Abstract
The tumor microenvironment is a pivotal factor in tumorigenesis, and especially in progression, as the pathogenesis of cancer critically depends on the complex interactions between various microenvironmental components. A key component of the tumor immunoenvironment is the infiltration of immune cells, which has been proven to play a dual role in tumor growth and progression. This Janus two-faced function of the tumor immunoenvironment is seen in tumor infiltration by T cells, which correlates with improved patient survival, but also with the homing of multiple subsets of immunoregulatory cells that inhibit the antitumor immune response. Regulatory dendritic cells (regDCs) have recently been shown to be induced by tumor-derived factors and represent a new and potentially important player in supporting tumor progression and suppressing the development of antitumor immune responses. Our recent data reveal that different tumor cell lines produce soluble factors that induce polarization of conventional DCs into regDCs, both in vitro and in vivo. These regDCs can suppress the proliferation of pre-activated T cells and are phenotypically and functionally different from their precursors as well as the classical immature conventional DCs. Understanding the biology of regDCs and the mechanisms of their formation in the tumor immunoenvironment will provide a new therapeutic target for re-polarizing protumorigenic immunoregulatory cells into proimmunogenic effector cells able to induce and support effective antitumor immunity.
Keywords: Dendritic cells, Tumor microenvironment, Immunosuppression, Immune tolerance, Regulatory myeloid cells, CITIM 2011
The tumor immunoenvironment
The tumor microenvironment is a pivotal factor in tumorigenesis and especially in tumor progression as the pathogenesis of cancer critically depends on the complex interactions between various microenvironmental components [1]. Most of the early studies on the tumor immunoenvironment focused on the identification and function of cellular and humoral immune components in the cancer milieu. It was found that immune cells, including T cells, B cells, NK cells, dendritic cells (DCs), and macrophages, have the capacity to infiltrate solid tumors in humans and animals [2, 3]. Numerous immunohistochemical studies have concluded that in the majority of solid tumors, the density of tumor-infiltrating leukocytes inversely correlated with the tumor pathologic grade and stage. That is, more leukocytes were present in well-differentiated and less invasive tumors. Although the present data are contradictory, tumor infiltration by lymphocytes and DCs positively correlates with favorable prognostic features, such as the absence of lymph node metastases, distant metastases, and overall survival [4–6]. For instance, extensive retrospective analyses provide evidence that tumor-infiltrating CD8+ lymphocytes have antitumor activity as judged by their favorable effect on clinical outcome and patient survival in ovarian, renal, lung, colorectal, esophageal, pancreatic, and breast tumors [7–9]. In addition to the data showing a prognostic significance for T cells and DC infiltrates of the primary tumors, new data demonstrate a strong association between the local immune cell profile and chemotherapy outcome in colorectal cancer liver metastases: the immune cell density showed a significant prognostic effect on the progression-free survival under chemotherapy [10].
The potential effects of the immune cell infiltrate in solid tumors are various and intricate. The accumulation of tumor-specific T cells at the tumor site, draining lymph nodes, and in the peripheral circulation suggests uptake of tumor antigens by antigen-presenting cells and T cell priming. Although there is strong evidence that immune effectors can control tumor growth under natural conditions or in response to therapy, it is clear that malignant cells efficiently escape immune surveillance. Interestingly, animal models and cancer patients do not show systemic immunosuppression, as they retain the ability to mount T cell-dependent immune responses to pathogens, model antigens, and experimental cancer vaccines [11]. However, specific antitumor immunity is inhibited, suggesting that tumors may utilize diverse pathways to suppress the antitumor immune response, induce tumor-specific tolerance, or polarize immune cells into protumorigenic immunoregulatory subsets [6, 12]. As a consequence, tumor-specific CTLs are unable to efficiently differentiate, activate, migrate, or respond to targeting signals and stimuli. For many tumors, the process of immune evasion is made evident by the accumulation of myeloid regulatory cells (MRCs) and regulatory T lymphocytes (Tregs) in the tumor tissue, lymph nodes or circulation. The presence of MRCs and Tregs correlates with advanced tumor progression and a poor prognosis.
Myeloid regulatory cells
Myeloid cell subpopulations, including polymorphonuclear neutrophils (PMNs), monocytes, macrophages (i.e., extravasated peripheral blood monocytes), and DCs, are key mediators of inflammatory and immune responses [13]. Myeloid cells play an important role in adaptive immunity. For example, antigen-presenting DCs activate antigen-specific T cells, while PMNs, macrophages, and some immature myeloid cells suppress T cell responses, which have led to the concept of myeloid-derived suppressor cells (MDSCs) [13]. Thus, diversity is a hallmark of tumor-associated myeloid-derived regulatory cells capable of suppressive activity in the blood, lymphoid tissues, and tumors [14]. Some MRC subpopulations, such as TAMs and MDSCs, are well described and in the focus of numerous research teams. Other MRC subsets, such as TIE2-expressing monocytes (TEMs) and N2 PMNs, have only recently attracted the attention of tumor immunologists and now are under intensive investigation. Finally, the availability of information about the newly described regulatory macrophages and regDCs is very restricted due to the lack of specific phenotypic markers, inconsistent terminology, and limited understanding of their role in tumor development and progression.
In the tumor microenvironment, myeloid regulatory and antigen-presenting cells play a primary role in the initiation, maintenance, and outcome of the antitumor immune response. Depending on their maturity, polarization status, activity, and location, myeloid cells can control the differentiation, expansion, and polarization of CD4+ Th1, Th2, Th17, and Treg cells or modulate CD8+ CTL proliferation and activity. Furthermore, tumor-associated myeloid cells may collectively exert both the stimulatory and inhibitory forces on the proliferative, angiogenic, and immunomodulating properties of the tumor, as well as its potential to spread and metastasize. MRCs can also directly affect tumor cell interaction with other critical intratumoral stromal elements, such as fibroblasts and the endothelial cellular network (Fig. 1). For instance, immunogenic (M1) and alternatively activated (M2) macrophages, Tie2-expressing monocytes, and the recently identified regulatory macrophages have all been reported to control both tumor progression and the development of the antitumor immunity [14–16]. More specifically, alternatively activated tumor-associated protumorigenic macrophages have been implicated in carcinogenesis through M-CSF, TNF-α, IL-10, and TGF-β; angiogenesis through VEGF; and local invasion and metastasis through cathepsins B and S. They also contribute trophic functions for the emergence of nascent tumor clones, phagocytose apoptotic tumor cells, recruit other hematopoietic cells, influence the tissue response to hypoxia, and work with Tregs to suppress Th1 and CTL antitumor responses [17].
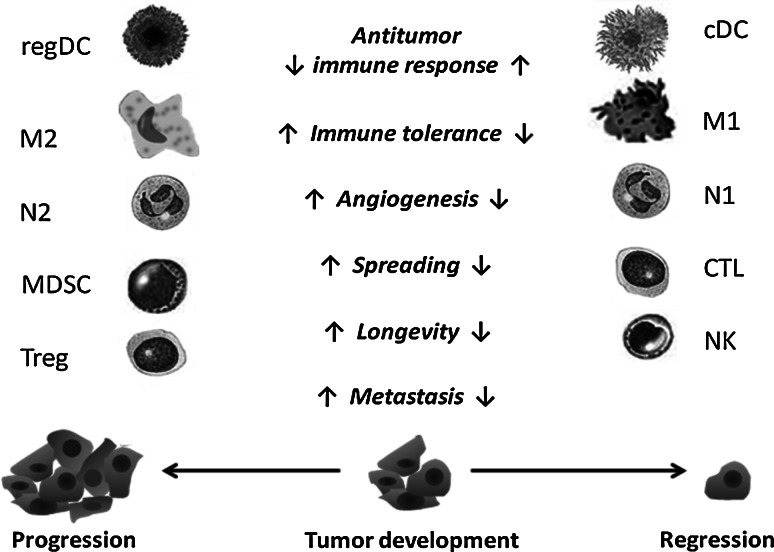
Fig. 1.
Immune effectors and immune regulators in the tumor immunoenvironment. Development and maintenance of the antitumor immune response is associated with the activity of antigen-presenting dendritic cells (conventional DCs or cDCs), cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, inflammatory M1 macrophages, and N1 neutrophils, which collectively induce and regulate antitumor immunity, kill tumor cells, suppress intratumoral angiogenesis, and control tumor spreading. However, in the tumor microenvironment, these cells or their precursors can be polarized into different subsets of immune regulators with protumorigenic activities. These cells include alternatively activated M2 tumor-associated macrophages (TAMs), N2 neutrophils, myeloid-derived suppressor cells (MDSCs), regulatory DCs (regDCs), and regulatory T cells (Tregs). Myeloid regulatory cells and Tregs inhibit the functional activity of CTLs, support polarization of immune effectors into regulatory cells, provide tumor trophic factors, and stimulate tumor invasiveness and metastasis. The functionality of the tumor immunoenvironment is tightly regulated by multiple factors expressed by tumor cells, immune cells, and other cell subsets within the microenvironment
PMN cells in the tumor milieu may be polarized as antitumoral or protumoral tumor-associated neutrophils (TANs). Certain neutrophils can counteract the progression of malignancies through tumor cytotoxicity, tumor rejection, and enhancement of antitumoral immune memory. These cells have recently been designated as “N1 neutrophils” [18]. With the opposite polarity, N2 neutrophils may be detrimental for the host and beneficial for tumor growth, invasion, and metastasis by proteolysis of extracellular matrix components, promotion of angiogenesis and intravasation, and mediation of immunosuppression [19, 20]. New studies indicate that cytokines, such as TGF-β and IFN-β, direct neutrophil polarization within the tumor microenvironment. For instance, TGF-β blockade has been reported to increase neutrophil-attracting chemokines, resulting in an influx of TANs that are hypersegmented, more cytotoxic to tumor cells, and expressing higher levels of proinflammatory cytokines. Accordingly, following TGF-β blockade, depletion of these neutrophils significantly blunted antitumor effects of treatment and reduced CD8+ T cell activation [21]. These data suggest that TGF-β within the tumor microenvironment induces a population of TANs with an N2 protumorigenic phenotype, whereas blocking TGF-β results in the recruitment and activation of TANs with an N1 antitumor phenotype.
A growing body of recent studies describes a heterogeneous population of myeloid cells, termed MDSCs, which are increased in the peripheral blood and tumor microenvironment of patients with various types of cancer. Accumulation of MDSCs in the peripheral circulation has been associated with the extent of disease and correlates with the tumor’s pathological stage. Furthermore, the MDSC level of patients was shown to be an independent prognostic factor for survival [22]. MDSCs have primarily been implicated in promoting tumor growth by inhibiting T cell, NK cell, NKT cell, and DC numbers and function, thus suppressing antitumor immunity. There is also compelling evidence that MDSCs directly facilitate tumor growth, angiogenesis, and metastasis [23]. Two main subsets of MDSCs have been identified in cancer patients: monocytic and granulocytic. Both subsets actively suppress immune cells through a variety of mechanisms such as arginase, NO, and reactive oxygen species [24]. Just as in humans, an accrual of monocytic and granulocytic MDSCs has been noted in the bone marrow, spleen, peripheral circulation, and tumors of tumor-bearing mice. Experimental and clinical tumors contain a large number of MDSCs, accounting for 30–70% of all tumor-infiltrating leukocytes, depending upon the size and type of the tumor [25]. The tumor-induced increase in MDSCs is due to augmented proliferation and differentiation in the bone marrow and subsequent migration and homing to both the tumor and secondary lymphoid tissues, where they regulate inflammatory and immune responses. This increased turnover of MDSCs is regulated by inflammatory mediators produced within the tumor, including cytokines, growth factors, and chemokines [13].
Tolerogenic dendritic cells
One of the most important findings about DCs is that they are not a single cell type, but rather a heterogeneous population of cells that arose from distinct bone marrow-derived hematopoietic lineages and are characterized by specific homing patterns and specialized immune functions. DCs are a highly plastic group of mostly myeloid, intrinsically efficient antigen-presenting cells, which includes tissue-resident DCs, migratory DCs, and inflammatory DCs. The latter are capable of eliciting lymphocyte responses by activating CD4+ Th1 and Th2 cells and CD8+ CTLs [26]. In contrast to these conventional DCs (cDCs), other DC subsets such as the plasmacytoid DCs (pDCs), immature conventional DCs, and regulatory DCs may exhibit potent immunosuppressive and tolerogenic properties in the tumor milieu by blocking proliferation of naïve and antigen-specific CD4+ and CD8+ T cells, supporting polarization and activation of Treg lymphocytes and stimulating intratumoral neovascularization [6, 27, 28].
DCs are released from the bone marrow as precursor cells and differentiate into immature, semimature, and mature DCs after migration into the peripheral tissues [29]. In mice, the lymph nodes contain type-I interferon-producing pDCs and two types of potent antigen-presenting DC subsets: tissue-derived migratory DCs and blood-derived resident DCs [30]. Phenotypically, pDCs are lineagenegCD11clowSiglec-H+. PDCA1 (CD317) is a useful marker of pDCs, but it may be upregulated on various cell types during inflammation. Migratory DCs are lineagenegSiglec-H−CD11clowMHCIIhi and resident DCs are lineagenegSiglec-H−CD11chiMHCIIint. Since migratory DCs and a proportion of resident DCs may express CD11b, a CD11cneg gate is required when identifying macrophages in peripheral lymphoid tissues [13]. However, in the spleen and lymph nodes, CD11chi mononuclear phagocytes are DCs rather than macrophages [17]. Therefore, there is a great deal of ambiguity in distinguishing between DCs, macrophages, and other subsets of MRCs. One source of confusion is the utilization of certain “specific” markers, which may be less “specific” in different microenvironments. For example, the conventional markers used to identify macrophages and DCs in mice, such as F4/80, CD11c, CD11b, and MHC class II, have turned out to be “less specific” than originally believed. For instance, CD11c, the privileged marker of DCs, has been shown to be expressed by tissue-resident macrophages. Consequently, cells that were morphologically and functionally active phagocytes were thought to “convert” into DCs. This led to the concept of the immature DC and then inflammatory or TNF and iNOS-producing DCs (TIP DCs) [17]. Furthermore, it has been proposed that mouse Gr1+ monocyte-derived TIP DCs that arise following infection or during myocardial damage resemble human M1 macrophages, whereas the mouse cells derived from Gr1− monocytes resemble human M2 macrophages [17, 31]. Although a recent study demonstrated that infection-associated CD11b+Ly-6C+CD11c+ inflammatory DCs are the main population of TIP DCs and are derived from CD11b+Ly-6C+ monocytes [32], the lineage relationships among inflammatory monocytes, resident monocytes, TIP DCs, and inflammatory DCs remain to be determined [13].
Another problem in the characterization of MRC subsets is due to two common assumptions: (1) the same markers or combination of markers that can distinguish myeloid cells in the circulation or lymphoid tissues may also exist in non-lymphoid tissues; and (2) the same set of markers are suitable for studying myeloid cells under normal and pathophysiological conditions, such as inflammation and cancer. For instance, mouse MDSCs have been characterized as CD11b+Gr1+ cells, including PMNs, monocytes/macrophages, DCs, and early myeloid precursors [13]. Although GR-1hi PMNs can be considered a major component of CD11b+Gr1+ MDSCs in the bone marrow, blood, and spleen, the majority of tumor-infiltrating MDSCs may, in fact, be Ly-6Chi macrophages [25]. However, blocking TGF-β increases neutrophil-attracting chemokines, resulting in an influx of CD11b+Ly-6C+ tumor-associated neutrophils that are hypersegmented, more cytotoxic to tumor cells and express higher levels of proinflammatory cytokines [21]. Ly-6Chi macrophages are selectively accumulated in tumors due to preferential recruitment by the tumor via the CCR2 signaling axis. Of note, splenic Gr1+ monocytes in tumor-bearing mice can also differentiate into MDSCs that promote the development of tumor-induced Treg cells and anergy [33].
Interdifferentiation of MRC subsets in the tumor microenvironment makes the process of their identification and characterization even more difficult. Regulatory DCs are not an exception. Thus, regDCs are a good example of the misused terminology, mixed phenotyping, and misunderstanding of the cell biology. This misinformation has led to very little data being collected on their function in the tumor microenvironment and, consequently, to insufficient appreciation of their role in tumor development and progression. Any DC subpopulation that is able to directly or indirectly suppress the immune response is commonly termed “regulatory DCs” or “tolerogenic DCs”, resulting in a great deal of uncertainty about their nature and biology. Immature DCs, “alternatively activated” DCs, DCs that have been exposed to immunosuppressive agents, pDCs, and DCs treated with tumor-derived factors or isolated from the tumor environment have all been classified as tolerogenic DCs or regDCs. Unfortunately, in many cases, the tolerogenic function of the DCs was not directly evaluated and the conclusion made based on the low ability of the tested DCs to stimulate T cell proliferation in an allogeneic mixed leukocyte reaction (MLR). It is important to realize that low activity of DCs in MLR does not reflect their ability to suppress T cells or induce tolerance; it only demonstrates an inability of DC to stimulate allogeneic T cell proliferation. In other words, these DCs are suppressed and not fully functional themselves, but they are not necessarily always immunosuppressive. For instance, we and others have reported that DCs generated from their precursors in the presence of tumor-derived factors display low activity in MLRs, low ability to process and present antigens to antigen-specific CD4+ and CD8+ T cells, low endocytic potential (i.e., are not immature DCs), low IL-12 and CD40 expression, altered motility, etc. [34–37]. Thus, tumor-treated DC precursors give rise to functionally deficient DCs with inhibited antigen-presenting function and decreased longevity that, however, did not inhibit proliferation of activated T cells. Therefore, these DCs should not be called tolerogenic or regulatory unless their ability to suppress effector T cell function or induce tolerance is directly verified.
One generally accepted paradigm is that functional properties of DCs are maturation dependent. Tissue-resident DCs receive different signals that drive maturation and differentiation. Upon encounter with T cells, tissue-resident DCs induce distinct responses, depending on their stage of maturation. In the steady state, immature DCs preferentially induce and maintain peripheral T cell tolerance instead of an inflammatory immune response. This functional property correlates with their weak immunostimulatory and migratory capacities. Inflammatory signals and microbial products induce the terminal differentiation of DCs, resulting in dramatic changes in morphology, phenotype, and function. The resulting mature DCs are potent stimulators of T cell immunity that initiate and boost effector T cell responses [38]. However, current evidence suggests that DCs can exist in a multitude of functional states other than simply immature or mature. Additionally, both phenotypically “immature” and “mature” DCs may be conditioned by its microenvironment to support either immune tolerance or immunosuppression [39]. One of the new models of DC maturation suggests that the quality of the maturation signals largely determines the polarization of tissue-resident immature DCs into either tolerogenic or immunogenic effector cells [38]. Interestingly, this model does not exclude an intrinsic tolerogenic function of immature DCs. However, this tolerogenic capacity of immature DCs is restricted to non-inflammatory situations and can easily be modulated in inflammatory or infectious conditions. The applicability of this model to the tumor microenvironment, where a variety of inflammatory, anti-inflammatory, and immunosuppressive signals co-exist, remains to be determined.
Regulatory dendritic cells
Strong evidence supports the presence of DCs with tolerogenic properties in different subsets, including immature and mature myeloid cells, conventional DCs, or pDCs [40, 41]. Thus, DCs with “tolerogenic” function exist in a particular environment under specific conditions. Thus, cDCs are functionally flexible in response to the environment in which they are activated or the type of antigen encountered. These cDCs can become activated DCs that prime effector responses or tolerogenic DCs that modulate and suppress effector responses [40]. The term “regulatory” DC is commonly used interchangeably with “tolerogenic” DC, creating massive controversy in understanding the nature of DCs with immunosuppressive function. For instance, regDCs have been described as cells expressing high levels of CD80 and CD86, producing IL-10 and able to induce differentiation of CD4+ Treg cells [42]. Other groups stated that regDCs express low levels of CD40, CD80, CD86, and MHC class II molecules and suppress the development of experimental allergic encephalomyelitis, allergic inflammation, and autoimmune gastritis [43, 44]. At the same time, Sato et al. [45] suggested that regDCs express high levels of MHC molecules and exceptionally low levels of costimulatory molecules, supporting the generation of CD4+ and CD8+ Treg cells and preventing GVHD. In contrast, Isomura et al. [46] reported that regDCs express high levels of costimulatory molecules CD80 and CD86 as well as high levels of inhibitory B7-H1, B7-DC, and B7-H3 molecules capable of blocking DTH induction. Furthermore, other studies demonstrated that regDCs had lower expression of MHC class II and CD86, but higher levels of CD80 and CD40; produced IL-10 and nitric oxide (NO), but not IL-12 and TGF-β; may activate naive T cells, but did not promote proliferation; and did not induce Treg development [47]. Moreover, Kwon et al. [48] reported that regDCs express high levels of IL-10, TGF-β, COX-2, and indoleamine 2,3-dioxygenase (IDO).
The multiple discrepancies and controversies in phenotypic and functional characterization of the so-called “regulatory” DCs suggest that the use of this term is excessively abused and unstandardized. In fact, conflicting reports further compound the difficulty in understanding the biology of DC subsets with immunosuppressive and/or tolerogenic properties. For example, one body of studies suggests that regDCs are mature cells that can be induced with TGF-β, IL-10, and IL-6 [47, 49, 50], while others find that regDCs remain immature after exposure to prednisolone or Vitamin D3 [51–53]. One should only assign the term “tolerogenic” to DCs that have been proven to induce immunological tolerance or, at the very least, encourage the formation of functional Tregs under various experimental and pathophysiological conditions. Similarly, to slow the growing perplexity, the term “regulatory” should be restricted to DC subsets with experimentally established immunosuppressive activity in the tumor microenvironment.
Using several murine tumor models, Norian et al. [54] found that MHCII+CD11b+CD11c+ tumor-infiltrating DCs may act as regDCs that suppress CD8+ T cell function and antitumor immunity in vivo. Stimulation of naïve T cells with these regDCs altered the T cell fate, resulting in minimal expansion, impaired IFN-γ production, and anergy. Several reports demonstrated that tumor-derived factors, including IL-10, TGF-β, VEGF, and PGE2, may condition DCs to a regulatory state, resulting in low expression of MHC and costimulatory molecules (i.e., CD40, CD80, CD86), leading to T cell anergy and Treg induction [55, 56]. Murine cancers could also drive DCs to differentiate into regDCs with a CD11cloCD11bhiIalo phenotype, high expression of IL-10, NO, VEGF, and arginase I, and an ability to suppress T cells [57]. Our results indicate that semimature DCs generated from their bone marrow precursors can be polarized into regDCs by tumor-conditioned medium, which contains tumor-derived factors. These regDCs actively suppressed proliferation of mitogen-activated T cells in vitro [28] and supported tumor formation in vivo. Tumor-associated regDCs express low level of CD11c, high level of CD11b, low levels of MHC II, and costimulatory molecules and do not express Gr1, PDCA-1, or F4/80. Since bone marrow-derived cDCs (excluding macrophages, granulocytes and MDSCs) can be polarized into regDCs in the tumor microenvironment, regDCs may represent a true subset of tumor-induced DCs that are immunosuppressive or tolerogenic. The unexpected role for DCs as enhancers of tumor progression raises questions about how regDCs convey their tumor-promoting effects and whether they can be harnessed to block tumor-supporting mechanisms while simultaneously activating antitumor immune responses. Our new data suggest that neutralization or prevention of regDC polarization in tumor-bearing mice results in a boosting of tumor-specific immune responses and inhibition of tumor growth.
In spite of the proven protumorigenic nature of tumor-associated regDCs, it is still unknown whether their recruitment and function depend on the tumor type, location, or specific tumor-derived factors. Further, it is still unknown how regDCs interact with MDSCs and macrophages in the tumor immunoenvironment or if regDCs can affect activation and function of cDCs. Additional studies are required for a systemic comparison of different DC subsets displaying a spectrum of immunosuppressive and tolerogenic properties in various environmental conditions. It is conceivable that DCs, as cells with high developmental plasticity, may acquire a tolerogenic or regulatory phenotype somewhat similar to immature DCs in response to specific environmental conditions such as the tumor milieu.
Conclusions
In short, myeloid regulatory cells are infamously heterogeneous, functioning in distinct differentiation and maturation stages such as monocytes, MDSCs, PMNs, macrophages, and DCs that adopt different activation and polarization forms in response to a changing microenvironment. Accumulating evidence shows that MRCs orchestrate the inflammatory events during de novo carcinogenesis, participate in tumor immunosurveillance and immune editing, and contribute to the progression of established tumors. At the tumor site, cells such as TAMs, TANs, and TADCs are confronted with different tumor microenvironment stimuli, leading to MRC subsets with diverse specialized functions and activities, such as regDCs. In spite of the growing controversy in phenotypic classification of and distinguishing between tumor-associated macrophages, granulocytes, DC and MDSC subsets, the role of regDCs in regulating tumor progression and response to therapy has been repeatedly proven and confirmed. However, in addition to verified role of tumor- and stroma-derived factors in attracting and activating MRCs, there are several supplementary pathways that may also participate in controlling the differentiation and function of MRC subsets in patients with cancer (Fig. 2). Many chronic inflammatory conditions and diseases, infections, psychological and mechanical stress of the disease and its treatment, aging, chemotherapy, and other types of therapies significantly alter the status of the immune system in patients with cancer, therefore influencing the interaction between immune cells, immunomodulating the properties of tumor and stroma cells, as well as regulating myelopoiesis and MRC differentiation and function. For instance, it has been recently shown in a randomized clinical trial that psychological intervention to reduce stress in patients with stage II and III breast cancer led to enhanced immune function, fewer recurrences, and improved overall survival. Interestingly, patients with high levels of stress had elevated levels of MDSCs compared to patients with lower stress [58]. Therefore, consideration of the different mechanisms and factors that alter the appearance and function of macrophages, PMNs, DCs, and their precursors in the tumor environment is crucially important for designing novel cellular and molecular-targeted approaches to cancer treatment.
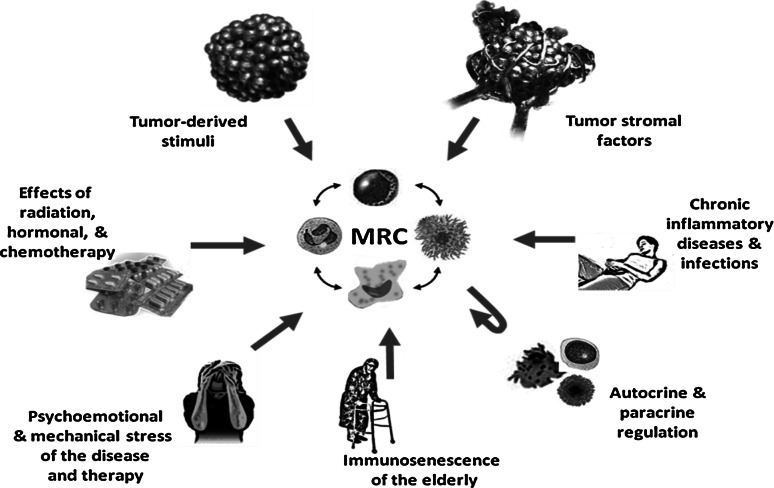
Fig. 2.
Regulation of myeloid regulatory cell polarization, differentiation, and inter-differentiation in patients with cancer. Myeloid regulatory cells (MRCs) represent a mixed population of macrophages, polymorphonuclear neutrophils (PMNs), dendritic cells, and immature myeloid cells at various stages of differentiation, polarization, and activation. A variety of tumor-derived and stroma-derived factors affect the attraction, homing, polarization, and activation of MRCs at the tumor site(s). Cytokines, chemokines, and growth factors produced by immune cells may also control MRC maturation and activity, as well as the distribution of MRCs in the tumor immunoenvironment. The balance between proinflammatory and anti-inflammatory cytokines can be markedly altered by concomitant immune-mediated inflammatory diseases, infections, therapeutic interventions, and age, which also significantly changes the migration and function of MRCs. Additionally, chronic and acute psychoemotional stress in patients with cancer is known to influence neuroendocrine pathways, thus participating in the overall regulation of the immune status and the interactions between individual MRCs as well as with other immune cells
Conflict of interest
In submitting this statement, as corresponding author, I hereby affirm the accuracy of all responses that were entered on line with submission of this manuscript and declare that there is no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Second International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2011), held in Budapest, Hungary, 2nd–5th May 2011. It is part of a CII series of Focussed Research Reviews and meeting report.
References
- 1.Witz IP. The tumor microenvironment: the making of a paradigm. Cancer Microenviron. 2009;1:9–17. doi: 10.1007/s12307-009-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalona WJ, Mann R, Nime F, Potvin C, Harty JI, Gomolka D, Eggleston JC. Identification of complement-receptor lymphocytes (B cells) in lymph nodes and tumor infiltrates. J Urol. 1975;114:915–921. doi: 10.1016/s0022-5347(17)67174-x. [DOI] [PubMed] [Google Scholar]
- 3.Kurihara K, Hashimoto N. The pathological significance of Langerhans cells in oral cancer. J Oral Pathol. 1985;14:289–298. doi: 10.1111/j.1600-0714.1985.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 4.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talmadge JE. Immune cell infiltration of primary and metastatic lesions: mechanisms and clinical impact. Semin Cancer Biol. 2011;21:131–138. doi: 10.1016/j.semcancer.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han B, Ferris RL. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 7.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 8.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, Pages F, Trajanoski Z, Galon J. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–1440. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 10.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel Doeberitz M, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 11.Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192–205. doi: 10.1111/j.1600-065X.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueha S, Shand FH, Matsushima K. Myeloid cell population dynamics in healthy and tumor-bearing mice. Int Immunopharmacol. 2011;11:783–788. doi: 10.1016/j.intimp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manrique SZ, Correa MA, Hoelzinger DB, Dominguez AL, Mirza N, Lin HH, Stein-Streilein J, Gordon S, Lustgarten J. Foxp3-positive macrophages display immunosuppressive properties and promote tumor growth. J Exp Med. 2011;208:1485–1499. doi: 10.1084/jem.20100730. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccard H, Muschel RJ, Opdenakker G (2011) On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol [Epub ahead of print] [DOI] [PubMed]
- 19.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 21.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW (2011) Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother (in press) [DOI] [PMC free article] [PubMed]
- 23.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11:816–826. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 26.Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tisch R. Immunogenic versus tolerogenic dendritic cells: a matter of maturation. Int Rev Immunol. 2010;29:111–118. doi: 10.3109/08830181003602515. [DOI] [PubMed] [Google Scholar]
- 28.Shurin MR, Naiditch H, Zhong H, Shurin GV. Regulatory dendritic cells: new targets for cancer immunotherapy. Cancer Biol Ther. 2011;11:988–992. doi: 10.4161/cbt.11.11.15543. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 32.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 34.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 35.Shurin MR, Yurkovetsky ZR, Tourkova IL, Balkir L, Shurin GV. Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer. 2002;101:61–68. doi: 10.1002/ijc.10576. [DOI] [PubMed] [Google Scholar]
- 36.Tourkova IL, Shurin GV, Wei S, Shurin MR. Small rho GTPases mediate tumor-induced inhibition of endocytic activity of dendritic cells. J Immunol. 2007;178:7787–7793. doi: 10.4049/jimmunol.178.12.7787. [DOI] [PubMed] [Google Scholar]
- 37.Tourkova IL, Shurin GV, Ferrone S, Shurin MR. Interferon regulatory factor 8 mediates tumor-induced inhibition of antigen processing and presentation by dendritic cells. Cancer Immunol Immunother. 2009;58:567–574. doi: 10.1007/s00262-008-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinbrink K, Mahnke K, Grabbe S, Enk AH, Jonuleit H. Myeloid dendritic cell: from sentinel of immunity to key player of peripheral tolerance? Hum Immunol. 2009;70:289–293. doi: 10.1016/j.humimm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Gregori S. Dendritic cells in networks of immunological tolerance. Tissue Antigens. 2011;77:89–99. doi: 10.1111/j.1399-0039.2010.01615.x. [DOI] [PubMed] [Google Scholar]
- 41.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbari O, Umetsu DT. Role of regulatory dendritic cells in allergy and asthma. Curr Allergy Asthma Rep. 2005;5:56–61. doi: 10.1007/s11882-005-0055-3. [DOI] [PubMed] [Google Scholar]
- 43.Torisu M, Murakami H, Akbar F, Matsui H, Hiasa Y, Matsuura B, Onji M. Protective role of interleukin-10-producing regulatory dendritic cells against murine autoimmune gastritis. J Gastroenterol. 2008;43:100–107. doi: 10.1007/s00535-007-2133-x. [DOI] [PubMed] [Google Scholar]
- 44.Fujita S, Yamashita N, Ishii Y, Sato Y, Sato K, Eizumi K, Fukaya T, Nozawa R, Takamoto Y, Yamashita N, Taniguchi M, Sato K. Regulatory dendritic cells protect against allergic airway inflammation in a murine asthmatic model. J Allergy Clin Immunol. 2008;121:95–104. doi: 10.1016/j.jaci.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 45.Sato K, Eizumi K, Fukaya T, Fujita S, Sato Y, Takagi H, Yamamoto M, Yamashita N, Hijikata A, Kitamura H, Ohara O, Yamasaki S, Saito T, Sato K. Naturally occurring regulatory dendritic cells regulate murine cutaneous chronic graft-versus-host disease. Blood. 2009;113:4780–4789. doi: 10.1182/blood-2008-10-183145. [DOI] [PubMed] [Google Scholar]
- 46.Isomura I, Shintani Y, Yasuda Y, Tsujimura K, Morita A. Induction of regulatory dendritic cells by topical application of NF-kappaB decoy oligodeoxynucleotides. Immunol Lett. 2008;119:49–56. doi: 10.1016/j.imlet.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 48.Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, Nam JH, Rhee JH, Hwang KC, Im SH. Generation of regulatory dendritic cells and CD4 + Foxp3 + T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107:2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, Sanchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4 + T cells. J Immunol. 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 50.Usui Y, Takeuchi M, Hattori T, Okunuki Y, Nagasawa K, Kezuka T, Okumura K, Yagita H, Akiba H, Goto H. Suppression of experimental autoimmune uveoretinitis by regulatory dendritic cells in mice. Arch Ophthalmol. 2009;127:514–519. doi: 10.1001/archophthalmol.2009.34. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen AW, Holmstrom K, Jensen SS, Fuchs D, Rasmussen S, Kvistborg P, Claesson MH, Zocca MB. Phenotypic and functional markers for 1alpha, 25-dihydroxyvitamin D(3)-modified regulatory dendritic cells. Clin Exp Immunol. 2009;157:48–59. doi: 10.1111/j.1365-2249.2009.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luther C, Adamopoulou E, Stoeckle C, Brucklacher-Waldert V, Rosenkranz D, Stoltze L, Lauer S, Poeschel S, Melms A, Tolosa E. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J Immunol. 2009;183:841–848. doi: 10.4049/jimmunol.0802046. [DOI] [PubMed] [Google Scholar]
- 53.Fu BM, He XS, Yu S, Hu AB, Zhang J, Ma Y, Tam NL, Huang JF. A tolerogenic semimature dendritic cells induce effector T-cell hyporesponsiveness by activation of antigen-specific CD4+ CD25+ T regulatory cells that promotes skin allograft survival in mice. Cell Immunol. 2010;261:69–76. doi: 10.1016/j.cellimm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via l-arginine metabolism. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4 + CD25 + Foxp3 + regulatory T cells. J Immunol. 2009;182:2795–2807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182:6207–6216. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 58.Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–87. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]