Abstract
Objective
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system. Circulating autoantibodies (NMO-IgG) against astrocyte water channel aquaporin-4 (AQP4) cause complement- and cell-mediated astrocyte damage with consequent neuroinflammation and demyelination. Current NMO therapies, which have limited efficacy, include immunosuppression and plasma exchange. The objective of this study was to develop a potential new NMO therapy based on blocking of pathogenic NMO-IgG to its target, AQP4.
Methods
We generated non-pathogenic recombinant monoclonal anti-AQP4 antibodies that selectively block NMO-IgG binding to AQP4. These antibodies comprise a tight-binding anti-AQP4 Fab and a mutated Fc that lacks functionality for complement- and cell-mediated cytotoxicity. The efficacy of the blocking antibodies was studied using cell culture, spinal cord slice and in vivo mouse models of NMO.
Results
In AQP4-expressing cell cultures, the non-pathogenic competing antibodies blocked binding of NMO-IgG in human sera, reducing to near zero complement- and cell-mediated cytotoxicity. The antibodies prevented the development of NMO lesions in an ex vivo spinal cord slice model of NMO and in an in vivo mouse model, without causing cytotoxicity.
Interpretation
Our results provide proof-of-concept for therapy of NMO with blocking antibodies. The broad efficacy of antibody inhibition is likely due to steric competition because of its large physical size compared to AQP4. Blocker therapy to prevent binding of pathogenic autoantibodies to their targets may be useful for treatment of other autoimmune diseases as well.
INTRODUCTION
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system (CNS) causing lesions mainly in the optic nerve and spinal cord.1,2 Within five years of diagnosis more than half of NMO patients become blind in one or both eyes or require ambulatory assistance, and about one-third die.3 Nearly all NMO patients are seropositive for autoantibodies (NMO-IgG) against extracellular epitope(s) on aquaporin-4 (AQP4),4,5 a water-selective channel expressed strongly at the plasma membrane of astrocyte foot processes throughout the CNS.6,7 A pathogenic role of NMO-IgG in NMO is supported by the high specificity of NMO-IgG seropositivity in NMO, correlations between NMO-IgG titers with disease activity, and the clinical benefit of NMO-IgG depletion.8,9 Additionally, administration of human NMO-IgG to naïve mice or to rats with pre-existing neuroinflammation produces NMO-like pathology.10–13 In cultured cells, including astrocytes, NMO-IgG binding to AQP4 causes complement activation and cytotoxicity.14 NMO-IgG binding to AQP4 in astrocytes in the CNS is thought to initiate a series of inflammatory events, including antibody-dependent complement and cell-mediated astrocyte damage, leukocyte recruitment, cytokine release and demyelination.14,15 Current NMO therapies, which have limited efficacy, include generalized immunosuppression, B-cell depletion and plasmapheresis.16,17
Here, we investigated the possibility of a selective blocker approach to treat NMO. The idea is that blocking of the binding of pathogenic NMO-IgG to astrocyte AQP4, or displacing AQP4-bound NMOIgG, would reduce NMO disease pathology. A recombinant monoclonal antibody approach was used to generate non-pathogenic, high-affinity, anti-AQP4 antibodies that blocked binding of pathogenic NMO-IgG in human NMO serum to extracellular epitope(s) on AQP4 and prevented consequent antibody-dependent complement- (CDC) and cell- (ADCC) mediated cytotoxicity. We present proof-of-concept data in cell culture, ex vivo spinal cord slice and in vivo mouse models for the utility of non pathogenic anti-AQP4 antibodies.
METHODS
Recombinant NMO-IgGs and NMO patient sera
Recombinant monoclonal NMO antibodies (rAbs) were generated from clonally-expanded plasma blasts in cerebrospinal fluid (CSF) as described.10 Point mutations were introduced into the IgG1Fc sequence to generate constructs deficient in CDC (mutation K322A), ADCC (mutations K326W/E333S) or both (mutations L234A/L235A).18–21 Mutated IgG1Fc constructs were subsequently subcloned into the pIgG1Flag vector containing the heavy-chain variable region sequence of rAb-53 to generate constructs encoding the non-pathogenic blocking antibodies. Divalent rAbs and blocking antibodies were generated as described.10 BSA was excluded from the storage solution for surface plasmon resonance measurements. NMO serum was obtained from a total of ten NMO-IgG seropositive individuals who met the revised diagnostic criteria for clinical disease.22 Control (non-NMO) human serum was obtained from a total of three non-NMO individuals, or purchased from the UCSF cell culture facility. For some studies total IgG was purified and concentrated from serum using a Melon Gel IgG Purification Kit (Thermo Fisher Scientific, Rockford, IL) and Amicon Ultra Centrifugal Filter Units (Millipore, Billerica, MA).
Cell culture and transfections
U87MG (ATCC HTB-14) and CHO-K1 (ATCC CCL-61) cells, without or with stable human AQP4 expression, were cultured using standard procedures. NK-92 cells expressing CD16 (Fox Chase Cancer Center) were cultured using standard procedures.
Surface plasmon resonance
Real-time binding of rAbs to AQP4 was measured by surface plasmon resonance at 25 °C using a Biacore T-100 instrument based on reported procedures.23 Purified recombinant human M1 AQP4 (provided by William Harries and Robert Stroud, UCSF) was reconstituted at 3% (wt/wt) in proteoliposomes containing 95:5 L-a-phosphatidylcholine : L-a-phosphatidylserine by detergent dialysis using b-octyl glucoside. Proteoliposomes (and protein-free liposomes as reference) were immobilized on a L1 sensor chip (Biacore) to give 6000 response units of proteoliposome immobilization. For binding measurements, rAbs in PBS were injected for 80 s followed by a 240 s washout period.
NMO-IgG binding to AQP4 in cells
The kinetics of rAb-53 binding to AQP4 was measured by quantitative imaging in U87MG cells stably expressing human AQP4-M23 as described.24 For some studies rAb-53 was fluorescently labeled with Cy3 using standard succinimidyl chemistry.
Complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC)
For assay of CDC, CHO cells expressing human AQP4 were incubated for 30 min with 12.5 μg/ml blocking antibody (or control IgG), then for 90 min at 37 °C with NMO-IgG (2.5 μg/ml) or control IgG and 5% human complement. Calcein-AM and ethidium-homodimer (Invitrogen) were added to stain live cells green and dead cells red. Complement-mediated cytotoxicity by 1–2% NMO patient sera (or control non-NMO sera) and 5% human complement were measured similarly, without vs. with 50–100 μg/ml blocking antibody. For assay of ADCC, NK-92 cells expressing CD16 were used as the effector cells. AQP4-expressing CHO cells were incubated for 30 min with 15 μg/ml blocking antibody (or control-IgG), then for 3 h at 37 °C with NMO-IgG (5 μg/ml) or control-IgG and effector cells (effector : target ratio 30:1).
In vivo NMO model
Adult mice (30–35 g) were anaesthetized and injected intracerebrally, as described,13 with purified total IgG (14 μL, 6–38 mg/mL) isolated NMO patient serum (5 different NMO seropositive patients studied) plus human complement (10 μL), without or with blocking antibody (10 μg). Controls included non-NMO human IgG, AQP4 null mice, and injection of blocking antibody alone. Mice were killed at 24 h after injection and paraffin blocks of brain were coronally at 1.6 mm from the frontal poles for staining with hematoxylin/eosin, Luxol Fast Blue (myelin), AQP4 antibody, and C5b-9. Micrographs were quantified for loss of AQP4 and myelin as described.13,25
Ex vivo NMO model
Postnatal day 7 mouse pups were decapitated and the spinal cord was removed and placed in ice-cold Hank's balanced salt solution (HBSS, pH 7.2), as described.26 Transverse slices of cervical spinal cord of thickness 300 μm were cut using a vibratome, and placed on transparent membrane inserts (Millipore, Millicell-CM 0.4 μm pores, 30 mm diameter) in 6-well plates containing 1 mL culture medium, with a thin film of culture medium covering the slices. Slices were cultured in 5% CO2 at 37 °C for 10 days in 50% minimum essential medium (MEM), 25% HBSS, 25% horse serum, 1% penicillin-streptomycin, glucose (0.65%) and HEPES (25 mM). On day 7, purified IgG (from NMO patient or control sera, 300 μg/ml) and human complement (10 %) were added to the culture medium, without or with blocking antibody (10 μg/ml). Slices were cultured for another 3 days, and immunostained for AQP4, GFAP and MBP. Sections were scored as follows: 0, intact slice with normal GFAP and AQP4 staining; 1, mild astrocyte swelling and/orseen reduced AQP4 staining; 2, at least one lesion with loss of GFAP and AQP4 staining; 3, multiple lesions affecting > 30 % of slice area; 4, lesions affecting > 80 % of slice area.
RESULTS
Generation and characterization of non-pathogenic anti-AQP4 antibodies
The rationale for blocking antibody therapy of NMO is depicted in Fig. 1a. Pathogenic autoantibodies that bind to extracellular epitopes on AQP4 (NMO-IgG) are substantially larger than AQP4 tetramers, preventing the simultaneous binding of more than one antibody. We reasoned, therefore, that a non-pathogenic antibody with high binding affinity and slow washout would compete with the binding of pathogenic antibodies and thus block downstream astrocyte damage and neuroinflammation.
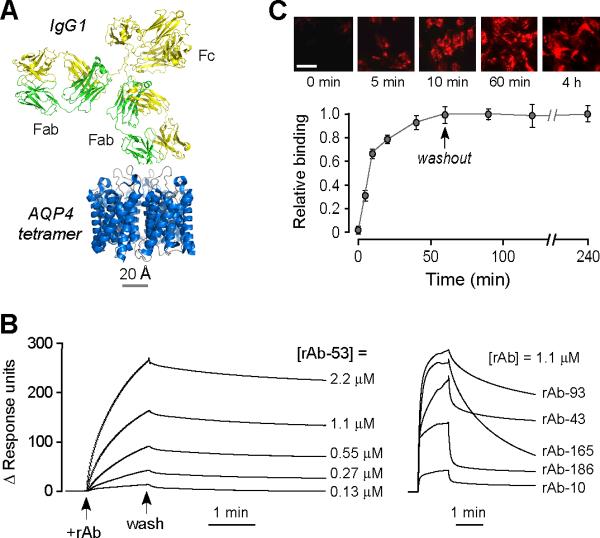
Figure 1. High-affinity monoclonal, recombinant anti-AQP4 antibody for antibody blocking therapy.
a. Crystal structure of AQP4 tetramer shown on the same scale with that of an IgG1 antibody. b. Surface plasmon resonance measurement of recombinant antibody binding to AQP4-reconstituted proteoliposomes showing binding / unbinding kinetics of rAb-53 (left) at different concentrations, and different NMO rAbs (right) at fixed concentration. c. Binding and unbinding kinetics rAb-53 (25 μg/ml) to AQP4-expressing U87MG cells. Binding measured by incubation with rAb-53 for specified times followed by rinsing, fixation and fluorescent secondary antibody addition. Washout measured after 60 min incubation with rAb-53 followed by washout with antibody-free buffer for specified times. Top: Representative micrographs showing cell surface staining by rAb-53 (red). Bottom: Averaged binding data (mean ± S.E., n=4).
In order to engineer suitable non-pathogenic AQP4 antibodies, we generated and screened ten recombinant monoclonal NMO-IgGs that were derived from clonally expanded plasma blast populations in the CSF of three NMO patients. Paired heavy and light chain variable region sequences from single cells were PCR-amplified, cloned into expression vectors containing heavy and light chain constant region sequences, coexpressed in HEK293 cells, and the recombinant IgG purified from supernatants. Binding of each monoclonal recombinant antibody to AQP4 in reconstituted proteoliposomes was measured by surface plasmon resonance. Of ten recombinant antibodies tested, we found highest affinity and slowest washout for antibody rAb-53 (Fig. 1b, left). Binding of rAb-53 to AQP4-proteoliposomes occurred within a few minutes (binding rate constant 1.4 × 104 M−s−1) and washout over many hours (off rate constant 3.8 × 10−4 s−1), with an apparent binding affinity of 27 nM. Other recombinant NMO antibodies had substantially more rapid washout and reduced binding affinity (examples shown in Fig. 1b, right). Slow rAb-53 washout was verified in live cells expressing AQP4. Fig. 1c shows rAb-53 binding over 5–10 minutes, without measurable washout over 3 hours.
Blocking antibodies compete with NMO-IgG binding to AQP4
Point mutations in the Fc portion of rAb-53 were introduced in order to inhibit CDC (K322A), ADCC (K326W/E333S) or both (L234A/L235A), while preserving the AQP4-binding Fab sequences (Fig. 2a). Introduction of these mutations did not affect antibody binding to AQP4, with representative surface plasmon resonance data for one of the mutated antibodies shown in Fig. 2b. As expected, the Fc mutations did not significantly alter on or off binding rate constants or reduce binding affinities.
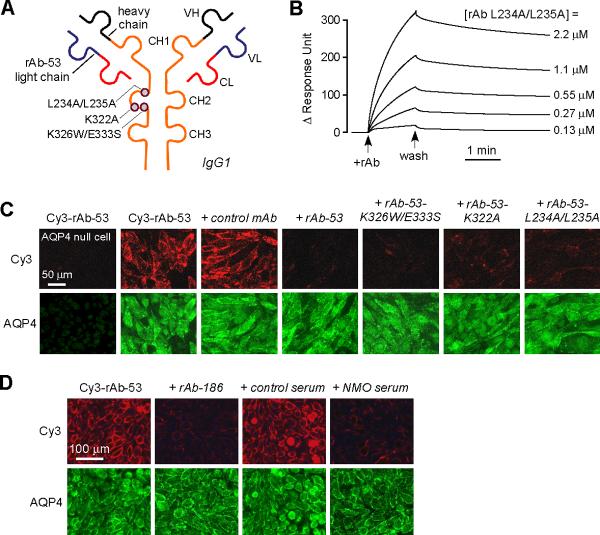
Figure 2. Mutated, non-pathogenic rAb-53 monoclonal antibodies blocks binding of pathogenic NMO-IgG to AQP4.
a. Schematic of rAb-53 showing heavy (VH) and light (VL) chain variable regions, light chain constant region (CL), and IgG1 heavy chain constant regions (CH1-CH3). Locations of amino acid mutations introduced in the CH2 domain to reduce CDC (K322A), ADCC (K326W/E333S) or both (L234A/L235A). b. Surface plasmon resonance measurements of binding and washout of a mutated rAb-53 (L234A/L235A) to AQP4-reconstituted proteoliposomes. c. Mutated rAb-53 blocks binding of Cy3-labeled (non-mutated) rAb-53 to AQP4-expressing cells. Cy3 fluorescence imaged in AQP4-null (left-most panel) or AQP4-expressing (other panels) cells incubated with 20 μg/ml Cy3-rAb-53 for 1 h in the absence or presence of indicated (unlabeled) antibodies at 100 μg/ml. d. Unrelated monoclonal NMO antibodies and human NMO serum block AQP4 binding of Cy3-labeled rAb-53. Cy3 fluorescence imaged in cells incubated with 20 μg/ml Cy3-rAb-53 for 1 h in the absence or presence of 10% control (non-NMO) or NMO patient serum, or 100 μg/ml recombinant NMO monoclonal antibody rAb-186.
To determine whether the mutated rAb-53 antibodies blocked binding of non-mutated rAb-53, rAb-53 was fluorescently labeled with Cy3 under conditions that did not affect binding to AQP4. Fig. 2c shows that a 5-fold excess of each of the mutated antibodies, as well as non-mutated rAb-53, blocked the binding of Cy3-labeled rAb-53 to AQP4-expressing cells. A non-AQP4-specific (isotype control) monoclonal recombinant antibody had no effect. Importantly, human NMO serum, which contains a polyclonal mixture of NMO-IgGs, blocked binding of Cy3-labeled rAb-53 (one of five representative human NMO sera is shown in Fig. 2d), as did other monoclonal NMO antibodies (rAb-186 is shown in Fig. 2d). Non-NMO (control) human serum had no effect. These data suggest competition among NMO autoantibodies for binding to surface epitopes on AQP4.
Blocking antibodies prevent cell killing by NMO-IgG
A major downstream consequence of NMO-IgG binding to cell surface AQP4 is complement-mediated cell killing. Fig. 3a shows a live/dead cell assay in which live cells are stained green and dead cells red. Incubation of AQP4-expressing cells with rAb-53 and complement together caused extensive cell killing. The rAb-53 mutants K322A and L234A/L235A, which are deficient in complement C1q activation, caused little cell killing, whereas K326W/E333S, which has intact complement binding, caused cell killing. In control studies, complement or rAb-53 alone did not cause cell killing, nor did rAb-53 and complement together when incubated with AQP4 null cells (not shown). Fig. 3b shows that a five-fold molar excess of K322A or L234A/L235A greatly reduced cell killing by rAb-53 with complement.
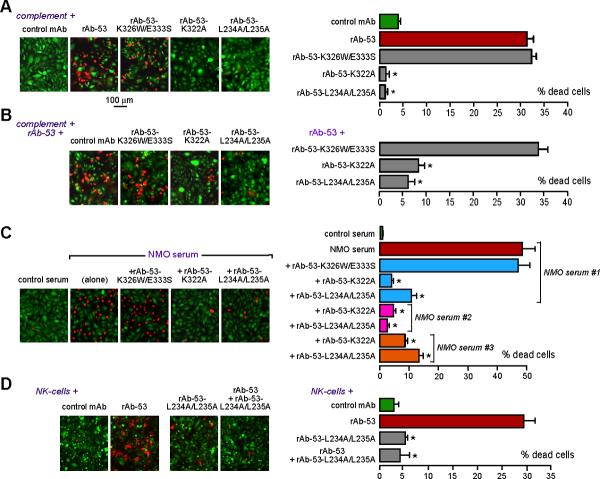
Figure 3. Mutated non-pathogenic rAb-53 prevents CDC and ADCC in NMO-IgG-exposed AQP4-expressing cells.
a. Live/dead cell assay after 90 min exposure of AQP4-expressing CHO cells to human complement together with control (non-NMO) mAb or rAb-53 (2.5 μg/ml, non-mutated or mutated). Percentage dead cells summarized at the right (mean ± S.E., n=4−6, * P < 0.001 compared to rAb-53 alone). b. Assay as in A done with complement + rAb-53, in the presence of 12.5 μg/ml of the indicated blocking antibodies. c. Live/dead cell assay after 60 min exposure to control (non-NMO) serum or NMO patient sera in the presence of complement, and the absence or presence of indicated blocking antibodies. d. ADCC assay done using AQP4-expressing CHO cells incubated with NK-cells together with control (non-NMO) mAb or rAb-53 or blocking antibodies (individually), or rAb-53 and blocking antibodies together.
The polyclonal mixture of NMO-IgGs in NMO patient serum is thought to recognize various overlapping 3-dimensional epitopes on the extracellular surface of AQP4. Fig. 3c shows that rAb-53 mutants K322A and L234A/L235A blocked complement-mediated cell killing by NMO sera from different NMO patients (representative data from 3 of 6 patient sera shown). Control (non-NMO) serum did not cause cell killing. Therefore, the blocking antibodies rAb53-K322A and L234A/L235A block binding of different NMO-IgGs and consequent cell killing, probably by steric hindrance at the AQP4 surface.
The ability of blocking antibodies to reduce NMO-IgG-dependent ADCC was also verified. AQP4-expressing cells were incubated with NK-cells in the absence or presence of rAb-53 and in the absence or presence of rAb-53 mutant L234A/L235A. Fig. 3d shows marked killing by NK-cells in the presence of rAb-53, with little killing by NK-cells in the presence of control or blocking antibody. Inclusion of blocking antibody L234A/L235A during the incubation with NK-cells and rAb-53 greatly reduced cell killing.
Blocking antibody reduces NMO lesions in an in vivo mouse model
Proof-of-concept studies were done in in vivo and ex vivo NMO models to investigate the efficacy of blocking antibody in reducing NMO lesions. NMO lesions were created in mouse brain in vivo by intraparenchymal injection of IgG purified from NMO serum, together with human complement.25 At 24 h after injection, there was marked inflammatory cell infiltration (primarily neutrophils), loss of AQP4 and myelin, and vasculocentric complement activation in the injected hemisphere (Fig. 4a). In control experiments, there was little or no inflammatory cell infiltration, loss of myelin or AQP4, or complement activation following intracerebral injection of: (i) control (non-NMO) human IgG with complement; (ii) NMO-IgG with complement in AQP4 null mice; or (iii) blocking antibody alone. Coinjection of NMO-IgG and complement with blocking antibody greatly reduced AQP4 and myelin loss, as quantified for a series mice in Fig. 4b. Fig. 4c shows data from five pairs of mice in which NMO-IgG from different seropositive NMO patients was injected with or without blocking antibody. Blocking antibody greatly reduced lesion size.
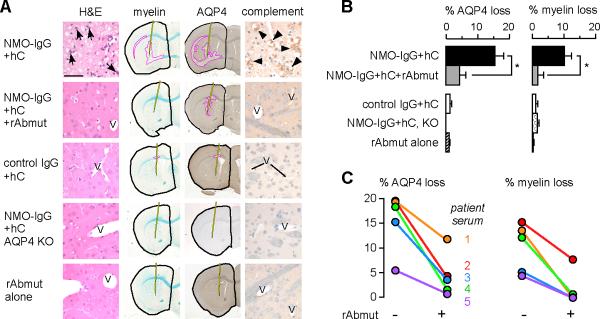
Figure 4. Blocking antibody reduces NMO-like lesions in mouse brain in vivo produced by intracerebral injection of NMO-IgG and human complement.
a. Panel of mouse brain sections at 24 h after intracerebral injection, stained with hematoxylin and eosin (H&E) and Luxol fast blue (myelin), and immunostained brown for AQP4 (AQP4) and C5b-9 (activated complement). Intracerebral injections were made of NMO-IgG (purified IgG from NMO serum) and human complement, without or with blocking antibody (rAbmut), with controls (control IgG, AQP4 knockout mice, rAbmut alone). Pink line indicates areas of absent Luxol fast blue staining or AQP4 immunoreactivity. Black line outlines the injected hemisphere and shows needle tract. Arrows, neutrophils; arrowheads, perivascular C5b-9 immunoreactivity; V, vessel. Bar, 50 μm. b. AQP4 and myelin loss quantified as % area outlined with pink / area outlined with black (S.E.M., 5 mice per group, * P < 0.01). c. % myelin and AQP4 loss shown for five pairs of mice, each pair injected with NMO-IgG from a different NMO patient with human complement, without or with rAbmut.
Blocking antibody reduces NMO lesions in an ex vivo spinal cord slice model
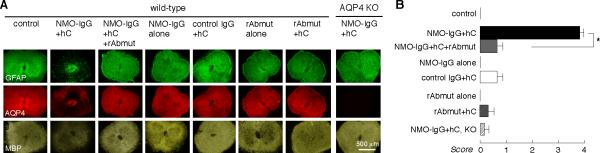
Studies were also done in an ex vivo spinal cord slice model of NMO in which spinal cord slices from mice were cultured for 7 days, and then incubated for 3 days with NMO-IgG (purified IgG from NMO patient serum) and human complement.26 This ex vivo model allows for exposure of CNS tissue to antibodies and complement under defined conditions. As shown in Fig. 5a, NMO-IgG and complement produced characteristic NMO lesions with marked loss of AQP4, GFAP and myelin immunofluorescence, which was not seen in the absence of complement or in spinal cord slices from AQP4 null mice. Inclusion of blocking antibody greatly reduced the severity of NMO lesions, with preservation of AQP4, GFAP and myelin. Incubation with blocking antibody alone or with complement produced little or no pathology. Fig. 5b summarizes histological scores of NMO lesion severity. Near complete protection by blocking antibody was also found against rAb-53 as well as NMO-IgG from two other NMO patients (data not shown).
Figure 5. Blocking antibody reduces NMO-like lesions produced by NMO-IgG and human complement in ex vivo spinal cord slice cultures.
a. Ex vivo spinal cord slice culture model in which slices were cultured for 7 days, followed by 3 days in the presence of NMO-IgG (purified IgG from NMO serum) and human complement, without or with blocking antibody (rAbmut). Immunostaining shown for AQP4, GFAP and myelin. Controls include non-NMO IgG, NMO-IgG or rAbmut alone, rAbmut with complement, and slice cultures from AQP4 null mice. b. NMO lesion scores (see Methods) (S.E.M., n=4−5, * P < 0.001).
DISCUSSION
Our data provide proof-of-concept for the potential utility of blocking antibodies for NMO therapy. The engineered high-affinity, non-pathogenic, recombinant monoclonal antibodies blocked cell surface AQP4 binding of polyclonal NMO-IgG in NMO patient sera in cell culture, ex vivo spinal cord and in vivo mouse models of NMO, preventing downstream cytotoxicity and NMO lesions. Further testing of blocking antibody therapy in new animal models of NMO is indicated as they become available.
Though monoclonal antibody therapy has been used for a wide variety of targets and diseases, the use of a non-pathogenic blocking monoclonal antibody is novel, as is the idea of targeting an autoantibody-antigen interaction for therapy of an autoimmune disease. NMO is a unique disease ideal for monoclonal antibody blocker therapy because the single target of pathogenic autoantibodies, AQP4, is a plasma membrane protein having a small extracellular footprint compared to antibody size, and pathology is dependent on antibody effector function.
Though mutated, complete IgG1 antibodies were used here for initial proof-of-concept studies, many modifications are possible to augment the therapeutic efficacy of blocking antibodies. Variations in antibody design, such as the use of single-chain antibodies or antibody conjugates,27 may increase antibody stability and CNS penetration,28 and mutagenesis of the variable domains may increase AQP4 binding avidity.29.30 Alternative antibody isotypes, such as IgG4, may increase therapeutic efficacy by eliminating residual effector function in the IgG1 Fc region.31 Intravenous blocking antibody therapy for NMO is potentially useful during acute disease exacerbations to reduce NMO pathology when the blood-brain barrier at the lesion site is open, and perhaps for maintenance therapy to reduce the frequency and severity of exacerbations. Intravitreal administration of blocking antibody may be efficacious in limiting retinal ganglion cell loss following optic neuritis in NMO.
It is important that the blocking antibody itself not produce CNS pathology. Pathology was not seen following blocking antibody incubation with spinal cord slices or direct intracerebral injection. Although the NMO attack severity has been correlated with the degree of complement activation,32 the possibility of complement- and cell-independent NMO pathology has been proposed.33 It has been suggested from data in a transfected cell model that NMO-IgG causes AQP4 and EAAT2 internalization,34 which may contribute to NMO pathology. If correct, similar internalization by blocking antibody is possible. However, we have found little or no NMO-IgG-induced loss or internalization of AQP4 in astrocytes in the intact CNS (unpublished data). Indeed, the complete absence of AQP4 in mice does not cause baseline abnormalities in CNS anatomy or function; only significant stresses produced phenotypes of altered water balance,6,35 neuroexcitation,36,37 glial scarring,38,39 and neuroinflammation.40 Though AQP4 is also expressed outside of the CNS in kidney, lung, stomach, skeletal muscle and exocrine glands, its deletion in mice does not produce pathology or significant functional impairment.41 It is thus unlikely that blocking antibody therapy would itself produce toxicity, though a full, formal evaluation of toxicity is needed for further pre-clinical development. As with any therapy involving monoclonal antibodies, there is a potential for the generation of anti-idiotypic antibodies directed against the monoclonal antibody. Such anti-idiotype antibodies may diminish the effectiveness of the anti-AQP4 monoclonal antibody or paradoxically enhance immune responses against CNS astrocytes by binding to astrocyte-bound therapeutic antibody and activating complement.
In conclusion, blocking of NMO-IgG interaction with AQP4 by non-pathogenic antibodies represents a novel approach for NMO therapy. Non-pathogenic blocking antibodies may have therapeutic utility in other autoimmune diseases as well.
Acknowledgments
This work was supported by grants from the Guthy-Jackson Charitable Foundation (A.S.V., M.C.P. and J.L.B.), grants EY13574, EB00415, DK35124, HL73856, DK86125 and DK72517 from the National Institutes of Health (to A.S.V.), and Grant RG4320 from the National Multiple Sclerosis Society (to J.L.B.).
Footnotes
Potential Conflicts of Interest. None to report.
REFERENCES
- 1.Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. doi: 10.1038/ncpneuro0764. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 3.Bizzoco E, Lolli F, Repice AM, et al. Prevalence of neuromyelitis optica spectrum disorder and phenotype distribution. J Neurol. 2009;256:1891–1898. doi: 10.1007/s00415-009-5171-x. [DOI] [PubMed] [Google Scholar]
- 4.Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131:3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mader S, Lutterotti A, Di Pauli F, et al. Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS One. 2010;5:e10455. doi: 10.1371/journal.pone.0010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, et al. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6:383–392. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- 9.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JL, Lam C, Kalluri SR, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradl M, Misu T, Takahashi T, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009;66:630–643. doi: 10.1002/ana.21837. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita M, Nakatsuji Y, Kimura T, et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009;386:623–627. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- 13.Saadoun S, Waters P, Bell BA, et al. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133:349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- 15.Parratt JD, Prineas JW. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler. 2010;16:1156–1172. doi: 10.1177/1352458510382324. [DOI] [PubMed] [Google Scholar]
- 16.Cree B. Neuromyelitis optica: diagnosis, pathogenesis, and treatment. Curr Neurol Neurosci Rep. 2008;8:427–433. doi: 10.1007/s11910-008-0066-2. [DOI] [PubMed] [Google Scholar]
- 17.Sellner J, Boggild M, Clanet M, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17:1019–1032. doi: 10.1111/j.1468-1331.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 18.Baudino L, Nimmerjahn F, Shinohara Y, et al. Impact of a three amino acid deletion in the CH2 domain of murine IgG1 on Fc-associated effector functions. J Immunol. 2008;181:4107–4112. doi: 10.4049/jimmunol.181.6.4107. [DOI] [PubMed] [Google Scholar]
- 19.Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 20.Hezareh M, Hessell AJ, Jensen RC, et al. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol. 2001;75:12161–12168. doi: 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idusogie EE, Wong PY, Presta LG, et al. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166:2571–2575. doi: 10.4049/jimmunol.166.4.2571. [DOI] [PubMed] [Google Scholar]
- 22.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 23.Patel AR, Kanazawa KK, Frank CW. Antibody binding to a tethered vesicle assembly using QCM-D. Anal Chem. 2009;81:6021–6029. doi: 10.1021/ac802756v. [DOI] [PubMed] [Google Scholar]
- 24.Crane JM, Lam C, Rossi A, et al. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011;286:16516–16524. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saadoun S, Waters P, MacDonald C, et al. T cell deficiency does not reduce lesions in mice produced by intracranial injection of NMO-IgG and complement. J Neuroimmunol. 2011;235:27–32. doi: 10.1016/j.jneuroim.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Bennett JL, Verkman AS. Aquaporin-4-dependent neuromyelitis optics lesions produced by NMO-IgG in an in vitro spinal cord slice culture model. Ann Neurol. 2011 doi: 10.1002/ana.22551. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagemeyer CE, von Zur Muhlen C, von Elverfeldt D, et al. Single-chain antibodies as diagnostic tools and therapeutic agents. Thromb Haemost. 2009;101:1012–1019. [PubMed] [Google Scholar]
- 28.Kontermann RE. Strategies to extend plasma half-lives of recombinant antibodies. BioDrugs. 2009;23:93–109. doi: 10.2165/00063030-200923020-00003. [DOI] [PubMed] [Google Scholar]
- 29.Igawa T, Tsunoda H, Kuramochi T, et al. Engineering the variable region of therapeutic IgG antibodies. MAbs. 2011;3 doi: 10.4161/mabs.3.3.15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieri P, Donadio E, Rossi S, et al. Antibodies for therapeutic uses and the evolution of biotechniques. Curr Med Chem. 2009;16:753–779. doi: 10.2174/092986709787458380. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko E, Niwa R. Optimizing therapeutic antibody function: progress with Fc domain engineering. BioDrugs. 2011;25:1–11. doi: 10.2165/11537830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Hinson SR, McKeon A, Fryer JP, et al. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch Neurol. 2009;66:1164–1167. doi: 10.1001/archneurol.2009.188. [DOI] [PubMed] [Google Scholar]
- 33.Marignier R, Nicolle A, Watrin C, et al. Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury. Brain. 2010;133:2578–2591. doi: 10.1093/brain/awq177. [DOI] [PubMed] [Google Scholar]
- 34.Hinson SR, Roemer SF, Lucchinetti CF, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008;205:2473–2481. doi: 10.1084/jem.20081241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulos MC, Manley GT, Krishna S, et al. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 36.Binder DK, Yao X, Zador Z, et al. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia. 2006;53:631–636. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- 37.Padmawar P, Yao X, Bloch O, et al. K+ waves in brain cortex visualized using a long-wavelength K+-sensing fluorescent indicator. Nat Methods. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- 38.Auguste KI, Jin S, Uchida K, et al. Greatly impaired migration of implanted aquaporin-4-deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- 39.Saadoun S, Papadopoulos MC, Watanabe H, et al. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Zhang H, Varrin-Doyer M, et al. Proinflammatory role of aquaporin-4 in autoimmune neuroinflammation. FASEB J. 2011;25:1556–1566. doi: 10.1096/fj.10-177279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verkman AS. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev Mol Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]