Abstract
Endothelial progenitor cells (EPCs) enter the systemic circulation in response to cues related to vascular damage and need for neovascularization. Thus, EPCs could become readily accessible informers of vascular status and enable the survey of vascular pathologies during preclinical stages. To identify EPC changes with biomarker potential, we investigated whether discrete EPC abnormalities were associated with early nonproliferative diabetic retinopathy (NPDR). Two EPC subtypes with different functions have been characterized to date—one solely committed to the endothelial lineage and the other carrying both endothelial and monocytic markers. We found that only the latter, colony-forming units (CFU)-Hill cells, manifested abnormalities in type 1 diabetic patients with NPDR compared with control subjects. The abnormalities consisted in an increased number of colonies formed in vitro and downregulation of the molecules that facilitate homing at sites of vascular injury. The abnormalities were absent in type 1 diabetic patients free of retinopathy and other complications, despite long diabetes duration, but were detected in some of the patients without clinical retinopathy after short diabetes duration. CFU-Hill cells are potential informers of diabetic microangiopathy but may be preempted from carrying out reparative functions if the molecular abnormalities compromise interactions with the damaged vascular wall.
In 1997, Asahara et al. (1) reported the isolation from human peripheral blood of “putative endothelial cell progenitors or angioblasts,” which were differentiated in vitro into endothelial cells and incorporated in vivo into sites of active angiogenesis. Grant et al. (2) showed that hematopoietic stem cells are recruited to the retina after ischemic injury and are incorporated into new vessels. Gunsilius et al. (3) detected in the endothelium of human vessels chromosomal markers of bone marrow–derived cells. These observations introduced the novel concept that cells derived from bone marrow can contribute in the adult organism to both the formation of new vessels and the homeostatic maintenance or repair of the vascular endothelium.
An additional new concept was that these bone marrow–derived cells, insofar as traveling in the peripheral circulation on their way to the wall of vessels, could become readily accessible biomarkers of vascular events or vascular status. Such biomarkers could change the approach to vascular pathologies with a long subclinical phase. The knowledge of endothelial progenitor cells (EPCs) is still evolving, and it has become clear that they are heterogeneous subpopulations of cells (4–8) and that different assays enumerate different subpopulations. Currently, circulating EPCs are enumerated either by flow cytometry on the basis of the combined expression of surface antigens characteristic of hematopoietic stem/progenitor cells (CD34) and endothelial cells (vascular endothelial growth factor [VEGF] receptor 2 [VEGFR-2]) or on the basis of clonogenicity (i.e., the capability to form colonies in vitro). There must be at least two types of circulating progenitor cells that form colonies in vitro. One type, named endothelial colony-forming cells or late outgrowth cells, gives rise to colonies within 7–21 days of plating (5,6). The cells of the colonies have the typical morphology and molecular specifications of endothelial cells (8) and can be transplanted to form chimeric blood vessels in vivo (5,6). The circulating EPCs shown to date to generate these late outgrowth endothelial cells consist of cells that are positive for CD34 and VEGFR-2 but negative for the endothelial precursor marker CD133 and the leukocyte marker CD45 (7). A second type of EPCs, not yet characterized antigenically in the circulation, has become known as colony-forming units (CFU)-Hill cells or early outgrowth endothelial cells (5,6). These cells form colonies within 5–7 days; the cells are round, or spindle shaped at the periphery of the colonies, and are positive for both endothelial markers and hematopoietic (CD45) and monocytic (CD14) lineage markers (5,8). These cells do not integrate into blood vessels but exert proangiogenic effects through paracrine mechanisms (5,8).
To date, the most extensive evaluation of EPCs as informer of vascular status has been in atherosclerotic vascular disease. A decreased number of circulating CD34+VEGFR-2+ cells was found to predict the occurrence of cardiovascular events and death (9,10), and a decreased number of CFU-Hill cells was found to be associated with a larger burden of cardiovascular risk factors (10,11). Insofar as both the atherosclerotic process and its risk factors cause endothelial injury, the above findings have been interpreted to indicate EPC depletion and senescence in atherosclerosis, as if a finite pool had been exhausted by the sustained demand for endothelial repair (9,11). Within this paradigm, exhaustion should be preceded by a phase of increased EPC mobilization at the time of the early acceleration of endothelial damage and death. Such a course of events could be relevant to diabetic retinopathy, in which accelerated death of capillary endothelial cells precedes the morphological vascular lesions (12). The ability to capture such death early could provide a time and a target for measures that may prevent progression to clinical retinopathy. Only a few studies provide information on EPC behavior in relation to the discrete stages of retinopathy. Two studies reported (13,14) an increased number of circulating CD34+ mononuclear cells in patients with proliferative diabetic retinopathy (PDR) compared with nondiabetic control subjects. In patients with nonproliferative diabetic retinopathy (NPDR), Lee et al. (13) reported increased number of circulating CD34+ cells versus nondiabetic control subjects, and Brunner et al. (15) noted a decrease versus diabetic patients without retinopathy (nondiabetic control subjects were not examined). Among the above studies, none addressed the CFU-Hill cells, only one specified the CD45 status of the EPCs enumerated (14), and two did not exclude participants taking drugs such as statins that affect EPCs (14,15).
We performed this study to learn whether discrete EPC subtypes manifest changes in association with early diabetic retinopathy, with a view to identify events that may generate biomarkers of retinopathy. To prevent the influence of confounders, we enrolled patients with type 1 diabetes who had no complications other than early NPDR and took no drugs known to affect EPC, and we studied them under conditions of near normoglycemia.
RESEARCH DESIGN AND METHODS
The ethics committee of the San Raffaele Hospital approved the study, and participants gave written informed consent. We recruited from the Diabetes Clinic of the San Raffaele Hospital three groups of patients with type 1 diabetes as follows: 1) a group with NPDR after ≤20 years of diabetes to provide information on the processes associated with early retinopathy, 2) a group with no clinical retinopathy after short diabetes duration (≤7 years) to provide information on processes that predate the appearance of clinical retinopathy, and 3) a group with no clinical retinopathy after long diabetes duration (≥23 years) to provide information on processes surrounding resistance to retinopathy and other complications of diabetes. Healthy volunteers with no history of diabetes were recruited concurrently and frequency matched with the diabetic patients for age and sex. Exclusion criteria were smoking, pregnancy, systemic diseases other than type 1 diabetes, retinal diseases other than diabetic retinopathy, hypertension, renal insufficiency (serum creatinine >1.5 mg/dL), neuropathy (symptoms), heart disease (symptoms and/or electrocardiogram changes), and peripheral vascular disease (symptoms and/or abnormal pulse exam). Additional exclusion criteria were the use of statins, ACE inhibitors, angiotensin receptor antagonists, aspirin or other antiplatelet drugs, and anti-inflammatory drugs on a chronic basis. Permitted medications were birth control pills, antidepressants, vitamins, and anti-inflammatory drugs on an occasional basis but not during the 2 weeks preceding the study.
The diabetic patients underwent a complete eye examination and photography. Early Treatment Diabetic Retinopathy Study (ETDRS) seven standard field stereoscopic color photographs of each eye were used to diagnose and grade retinopathy. The retinopathy grade assigned to the individual patient was that for the eye with the more advanced level of retinopathy. Patients were excluded if they had severe NPDR or PDR.
EPC profile.
The number of circulating CD45−CD34+VEGFR-2+ cells, the number of CFU-Hill cells, and the plasma levels of VEGF and stromal cell–derived factor 1 (SDF-1) were measured to evaluate the EPC profile of each study subject. VEGF and SDF-1 are major regulators of EPC recruitment and homing (16). Peripheral blood was collected in EDTA in the fasting state between 9:00 a.m. and 10:00 a.m.; in the diabetic patients, the blood was drawn when capillary blood glucose levels were between 60 and 200 mg/dL.
Circulating CD34+VEGFR-2+ cells were enumerated by flow cytometry using a hierarchical gating strategy to count cells negative for the pan-hematopoietic marker CD45 or expressing only very low levels (CD45dim). In the literature, these cells are referred to as CD45− or CD45dim interchangeably (4,17,18), but we reported previously that CD34+ cells completely negative for CD45 were virtually absent (18). The counterstaining of the CD34+ cells by CD45 monoclonal antibody was performed following established protocols that permit accurate separation of EPCs that express low levels of CD45 on their surface and have low orthogonal side scatter (CD45dim and SSClow) from lymphocytes and monocytes, which express higher levels (19). Thus, the combination of antigenic characteristics CD45dimCD34+VEGFR-2+ identifies EPCs as opposed to both hematopoietic precursors and mature circulating endothelial cells (4).
In brief, 100 μL peripheral blood was incubated with the following monoclonal antibodies: PE-conjugated anti-human CD34 (BD, Franklin Lakes, NJ), allophycocyanin-conjugated anti-human VEGFR-2 (R&D Systems, Minneapolis, MN), and PE-Cy7–conjugated anti-human CD45 (Beckman Coulter, Brea, CA). After incubation, 100 μL Flow-Count beads (Beckman Coulter) was added to the stained whole blood. To avoid the loss of cells and/or counting beads, a lyse-no-wash technique was used as follows: erythrocytes were lysed with ammonium chloride buffer (155 mmol/L ammonium chloride, 10 mmol/L potassium bicarbonate, and 0.1 mmol/L EDTA), and the sample was analyzed immediately on a FACSCanto II flow cytometer with FACSDiva software (BD Biosciences, San Jose, CA). The listmode files obtained from these analyses were subsequently processed with FCS Express (De Novo Software, Los Angeles, CA), and the number of CD45dimCD34+VEGFR-2+ was directly determined and expressed per milliliter of blood.
The number of CFU-Hill cells was measured as described by Hill et al. (11) with minor modifications. Peripheral blood mononuclear cells isolated by Ficoll density gradient centrifugation of 30 mL blood were washed twice with PBS and seeded into plates previously coated with endothelial cell attachment factor (Sigma-Aldrich, St. Louis, MO). Cells were cultured in medium 199 supplemented with 20% FBS. After 48 h, the nonadherent cells were collected, sedimented by centrifugation, resuspended in culture medium, and reseeded at 1 × 106/well in 24-well plates previously coated with endothelial cell attachment factor. The number of colonies formed in 12 wells for each sample was counted on day 7 to obtain the average number of colonies per well.
The plasma levels of VEGF and SDF-1 were measured by ELISA (Quantikine Immunoassay; R&D Systems). Plasma was separated within 30 min of blood collection and stored at −20°C until used for the assay.
Gene expression profile of CFU-Hill cells.
Total RNA was isolated from the colonies formed on day 7 using the RNeasy Mini Kit (QIAGEN, Valencia, CA) with on-column DNase I digestion. Next, 2 μg total RNA from each sample were reverse transcribed using the RT2 First Strand kit (C-03; SABiosciences, Frederick, MD). We used the Human Embryonic Stem Cell RT2 Profiler PCR Array (PAHS-081; SABiosciences) to test directions of differentiation of CFU-Hill cells and the Human Endothelial Cell Biology RT2 Profiler PCR Array (PAHS-015; SABiosciences) to examine comprehensively endothelial specifications. The Profiler PCR Arrays (SABiosciences) measure quantitatively the expression of a panel of genes using SYBR Green-based real-time PCR. To obtain a reference for the level of expression of endothelial-specific genes, we also tested human umbilical vein endothelial cells (HUVECs). HUVECs harvested as previously described (20) were used for this experiment at passage 5 and cultured in EGM-2MV BulletKit (Lonza, Basel, Switzerland). At confluence, RNA was isolated and processed as described for the CFU-Hill cell colonies.
Quantitative real-time PCR was used to measure the expression of CXCR4, CXCR2, CD45, and CD14. The measurements were performed in the same RNA samples tested in the PCR arrays. The primer sequences are listed in Supplementary Table 1. The PCR mix for SYBR Green assay was prepared using 10 μL SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), 1 μL (1 pmol) sense primers, 1 μL (1 pmol) antisense primers, and 100 ng cDNA in a final reaction volume of 20 μL. The real-time PCR was performed in a Model 7900HT Fast Real-Time PCR System (Applied Biosystems) under the universal cycling conditions consisting of one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and at 60°C for 1 min. The level of β-actin expression, known from the PCR arrays to be similar in all study groups, was used to normalize the amount of cDNA added to each reaction and obtain ΔCT (cycle threshold). The mean ΔCT of samples from control subjects was used as the calibrator to calculate the ΔΔCT. The relative expression of each transcript was calculated by the comparative CT method (21).
Statistical analyses.
Data are summarized with the mean ± SD or median and interquartile range for values nonnormally distributed. The variables in diabetic patients and respective control groups were compared using a two-tailed t test or ANOVA followed by Fisher protected least significant difference test for more than two groups. For values nonnormally distributed, the Mann-Whitney U test was used. Longitudinal data in the same individuals were compared using the paired t test. The relationship between two variables was tested by linear regression.
The data of the PCR arrays were analyzed using the web-based PCR analysis software dedicated to the RT2 Profiler PCR Arrays, which automatically performs all ΔΔCT-based fold-change calculations from the uploaded raw CT data. The data were first surveyed for genomic DNA contamination, efficiency of reverse transcription, and PCR controls. Pairwise comparisons between the control CFU-Hill cells and HUVECs, and between the diabetic and control CFU-Hill cells, were performed to identify the genes manifesting a twofold or greater change in the level of expression at the P ≤ 0.05 level.
RESULTS
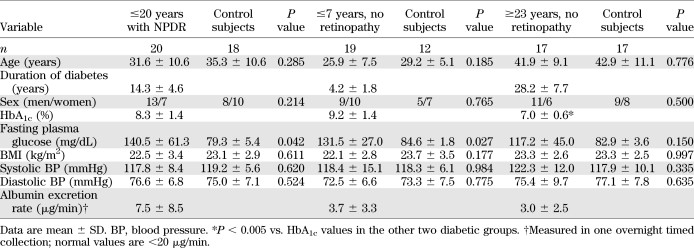
Table 1 presents the clinical characteristics of the three groups of diabetic patients studied. Because age was expected to differ among groups, the patients in each group were frequency matched for age and sex with nondiabetic control groups. For all groups of participants, the mean BMI was within the healthy reference range for white adults (22) and systemic blood pressure was normal. In the group of patients with ≥23 years of diabetes and no complications, the HbA1c was lower than in the other two diabetic groups. Among the 20 patients with NPDR, 5 had level 20 (microaneurysms only) in the ETDRS scale (23), 13 had level 35 (microaneurysms and a defined limited severity and extent of one or more of the following: hemorrhages, hard exudates, soft exudates, and intraretinal microvascular abnormalities), and 2 had level 43 (same abnormalities as for level 35 but greater extent). The diabetic patients had albumin excretion rates within the normal range.
TABLE 1.
Clinical characteristics of the three groups of type 1 diabetic patients studied and respective nondiabetic control subjects
No participant practiced extreme sports, and none had exercised before the blood drawing. Regular exercise of moderate-to-vigorous intensity (calisthenics, tennis, swimming, and running) was reported by 48% of the control subjects, 45% of the patients with NPDR, 37% of the patients with ≤7 years of diabetes, and 47% of the patients with ≥23 years of diabetes.
CFU-Hill cells are selectively affected in NPDR patients.
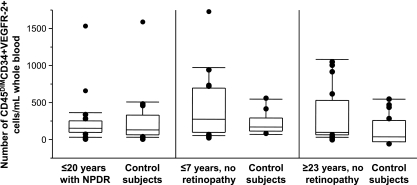
The median number (interquartile range) of circulating CD45dimCD34+VEGFR-2+cells was 118/mL blood (59–217) in the NPDR patients and 95 (32–296) in their control subjects (P = 0.9), 236 (62–664) in the group with ≤7 years of diabetes and 95 (35–214) in their control subjects (P = 0.4), and 56 (23–497) in the group with ≥23 years of diabetes and 69 (3–273) in their control subjects (P = 0.7) (Fig. 1). The levels of circulating VEGF and SDF-1 were also similar in all groups. They were, respectively, 43 ± 31 and 2,406 ± 458 pg/mL in the group with NPDR (vs. 38 ± 13 and 2,451 ± 560 in their control subjects), 34 ± 18 and 2,354 ± 485 pg/mL in the group with ≤7 years of diabetes (vs. 37 ± 15 and 2,506 ± 656 in their control subjects), and 38 ± 9 and 2,288 ± 288 pg/mL in the group with ≥23 years of diabetes (vs. 34 ± 17 and 2,411 ± 611 in their control subjects).
FIG. 1.
The number of circulating CD45dim EPCs is similar in type 1 diabetic patients with NPDR (n = 20) or without NPDR after short (≤7 years, n = 19) or long (≥23 years, n = 17) diabetes duration and age- and sex-matched control subjects. The cells were enumerated by flow cytometry using a hierarchical gating strategy to count only CD34+VEGFR-2+ cells negative for the pan-hematopoietic cell marker CD45 or expressing only low levels (CD45dim). Each box plot shows the 10th, 25th, 50th (median), 75th, and 90th percentiles of the counts obtained in the respective group. Values >90th and <10th percentile are plotted as individual points.
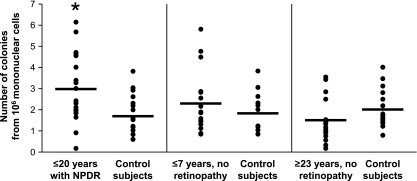
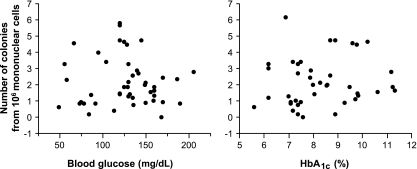
The number of colonies formed in the Hill assay from 106 mononuclear cells was in the range previously reported (5) and was increased in patients with NPDR when compared with their control subjects (P = 0.01) (Fig. 2). Patients with the milder retinopathy (ETDRS 20, n = 5) tended to form more colonies (4.1 ± 1.7 colonies from 106 mononuclear cells) than patients with more severe retinopathy (2.6 ± 1.4, n = 15; P = 0.078). Some of the patients with ≤7 years of diabetes and no retinopathy showed colony counts in the high range observed among NPDR patients, but the group data did not differ from matched control subjects (P = 0.42). In the patients with ≥23 years of diabetes and no retinopathy, the colony counts were similar to control subjects (P = 0.16). We had 80% power (α = 0.05) to detect differences between experimental groups and their control subjects equal to 1 SD. In addition, the colony counts were significantly higher in the NPDR patients than in the patients with ≥23 years of diabetes and no retinopathy (P = 0.0008), indicating that higher colony counts were associated with retinopathy rather than diabetes per se. Indeed, the number of colonies in the diabetic patients showed no relationship with HbA1c or blood glucose levels measured at the time of blood collection (Fig. 3).
FIG. 2.
The number of CFU-Hill cells in type 1 diabetic patients with NPDR (n = 20) is increased when compared with age- and sex-matched control subjects. The CFU-Hill cells were detected by performing the Hill assay as described in research design and methods and enumerated as the number of colonies formed in vitro by 106 circulating mononuclear cells. Each point in the scattergrams corresponds to the number of colonies (average of the number of colonies in 12 wells) counted on day 7 for each individual subject; the horizontal lines represent the group means. The cells of the colonies expressed CD45 and CD14 in addition to endothelial-specific transcripts as described in text. *P = 0.01 vs. control subjects.
FIG. 3.
The number of CFU-Hill cells does not show association in individual diabetic patients with the blood glucose or the HbA1c levels measured at the time of blood collection. The CFU-Hill cells were enumerated as the number of colonies formed in vitro by 106 circulating mononuclear cells.
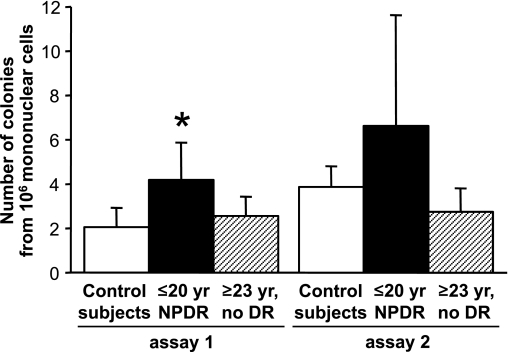
In a subset of the diabetic patients and control subjects, a second Hill assay was performed 1.5–2 years after the first for the purpose of measuring gene expression (see below). Comparison of the two consecutive assays (Fig. 4) showed reproducibility within each group of subjects, and the colony count tended to remain consistently higher in the NPDR group as compared with the other groups.
FIG. 4.
The number and patterns of CFU-Hill cells show consistency over time. In a subset of diabetic patients and control subjects (n = 6 in each group), a second Hill assay (assay 2) was performed 1.5–2 years after the first (assay 1). CFU-Hill cells were enumerated as the number of colonies formed in vitro by 106 circulating mononuclear cells. Within each group, the colony counts obtained in assay 2 were not different from the counts obtained in assay 1 (paired analysis). In patients with NPDR, the colony count in assay 1 was higher than in control subjects (*P = 0.03) and tended to be higher than in patients with ≥23 years (yr) of diabetes and no diabetic retinopathy (DR; P = 0.06); the tendency persisted in assay 2. The bar graphs show the mean ± SD of the colony counts.
CFU-Hill cells show altered gene expression in NPDR patients.
The expression of embryonic genes and genes of specific differentiation lineages did not differ in the CFU-Hill cells from NPDR patients and nondiabetic control subjects. The cells expressed the transcript for c-KIT and NANOG, but the mRNAs for many other transcription factors that maintain stemness (FOXD3, GATA6, GBX2, NR5A2, SOX2, and ZFP42) were undetectable. The colonies expressed all endothelial lineage transcripts detectable with the embryonic profiler: vascular endothelium–cadherin and VEGFR-1 at low levels and platelet-endothelial cells adhesion molecule 1 more robustly.
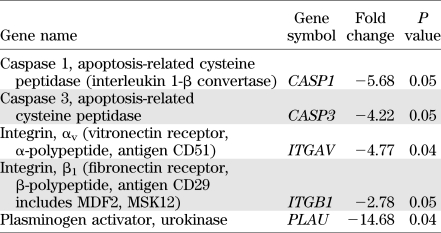
The endothelial cell biology profiler showed that the CFU-Hill cells from control subjects expressed most endothelial-specific genes. However, the level of expression of vascular endothelium–cadherin, von Willebrand factor, endothelin-1, and the Tie2 receptor was 100-fold lower than in HUVECs. We compared the expression of endothelial genes in the colonies from NPDR patients (age 38 ± 16 years, duration of diabetes 14 ± 4 years), patients with ≥23 years of diabetes and no retinopathy (age 59 ± 18 years, duration of diabetes 38 ± 13 years), and control subjects (age 49 ± 14 years, chosen to be equidistant from the ages of the two diabetic groups and not statistically different from either). The gene expression of colonies from patients with ≥23 years of diabetes and no retinopathy was similar to that of the control subjects. In contrast, the colonies from NPDR patients showed a significantly lower expression of genes encoding regulators of apoptosis and cell-matrix interactions (Table 2). In particular, the lower expression of integrins that regulate cell adhesion, and of urokinase plasminogen activator that is involved in the degradation of the extracellular matrix, suggested a defect in the specifications that mediate EPC homing at sites of endothelial injury.
TABLE 2.
Genes differentially expressed in colonies of CFU-Hill cells from type 1 diabetic patients with NPDR (n = 6) compared with nondiabetic control subjects (n = 6)
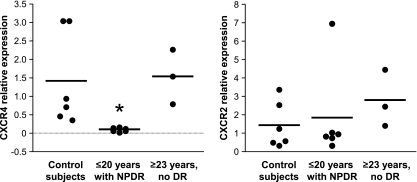
We thus tested the expression of homing receptors (Fig. 5). In the CFU-Hill cells of NPDR patients, the relative expression of CXCR4 (the receptor for SDF-1) was remarkably low (0.1 ± 0.06) when compared with both control subjects (1.4 ± 1.2, P = 0.02) and patients with ≥23 years of diabetes and no retinopathy (1.5 ± 0.7, P = 0.03). The relative expression of CXCR2 (the receptor for other angiogenic chemokines) (24) did not differ among the three groups.
FIG. 5.
The expression of the homing receptor CXCR4 is downregulated in CFU-Hill cells from NPDR patients. The relative expression of CXCR4 and CXCR2 were measured by quantitative real-time PCR in the colonies formed by CFU-Hill cells of control subjects (n = 6), NPDR patients (n = 6), and patients with ≥23 years of diabetes and no retinopathy (n = 3). *P < 0.03 vs. the other two groups.
In agreement with previous observations (5,8), the colonies formed by CFU-Hill cells expressed both CD45 and CD14; the levels of expression were similar in the colonies from diabetic and control subjects.
DISCUSSION
Our observations reveal that in patients with type 1 diabetes, the CFU-Hill cells, a discrete subpopulation of circulating EPCs that is enumerated via its early clonogenic activity and that carries both endothelial and monocytic markers—agreeing with previous studies (5,8) and this study—manifest abnormalities in association with the presence of NPDR and possibly before clinical evidence of retinopathy. The abnormalities consist in a greater than normal number of colonies formed in vitro and reduced expression of the molecules that guide and facilitate EPC homing. If occurring in vivo, the latter abnormalities may preempt this subpopulation of EPCs from being recruited to sites of vascular lesions and carrying on their putative reparative role.
This is, to our knowledge, the first study that compares and contrasts in a clinical condition the behavior of EPC subtypes belonging to different lineages and finds that one of the subpopulations is selectively or preferentially abnormal. In most studies, investigators enumerate EPCs by only one method and/or seldom specify the CD45 status of the circulating CD34+VEGFR-2+ cells, leaving uncertainty about the EPC subtype being examined. In this study, the EPCs enumerated by flow cytometry excluded CD45+ cells (18), and the behavior of these EPCs was compared with that of the CFU-Hill cells that express CD45. EPC subtypes differing for myeloid markers are not related (5), and our findings suggest that they can be subject to different regulation. The regulation may be responsive to the nature and severity of vascular changes because CD45−CD34+ cells, circulating in normal number in our NPDR patients, were increased in patients with PDR when compared with control subjects (14).
Do the lesions of NPDR signal to CFU-Hill cells? Although we have no proof that the abnormalities of CFU-Hill cells relate to the retinal microangiopathy, the combination of experimental design and findings is consistent with this possibility. We enrolled patients who were healthy except for their type 1 diabetes, were nonsmokers, took no drugs known to affect EPCs, and engaged in regular physical activity in the same proportion as the nondiabetic control subjects. We tested the patients when blood glucose was within a predefined range that excluded severe hyperglycemia and at the same time of day to prevent confounding influences from circadian release of EPCs (25). The patients with no retinopathy after an average of 28 years of diabetes and, thus, arguably protected from retinopathy, showed no EPC abnormality individually or as a group. These patients also had the best glycemic control among the diabetic groups, but it must be noted that the diabetic group with the worst glycemic control also had no EPC abnormality. However, this latter group—consisting of the patients with absent retinopathy after a short duration of diabetes—included some individuals with a number of CFU-Hill cell colonies in the range of NPDR patients. This is the expected pattern if the group is at risk for developing retinopathy and incipient but still subclinical vascular abnormalities signal to CFU-Hill cells. If indeed the number of colonies formed in vitro corresponds to the number of circulating CFU-Hill cells (11), then NPDR sends signals to increase the number of circulating CFU-Hill cells. The signals did not appear to be transmitted via changes in the circulating levels of VEGF or SDF-1, but our cross-sectional measurements may have failed to identify small increments sufficient to signal. Longitudinal measurements performed in the same patients will be required to address this possibility. Any initial stimulus to the bone marrow to increase mobilization may be potentiated by stimuli deriving from the decreased homing efficiency and the attempt to compensate for functional failure. The homing difficulties may also contribute to an increased number of circulating CFU-Hill cells on account of the longer time in the circulation.
The angiogenic and reparative functions of EPCs require interactions of these cells with the vessel wall. Three main types of molecules regulate or facilitate adhesion and homing: angiogenic chemokines and their receptors, integrins, and matrix proteases (16,26). The reduced expression of critical members of these families of molecules may thus jeopardize EPC functions in NPDR patients. EPCs carrying both endothelial and monocytic markers have mostly been reported to facilitate angiogenesis by providing paracrine signals, as opposed to merging into the wall of new vessels (5,17,27). However, when examining endothelial repair as opposed to neovascularization, it was noted that the human EPCs that best adhered to injured carotid arteries and incorporated at sites of denudation were CD14+ (i.e., of monocytic lineage) (16,24). Within this context, one could speculate that the reduced expression of adhesive molecules by the CFU-Hill cells of NPDR patients may have consequences facilitating the progression of retinopathy. Migratory and reparative defects of circulating CD34+ cells have been reported in patients with type 2 diabetes (28), and because the CD34+ cells were unfractionated, would have included CFU-Hill cells, possibly carrying molecular abnormalities similar to those we observed. However, the findings in the type 2 diabetic patients may or may not relate directly to retinopathy because the group included patients with retinopathy but also with cardiovascular disease and taking statins (28), characteristics that affect CFU-Hill cells and possibly other EPCs in divergent directions (10,11).
The next steps of the investigation must address whether the abnormalities of the CFU-Hill cells found associated with NPDR are mechanistically linked to the incipient microangiopathy. It should first be determined whether the profoundly reduced expression of the homing receptor CXCR4 and integrins deprives the cells of adhesive and migratory functionality and, thus, limits interactions with the vascular wall. The abnormalities must be carried over from the circulating parent cells, perhaps as a manifestation of “memory” of the diabetic state (20), because the clonogenic assay is performed in medium containing normal glucose. Thus, the information is likely to be relevant to events occurring in vivo. Statins may be useful probes to address functionality because when applied in vitro to human EPCs, they increase the EPC expression of integrins and adhesiveness (29). Once adhesive functionality is documented, it should be possible to determine if, in diabetes, the CFU-Hill cells are geared to exert reparative functions. However, clinically usable information on the role of these cells in early retinopathy will ultimately require the identification of their parent cells, the ones that actually circulate and interact with the vessels.
At this time, our findings deliver a candidate informer of preclinical abnormalities in diabetic retinal vessels and new lines of inquiry into the mechanisms operative in retinopathy progression; these may in turn deliver new targets for early intervention.
Supplementary Material
ACKNOWLEDGMENTS
The work was supported by grants from the Juvenile Diabetes Research Foundation (1-2007-16 to G.Z.) and the National Institutes of Health (R01-EY-017637 to C.G. and M.L.).
No potential conflicts of interest relevant to this article were reported.
G.Z. originated the project, researched data, contributed to discussion, and reviewed and edited the manuscript. A.M., A.P., G.T., and R.L. researched data, contributed to discussion, and reviewed and edited the manuscript. S.M. researched data. M.R.P., A.S., and R.B. researched data and reviewed the manuscript. C.G. contributed to discussion and reviewed and edited the manuscript. M.L. originated the project, researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. G.Z. and M.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 45th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 29 September–2 October 2009.
The authors thank Elisa Giglio and Anna Pulcina, medical students at the University Vita-Salute San Raffaele, who provided important contributions to patient recruitment.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1197/-/DC1.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 2.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002;8:607–612 [DOI] [PubMed] [Google Scholar]
- 3.Gunsilius E, Duba HC, Petzer AL, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet 2000;355:1688–1691 [DOI] [PubMed] [Google Scholar]
- 4.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 2006;6:835–845 [DOI] [PubMed] [Google Scholar]
- 5.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007;109:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia 2007;21:1141–1149 [DOI] [PubMed] [Google Scholar]
- 7.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol 2007;27:1572–1579 [DOI] [PubMed] [Google Scholar]
- 8.Medina RJ, O’Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 2010;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt-Lucke C, Rössig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005;111:2981–2987 [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005;353:999–1007 [DOI] [PubMed] [Google Scholar]
- 11.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600 [DOI] [PubMed] [Google Scholar]
- 12.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye (Lond) 2006;20:546–552 [DOI] [PubMed] [Google Scholar]
- 14.Tan K, Lessieur E, Cutler A, et al. Impaired function of circulating CD34(+) CD45(−) cells in patients with proliferative diabetic retinopathy. Exp Eye Res 2010;91:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner S, Schernthaner GH, Satler M, et al. Correlation of different circulating endothelial progenitor cells to stages of diabetic retinopathy: first in vivo data. Invest Ophthalmol Vis Sci 2009;50:392–398 [DOI] [PubMed] [Google Scholar]
- 16.Hristov M, Zernecke A, Liehn EA, Weber C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb Haemost 2007;98:274–277 [PubMed] [Google Scholar]
- 17.Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerbini G, Lorenzi M, Palini A. doi: 10.1056/NEJMc081278. Tumor angiogenesis [comment on N Engl J Med 2008;358:2039–2049]. N Engl J Med 2008;359:763; author reply 764. [DOI] [PubMed] [Google Scholar]
- 19.Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR; International Society of Hematotherapy and Graft Engineering Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. Cytometry 1998;34:61–70 [PubMed] [Google Scholar]
- 20.Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A 1990;87:404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhardinger C, Costa MB, Coulombe MC, Toth I, Hoehn T, Grosu P. Expression of acute-phase response proteins in retinal Müller cells in diabetes. Invest Ophthalmol Vis Sci 2005;46:349–357 [DOI] [PubMed] [Google Scholar]
- 22.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 2010;363:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 24.Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res 2007;100:590–597 [DOI] [PubMed] [Google Scholar]
- 25.Busik JV, Tikhonenko M, Bhatwadekar A, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med 2009;206:2897–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hristov M, Weber C. Progenitor cell trafficking in the vascular wall. J Thromb Haemost 2009;7(Suppl. 1):31–34 [DOI] [PubMed] [Google Scholar]
- 27.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005;8:211–226 [DOI] [PubMed] [Google Scholar]
- 28.Caballero S, Sengupta N, Afzal A, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007;56:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002;105:3017–3024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.