Abstract
The role of brain insulin signaling in the control of food intake in humans has not been thoroughly defined. We hypothesized that the hormone contributes to the postprandial regulation of appetite for palatable food, and assessed the effects on appetite and snack intake of postprandial versus fasted intranasal insulin administration to the brain in healthy women. Two groups of subjects were intranasally administered 160 IU insulin or vehicle after lunch. Two hours later, consumption of cookies of varying palatability was measured under the pretext of a taste test. In a control study, the effects of intranasal insulin administered to fasted female subjects were assessed. Compared with placebo, insulin administration in the postprandial but not in the fasted state decreased appetite as well as intake and rated palatability of chocolate chip cookies (the most palatable snack offered). In both experiments, intranasal insulin induced a slight decrease in plasma glucose but did not affect serum insulin concentrations. Data indicate that brain insulin acts as a relevant satiety signal during the postprandial period, in particular reducing the intake of highly palatable food, and impacts peripheral glucose homeostasis. Postprandial intranasal insulin administration might be useful in curtailing overconsumption of snacks with accentuated rewarding value.
Observations in animals that the central nervous application of insulin decreases food intake (1,2) have led to the current concept that insulin, which reaches the brain via a receptor-mediated saturable transport (3), acts as a negative feedback signal in the homeostatic regulation of body weight (4). In humans, intranasal administration of the hormone enables the assessment of brain insulin effects in the absence of relevant systemic absorption (5). Thus, intranasal insulin has been shown to reduce food intake after acute administration (6) and to decrease body fat after long-term treatment (7). These effects were observed in men but not in women, which is in accordance with animal studies in which male but not female rats decreased food intake and lost body weight during 24 h of intracerebroventricular insulin administration (2,8). This pattern suggests that the contribution of brain insulin to the control of energy intake displays a certain degree of sex specificity. However, neuroimaging experiments have yielded evidence for food-related central nervous effects of insulin in women (9–11). These conflicting results highlight the fact that the preconditions and mechanisms of the anorexigenic impact of brain insulin signaling in humans are poorly understood. Notably, the acute reduction in food intake elicited by intranasal insulin administration (6) but also intravenous infusion of the insulin analog detemir (12) in the fasted state was not preceded by changes in self-rated hunger, implying that central nervous insulin exerts its anorexigenic effects via signals that contribute to meal termination and satiety rather than by reducing hunger motivation in fasted subjects (13).
Recent evidence indicates that in addition to acting on homeostatic, i.e., primarily hypothalamic, networks of food intake control, insulin modulates extrahypothalamic neural pathways processing the rewarding aspects of energy intake (14). Also, recalling previous lunch decreases afternoon snack intake in women (15,16), suggesting that the reward component of insulin’s satiating impact might be further promoted by the memory-improving effect of the hormone (17). Against this background, we hypothesized that intranasal insulin administration in the postprandial but not in the fasted state decreases subsequent intake of palatable snacks in women, who in this context also served as a model of moderate central nervous insulin sensitivity. We also assumed that the satiating impact of the hormone might be associated with improved recall of previous lunch intake.
RESEARCH DESIGN AND METHODS
Subjects were young healthy women who were taking oral (estrogen dominant, single-phase) contraceptives but were otherwise free of medication and were nonsmokers. All relevant illness was excluded by clinical examination. Habitual eating behavior was assessed via a lifestyle questionnaire on dietary restraint and tendency toward disinhibition (15). In brief, dietary restraint, i.e., the conscious effort to restrict calorie intake to control body weight was assessed using the restraint scale of the Dutch Eating Behavior Questionnaire (18). Only subjects with a score of 2.3, i.e., the median score for European populations (19), or less were included. Tendency toward disinhibition was assessed with the disinhibition scale of the Three Factor Eating Questionnaire (20), with an inclusion score of 8 or less. Subjects were kept unaware of hypothesized treatment effects on food intake and were informed that the experiments concerned the effect of insulin on taste preferences. Participants gave written informed consent to the studies, which conformed to the Declaration of Helsinki and were approved by the local ethics committee. All experiments were performed in a double-blind fashion.
Design and procedure of experiments I and II.
For experiment I (Fig. 1A), 30 women were randomly assigned to two groups, insulin and placebo, that were closely comparable regarding age (22.27 ± 0.73 vs. 23.13 ± 0.99 years, P = 0.49), BMI (21.47 ± 0.37 vs. 21.13 ± 0.36 kg/m2, P = 0.51), as well as prescreening scores of dietary restraint (1.71 ± 0.09 vs. 1.68 ± 0.08, P = 0.59) and disinhibition tendency (4.47 ± 0.41 vs. 4.87 ± 0.49, P = 0.54). Each woman participated in one individual experimental session (one participant per session) scheduled not to take place during her menstruation phase. Participants were instructed to abstain from caffeinated and alcoholic beverages after 2000 h on the day preceding the experiment, to have regular breakfast before 0800 h of the experimental day and to stay fasted afterward. After arrival at the laboratory around 1100 h, a venous cannula was inserted into the subject’s nondominant arm, which was positioned in a heated box (55°C) to enable drawing of arterialized venous blood.
FIG. 1.
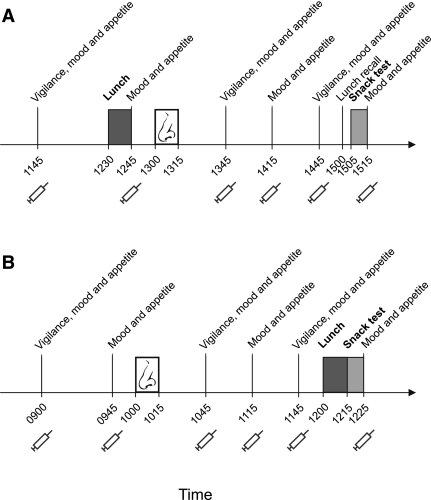
Experimental procedures. A: In experiment I, healthy young women consumed lunch at 1230 h and were intranasally administered insulin (160 IU; n = 15) or placebo (n = 15) at 1300 h. At 1500 h, free lunch recall took place, and 5 min later, snack intake was assessed with the participants assuming a taste rating task. Throughout the session, vigilance, mood, appetite, and thirst were assessed, and blood samples were taken (syringe symbols). B: In experiment II, insulin and placebo administration, respectively, took place at 1000 h and snack intake was assessed at 1215 h immediately after lunch serving as a preload (n = 13). The interval between insulin administration and snack intake assessment was kept constant between both experiments.
Experimental sessions started around 1145 h with baseline blood sampling and assessments of vigilance, mood, appetite, and thirst. From 1230–1245 h lunch was served, followed at 1300 h by the intranasal administration of 16 0.1-mL puffs (8 per nostril) of insulin or placebo at 60-s intervals, amounting to a total dose of 1.6 mL insulin (160 IU; Insulin Actrapid; Novo Nordisk, Mainz, Germany) or vehicle (6,21). At 1500 h, after a further 2 h of repeated blood sampling and vigilance, mood, appetite, and thirst assessments, participants of both groups were asked to write down as precisely and completely as possible what they had had for lunch. They were left alone for 5 min to do this. Free lunch recall protocols were quantified offline by a person blinded to the respective experimental group. Immediately after lunch recall, snack intake was assessed under the pretext of a cookie taste test. The experiment ended with another assessment of vigilance, mood, appetite, and thirst.
Experiment II (Fig. 1B) was carried out to assess whether the insulin effects observed in experiment I are specific to the postprandial state. A group of 13 women (age, 22.77 ± 0.61 years; BMI, 22.89 ± 0.52 kg/m2; restraint, 2.20 ± 0.07; disinhibition, 3.92 ± 0.49) participated in two conditions (insulin and placebo) spaced apart 28 days, ensuring participation on identical days in the menstrual cycle (with the exception of the menstruation phase). The order of conditions was balanced across subjects. After an overnight fast, subjects arrived at the laboratory around 0815 h and, after preparation of blood sampling and baseline measurements, were intranasally administered 160 IU insulin and placebo, respectively, at 1000 h, i.e., in the fasted state. Two hours later, snack intake was assessed following lunch, which in this instance served as a caloric preload. Substance administration, lunch procedure, snack intake assessment, repeated blood sampling, and behavioral assessments of vigilance, mood, appetite, and thirst were identical to experiment I. In both experiments, interviews at the end of the sessions confirmed that none of the participants had been aware of the purpose of the study.
Lunch and assessment of snack intake.
For lunch, the participant was presented with six hot, freshly baked mini pizzas (∼400 kcal; flavors “Hawaiian,” bacon, cheese, and salami) each cut into quarters to conceal portion size, yielding 24 pieces of pizza. A bottle of still mineral water was also provided. The participant was asked to rate the palatability of the pizzas on a visual analog scale (VAS) anchored at 0 (not palatable) and 100 (highly palatable). Each participant was asked to consume the whole meal, being told that this was “to make the ratings fair.”
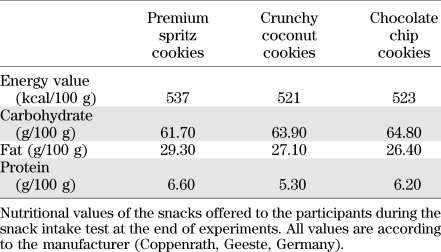
The snack test at the end of experiments was based on the procedure used by Rogers and Hill (22) and by Higgs and colleagues (15,16). Three plates of cookies were placed on the table, each containing a different variety and labeled Cookie A, B, and C, respectively. The three types were premium spritz cookies, crunchy coconut cookies, and chocolate chip cookies, respectively (Coppenrath, Geeste, Germany; Table 1). Of each variety, 15 cookies broken into bite-sized pieces were provided, allowing for a considerable amount to be eaten without the plates appearing empty to ensure that participants would not restrict cookie intake based on whether the experimenter could see how much had been consumed. In addition, a glass of still mineral water was provided. The participant was instructed to taste and rate each type of cookie on a VAS assessing palatability (0–100). The importance of giving accurate ratings was emphasized and subjects were informed that during and after completion of the rating task they could eat as many cookies as they liked because any remaining cookies would be discarded, and then they were left alone for 10 min. Cookie intake was measured by weighing the cookies before and after the cookie taste test.
TABLE 1.
Snack test cookies
Measurements of vigilance, mood, appetite, and thirst.
During both experiments, subjects repeatedly performed a simple 5-min PC-based vigilance task. In this task, a digital millisecond counter appeared at random intervals in the middle of the screen, starting at 0 ms to count upwards, and subjects were required to press a key as fast as possible, receiving immediate feedback in the form of the reaction time. For each 5-min task, mean reaction time was registered. Self-reported mood was assessed with 5-point scales covering the categories good/bad mood, alertness/sleepiness, and calmness/agitation (MDBF; 23), and with a checklist containing 123 adjectives assessing mood on 14 dimensions (EWL-K; 24). Appetite and thirst were rated on VASs anchored at 0 and 100. Heart rate and blood pressure were monitored throughout the experimental sessions.
Plasma glucose and hormone concentrations.
In both experiments, blood glucose concentrations were monitored online using the HemoCue B-Glucose Analyzer (Ängelholm, Sweden). Blood samples for the subsequent assessment of plasma glucose, serum insulin, and C-peptide (in both experiments) as well as of plasma ACTH and ghrelin and serum cortisol and leptin (experiment I) were centrifuged immediately, and serum and plasma were stored at –20°C. Routine assays were used to determine concentrations of plasma glucose (measured in fluoride plasma according to the hexokinase method [Aeroset; Abbott Diagnostics, North Chicago, IL]); insulin, C-peptide, ACTH, cortisol (all Immulite; DPC, Los Angeles, CA); and total ghrelin and leptin (radioimmunoassay; Millipore, Billerica, MA).
Statistical analysis.
Comparisons between the effects of insulin and placebo were based on ANOVA with the between-subjects factor “group” (experiment I) and the within-subjects factor “treatment” (experiment II), respectively, and the factors time or cookie type as appropriate. Significant interaction effects were specified by pairwise t tests. All data are presented as means ± SE. A P value < 0.05 was considered significant.
RESULTS
Experiment I: postprandial intranasal insulin administration reduces appetite and intake of palatable snacks.
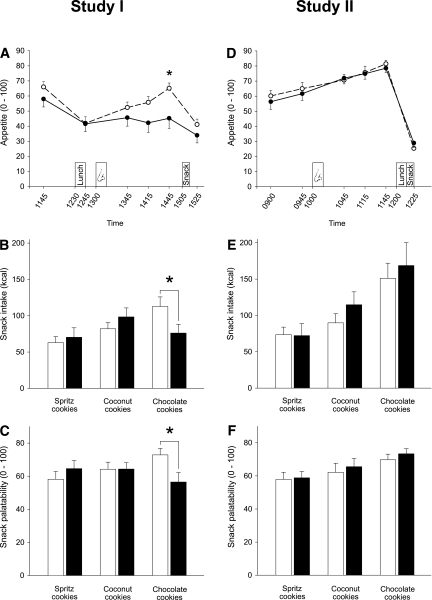
In experiment I, lunch intake induced a sharp decline in rated appetite in both groups (P < 0.001 for time; Fig. 2A). Postprandial administration of insulin abrogated the subsequent rise in appetite ratings that slowly emerged in the control group before the snack test (P = 0.04 for group × time). Total cookie intake at 1505 h did not differ between groups (insulin, 244.75 ± 22.94 kcal; placebo, 257.73 ± 17.09 kcal; P = 0.65). However, intake of chocolate chip cookies was significantly reduced in the insulin compared with the placebo group (76.11 ± 12.01 vs. 112.74 ± 13.15 kcal, P = 0.049; P = 0.049 for group × cookie type), whereas consumption of spritz cookies and coconut cookies was not affected (both P > 0.29; Fig. 2B). Accordingly, although the placebo group consumed more chocolate chip than other cookies (P = 0.016), this relation was absent in the insulin group (P = 0.55; P = 0.021 for the respective group effect). A corresponding pattern was revealed for snack palatability ratings, which globally did not differ between groups (P = 0.39; Fig. 2C) but with regard to chocolate chip cookies were distinctly reduced after insulin compared with placebo administration (56.48 ± 5.70 vs. 72.88 ± 3.92, P = 0.025; P = 0.049 for group × cookie type). Thus, chocolate chip cookies were rated significantly more palatable than the remaining cookie types in the placebo (P = 0.015) but not in the insulin group (P = 0.22; P = 0.014 for group effect).
FIG. 2.
Appetite and snack intake in experiments I and II. A: Appetite rated on visual analog scales anchored at 0 and 100 throughout experiment I in a group of subjects who were intranasally administered insulin (160 IU; black dots and solid lines; n = 15) at 1300 h (nose symbol) and a placebo control group (white dots and dashed lines; n = 15). Lunch was consumed at 1230 h and snacks were offered at 1505 h. B: Snack intake (kcal) assessed at 1505 h under the pretext of a taste rating session in the placebo group (white bars) and the insulin group (black bars) of experiment I. Three different types of cookies were offered. C: Snack palatability rated on visual analog scales anchored at 0 (not palatable) and 100 (highly palatable) during the snack test at 1505 h (experiment I). *P < 0.05 for comparisons between groups (t tests). D–F: Respective results obtained in the 13 subjects of experiment II who were intranasally administered insulin (160 IU; black dots, solid lines) (D; nose symbol) and placebo (white dots, dashed lines), respectively, at 1000 h. The snack test took place at 1215 h. Values are means ± SE.
Palatability ratings of the mini pizzas offered for lunch were comparable between the placebo (74.27 ± 5.75) and the insulin groups (75.67 ± 3.48, P = 0.84). Protocols of free lunch recall yielded full data sets for the categories “number of consumed mini pizzas” and “number of pizza types.” Both scores did not differ between groups (placebo vs. insulin, 5.92 ± 0.33 vs. 5.33 ± 0.19, P = 0.12; and 2.85 ± 0.22 vs. 3.00 ± 0.20, P = 0.61, respectively). Throughout the experimental sessions, thirst ratings (P = 0.50) and mood according to MDBF (all P > 0.33) and EWL-K scales (P > 0.13) were comparable between groups, as were reaction times in the vigilance task (P > 0.68), heart rate (P > 0.28), and blood pressure (P > 0.53).
Experiment II: intranasal insulin administration in the fasted state does not affect appetite and snack intake.
In experiment II, appetite ratings increased until they dropped after pizza and snack intake, with no differences between conditions (P = 0.59 for treatment × time; Fig. 2D). Snack consumption at 1215 h did not differ regarding total intake (insulin, 355.61 ± 59.12 kcal; placebo, 314.24 ± 30.48 kcal; P = 0.33) and intake according to cookie type (all P > 0.14; Fig. 2E). Intranasal insulin did not affect palatability ratings globally (P = 0.12) nor according to cookie type (P = 0.74; Fig. 2F). Across conditions, intake (P = 0.003) and palatability ratings (P = 0.039) of chocolate chip cookies exceeded those of the remaining types. Palatability ratings of the pizza lunch were comparable between the placebo (75.62 ± 6.59) and the insulin conditions (79.69 ± 2.82, P = 0.59). Insulin treatment likewise did not alter thirst ratings (P = 0.78), mood according to MDBF (all P > 0.28) and EWL-K scales (P > 0.09), vigilance (P > 0.26), heart rate (P > 0.29), or blood pressure (P > 0.73).
Exploratory comparisons between both experiments revealed that neither snack intake (P = 0.068) nor rated snack palatability (P = 0.59) generally differed between the two groups of experiment I and the subjects of experiment II (collapsed conditions). In both experiments, subjects could not correctly indicate at the end of the session whether they had received insulin or placebo (experiment I, P = 0.12; experiment II, P = 0.69; χ2 tests).
Plasma glucose and endocrine parameters.
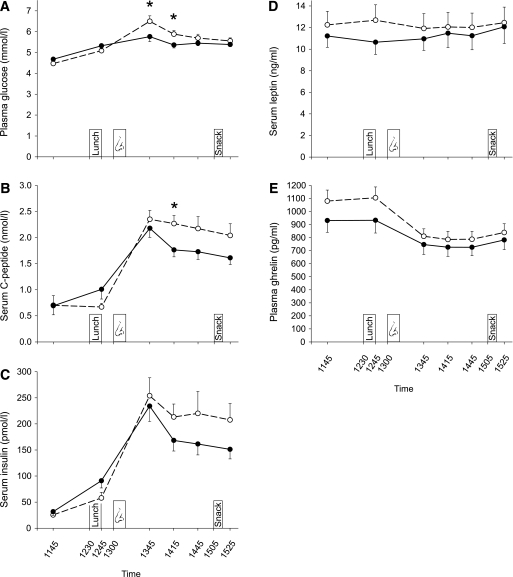
In both experiments, blood parameters did not differ during baseline (all P > 0.07). In experiment I, concentrations of plasma glucose (P = 0.016 for group × time) and serum C-peptide (P = 0.021) displayed slight reductions in the insulin compared with the placebo group that emerged after intranasal insulin administration but were no longer detectable before the snack test (Fig. 3A and B). Serum insulin (P = 0.12) and leptin (P = 0.11) were not affected by insulin administration; Fig. 3C and D). Plasma ghrelin concentrations showed the expected postprandial decrease with no significant differences between groups (P = 0.077; Fig. 3E). Likewise, there were no treatment effects on plasma ACTH (P = 0.42) and serum cortisol (P = 0.70). In line with experiment I, in experiment II plasma glucose concentrations were slightly reduced following intranasal insulin (P = 0.031 for treatment × time), averaging 4.36 ± 0.09 mmol/L in the insulin and 4.63 ± 0.09 mmol/L in the placebo condition between 1045 h and 1145 h (P = 0.002). C-peptide concentrations also decreased following intranasal insulin (P = 0.001 for treatment × time; 0.30 ± 0.03 vs. 0.42 ± 0.03 nmol/L, P = 0.001), whereas serum insulin levels did not differ between conditions (P = 0.57).
FIG. 3.
Plasma glucose and hormonal parameters in experiment I. Concentrations of plasma glucose (A), serum C-peptide (B), serum insulin (C), serum leptin (D), and plasma ghrelin (E) measured throughout experiment I in a group of subjects who were intranasally administered insulin (160 IU; black dots and solid lines; n = 15) at 1300 h (nose symbol) and a placebo control group (white dots and dashed lines; n = 15). Lunch was consumed at 1230 h, and snacks were offered at 1505 h. Values are means ± SE. *P < 0.05 for comparisons between groups (t tests).
DISCUSSION
Intranasal insulin administration to the brain has anorexigenic and catabolic properties in male subjects (6,7), but the precise role of the hormone in the acute regulation of food intake in humans has not yet been characterized. By demonstrating that intranasal insulin administration in the postprandial but not in the fasted state reduces appetite and snack intake in women, we provide evidence for the notion that insulin acts as a satiety signal in humans. As the insulin-induced reduction in snack intake was only found for hedonically salient but not for less palatable snacks, our findings moreover suggest that postprandial insulin in particular modulates the reward-related, nonhomeostatic control of food intake.
In line with previous results (6,21,25), in both experiments plasma glucose concentrations slightly decreased immediately after intranasal insulin administration but clearly remained within the euglycemic range. It might be speculated that intranasally administered insulin accessing relevant hypothalamic structures acted on the glucoregulatory brain-liver axis. By opening ATP-sensitive K+ channels of glucose-responsive hypothalamic neurons (26,27), intracerebroventricularly administered insulin can decrease hepatic glucose production by more than 40% (28) and plasma glucose concentrations by more than 1 mmol/L (27) in rats. These findings are in line with related results in canines (29,30), but discordant canine data (31,32) have sparked controversy about the relevance of brain insulin for peripheral glucose homeostasis in different species. Remarkably, activation of ATP-sensitive K+ channels by oral diazoxide has most recently been reported to suppress endogenous glucose production in healthy humans (33). Although against this background a centrally mediated effect of intranasal insulin on plasma glucose appears likely (25), more refined measures of peripheral glucose metabolism will be needed to substantiate this conclusion. Also, the decrease in C-peptide concentrations might reflect attenuated secretion of endogenous insulin due to a small ratio of intranasal insulin entering the blood stream via the nasal mucosa (10).
In experiment I, intranasal insulin administered immediately after lunch markedly enhanced the satiating effect of food intake, keeping appetite ratings at postlunch levels, whereas they slowly rose again in the control group, and reducing snack intake from chocolate chip cookies. In contrast, neither appetite nor snacking were affected by insulin administered in the fasted state in experiment II, a finding that replicates our previous results in women (6,21). Importantly, rated lunch and snack palatability as well as overall snack intake did not differ between both experiments, identifying the postprandial timing as the critical determinant of insulin’s impact on chocolate chip cookie intake. Thus, although in accordance with experiments in rodents (2,8), women per se are less sensitive to the anorexigenic impact of brain insulin than men who decrease food intake in response to intranasal insulin administration in the fasted state (6), brain insulin feedback can be assumed to exert a satiating effect not only in males but also in females. Although intranasal insulin administration may have enhanced and prolonged the postprandial brain insulin signal (5,34), the sharp postlunch decline in appetite in conjunction with meal-related insulin secretion observed in the placebo group of experiment I is in accordance with the notion of postprandial insulin acting as a physiological satiating factor. Support for this assumption comes from studies showing that the postprandial surge in circulating insulin is associated with increased satiety and reduced energy intake (35) as well as a reduction in regional cerebral blood flow in the orbitofrontal cortex (36) whose activity predicts feeding behavior, particularly in the fed state (37). Considering that remembering a preceding meal has been previously shown to decrease snack consumption (15,16) and that intranasal insulin improves declarative, hippocampus-dependent memory functions (6,17), experiment I included a free recall of lunch memory which, however, did not yield any signs of insulin-improved meal memory. Although more subtle insulin-induced cognitive changes might have eluded our attention, this finding does not support the assumption that the satiating impact of insulin depends on an improvement of food-specific memory functions.
Of note, the insulin-induced reduction in rated palatability and intake of snacks was restricted to chocolate chip cookies, which according to palatability ratings had the strongest rewarding quality but regarding nutritional values were comparable to the other snacks. This pattern suggests that in addition to promoting satiety, e.g., by enhancing the sensitivity to signals such as cholecystokinin (38), insulin administration also affected nonhomeostatic pathways that mediate reward-related “hedonic” aspects of food intake. Accordingly, in neuroimaging studies in fasted men and women who were presented pictures of food stimuli, intranasal insulin reduced activity of the fusiform gyrus (10), an area that also displays reduced activity during satiation (39) but increased activation when subjects experience food liking or food craving (40). Insulin did not affect general mood in our participants, indicating that the attenuating impact on hedonic processing is food-specific. Although the suppressive effect of central nervous insulin on food intake in general has been repeatedly demonstrated in animals (e.g., 1,2,8), recent experiments have provided a neurophysiological framework for a role of the hormone in hedonic food processing (rev. in 14). Thus, insulin receptors are expressed in dopaminergic neurons of the ventral tegmental area and substantia nigra (41), and brain administration of insulin decreases the rewarding quality of food (42–44) presumably by suppressing mesolimbic dopaminergic signaling (45). Respective experimental paradigms simulate a dessert or between-meal snack experience (46) similar to the present experiments, and it will be important to examine which types of palatable food are subject to insulin’s “anhedonic” impact in humans.
In summary, we demonstrate that postprandially administered intranasal insulin enhances the satiating effect of meals and reduces palatable snack intake, suggesting that insulin acts as a relevant signal in the short-term regulation of satiety in humans. Our results were obtained in women, who in comparison with men display generally reduced sensitivity to insulin’s anorexigenic brain effect (6,7,21). Thus, postprandial insulin administration might be speculated to also decrease intake of palatable snacks in obesity, which is characterized by central nervous insulin resistance (9,11,47) and a blunted association between postprandial insulin secretion and satiety (35). Considering that the rewarding effect of palatable food overriding the homeostatic control of energy intake may promote obesity (48), insulin’s potential to curb the appetite for hedonically salient, calorie-rich food deserves particular attention.
ACKNOWLEDGMENTS
This study was supported by Deutsche Forschungsgemeinschaft (KFO 126/B5). The funding source had no input in the design and conduct of this study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the article.
No potential conflicts of interest relevant to this article were reported.
M.H., S.H., and H.L. designed the study. M.H., S.H., M.T., and V.O. analyzed the data. M.H. enrolled students and collected data or did experiments for the study. M.H., S.H., M.T., V.O., and H.L. discussed the results and contributed to writing the manuscript. M.H. and S.H. wrote the manuscript. All authors take full responsibility for the contents of the article. M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Monique Friedrich, Meike Gaul, Anne Martin (all from the Department of Neuroendocrinology, University of Lübeck, Lübeck, Germany), and Kirstin Nordhausen (Internal Medicine I, University of Lübeck) for their expert technical assistance and Martina Grohs, Heidi Ruf, Ingrid von Lützau (all from the Department of Neuroendocrinology, University of Lübeck), and Jutta Schwanbom (Internal Medicine I, University of Lübeck) for their invaluable laboratory work.
Footnotes
See accompanying commentary, p. 773.
REFERENCES
- 1.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979;282:503–505 [DOI] [PubMed] [Google Scholar]
- 2.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 2003;52:682–687 [DOI] [PubMed] [Google Scholar]
- 3.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des 2003;9:795–800 [DOI] [PubMed] [Google Scholar]
- 4.Belgardt BF, Brüning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 2010;1212:97–113 [DOI] [PubMed] [Google Scholar]
- 5.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002;5:514–516 [DOI] [PubMed] [Google Scholar]
- 6.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008;93:1339–1344 [DOI] [PubMed] [Google Scholar]
- 7.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 2004;53:3024–3029 [DOI] [PubMed] [Google Scholar]
- 8.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 2006;55:978–987 [DOI] [PubMed] [Google Scholar]
- 9.Tschritter O, Preissl H, Hennige AM, et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA 2006;103:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guthoff M, Grichisch Y, Canova C, et al. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab 2010;95:748–755 [DOI] [PubMed] [Google Scholar]
- 11.Guthoff M, Stingl KT, Tschritter O, et al. The insulin-mediated modulation of visually evoked magnetic fields is reduced in obese subjects. PLoS ONE 2011;6:e19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallschmid M, Jauch-Chara K, Korn O, et al. Euglycemic infusion of insulin detemir compared with human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects. Diabetes 2010;59:1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science 1998;280:1378–1383 [DOI] [PubMed] [Google Scholar]
- 14.Könner AC, Klöckener T, Brüning JC. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol Behav 2009;97:632–638 [DOI] [PubMed] [Google Scholar]
- 15.Higgs S. Memory for recent eating and its influence on subsequent food intake. Appetite 2002;39:159–166 [DOI] [PubMed] [Google Scholar]
- 16.Higgs S, Williamson AC, Attwood AS. Recall of recent lunch and its effect on subsequent snack intake. Physiol Behav 2008;94:454–462 [DOI] [PubMed] [Google Scholar]
- 17.Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004;29:1326–1334 [DOI] [PubMed] [Google Scholar]
- 18.van Strien T, Frijters JER, van Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. Int J Eat Disord 1986;5:747–755 [Google Scholar]
- 19.Gorman BS, Allison DB. Measures of restrained eating. In Handbook of Assessment Methods for Eating Behaviours and Weight-Related Problems. Allison DB, Ed. Thousand Oak, CA, Sage, 1995, p. 149–184 [Google Scholar]
- 20.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 21.Krug R, Benedict C, Born J, Hallschmid M. Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory. J Clin Endocrinol Metab 2010;95:E468–E472 [DOI] [PubMed] [Google Scholar]
- 22.Rogers PJ, Hill AJ. Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict Behav 1989;14:387–397 [DOI] [PubMed] [Google Scholar]
- 23.Steyer R, Schwenkmezger P, Notz P, Eid M. Der mehrdimensionale Befindlichkeitsfragebogen (MDBF). Handanweisung. Göttingen, Hogrefe, 1997 [Google Scholar]
- 24.Janke W, Debus G. Die Eigenschaftswörterliste (EWL). Göttingen, Hogrefe, 1978 [Google Scholar]
- 25.Benedict C, Brede S, Schiöth HB, et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 2011;60:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 2000;3:757–758 [DOI] [PubMed] [Google Scholar]
- 27.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005;434:1026–1031 [DOI] [PubMed] [Google Scholar]
- 28.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 29.Agarwala GC, Mittal RK, Bapat SK, Bhardwaj UR. Effect of centrally administered insulin on blood glucose levels in dogs. Indian J Physiol Pharmacol 1977;21:11–18 [PubMed] [Google Scholar]
- 30.Chowers I, Lavy S, Halpern L. Effect of insulin administered intracisternally on the glucose level of the blood and the cerebrospinal fluid in vagotomized dogs. Exp Neurol 1966;14:383–389 [DOI] [PubMed] [Google Scholar]
- 31.Edgerton DS, Lautz M, Scott M, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest 2006;116:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramnanan CJ, Saraswathi V, Smith MS, et al. Brain insulin action augments hepatic glycogen synthesis without suppressing glucose production or gluconeogenesis in dogs. J Clin Invest 2011;121:3713–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishore P, Boucai L, Zhang K, et al. Activation of KATP channels suppresses glucose production in humans. J Clin Invest 2011;121:4916–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallschmid M, Schultes B, Marshall L, et al. Transcortical direct current potential shift reflects immediate signaling of systemic insulin to the human brain. Diabetes 2004;53:2202–2208 [DOI] [PubMed] [Google Scholar]
- 35.Flint A, Gregersen NT, Gluud LL, et al. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr 2007;98:17–25 [DOI] [PubMed] [Google Scholar]
- 36.Tataranni PA, Gautier JF, Chen K, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 1999;96:4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007;450:106–109 [DOI] [PubMed] [Google Scholar]
- 38.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinintranasal Physiol Behav 1995;58:755–760 [DOI] [PubMed] [Google Scholar]
- 39.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001;115:493–500 [DOI] [PubMed] [Google Scholar]
- 40.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage 2004;23:1486–1493 [DOI] [PubMed] [Google Scholar]
- 41.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 2003;964:107–115 [DOI] [PubMed] [Google Scholar]
- 42.Sipols AJ, Stuber GD, Klein SN, Higgins MS, Figlewicz DP. Insulin and raclopride combine to decrease short-term intake of sucrose solutions. Peptides 2000;21:1361–1367 [DOI] [PubMed] [Google Scholar]
- 43.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci 2004;118:479–487 [DOI] [PubMed] [Google Scholar]
- 44.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol 2009;296:R9–R19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figlewicz DP, Szot P, Chavez M, Woods SC, Veith RC. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res 1994;644:331–334 [DOI] [PubMed] [Google Scholar]
- 46.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol 2008;295:R388–R394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 2008;32:275–282 [DOI] [PubMed] [Google Scholar]
- 48.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron 2011;69:664–679 [DOI] [PMC free article] [PubMed] [Google Scholar]