Abstract
Induction of heat shock protein (HSP) 72 improves metabolic profiles in diabetic model mice. However, its effect on pancreatic β-cells is not known. The current study investigated whether HSP72 induction can reduce β-cell stress signaling and apoptosis and preserve β-cell mass. MIN6 cells and db/db mice were sham-treated or treated with heat shock (HS) and mild electrical stimulation (MES) (HS+MES) to induce HSP72. Several cellular markers, metabolic parameters, and β-cell mass were evaluated. HS+MES treatment or HSP72 overexpression increased HSP72 protein levels and decreased tumor necrosis factor (TNF)-α–induced Jun NH2-terminal kinase (JNK) phosphorylation, endoplasmic reticulum (ER) stress, and proapoptotic signal in MIN6 cells. In db/db mice, HS+MES treatment for 12 weeks significantly improved insulin sensitivity and glucose homeostasis. Upon glucose challenge, a significant increase in insulin secretion was observed in vivo. Compared with sham treatment, levels of HSP72, insulin, pancreatic duodenal homeobox-1, GLUT2, and insulin receptor substrate-2 were upregulated in the pancreatic islets of HS+MES-treated mice, whereas JNK phosphorylation, nuclear translocation of forkhead box class O-1, and nuclear factor-κB p65 were reduced. Apoptotic signals, ER stress, and oxidative stress markers were attenuated. Thus, HSP72 induction by HS+MES treatment protects β-cells from apoptosis by attenuating JNK activation and cell stresses. HS+MES combination therapy may preserve pancreatic β-cell volume to ameliorate glucose homeostasis in diabetes.
Type 2 diabetes (T2D) is one of the major causes of morbidity and mortality worldwide (1). Insulin resistance and pancreatic β-cell dysfunction are the main pathophysiologic features of T2D. Current lines of evidence suggest that visceral fat accumulation is strongly associated with the pathogenesis of metabolic syndrome (2) and also with T2D and insulin resistance (3).
Cellular stresses, such as oxidative stress and endoplasmic reticulum (ER) stress, have been considered to be critical factors that cause or worsen insulin resistance as well as β-cell dysfunction in T2D (4–7). These stress pathways can be activated by metabolic alterations in diabetes and metabolic syndrome, resulting in augmentations to further deteriorate metabolic abnormalities. Recent studies have shown that obesity, compromising ER function, results in insulin resistance and T2D that are partially dependent on Jun NH2-terminal kinase (JNK) activation (8).
Heat shock protein (HSP) 72 is a major inducible molecular chaperone and plays central roles in protein synthesis, folding, refolding, and transport (9). Constitutive overexpression of HSP72 blocks the apoptotic cell death initiated by cellular stresses such as heat shock (HS), ceramide, ethanol, ionizing irradiation, tumor necrosis factor-α (TNF-α), and ischemia (10). Whole-body hyperthermia causes HSP72 overexpression in the heart, resulting in phosphatidylinositol 3-kinase (PI-3 K)–dependent activation of Akt in association with protection against cardiac ischemia–reperfusion injury (11). Hyperthermia is also known to activate Akt in PI-3K–dependent and –independent manners (12).
Decreased expression of HSP72 in the skeletal muscle of T2D patients has been reported, and this reduction is correlated with the degree of insulin resistance (13–15). In fact, induction of HSP72 by any means, such as whole-body hyperthermia, transgenic overexpression of HSP72 in muscle, or administration of an HSP72 coinducer, is beneficial for treating hyperglycemia in diabetic humans and animal models (15–20). Indeed, HSP72 has been postulated to attenuate the activation of the JNK pathway, which is involved in the pathogenesis of both insulin resistance and β-cell failure (15,21). Moreover, we previously reported that the combination of heat shock (HS) and mild electrical stimulation (MES) (HS+MES) ameliorates insulin resistance in high-fat-fed diabetic mice (22,23). In addition to hyperthermia, MES directly activates Akt in muscle cells (24). This combined HS+MES therapy significantly reduces visceral fat accumulation and ameliorates glucose homeostasis in high-fat-fed mice with restoration of insulin signaling (22).
In this study, we used MIN6 cells and db/db mice to assess whether HSP72 induction by HS+MES treatment can improve β-cell function in vitro and in vivo. Our results showed that HS+MES significantly increased HSP72 protein levels in MIN6 cells and reduced JNK activation, ER stress, and the proapoptotic signal induced by TNF-α. Furthermore, HS+MES treatment increased the insulin contents, reduced apoptotic signals, and reduced cellular stress markers, in β-cells of db/db mice. Thus, induction of HSP72 by HS+MES treatment may protect pancreatic β-cells against apoptosis through inhibition of JNK and ameliorate glucose homeostasis in diabetes.
RESEARCH DESIGN AND METHODS
Animals.
Six-week-old male db/db mice (BKS.Cg-m+/+Leprdb/J: Leprdb/Leprdb mice) or wild-type (WT) littermates were obtained from Charles River Laboratories Inc. (Kanagawa, Japan) and housed in a vivarium, in accordance with the guidelines of the Kumamoto University Animal Facility Center. The mice were maintained on standard chow and water ad libitum. All procedures were approved by the Kumamoto University Animal Care and Use Committee.
HS+MES treatment.
The HS+MES treatment was performed as described in elsewhere (22). Briefly, mice were treated with or sham-treated with HS+MES twice a week for 12 weeks. HS (42°C)+MES (0.6 V/cm, 55 pulses/s, 0.1-ms duration) were delivered to the mice through rubber pads with a Biometronome generator (Tsuchiya Gum Co., Ltd., Kumamoto, Japan).
Glucose tolerance test.
An intraperitoneal glucose tolerance test (IPGTT) was performed as described elsewhere (22).
Immunohistochemistry.
Antibodies (summarized in Supplementary Data 1) were used to investigate individual protein expression by immunohistochemistry of frozen pancreatic sections. The practical method of immunohistochemical analysis was described elsewhere (22). The insulin-positive areas and islet sizes were evaluated using a BZ-II Analyzer (Keyence). At least 20 fields from three animals were randomly examined.
Laser capture microdissection and total RNA isolation.
Pancreatic sections were prepared at 10-μm thickness using a cryostat (Leica, Wetzlar, Germany) and mounted on RNase-free treated glass slides. For laser capture microdissection (LCM), a HistoGene LCM Frozen Section Staining Kit (Arcturus, Mountain View, CA) was used to stain the tissue, and the islets were irradiated with a laser using the PixCell system (Arcturus). The peripheral area was removed, and the β-cell–rich core area was collected. For each specimen, at least 500 hits were used to obtain enough RNA for amplification.
Quantitative real time RT-PCR.
Total RNA was extracted from LCM dissected islets using NucleoSpin micro RNA (MACHEREY-NAGEL, Düren, Germany). The first-strand complementary DNA (cDNA) synthesis from 1 μg total RNA was primed with oligo (dT) (Takara, Tokyo, Japan). A LightCycler System (Roche Diagnostics, Meylan, France) was used to quantify the transcripts. The quantitative results for these messenger RNA (mRNA) levels were normalized by the β-actin mRNA levels. The sequence of primers used is indicated in Supplementary Data 2.
Insulin content.
To assess the insulin content, the pancreas was rapidly removed, homogenized, and extracted in acid ethanol overnight at 4°C. The insulin contents were measured by using an enzyme-linked immunosorbent assay (ELISA) kit (Linco Research Inc., St. Charles, MO).
Culture of MIN6 cells.
MIN6 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 25 mmol/L glucose, 15% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 5 μL/L β-mercaptoethanol at 37°C and 5% CO2. After reaching 60–80% confluence, the cells were treated with MES (0.6 V/cm, 55 pulses/s, 0.1-ms duration) and HS (42°C) for 10 min (22,23). At 10 h after the treatment, whole cell lysates were extracted.
TNF-α treatment and HSP72 overexpression in MIN6 cells.
MIN6 cells were treated with MES and/or HS for 10 min. At 10 h after the treatment, the cells were incubated with or without recombinant mouse TNF-α (25 ng/mL) for 6 h, and then lysed. The lysates were extracted for Western blotting. The relative intensities of the protein expression levels were analyzed using Image J software (National Institutes of Health, Bethesda, MD). For HSP72 overexpression, an Hsp72 cDNA-containing plasmid (22) was transfected using TransIT-LT1 (Takara, Tokyo, Japan).
Statistical analyses.
Quantitative data are presented as the means ± SD of at least three independent experiments. Statistical analyses were based on the Student t test for paired or unpaired data, as appropriate. Comparisons with multiple groups were assessed with ANOVA. Values of P < 0.05 were considered to indicate statistical significance.
RESULTS
HS+MES treatment increases HSP72 protein and decreases phospho-JNK, ER stress, and proapoptotic signal in MIN6 cells.
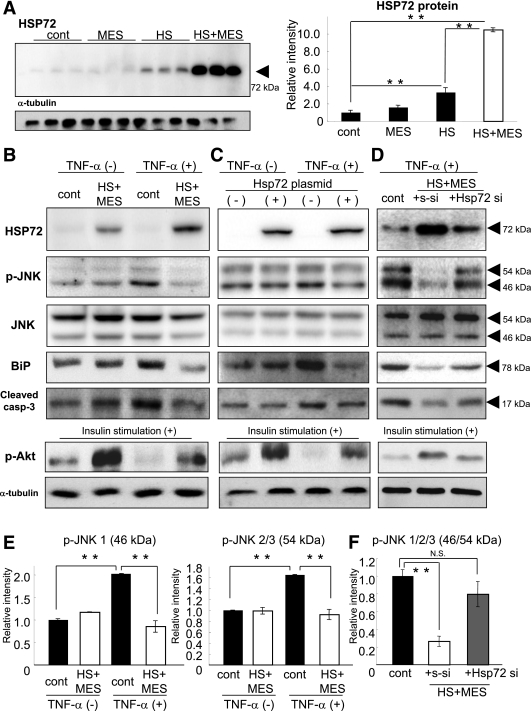
To examine the effects of HS+MES on pancreatic β-cells, we determined the HSP72 protein levels in MIN6 cells at 10 h after a 10-min treatment with HS (42°C) and/or MES (0.6 V/cm, 55 pulses/s, 0.1-ms duration). HSP72 protein expression was dramatically induced by HS+MES (10.5-fold increase compared with control cells, P < 0.000001), whereas HS alone caused a mild increase in HSP72 (3.3-fold increase, P < 0.0001) and MES alone did not (Fig. 1A).
FIG. 1.
HS+MES treatment induces HSP72 and suppresses p-JNK in MIN6 cells. A: MIN6 cells were treated with HS and/or MES for 10 min. At 10 h after the treatment, the cells were harvested, and the HSP72 protein levels were determined by Western blotting. B: After HS+MES treatment, MIN6 cells were incubated with or without TNF-α (25 ng/mL) for 6 h. The expression levels of HSP72, JNK, p-JNK, BiP, and cleaved caspase-3 were determined by Western blotting. Upon insulin stimulation, p-Akt levels were also investigated. C: HSP72 protein was overexpressed by transfection of an Hsp72 cDNA-containing plasmid. TNF-α stimulation was carried out and the expression levels of HSP72, JNK, p-JNK, BiP, and cleaved caspase-3 were determined as described for B. D: Upon HS+MES with TNF-α treatment, s-siRNA or Hsp72 si-RNA was transfected. HSP72, JNK, p-JNK, BiP, and cleaved caspase-3 were determined by Western blotting. E: Quantitative results of p-JNK levels in the data shown in B. F: Quantitative results of p-JNK levels in the data shown in D. **P < 0.01; NS vs. indicated group.
To examine whether the induced HSP72 could suppress JNK activation, the effects of HS+MES treatment on the activation of JNK induced by TNF-α (25 ng/mL) were investigated. TNF-α stimulation caused significant increases in the phospho-JNK (p-JNK) levels, and these increases were completely suppressed by HS+MES treatment to comparable levels to TNF-α unstimulated cells (61.3% reduction in p-JNK1; 43.1% reduction in p-JNK2/3; Fig. 1B and E). To confirm whether these effects were mediated by HSP72, an HSP72 expression vector (22) was used to overexpress HSP72 protein in MIN6 cells. After HSP72 overexpression by plasmid transfection, TNF-α–induced JNK phosphorylation was similarly attenuated to the levels as in the HS+MES treatment (55.8% reduction in p-JNK1; 28.4% reduction in p-JNK2/3; Fig. 1C), suggesting that HS+MES attenuates JNK activation upon TNF-α stimulation mainly through HSP72 induction.
To further confirm whether the inhibition of HSP72 cause opposite phenomenon, Hsp72 small interfering (siRNA) treatment was superimposed on HS+MES. HS+MES with scrambled siRNA (s-siRNA; Fig. 1D) increased HSP72 levels and reduced p-JNK (Fig. 1B). HS+MES with Hsp72 siRNA treatment partially decreased HSP72 expression and restored p-JNK activation (Fig. 1D and F). ER stress marker, binding immunoglobulin protein (BiP), and proapoptotic signal, and cleaved caspase-3 levels were also determined in the same experimental system. The alterations of these markers were quite similar as observed in p-JNK changes (Fig. 1B–D). These data indicate that HSP72 induced by HS+MES and JNK activation, ER stress, and proapoptotic signal were inversely correlated.
Because HSP72 and PI-3 K/Akt signal suppress the JNK signaling (11), Akt phosphorylation was investigated. When MIN6 cells were stimulated with insulin, increased p-Akt was observed in HS+MES or overexpression of HSP72 compared with nontreatment. HS+MES or HSP72 induction partially but significantly restored p-Akt levels under TNF-α stimulation (Fig. 1B and C). This effect was cancelled by Hsp72 siRNA treatment (Fig. 1D).
HS+MES treatment ameliorates glucose homeostasis and increases insulin secretion in db/db mice.
We previously showed that HS+MES treatment ameliorates insulin resistance and glucose homeostasis in high-fat-fed mice (22). We then examined whether HS+MES treatment exerts similar effects in insulin-deficient db/db mice (25). From 6 weeks of age, db/db mice received MES treatment (0.6 V/cm, 0.1-ms duration, 55 pulses/s) and HS (42°C) for 10 min twice a week for 12 weeks (22). Untreated db/db mice were sham-treated without HS or MES.
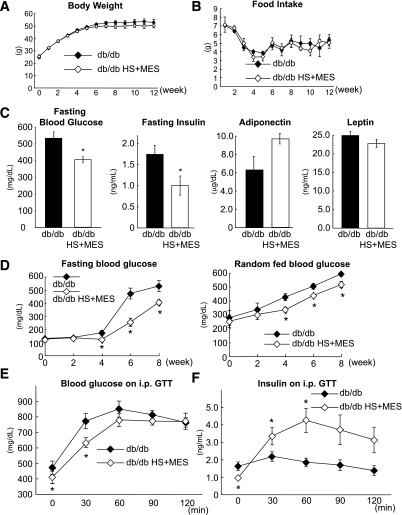
Until week 12 of the treatment, there were no detectable differences in the body weights and food intakes between the two groups (Fig. 2A and B). After 8 weeks of treatment, however, the fasting blood glucose level was significantly lower in the HS+MES-treated mice than in the control mice (527.0 ± 43.2 vs. 403.8 ± 18.6 mg/dL, P = 0.03; Fig. 2C). The fasting serum insulin level was also significantly decreased (1.75 ± 0.23 vs. 0.98 ± 0.25 ng/mL, P = 0.03; Fig. 2C), whereas the serum adiponectin and leptin levels were indistinguishable between the two groups (Fig. 2C). The fasting and random-fed blood glucose levels were both significantly lower as early as week 4 of the treatment, and these effects were sustained until week 8 of the treatment (Fig. 2D). On an IPGTT at week 8 of the treatment, significant suppression of the blood glucose excursion was observed at 30 min (Fig. 2E). The serum insulin levels during the IPGTT showed significant increases at 30 and 60 min (Fig. 2F).
FIG. 2.
HS+MES treatment improves glucose tolerance and insulin secretion in db/db mice. Weekly body weight increments (A) and food intakes (B) were measured during HS+MES treatment or sham-treatment in db/db mice. C: The fasting blood glucose, fasting insulin, adiponectin, and leptin levels were measured after 8 weeks of treatment. D: The changes in the fasting and random fed glucose levels were plotted. The glucose tolerance (E) and insulin secretion capability (F) were evaluated by intraperitoneal injection of glucose (1 g/kg body weight) in HS+MES-treated and sham-treated db/db mice. Whole blood was collected from the tail vein at the indicated times for blood glucose and insulin measurements. Values are means ± SEM (n = 4–6). *P < 0.05 vs. sham-treated db/db control mice.
HS+MES treatment increases HSP72 and insulin expression levels in pancreatic β-cells of db/db islets.
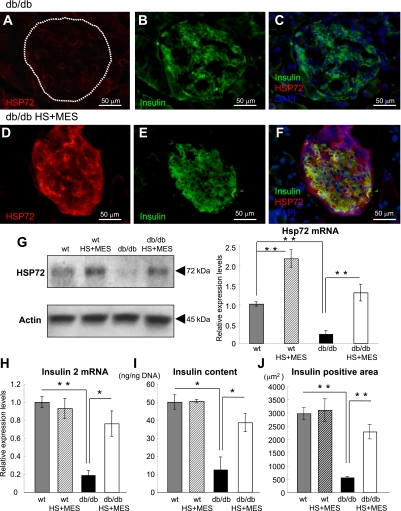
We previously reported that the combination of HS+MES effectively induces HSP72 expression in various tissues in vivo (22). In the current study, we consistently observed that cytoplasmic HSP72 protein expression was increased in the islets of treated db/db mice (Fig. 3D) compared with the islets of sham-treated db/db mice (Fig. 3A) at 12 weeks of treatment. Western blotting analyses revealed that the HSP72 protein levels were increased 5.7-fold (P = 0.0029) in the HS+MES group (Fig. 3G: left) compared with sham-treated mice. Hsp72 mRNA levels were also increased 5.4-fold compared with sham-treated db/db islets (Fig. 3G: right; P = 0.023), as assessed by quantitative real-time RT-PCR (qRT-PCR) using LCM.
FIG. 3.
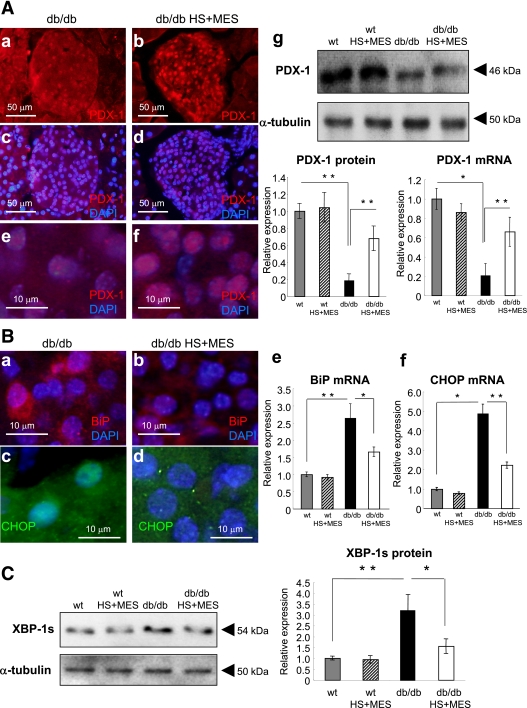
Increased expression of HSP72 and insulin in the pancreas of HS+MES-treated db/db mice. A–F: Sections of pancreas were immunostained for HSP72 and insulin with DAPI staining. Representative pancreatic sections from sham-treated db/db control and HS+MES-treated mice are shown. G: Western blotting of HSP72 and actin using whole pancreatic tissues (left panel) and Hsp72 mRNA was measured by qRT-PCR (right panel). Islet samples were isolated using LCM as described in research design and methods. Insulin-2 mRNA by qRT-PCR (H) using LCM and the insulin content per nanogram of DNA (I) were measured in islets isolated from HS+MES-treated and sham-treated db/db mice. J: Insulin-positive area was determined using fluorescent microscope BZ-8100. Values are means ± SEM (n = 50) islets each from three mice of each genotype. *P < 0.05, **P < 0.01 vs. indicated group. (A high-quality digital representation of this figure is available in the online issue.)
In parallel with the decreased insulin secretion, sham-treated db/db mice showed reduced numbers of insulin-positive cells. The insulin protein expression in each cell was also decreased (Fig. 3B). However, when the mice were treated with HS+MES, the number of insulin-positive cells and the intensity of the insulin immunoreactivity in each cell were greatly increased in the treated islets (Fig. 3E). Most insulin-positive cells coexpressed HSP72 in the treated islets (Fig. 3F). The insulin-2 mRNA levels from islets dissected by LCM and insulin contents in the islets of the treated mice were also significantly increased compared with those in sham-treated db/db mice (Fig. 3H and I). The insulin-positive area determined by fluorescent microscope in each islet of HS+MES treated db/db mice was increased by almost fourfold compared with sham-treated db/db islet (WT: 2,969.4 ± 226.4 μm2, WT HS+MES: 3,097.2 ± 431.8 μm2, sham-treated db/db: 561.0 ± 42.4 μm2 [P = 0.0021 vs. WT], db/db HS+MES: 2,284.9 ± 272.1 μm2 [P = 0.00004 vs. sham-treated db/db], n = 4 for each group), whereas the islet sizes were comparable between the two groups (sham-treated db/db: 3,028.1 ± 153.6 μm2 vs. db/db HS+MES: 2,987 ± 220.4 μm2, P = 0.83).
Thus, HS+MES treatment preserved β-cell mass and insulin secretion in vivo. However, in vitro experiments in MIN6 cells showed different effects. Insulin secretion in MIN6 cells at 6 and 10 h after HS+MES treatment was decreased by ∼30% compared with control (Supplementary Fig. 1A). This reduction in insulin secretion inversely paralleled with AMP-kinase (AMPK) activation (Supplementary Fig. 1B). When MIN6 cells were treated with HS+MES, p-AMPK levels were significantly upregulated at 1 h after treatment and diminished thereafter. Overexpression of dominant negative-AMPK completely cancelled HS+MES induced reduction of insulin secretion (data not shown).
JNK phosphorylation and forkhead box class O (FOXO) 1 nucleocytoplasmic translocation are reduced in HS+MES-treated db/db islets.
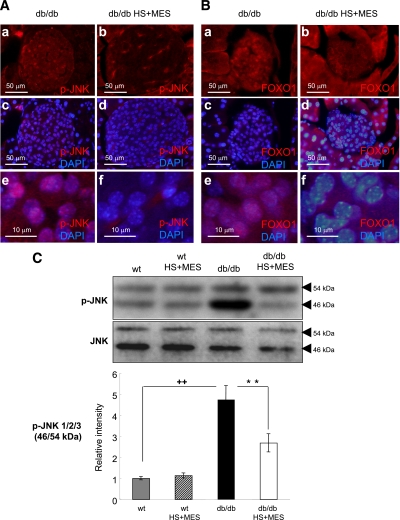
The JNK pathway, which is known to being activated by variety of stress signals, is reported to be abnormally accelerated in various tissues under diabetic conditions (26,27). When JNK is activated, dimerized JNK translocates into the nucleus and activates downstream targets such as c-jun. To analyze the subcellular localization of JNK, we performed immunohistochemical staining with an anti–p-JNK antibody. The results revealed that p-JNK was mainly localized in the nuclei in sham-treated db/db islets (Fig. 4A: a, c, and e), whereas the HS+MES-treated db/db islets showed drastic suppression of the nuclear accumulation of p-JNK (Fig. 4A: b, d, and f). P-JNK was also confirmed to being decreased by Western blotting (p-JNK1: 37 ± 7% reduction vs. sham-treated db/db mice [P < 0.0001]; p-JNK2/3: 48 ± 6% reduction vs. sham-treated db/db mice [P = 0.003]), whereas the JNK protein expression levels remained unaltered (Fig. 3C). These observations suggest that the JNK activation and cellular stress milieu were attenuated by the HS+MES treatment.
FIG. 4.
HS+MES treatment suppresses JNK activation and FOXO1 nuclear expression in the pancreas of db/db mice. Sections of pancreas were immunostained for pJNK (A: a–f) and FOXO1 (B: a–f). Representative pancreatic sections from sham-treated db/db and HS+MES-treated mice are shown. C: The levels of total JNK (46 kDa) and p-JNK (54 kDa) were determined by Western blotting in sham-treated and HS+MES-treated db/db mice. The expression levels of p-JNK were quantified as relative to the respective total JNK. Values are means ± SEM (n = 4–6). ++P < 0.01 vs. untreated-WT control mice. **P < 0.01 vs. sham-treated db/db mice. (A high-quality digital representation of this figure is available in the online issue.)
The forkhead transcription factor FOXO1 is an important transcription factor that plays key roles in apoptosis, cellular proliferation, differentiation, and glucose metabolism (28). To investigate the expression and intracellular localization of FOXO1, we carried out immunostaining with an anti-FOXO1 antibody. In sham-treated db/db islets, nuclear accumulation of FOXO1 was observed (Fig. 4B: a, c, and e). FOXO1 changed its intracellular localization from the nucleus to the cytoplasm upon HS+MES treatment (Fig. 4B: b, d, and f).
Pancreatic duodenal homeobox nuclear localization and ER stress markers in HS+MES-treated db/db islets.
JNK activation was reported to induce the nucleocytoplasmic translocation of the important transcription factor pancreatic duodenal homeobox (PDX-1) (29). Although PDX-1 was expressed at lower levels in nuclei in sham-treated db/db islets (Fig. 5A: a, c, and e), the number of signals and intensity of PDX-1 expression were dramatically increased in the HS+MES-treated group (Fig. 5A: b, d, and f). PDX-1 protein expression was decreased in sham-treated db/db mice compared with WT mice with or without HS+MES treatment. Upon HS+MES treatment, PDX-1 expression was partially recovered (Fig. 5A, g: 3.6-fold increase. P = 0.0082). PDX-1 mRNA expression in isolated islets by LCM showed significant increase in HS+MES-treated group (Fig. 5A, g: 3.1-fold increase. P = 0.016).
FIG. 5.
PDX-1 expression and ER stress signals in the pancreas. A: Sections of pancreatic specimen were immunostained for PDX-1 (a–f). Representative pancreatic sections from sham-treated and HS+MES-treated db/db mice are shown. PDX-1 protein expression assessed by Western blotting and PDX-1 mRNA expression measured by qRT-PCR were investigated (g). Relative PDX-1 protein and mRNA expression were quantified and corrected using respective internal controls (α-tubulin for protein, β-actin for mRNA) (g). B: Sections of pancreatic specimen were immunostained for BiP (a and b) and CHOP (c and d). Representative pancreatic sections from sham-treated and HS+MES-treated db/db mice are shown. BiP mRNA (e) and CHOP mRNA (f) were quantified by qRT-PCR using LCM. C: XBP-1s protein expression (identified as 54-kDa band) was determined by Western blotting, and the expression levels were corrected using α-tubulin. *P < 0.05, **P < 0.01 vs. indicated group. (A high-quality digital representation of this figure is available in the online issue.)
ER stress is one of the key mediators of β-cell dysfunction (30,31). The expression of an early ER stress marker, BiP, was suppressed in the islets of HS+MES treated db/db mice, in contrast to its upregulation in the sham-treated db/db islets (Fig. 5B: a, b, and e). Because excessive ER stress signals cause β-cell apoptosis in vivo, we investigated the apoptotic signals. The proapoptotic transcription factor C/EBP homolog protein (CHOP) is activated under severe ER stress conditions to induce cell elimination by apoptosis (30,31). Nuclear induction of CHOP was considerably suppressed in the HS+MES-treated db/db mice islets (Fig. 5B: c and d). CHOP mRNA expression was increased by 4.9-fold in the islets of sham-treated db/db mice compared with WT with or without HS+MES. CHOP mRNA in the islets of HS+MES-treated db/db mice was significantly decreased by 56 ± 7% (Fig. 5B, f, P = 0.0006). ER stress milieu was also investigated by alternative splicing of X-box binding protein (XBP)-1. Spliced form of XBP-1 (XBP-1s) protein was determined by Western blotting. XBP-1s level was increased by 3.4-fold (P = 0.0021) in sham-treated db/db mice compared with WT, and was significantly decreased by 52.2% (P = 0.015) upon HS+MES treatment (Fig. 5C).
Another molecular markers in β-cells treated with HS+MES.
Calcineurin/nuclear factor of activated T-cells (NFAT) signaling was recently reported to regulate β-cell growth and appropriate β-cell functions (32). To assess the effects of HS+MES treatment on the calcineurin/NFAT pathway, NFAT and one of its downstream targets, GLUT2, were visualized by immunohistochemistry. In sham-treated db/db islets, NFAT was predominantly located in the cytoplasm of cells with lower expression of insulin (Supplementary Fig. 2a). Upon HS+MES treatment, nuclear translocation of NFAT was observed in cells with higher insulin expression (Supplementary Fig. 2b). GLUT2, which participates in glucose sensing in β-cells, was increased in the membrane fraction of treated db/db islets compared with sham-treated db/db islets (Supplementary Fig. 2c and d). GLUT2 protein expression was decreased by 83 ± 12% in sham-treated db/db mice compared with WT. Upon HS+MES treatment in db/db mice, GLUT2 expression was almost restored (78 ± 9% of WT; Supplementary Fig. 2g). Insulin receptor substrate (IRS)-2, an important insulin-signaling molecule for β-cell growth (33), showed lower expression in sham-treated db/db islets and was greatly increased by HS+MES treatment (Supplementary Fig. 2: e, f, and h). Cleaved-caspase-3 was also downregulated in the HS+MES islets (Supplementary Fig 3A: a and b). Another early apoptotic marker, annexin V, displayed similar a trend (Supplementary Fig. 3A: c and d).
Nuclear factor-κB (NF-κB) signaling is crucial for transmitting cellular stresses (34). Nuclear localization of NF-κB p65 was predominant in sham-treated db/db islets, but decreased in treated islets (Supplementary Fig 3B: a and b). The oxidative stress marker, 8-hydroxy-2′-deoxyguanosine (8-OHdG), was increased in sham-treated db/db islets, but was attenuated in HS+MES-treated db/db islets (Supplementary Fig. 3B: c and d).
DISCUSSION
Cellular stress signaling pathways, such as ER stress and oxidative stress, have recently been recognized as important factors for the development and progression of T2D. Cell protection is one of the key ideas for preserving or even restoring pancreatic β-cell function under diabetic conditions. β-Cells are considered to be relatively sensitive to these stresses because the cells are continuously stimulated to produce insulin to overcome the demand for insulin in the state of insulin resistance (31). In the current study, we focused on the effects of HS+MES on β-cell protection.
HSP72 is one of the major inducible HSPs and plays significant roles in protein synthesis, folding, and cell survival (9). In response to stresses, which lead to denaturation of proteins, HSP72 prevents protein aggregation and induces refolding of damaged proteins into their native states (35). More important, it has been revealed that HSP72 can modulate the functions of several molecules, including JNK (36). HSP72 interacts physically with JNK, thereby suppressing JNK activation and JNK-mediated cell death (37). The JNK pathway is known to be activated under diabetic conditions and to be passively involved in the progression of insulin resistance and suppression of insulin biosynthesis (27). JNK-inhibitory peptide treatment was reported to improve insulin resistance and ameliorate glucose tolerance in diabetic mice (21).
We previously reported that HSP72 was greatly induced by HS+MES in various tissues (22). Using this combined treatment, we found that HSP72 was considerably increased in db/db mice islets and in MIN6 cells in the current study. Upregulation of HSP72 appears to be very effective for suppressing the JNK pathway. Recently, HSP72 induction by various methods, such as long-term hyperthermia, muscle-specific transgenic of HSP72 and HSP72 coinducer O-(3-piperidino-2-hydroxy-1-propyl)nicotinic amidoxime (BGP-15) treatment with hyperthermia, were also reported to protect against visceral adiposity and insulin resistance (15,17). We and others also found that geranylgeranyl acetone, another HSP72-inducer, displays similar metabolic advantages to HS+MES treatment (19,20), suggesting that induction of HSP72 may be beneficial for improving diabetic pathophysiology.
To further investigate the mechanisms of the preservation of β-cells through HSP72 induction by HS+MES, we focused on the JNK pathway. PI-3 K/Akt suppresses the JNK in islets, and JNK inhibition increases the viability of human islets (38). The activation of JNK suppresses insulin biosynthesis and also interferes with the actions of insulin. PDX-1 is a homeodomain-containing transcription factor that plays pivotal roles in the development and differentiation of β-cells (39). Oxidative stress or activation of JNK decreases the activity of Akt in SV-40–transformed hamster–derived β-cell line HIT-T15 cells, leading to decreased phosphorylation and increased nuclear localization of FOXO1, which is followed by enhanced cytoplasmic translocation of PDX-1 and finally results in suppression of insulin biosynthesis (40). PDX-1 has also been suggested to be involved in glucokinase and GLUT2 gene expression. In the current study, we have revealed that HS+MES-treated db/db islets show inhibition of p-JNK and nucleocytoplasmic translocation of FOXO1, and increased PDX-1 intensity in their nuclei, indicating that this combined treatment ameliorates β-cell integrity in diabetic animals, at least in part through inhibition of JNK. Indeed, whole-body hyperthermia in rats attenuates the inhibition of glucose-induced insulin release by lipopolysaccharide stimulation (41). Induction of HSP72 by glutamine supplementation has also been reported to decrease ischemic damage in rat islets (42).
Recent evidence has indicated that the calcineurin/NFAT pathway regulates multiple factors that control β-cell growth and appropriate endocrine functions (32). In the HS+MES-treated mice, NFAT was translocated into the nucleus, and GLUT2, a downstream target of NFAT, was upregulated, suggesting that NFAT pathway are involved in the improved β-cell function as well as JNK pathway.
Pancreatic β-cell apoptosis upon ER stress is mainly regulated by the inductions of CHOP and caspase-3 (31), which were significantly decreased by HS+MES treatment. As ER stress markers, BiP and XBP-1s expression were both downregulated by the HS+MES treatment, suggesting that the induction of HSP72 by HS+MES may attenuate the ER stress milieu as well as oxidative stress signaling confirmed by 8-OHdG reduction. These stress signals can also be activated by chronic inflammatory signals, such as NF-κB. Nuclear translocation of NF-κB increases TNF-α mRNA expression. Although we have not examined the local expression of TNF-α, NF-κB p65 nuclear translocation was attenuated by this treatment.
We also found that IRS-2, an important molecule for insulin signaling and β-cell proliferation (43), was upregulated in the treated islets. This effect may also contribute to the protection of the β-cells against apoptosis; however, the mechanisms of IRS-2 upregulation need to be studied.
The preventive effect of HS+MES on β-cell degradation in db/db mice may be mainly caused by the suppression of apoptosis through JNK inactivation. The glucose-lowering effect of HS+MES seems to be due primarily to preserving insulin production rather than preventing insulin resistance, because significant upregulation of insulin secretion in HS+MES-treated db/db mice showed a relatively small improvement of glucose excursion on the IPGTT (Fig. 2E and F). However, it remains uncertain whether the improved β-cell function observed in vivo is directly caused by HS+MES or through a secondary effect of reduced systemic glucose toxicity and insulin resistance. Because HS+MES may modulate certain numbers of molecules, collective analyses of gene and/or protein expressions need to be done for further elucidation.
Thus, HS+MES certainly prevents β-cell apoptosis and preserves β-cell mass in vivo; however, in vitro experiments using MIN6 cells repeatedly show decreased insulin secretion (∼30%) by HS+MES. This phenomenon may be explained by transient activation of AMPK by HS+MES because AMPK activation reduces insulin secretion (44). HS+MES activated AMPK at 1 h after the treatment and was diminished thereafter. We believe that preserved β-cell mass (four times greater than that of sham-treated db/db islets) can respond to a glucose challenge even though the insulin secretion capability of each cell is attenuated. Alternatively, transient activation of AMPK may decrease insulin secretion but does not last long in vivo. Activation of AMPK may also possibly contribute to protect β-cells (45).
In conclusion, we have revealed the induction of HSP72 in db/db islets by HS+MES, which results in inactivation of JNK and enhancement of PDX-1 nuclear translocation, thereby leading to increased insulin biosynthesis, accompanied by a reduced ER stress and oxidative stress milieu. Taken together, our findings indicate that HS+MES treatment not only improves insulin resistance in insulin-sensitive tissues but also contributes to the survival of β-cells in T2D. This treatment may hold promise as a novel therapeutic approach to human diabetes and metabolic syndrome.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Ministry of Education, Science, Sports and Culture of Japan (No. 19591058) to T.K., a grant from the Suzuken Memorial Foundation to T.K., and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, Japan (No. 20390259 and 23390243) to E.A.
No potential conflicts of interest relevant to this article were reported.
T.K. researched data, contributed to discussion, and wrote the manuscript. K.S. and R.M. researched data. S.M.-K. researched data and contributed to discussion. H.A. researched data. M.A.S., H.K., and E.A. reviewed and edited the manuscript. J.K., H.M., and N.F. contributed to discussion. T.K. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of the data and the accuracy of data analysis.
The authors appreciate the helpful advice and assistance of Kenshi Ichinose in their laboratory. The device used for the HS+MES treatment was kindly provided by Tsuchiya Rubber Co. Ltd., Kumamoto, Japan.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1098/-/DC1.
REFERENCES
- 1.Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Preventing diabetes—applying pathophysiological and epidemiological evidence. Br J Nutr 2000;84(Suppl. 2):S173–S176 [DOI] [PubMed] [Google Scholar]
- 2.Mori Y, Hoshino K, Yokota K, Itoh Y, Tajima N. Differences in the pathology of the metabolic syndrome with or without visceral fat accumulation: a study in pre-diabetic Japanese middle-aged men. Endocrine 2006;29:149–153 [DOI] [PubMed] [Google Scholar]
- 3.Basat O, Ucak S, Ozkurt H, Basak M, Seber S, Altuntas Y. Visceral adipose tissue as an indicator of insulin resistance in nonobese patients with new onset type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2006;114:58–62 [DOI] [PubMed] [Google Scholar]
- 4.Imoto K, Kukidome D, Nishikawa T, et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes 2006;55:1197–1204 [DOI] [PubMed] [Google Scholar]
- 5.Sakai K, Matsumoto K, Nishikawa T, et al. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun 2003;300:216–222 [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790 [DOI] [PubMed] [Google Scholar]
- 7.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl. 7):S52–S54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cell Biol 1985;5:330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp FR, Massa SM, Swanson RA. Heat-shock protein protection. Trends Neurosci 1999;22:97–99 [DOI] [PubMed] [Google Scholar]
- 11.Shinohara T, Takahashi N, Ooie T, et al. Phosphatidylinositol 3-kinase-dependent activation of akt, an essential signal for hyperthermia-induced heat-shock protein 72, is attenuated in streptozotocin-induced diabetic heart. Diabetes 2006;55:1307–1315 [DOI] [PubMed] [Google Scholar]
- 12.Maroni P, Bendinelli P, Tiberio L, Rovetta F, Piccoletti R, Schiaffonati L. In vivo heat-shock response in the brain: signalling pathway and transcription factor activation. Brain Res Mol Brain Res 2003;119:90–99 [DOI] [PubMed] [Google Scholar]
- 13.Kurucz I, Morva A, Vaag A, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 2002;51:1102–1109 [DOI] [PubMed] [Google Scholar]
- 14.Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 2003;52:2338–2345 [DOI] [PubMed] [Google Scholar]
- 15.Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A, 2008;105:1739–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 1999;341:924–925 [DOI] [PubMed] [Google Scholar]
- 17.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 2009;58:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Literáti-Nagy B, Kulcsár E, Literáti-Nagy Z, et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res 2009;41:374–380 [DOI] [PubMed] [Google Scholar]
- 19.Adachi H, Kondo T, Ogawa R, et al. An acylic polyisoprenoid derivative, geranylgeranylacetone protects against visceral adiposity and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab 2010;299:E764–E771 [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab 2011;300:E894–E901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneto H, Nakatani Y, Miyatsuka T, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med 2004;10:1128–1132 [DOI] [PubMed] [Google Scholar]
- 22.Morino S, Kondo T, Sasaki K, et al. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS ONE 2008;3:e4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morino S, Suico MA, Kondo T, et al. Mild electrical stimulation increases ubiquitinated proteins and Hsp72 in A549 cells via attenuation of proteasomal degradation. J Pharmacol Sci 2008;108:222–226 [DOI] [PubMed] [Google Scholar]
- 24.Yano S, Morino-Koga S, Kondo T, et al. Glucose uptake in rat skeletal muscle L6 cells is increased by low-intensity electrical current through the activation of the phosphatidylinositol-3-OH kinase (PI-3K) / Akt pathway. J Pharmacol Sci 2011;115:94–98 [DOI] [PubMed] [Google Scholar]
- 25.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996;379:632–635 [DOI] [PubMed] [Google Scholar]
- 26.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336 [DOI] [PubMed] [Google Scholar]
- 27.Kaneto H, Nakatani Y, Kawamori D, et al. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int J Biochem Cell Biol 2006;38:782–793 [DOI] [PubMed] [Google Scholar]
- 28.Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 2008;57:846–859 [DOI] [PubMed] [Google Scholar]
- 29.Kawamori D, Kajimoto Y, Kaneto H, et al. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 2003;52:2896–2904 [DOI] [PubMed] [Google Scholar]
- 30.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 2002;109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araki E, Oyadomari S, Mori M. Impact of endoplasmic reticulum stress pathway on pancreatic beta-cells and diabetes mellitus. Exp Biol Med (Maywood) 2003;228:1213–1217 [DOI] [PubMed] [Google Scholar]
- 32.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 2006;443:345–349 [DOI] [PubMed] [Google Scholar]
- 33.Burks DJ, White MF. IRS proteins and beta-cell function. Diabetes 2001;50(Suppl. 1):S140–S145 [DOI] [PubMed] [Google Scholar]
- 34.Dai T, Patel-Chamberlin M, Natarajan R, et al. Heat shock protein 27 overexpression mitigates cytokine-induced islet apoptosis and streptozotocin-induced diabetes . Endocrinology 2009;150:3031–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minami Y, Höhfeld J, Ohtsuka K, Hartl FU. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem 1996;271:19617–19624 [DOI] [PubMed] [Google Scholar]
- 36.Gabai VL, Meriin AB, Yaglom JA, Wei JY, Mosser DD, Sherman MY. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stewss-induced inhibition of JNK dephosphorylation. J Biol Chem 2000;275:38088–38094 [DOI] [PubMed] [Google Scholar]
- 37.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 2001;20:446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology 2004;145:4522–4531 [DOI] [PubMed] [Google Scholar]
- 39.Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians 1998;110:12–21 [PubMed] [Google Scholar]
- 40.Kawamori D, Kaneto H, Nakatani Y, et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem 2006;281:1091–1098 [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara S, Iwasaka H, Shingu C, et al. Heat shock protein 72 protects insulin-secreting beta cells from lipopolysaccharide-induced endoplasmic reticulum stress. Int J Hyperthermia 2009;25:626–633 [DOI] [PubMed] [Google Scholar]
- 42.Jang HJ, Kwak JH, Cho EY, et al. Glutamine induces heat-shock protein-70 and glutathione expression and attenuates ischemic damage in rat islets. Transplant Proc 2008;40:2581–2584 [DOI] [PubMed] [Google Scholar]
- 43.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 2002;283:E413–E422 [DOI] [PubMed] [Google Scholar]
- 44.da Silva Xavier G, Leclerc I, Salt IP, et al. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci USA 2000;97:4023–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyblom HK, Sargsyan E, Bergsten P. AMP-activated protein kinase agonist dose dependently improves function and reduces apoptosis in glucotoxic beta-cells without changing triglyceride levels. J Mol Endocrinol 2008;41:187–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.