistochemical analysis.
The immunostaining density of CGRP-like immunoreactive (CGRP-LI) nerve fibers was analyzed using a method described previously (10). All counts and measurements were performed by an investigator blinded to the source material, including the treatment of rats. For quantitative evaluation of CGRP-LI, confocal projection images of CGRP immunostaining, which consisted of 8–10 overlapping images (0.1 μm scanning) patched together, were magnified 20× and digitized as TIF images using a digital camera system (Olympus SP-1000; Olympus, Tokyo, Japan) and imported into a Windows XP computer (Toshiba, Tokyo, Japan). The stored digital images were analyzed using image-processing software (Simple PCI; Compix Imaging Systems, Cranberry Township, PA). The extraction of specific color and measured field commands were used to extract the CGRP-LI areas (which were stained green). Extraction of the signal was carried out using specific protocols based on the hue, lightness, and saturation color parameters. A measured field of 100 μm × 100 μm (10,000 μm2, which contained the adventitia layer [including immunostained perivascular nerve fibers]) was randomly selected on magnified images of the whole mount artery. The objective areas command was used to calculate the percentage of the CGRP-LI–positive area. The intensity of staining was estimated using a point-counting computer program, and the background level was subtracted from the experimental value to yield the corrected intensity. The average density in three arteries was taken as the nerve density per animal.
Preparation of DRG cultures.
Rat DRG neurons were isolated from 10-week-old rats. Under anesthesia with sodium pentobarbital (50 mg/kg i.p.), the DRG was dissected, isolated, and rapidly incubated in collagenase (5 mg/mL for 30 min at 37°C; Sigma Aldrich, Tokyo, Japan) followed by incubation for 30 min at 37°C with 1.25 mg/mL trypsin (Invitrogen, Tokyo, Japan) and 2.5 mg/mL collagenase. The tissue was resuspended PBS, collected by centrifugation, and mechanically dissociated by pipetting with Dulbecco’s modified Eagle’s medium (Gibco Invitrogen, Tokyo, Japan) containing 10% (v/v) fetal bovine serum (Gibco Invitrogen), 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco Invitrogen). The resulting cell suspensions were plated onto 22.2-mm culture dishes and maintained for 5 days at 37°C in a humidified incubator with 5% CO2 and air.
To examine neurite outgrowth on DRG cells, cells were treated in the absence or in the presence of CGP42112 (10 nmol/L; Sigma Aldrich), LY294002 (50 μmol/L; Sigma Aldrich), and YS49 (100 nmol/L and 1 μmol/L; Sigma Aldrich). The drugs were added to cells at 2 days in vitro for 4 consecutive days, and the medium was replaced every day.
Immunocytochemistry.
For immunocytochemistry, cells were fixed with 10% (v/v) formalin for 20 min and incubated with 0.5% (v/v) Triton X-100 for 10 min. After being washed with PBS, cells were incubated with 10% (v/v) normal goat serum containing 1% (w/v) BSA for 30 min at room temperature. Thereafter, cells were incubated with rabbit anti-CGRP (Enzo Life Sciences), diluted 1:500, and left overnight at 4°C. After being washed, cells were incubated for 60 min at room temperature with horseradish peroxidase–labeled goat anti–rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:1,000 in PBS containing 1% (w/v) BSA. After several washes in PBS, cells were incubated in diaminobenzidine solution.
To quantify neurite outgrowth in neurons, images of randomly chosen fields of neuron cultures were obtained with an Olympus IX50 camera (Olympus). To assess neurite number, a circle 120 μm in diameter was pulled around the perikaryon of a single neuron and the number of CGRP-positive neurites that crossed the drawn circle was counted.
Western blotting.
For Western blotting analysis, the DRG was isolated from the rat (thoracic spinal cord Th1–12) or primary FDR DRG neuron cultures, which were incubated with CGP42112 alone or CGP42112 + YS49 for 4 days and minced or detached in PBS. Samples (5 μg) were loaded on 10% (w/v) SDS-acrylamide gels. After transfer onto polyvinylidene difluoride membranes (Hybond P; GE Healthcare, Tokyo, Japan), membranes were blocked with blocking buffer consisting of Tris-buffered saline (TBS) containing 0.1% (v/v) Tween 20 (TBS-T) and 2.5% (v/v) membrane blocking agent (GE Healthcare) at room temperature for 1 h. The membranes were then probed overnight at 4°C with rabbit anti–AT2R polyclonal antibody (Santa Cruz Biotechnology; 1:500), rabbit antiphospho-Akt (Ser473) antibody (Cell Signaling Technology, Tokyo, Japan; 1:3,000), or rabbit anti–Akt antibody (Cell Signaling; 1:3,000) in blocking buffer. After the membranes were washed four times in TBS-T, the membranes were incubated with goat anti-rabbit horseradish peroxidase–linked IgG (Santa Cruz) (1:5,000) in blocking buffer for 1 h at room temperature. The polyvinylidene difluoride membranes were then washed four times with TBS-T, and the bound antibodies were detected using a chemiluminescent substrate kit (GE Healthcare). Bands were analyzed by densitometry using FluorchemTM8800 (α-Innotech, San Leandro, CA), and the content of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was detected using a rabbit anti–GAPDH antibody (1:10,000; Sigma), was used as a control to ensure that the same amount of protein was loaded in each lane.
Statistical analysis.
All data are expressed as the mean ± SEM. Comparisons between two values were analyzed using the Student t test. Analysis of variance followed by Tukey’s test was used to determine statistical significance where appropriate. A P value <0.05 was considered statistically significant.
RESULTS
Plasma glucose and insulin levels.
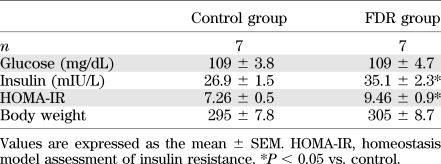
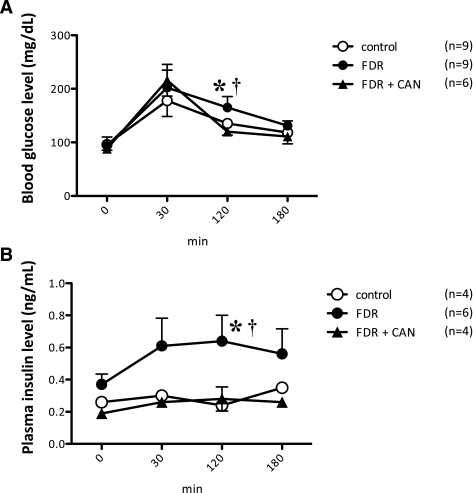
There was no significant difference in body weight and fasting blood glucose levels between the control and FDR groups (Table 1). However, plasma insulin levels were significantly higher in the FDR group when compared with the control (Table 1; P = 0.0060). The homeostasis model assessment insulin resistance also significantly increased in the FDR group (Table 1; P = 0.0319). These data indicate that fructose treatment induced insulin resistance as described previously (8,9). Blood glucose levels during the oral glucose tolerance test are shown in Fig. 1A. After the administration of glucose at 120 min, blood glucose levels in the FDR group were significantly higher than the control or FDR + CAN (AT1R antagonist candesartan cilexetil) group. Plasma insulin levels were also much higher in the FDR group when compared with the control and FDR + CAN group (Fig. 1B). These results indicate that the FDR group had hyperinsulinemia and that AT1R blockade induced the alleviation of hyperglycemia and hyperinsulinemia.
TABLE 1.
Physiological parameters determined in rats after administration of water (control) or 15% (w/v) fructose (FDR) for 4 weeks
FIG. 1.
Metabolic characteristics. Time course of blood glucose (A) and plasma insulin (B) levels during the oral glucose tolerance test. ○, control; ●, FDR; ▲, FDR + CAN. Each value indicates the mean ± SEM; n = 4–9. *P < 0.05 vs. control. †P < 0.05 vs. FDR + CAN.
Effect of AT1R blockade on thermal nociception and innervation of CGRP-LI fibers in mesenteric arteries.
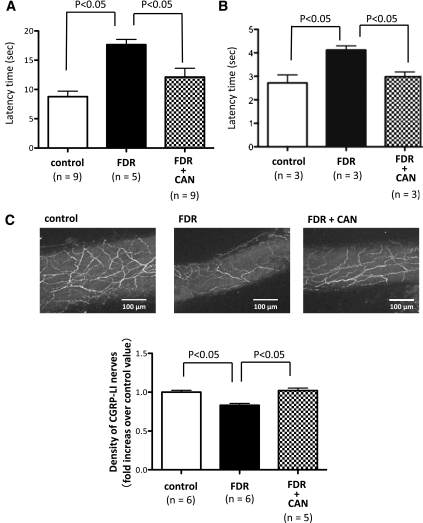
During the hot-plate test, licking or jumping responses were considered to be a result of supraspinal sensory integration (12). To test sensory performance at the supraspinal level, we measured the latency to respond when the hot plate was set at 55°C (Fig. 2A). The cutoff time was set at 30 s. Furthermore, the tail immersion test was performed for the assessment of thermal nociception (Fig. 2B). A significant increase in latency was observed in the FDR group compared with control rats in both tests, indicating that the FDR group had reduced sensitivity to noxious thermal stimuli. Administration of the AT1R antagonist candesartan cilexetil improved the response to thermal nociception in the FDR group.
FIG. 2.
AT1R blockade improved nociception responses and innervation of sensory nerve fibers. A: Nociceptive response of the control (white bar), FDR (black bar), and treatment with candesartan cilexetil FDR (FDR + CAN; checked bar) groups on the hot-plate test. Each bar indicates the mean ± SEM; n = 5–9. B: Nociceptive response of the control (white bar), FDR (black bar), and treatment with candesartan cilexetil FDR (FDR + CAN; checked bar) groups on the tail immersion test. Each bar indicates the mean ± SEM; n = 3. C: (Top panel) Representative confocal laser micrographs showing changes in the density of CGRP-like immunoreactive LI-containing nerve fibers in mesenteric arteries. A horizontal bar in the right lower corner of each panel indicates 100 µm. Bottom panel: Changes in the density of CGRP-containing nerve fibers in distal mesenteric arteries. Each bar indicates the mean ± SEM; n = 5 to 6.
Typical patterns of CGRP-LI nerve fibers in the small mesenteric arteries of control rats and the FDR group were observed. In addition, the distal mesenteric artery was densely innervated by CGRP-LI nerve fibers (Fig. 2C, top). The density of CGRP-LI nerve fibers in the FDR group was markedly decreased by ∼20% compared with age-matched control rats. In contrast, administration of the AT1R antagonist candesartan cilexetil increased the density of CGRP-LI nerve fibers to control levels (Fig. 2C, bottom). Thus AT1R inhibition in the FDR group includes both an improvement in sensory peripheral neuropathy and prevents a decrease of CGRP-LI nerve fibers.
Effect of AT1R blockade on AT2R expression and function in DRG cells.
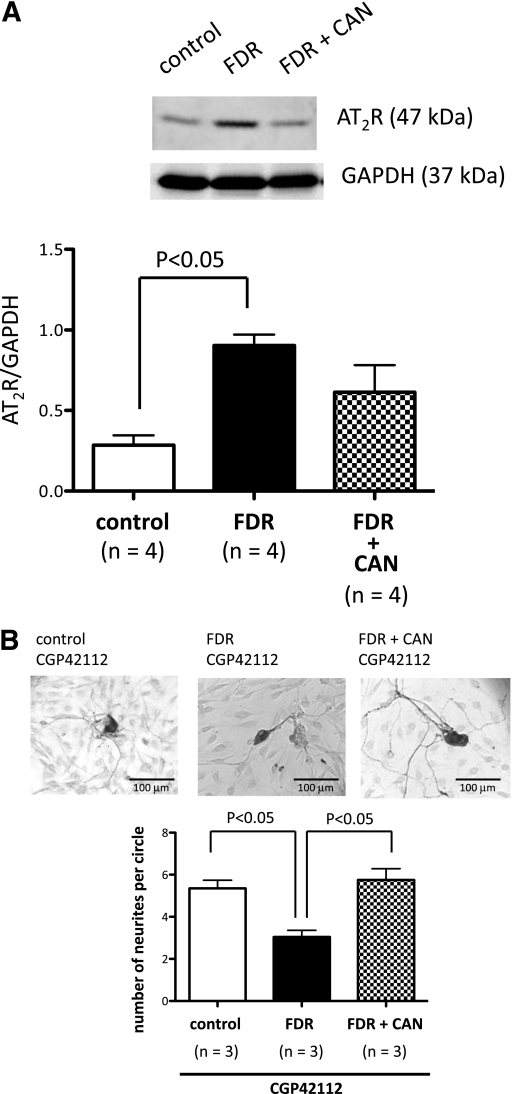
We next questioned why AT1R blockade improved peripheral nerve disorder in the FDR group. For this, we focused on AT2R expression and function in the DRG isolated from control rats, the FDR group, and FDR + CAN. According to our previous report (10), AT2R stimulation facilitated reinnervation of mesenteric perivascular CGRP containing nerves in rats. AT2Rs were detected as membrane proteins with a molecular weight of 47 kDa (Fig. 3A, top). In the FDR group, AT2R expression was significantly higher than control rats. Candesartan cilexetil administration led to the suppression of high AT2R expression (Fig. 3A, bottom). To determine the function of AT2Rs in the DRG, we examined whether AT2R activation could affect the neuronal sprouting response in DRG cells from the control, FDR, and FDR + CAN groups. Immunostaining showed CGRP-LI–positive neurons and neurite outgrowth (Fig. 3B, top). To activate the AT2R, we used 10 nmol/L CGP42112, which is a selective agonist of AT2Rs. The representative images showed different neurite growth patterns after 4 days of treatment with CGP42112. In the DRG of the FDR group, AT2R activation did not increase neurite outgrowth compared with the DRG of the control group, whereas DRG cells isolated from the FDR + CAN group showed an increase in neurite outgrowth similar to control DRG cells (Fig. 3B, bottom).
FIG. 3.
Effect of AT1R blockade on AT2R expression and function in DRG neurons. A: Representative Western blots of AT2R and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from DRG cells isolated from control, FDR, and treatment with candesartan cilexetil FDR (FDR + CAN) groups. Data are the mean ± SEM of normalized densitometry measurements from Western blots of AT2R compared with GAPDH (n = 4). B: (Top panel) Representative images showing neurite outgrowth of CGRP-immunopositive neurons in the control, FDR, and FDR + CAN groups after AT2R activator CGP42112 (10 nmol/L) treatment. A horizontal bar in the right lower corner of each panel indicates 100 µm. Bottom panel: Bar graph shows the effect of treatment with CGP42112 on synapse formation in DRG cells. Each bar indicates the mean ± SEM of three independent experiments.
Effect of the PI3K inhibitor on AT2R-mediated neurite outgrowth in cultured control DRG neurons.
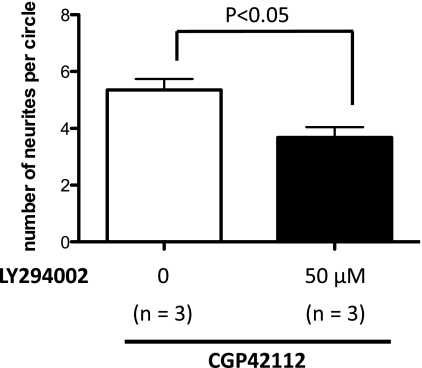
Next, we asked if insulin resistance induces the downregulation of AT2R function. We focused on the PI3K-Akt pathway. Because we found AT2R-mediated neurite outgrowth function was downregulated in the DRG of the FDR group, we examined whether PI3K inhibition could affect AT2R function in control rat DRG cells. Treatment with the PI3K inhibitor LY294002 (50 μmol/L) significantly inhibited neurite elongation after AT2R activation with CGP42112 similar to AT2R-mediated neurite outgrowth in cultured FDR DRG cells (Fig. 4; P = 0.0013).
FIG. 4.
PI3K inhibition abolished AT2R-mediated neurite outgrowth in cultured control rat DRG neurons. Primary control rat DRG neuron cultures were incubated with the AT2R activator CGP42112 (10 nmol/L; white bar) only or CGP42112 + the PI3K inhibitor LY294002 (50 μmol/L; black bar) for 4 days. The bar graph represents the change in mean neurite length after treatment with each drug. Each bar indicates the mean ± SEM of three independent experiments.
Insulin resistance downregulates Akt activity via a PI3K-dependent pathway in DRG neurons.
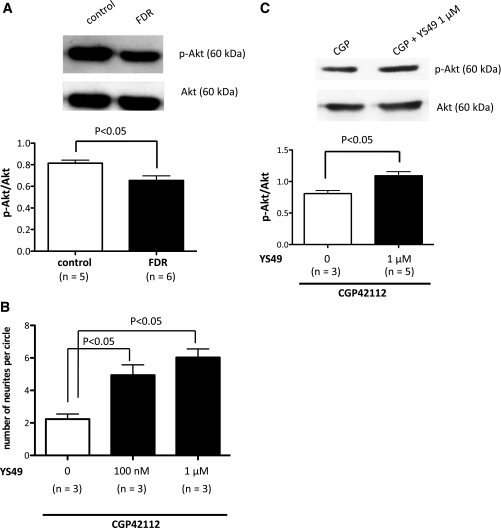
Because PI3K inhibition, which results in insulin resistance (13), was able to inhibit the function of AT2Rs, we next aimed to determine the mechanism. Akt is activated by insulin or various growth factors to function in a PI3K-sensitive pathway. Therefore, we examined the expression of phosphorylated Akt in control rats and FDR DRG neurons. We found that expression of phosphorylated Akt in FDR cells was significantly reduced by ∼20% when compared with control rats (Fig. 5A; P = 0.0079).
FIG. 5.
Insulin resistance downregulates Akt activity via a PI3K-dependent pathway in DRG neurons. A: Representative Western blots of Akt phosphorylation (p-Akt) and Akt isolated from DRG cells of the control (white bar) and FDR (black bar) groups. Data are the mean ± SEM of normalized densitometry measurements from Western blots of p-Akt compared with Akt (n = 5 to 6). B: Primary FDR DRG neuron cultures were incubated with the AT2R activator CGP42112 (10 nmol/L) only (white bar) or CGP42112 + the PI3K activator YS49 (100 nmol/L or 1 μmol/L; black bars) for 4 days. The bar graph represents the change in mean neurite length after treatment with each drug. Each bar indicates the mean ± SEM of three independent experiments. C: Representative Western blots of p-Akt and Akt from primary FDR DRG neuron cultures, which were incubated with CGP42112 (10 nmol/L) alone (white bar) or CGP42112 + YS49 (1 μmol/L; black bar) for 4 days. Data are the mean ± SEM of normalized densitometry measurements from Western blots of p-Akt compared with Akt (n = 3–5).
To confirm the involvement of PI3K, we examined the effect of the PI3K activator YS49 (100 nmol/L and 1 μmol/L) on AT2R-mediated neurite outgrowth in cultured DRG neurons isolated from the FDR group. The PI3K activator YS49 (100 nmol/L and 1 μmol/L) slightly increased neurite outgrowth after AT2R activation with CGP42112 (10 nmol/L) in cultured DRG cells isolated from the FDR group (Fig. 5B). Furthermore, the level of phosphorylated Akt expression in cultured FDR DRG cells subjected to AT2R activation using CGP42112 (10 nmol/L) in the presence of the PI3K activator YS49 (1 μmol/L) was significantly increased by ∼35% in its absence (Fig. 5C; P = 0.0112).
AT1R blockade increases phosphorylated Akt expression in DRG cells.
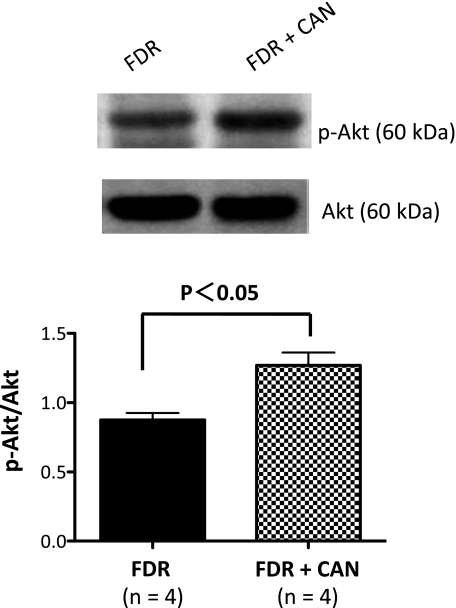
We next determined whether blockade of the AT1R prevented a decrease in phosphorylated Akt in the FDR group. Our results showed that DRG cells isolated from the FDR group had reduced expression of phosphorylated Akt compared with the control (see Fig. 5A). Administration of the AT1R antagonist candesartan cilexetil in the FDR group significantly increased phosphorylated Akt expression by ∼44% (Fig. 6; P = 0.0453). Based on these results, we propose that blockade of the AT1R induces phosphorylation of Akt and improves AT2R function.
FIG. 6.
AT1R blockade increases Akt phosphorylation (p-Akt) expression in the DRG. Representative Western blots of p-Akt and Akt isolated from the DRG of the FDR (black bar) and FDR + CAN (checked bar) groups are shown. Data are the mean ± SEM of normalized densitometry measurements from Western blots of p-Akt compared with Akt (n = 4).
DISCUSSION
In the current study, we demonstrated that insulin resistance induced peripheral sensory nerve defects, delayed the response to noxious thermal stimuli, and reduced the density of CGRP-LI nerve fibers in mesenteric arteries, which were improved after administration of the AT1R antagonist candesartan cilexetil. We also showed that insulin resistance resulted in an increase of AT2R expression and dysfunction, which is related to depressed PI3K-dependent Akt activation.
In the current study, we used FDR, which have been established as an animal model of insulin resistance, and it mimics human metabolic syndrome in many aspects, including hyperinsulinemia and hypertriglyceridemia (14). In our protocol, 6-week-old rats were given 15% (w/v) fructose solution with or without candesartan cilexetil for 4 weeks. At 10 weeks, when compared with age-matched control rats, fasting blood glucose levels were not different but plasma insulin levels were significantly higher in the FDR group (see Table 1). Moreover, during the oral glucose tolerance test, increases in blood glucose and insulin levels were reduced to control rat levels after candesartan cilexetil treatment. These findings suggest that AT1R blockade improved insulin sensitivity in fructose-induced insulin-resistant rats.
Hyperglycemia is known to contribute significantly to damage of nerve tissue in diabetes by the increased flux of glucose through the sorbitol/aldose reductase pathway. However, as shown by our present study, thermal hypoalgesia and a decrease of CGRP-LI sensory nerve innervation were observed in the FDR group, which had hyperinsulinemia but not hyperglycemia. Our laboratory has reported previously a reduction in microvascular function of rat mesenteric arteries and perivascular nerve dysfunction in FDR (9). Moreover, recent evidence demonstrated that obese Zucker diabetic fatty rats, a model for type 2 diabetes, developed vascular and neural impairment independently of hyperglycemia (15). Consistent with this, the current study suggests that not only hyperglycemia but also insulin resistance may contribute to diabetes-like neuropathy.
In the current study, we did not measure plasma triglycerides level. There is one possibility that hyperlipidemia could be associated with the insulin resistance–mediated nerve dysfunction through circulating. Another related study has demonstrated that irbesartan (AT1R blocker) reduced triglycerides level in the insulin-resistant rat (16) but another study has shown that olmesartan (AT1R blocker) did not influence the plasma triglycerides level in Zucker fatty rats (17). Further study will be needed to clarify the effect of candesartan treatment on triglycerides level and whether hyperlipidemia is involved in sensory nerve defect by insulin resistance or not.
The AT2R is known to mediate the effects of nerve regeneration. Our previous report suggested that AT2R activation facilitated the density of CGRP-LI nerves in rat mesenteric arteries (10). In the current study, we showed that insulin resistance induced the impairment of AT2R-mediated neurite outgrowth function. Of interest, treatment with the PI3K activator YS49 resulted in recovered downregulation of AT2R neuronal function in insulin-resistant FDR DRG neurons. Furthermore, the PI3K activator YS49 increased phosphorylation of Akt with AT2R activation in FDR DRG neurons. These results suggest that AT2R-mediated neurite outgrowth function may be required for PI3K and Akt activation. However, Cui et al. (18) demonstrated that AT2R activation inhibited insulin-induced PI3K activation and Akt phosphorylation inducing apoptosis in PC12 cells. The discrepancy between their results and ours may be caused by differences in experimental conditions (cell line or primary cell culture) and/or AT2R function (apoptosis or neurite outgrowth).
We have demonstrated for the first time that candesartan cilexetil treatment improves the hypoalgesic response to a thermal stimulus, innervation of CGRP-LI sensory nerves, and AT2R downregulation in a rat model of fructose-induced insulin resistance. In both clinical and experimental studies, AT1R antagonists have been shown to improve insulin resistance (19–22). Ang II is a well-known inhibitor of insulin signaling at various levels, including insulin receptor substrates and PI3K-Akt in vascular smooth muscle cells or skeletal muscle (19,23,24). Another related study demonstrated that candesartan treatment restored insulin-mediated PI3K/Akt phosphorylation in Dahl salt-sensitive rat aortas (25). Moreover, the PI3K-Akt signaling pathway promotes neurite elongation in PC12 cells (26,27). Our data showed that administration of candesartan cilexetil increased the expression of phosphorylated Akt in FDR DRG neurons to control levels. These results suggest that PI3K-dependent Akt expression plays an important role in AT1R-mediated blockade of neuronal improvement.
However, the exact mechanism of how AT1R blockade restores AT2R expression and function in FDR remains unclear. You et al. (28) showed that candesartan treatment restored AT2R expression and vasodilator function in spontaneously hypertensive rats. One possible mechanism involves blockade of the AT1R, which leaves the AT2R open to stimulation by Ang II, suggesting that preventing the action of Ang II via the AT1R allows free AT2Rs to respond to Ang II during neurite outgrowth. AT2Rs have two neuronal differentiation pathways; one is the NO-cGMP-PKG pathway and the other is the Rap-Raf-MEK-ERK cascade (29). Akt is known to regulate the phosphorylation of Raf (30). Our data showed an increase of AT2R expression, but function was attenuated in FDR, indicating that FDR had an impaired AT2R signaling cascade. Therefore, we focused on PI3K-Akt pathway. Based on our present data demonstrating increasing Akt phosphorylation in DRG neurons after candesartan treatment, we propose that Akt activity induces the improvement of the AT2R neuronal elongation pathway (Raf-MEK-ERK) and thus improves AT2R-mediated neurite outgrowth and peripheral nerve disorders in FDR.
In conclusion, AT2R-mediated neurite outgrowth function was attenuated in fructose-induced insulin-resistant rats. The AT1R blocker candesartan cilexetil improved sensory nerve defects and insulin sensitivity in the FDR group, which was associated with the restoration of Akt activation and AT2R function.
ACKNOWLEDGMENTS
This study was supported in part by a grant-in-aid for Scientific Research (KAKENHI) (No. 21770192) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
No potential conflicts of interest relevant to this article were reported.
N.H.-H. designed and conducted experiments, analyzed data, and wrote the manuscript. N.H. analyzed the data, contributed to discussions, and reviewed the manuscript. Y.I. performed animal treatments. H.S. provided technical assistance. Y.Z. analyzed the data and contributed to discussions. S.T. and H.K. reviewed the manuscript. N.H.-H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Shinonaga of Edanz Corporation and American Journal Experts for help in the preparation of the manuscript.
REFERENCES
- 1.Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 1997;100:2158–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiuchi T, Iwai M, Li HS, et al. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 2004;43:1003–1010 [DOI] [PubMed] [Google Scholar]
- 3.Wei Y, Sowers JR, Nistala R, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 2006;281:35137–35146 [DOI] [PubMed] [Google Scholar]
- 4.Igarashi M, Hirata A, Nozaki H, Kadomoto-Antsuki Y, Tominaga M. Role of angiotensin II type-1 and type-2 receptors on vascular smooth muscle cell growth and glucose metabolism in diabetic rats. Diabetes Res Clin Pract 2007;75:267–277 [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam S, Corvol P, Gasc JM. Angiotensin II type 2 receptor mRNA expression in the developing cardiopulmonary system of the rat. Hypertension 1996;28:91–97 [DOI] [PubMed] [Google Scholar]
- 6.Sales VL, Sukhova GK, Lopez-Ilasaca MA, Libby P, Dzau VJ, Pratt RE. Angiotensin type 2 receptor is expressed in murine atherosclerotic lesions and modulates lesion evolution. Circulation 2005;112:3328–3336 [DOI] [PubMed] [Google Scholar]
- 7.Stroth U, Meffert S, Gallinat S, Unger T. Angiotensin II and NGF differentially influence microtubule proteins in PC12W cells: role of the AT2 receptor. Brain Res Mol Brain Res 1998;53:187–195 [DOI] [PubMed] [Google Scholar]
- 8.Takatori S, Zamami Y, Yabumae N, et al. Pioglitazone opposes neurogenic vascular dysfunction associated with chronic hyperinsulinaemia. Br J Pharmacol 2008;153:1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamami Y, Takatori S, Hobara N, et al. Hyperinsulinemia induces hypertension associated with neurogenic vascular dysfunction resulting from abnormal perivascular innervations in rat mesenteric resistance arteries. Hypertens Res 2011;34:1190–1196 [DOI] [PubMed] [Google Scholar]
- 10.Hobara N, Goda M, Yoshida N, et al. Angiotensin II type 2 receptors facilitate reinnervation of phenol-lesioned vascular calcitonin gene-related peptide-containing nerves in rat mesenteric arteries. Neuroscience 2007;150:730–741 [DOI] [PubMed] [Google Scholar]
- 11.Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci 2009;66:2975–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther 1953;107:385–393 [PubMed] [Google Scholar]
- 13.Zawalich WS, Zawalich KC. A link between insulin resistance and hyperinsulinemia: inhibitors of phosphatidylinositol 3-kinase augment glucose-induced insulin secretion from islets of lean, but not obese, rats. Endocrinology 2000;141:3287–3295 [DOI] [PubMed] [Google Scholar]
- 14.Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr 1989;49:1155–1163 [DOI] [PubMed] [Google Scholar]
- 15.Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab 2005;289:E113–E122 [DOI] [PubMed] [Google Scholar]
- 16.Russell JC, Kelly SE, Vine DF, Proctor SD. Irbesartan-mediated reduction of renal and cardiac damage in insulin resistant JCR : LA-cp rats. Br J Pharmacol 2009;158:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ran J, Hirano T, Adachi M. Angiotensin II type 1 receptor blocker ameliorates overproduction and accumulation of triglyceride in the liver of Zucker fatty rats. Am J Physiol Endocrinol Metab 2004;287:E227–E232 [DOI] [PubMed] [Google Scholar]
- 18.Cui TX, Nakagami H, Nahmias C, et al. Angiotensin II subtype 2 receptor activation inhibits insulin-induced phosphoinositide 3-kinase and Akt and induces apoptosis in PC12W cells. Mol Endocrinol 2002;16:2113–2123 [DOI] [PubMed] [Google Scholar]
- 19.Higashiura K, Ura N, Miyazaki Y, Shimamoto K. Effect of an angiotensin II receptor antagonist, candesartan, on insulin resistance and pressor mechanisms in essential hypertension. J Hum Hypertens 1999;13(Suppl. 1):S71–S74 [DOI] [PubMed] [Google Scholar]
- 20.Clasen R, Schupp M, Foryst-Ludwig A, et al. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension 2005;46:137–143 [DOI] [PubMed] [Google Scholar]
- 21.Pscherer S, Heemann U, Frank H. Effect of renin-angiotensin system blockade on insulin resistance and inflammatory parameters in patients with impaired glucose tolerance. Diabetes Care 2010;33:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suksomboon N, Poolsup N, Prasit T. Systematic review of the effect of telmisartan on insulin sensitivity in hypertensive patients with insulin resistance or diabetes. J Clin Pharm Ther. 17 August 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol 2005;25:1142–1147 [DOI] [PubMed] [Google Scholar]
- 24.Csibi A, Communi D, Müller N, Bottari SP. Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS ONE 2010;5:e10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou MS, Schulman IH, Raij L. Role of angiotensin II and oxidative stress in vascular insulin resistance linked to hypertension. Am J Physiol Heart Circ Physiol 2009;296:H833–H839 [DOI] [PubMed] [Google Scholar]
- 26.Kimura K, Hattori S, Kabuyama Y, et al. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem 1994;269:18961–18967 [PubMed] [Google Scholar]
- 27.Kim Y, Seger R, Suresh Babu CV, Hwang SY, Yoo YS. A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol Cells 2004;18:353–359 [PubMed] [Google Scholar]
- 28.You D, Loufrani L, Baron C, Levy BI, Widdop RE, Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation 2005;111:1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendron L, Payet MD, Gallo-Payet N. The angiotensin type 2 receptor of angiotensin II and neuronal differentiation: from observations to mechanisms. J Mol Endocrinol 2003;31:359–372 [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 1999;286:1741–1744 [DOI] [PubMed] [Google Scholar]