Abstract
Applied molecular evolution is a rapidly developing technology that can be used to create and identify novel enzymes that nature has not selected. An important application of this technology is the creation of highly drug-resistant enzymes for cancer gene therapy. Seventeen O6-alkylguanine-DNA alkyltransferase (AGT) mutants highly resistant to O6-benzylguanine (BG) were identified previously by screening 8 million variants, using genetic complementation in Escherichia coli. To examine the potential of these mutants for use in humans, the sublibrary of AGT clones was introduced to human hematopoietic cells and stringently selected for resistance to killing by the combination of BG and 1,3-bis(2-chloroethyl)-1-nitrosourea. This competitive analysis between the mutants in human cells revealed three AGT mutants that conferred remarkable resistance to the combination of BG and 1,3-bis(2-chloroethyl)-1-nitrosourea. Of these, one was recovered significantly more frequently than the others. Upon further analysis, this mutant displayed a level of BG resistance in human hematopoietic cells greater than that of any previously reported mutant.
Keywords: human gene therapy; O6-alkylguanine; 1,3-bis(2-chloroethyl)-1-nitrosourea
Recent advances in directed molecular evolution enable the production of large libraries of randomized enzymes. These libraries can contain nucleotide sequences that encode active enzymes that nature has not selected. Entire genes or sequences within a gene can be randomized by multiple techniques, including substitution with random nucleotide oligomers, error-prone PCR, or methods involving in vitro recombination between homologous sequences (1–4). Enzymes with improved activity then can be identified by genetic complementation or high-throughput screening. Mutant enzymes created in this manner include herpes simplex virus thymidine kinase (5), glutathione S-transferase (6), thymidylate synthase (7), and O6-methylguanine-DNA methyltransferase (MGMT) (8–11). In these examples, selected mutants possessed altered substrate specificity, increased catalytic activity, or improved inhibitor resistance, resulting in proteins with more favorable characteristics for gene therapy.
In this report, we sought to identify novel human MGMT sequences that provide resistance to the clinically relevant combination of O6-benzylguanine (BG) and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in human hematopoietic cells. The wild-type (wt) O6-alkylguanine-DNA alkyltransferase protein (AGT), encoded by the MGMT gene, repairs O6-guanine alkylation damage formed by a variety of methylating agents [e.g., N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) and the therapeutic agent temozolomide] and clinically used chloroethylating agents such as BCNU. During DNA repair, the transfer of the alkyl adduct from DNA to a reactive cysteine within AGT inactivates the protein (12–14). The pseudosubstrate BG also reacts with the active-site cysteine to inactivate AGT. BG potentiates alkylating agent toxicity to tumors by depletion of AGT. BG currently is in clinical trials for combination use with BCNU (15).
The dose-limiting toxicity of alkylating agent chemotherapy is myelosuppression, as hematopoietic cells have low endogenous AGT levels. Because BG enhances the myelosuppressive effect of alkylating agents (15, 16, 18, 19)‖, mutant AGTs that are resistant to BG inactivation are candidates for gene therapy-mediated protection of hematopoietic cells. Mutant versions of AGT have been identified that are resistant to BG inactivation (9, 10, 20–24), and hematopoietic gene transfer of some of these mutants has been shown to protect mice from BG and alkylating agent toxicity (17, 25, 26). Effective MGMT gene therapy into hematopoietic cells requires the use of AGT proteins that remain active in concentrations of BG that inactivate wt AGT to ensure that hematopoietic cells are protected from BG at concentrations that deplete AGT in tumors and thus enhance sensitivity to alkylating agents. A second application of BG-resistant MGMT gene transfer is for in vivo selection of transduced cells. The sensitivity of hematopoietic stem cells to BG and BCNU provides powerful in vivo selection pressure favoring the proliferation of BG-resistant MGMT-transduced hematopoietic cells in treated individuals (17, 19).
From an initial library of 8 million randomized MGMT sequences, we selected 17 highly BG-resistant mutants in Escherichia coli (8–10). Because proteins may exhibit differential stability, folding characteristics, and compartmentalization in bacteria vs. human cells, we evaluated the survival of a human hematopoietic cell line harboring these mutants. The sublibrary of 17 mutants was introduced en masse to the human cells by retroviral gene transfer, and, after transduction, the sublibrary was selected under high-stringency BG plus BCNU for surviving clones. These experiments identified a single mutant AGT that was most effective in protecting hematopoietic cells.
Materials and Methods
Generation of Randomized AGTs.
Using random mutagenesis, a large library (1.5 × 106 clones) of AGTs containing mutations downstream from the active-site cysteine (amino acids 150–172) was selected for dual resistance to MNNG and BG, from which nine clones highly resistant to the two compounds were identified (10). Rational screening of other constructed mutants identified three additional clones resistant to BG (L.P.E. and L.A.L., unpublished data). A separate library of mutants (6.5 × 106 clones) containing upstream substitutions (amino acids 137–149) also was used to identify mutant AGTs resistant to BG. In this library, V139 was fixed as F, the active-site region between codons 143–147 (IPCHR) was left unchanged, and all other residues in the region were randomized (9). Among 37 mutants selected from this library that were capable of protecting cells against MNNG, five mutants were highly resistant to BG and MNNG.
Viral Transduction of K562 Cells.
Eighteen MGMT genes, composed of the 17 BG-resistant mutants plus wt AGT, were subcloned individually into the MFG retroviral vector by using a unique NcoI site at the 5′ end and a BamHI site at the 3′ end. The resultant plasmids were mixed at equimolar ratios and transfected into Phoenix retroviral producers (kindly provided by Gary Nolan, Stanford University, Stanford, CA) by using the calcium phosphate protocol found at www.stanford.edu/ group/nolan/protocols/pro_helper_dep.html; the transfection efficiency was 16%. The mixture of recombinant retrovirus was used to infect the human erythroleukemia cell line, K562, at a low multiplicity of infection to ensure only one mutant per cell. Virus produced had a K562 transduction efficiency of 5%.
BG and BCNU Selection of Random Mutants.
In all cases, K562 cells were exposed to 0–800 μM of BG for 1 h before treatment with 20 μM BCNU for 2 h. In the low-stringency selection, K562 cells were selected in 10 μM BG plus BCNU, a combination that kills 99% of untransduced K562 cells. After a 2-week recovery period, the cells were treated with 50 μM BG plus BCNU. In the high-stringency selection, K562 cells initially were selected in 80 μM BG plus 20 μM BCNU and then 2 weeks later were treated with 800 μM BG plus 20 μM BCNU. After recovery, a third treatment of 400 μM BG plus 20 μM BCNU was performed. Surviving cells were plated in methylcellulose, and individual colonies were sequenced for MGMT. In the second selection, the four MGMT sequences were transfected into Phoenix cells in an equimolar mixture before transduction of K562. Transduced K562 first were selected in 10 μM BG and BCNU to deplete untransduced cells from the population and then were treated further with 200 μM BG and BCNU and, 2 weeks later, with 800 μM BG and BCNU. Surviving cells were plated in methylcellulose, and MGMT in individual colonies was sequenced.
BG and BCNU Resistance.
Cells were treated with 0–800 μM BG for 1 h before the addition of 0–80 μM BCNU. After two additional hours in BG and BCNU, cells were washed free of drug and then cultured overnight (14–16 h) in fresh medium containing BG at the initial concentration.
AGT Activity Assay.
Alkyltransferase activity was measured as fmol of labeled methyl released from [3H]MNU-treated substrate DNA. AGT protein expression levels were determined by Western blotting and were used for normalization of activity. BG resistance was determined by 30-min incubation of cell extracts with BG followed by quantitation of alkyltransferase activity.
AGT Protein Stability.
Transduced K562 cells were cultured in complete medium plus 50 μg/ml cycloheximide for 0 or 12 h. The cells then were washed free of drug, resuspended in cell extract buffer (16), and sonicated. Total protein in the cell extracts was quantitated, and 20 μg was resolved by SDS/PAGE (12% gel) and transferred to Immobilon-P nitrocellulose membrane (Millipore). Protein levels were measured by staining with the human AGT-specific mAb, mt3.1 (kindly provided by T. Brent and D. Bigner, St. Jude Children's Research Hospital, Memphis, TN). Band intensities were determined by averaging densitometry values for three different exposures. Protein stability was determined by comparing the 12-h time point to the 0-h time point.
Results
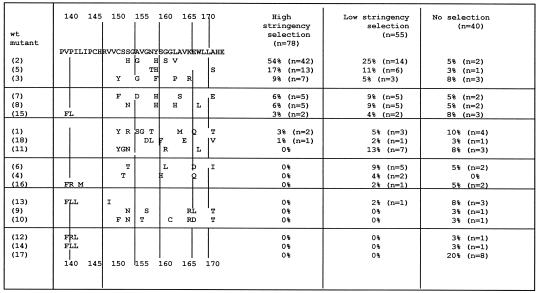
Transduced K562 cells expressing the randomized MGMT sequences were selected in either low-stringency (highest dose, 50 μM BG plus 20 μM BCNU) or high-stringency (highest dose, 800 μM BG plus 20 μM BCNU) conditions and then plated in methylcellulose for clonogenic growth as described in Materials and Methods. After selection, DNA from 55 low-stringency colonies and 78 high-stringency colonies was isolated and the MGMT genes were sequenced (see Fig. 1). Wild-type MGMT was not recovered after either low- or high-stringency selection. Thirteen of the initial 18 sequences were recovered after low-stringency selection. MGMT-2 appeared twice as frequently as the next most frequent sequence, MGMT-11. After high-stringency selection, only eight of the original 18 sequences were recovered. MGMT-11 was selected against after high-stringency selection, suggesting that it had only moderate BG resistance. Strikingly, sequence MGMT-2 was recovered in 54% of the clones and was significantly more prevalent than the next two most common mutants, MGMT-5 (17%) and MGMT-3 (9%) (P < 0.0001, Fisher's exact test).
Figure 1.
Randomized MGMT library used for competitive BG and BCNU selection. The specific substitutions in each randomized sequence are shown on the left. Mutants are arranged in order of their frequency after selection. The number in parentheses identifies the MGMT mutant. All mutants were obtained originally from BG and MNNG selection in E. coli except MGMT-9, MGMT-10, and MGMT-18, which were selected in MNNG alone. MGMT-17 is the wt MGMT sequence and has no substitutions. The table on the right shows the frequency of recovery for each mutant. Pooled K562 cells expressing individual mutants were selected in high-stringency conditions (highest dose of 800 μM BG plus 20 μM BCNU) or low-stringency conditions (highest dose of 50 μM BG plus 20 μM BCNU). The column labeled “No selection” represents the baseline frequency in the library transfected into the retroviral producer cells. Although the library was slightly biased toward wt MGMT, each of the mutants was represented equally.
To confirm that the competition experiment identified the mutant MGMT sequences with the greatest BG and BCNU resistance, we repeated the experiment with only four mutants in the mixture. The apparent “winner” from the first experiment, MGMT-2, was allowed to compete among the two next best mutants, MGMT-3 and 5, along with a mutant that did not survive the high-stringency selection but was frequent after low-stringency selection (MGMT-11). A pool of the four plasmids was transfected into Phoenix cells for K562 cell transduction, and high-stringency BG and BCNU selection was repeated. MGMT-2 and -5 were recovered significantly more frequently (52% and 43%, respectively; n = 21) than MGMT-3 (5%) and MGMT-11 (0%) (P < 0.05, Fisher's exact test). These results demonstrate that selection was reproducible and suggests that MGMT-2 and -5 conferred the strongest BG and BCNU resistance among the 17 mutant sequences tested.
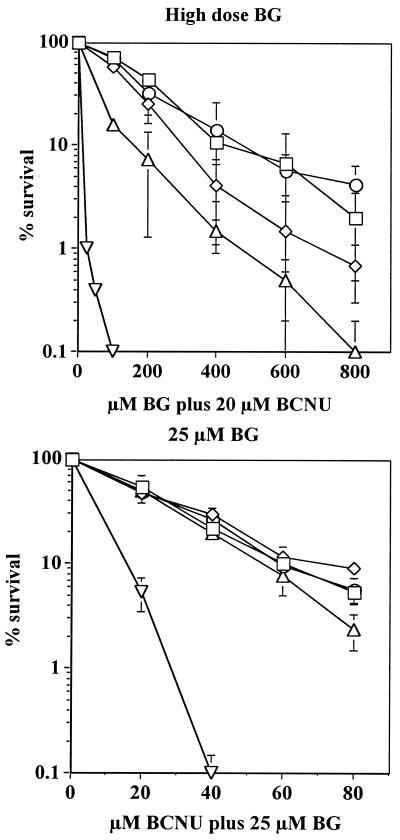
We next measured BG and BCNU resistance conferred to K562 by wt MGMT and each of the four mutants, an essential step in evaluating their potential for drug resistance gene therapy. Cells were treated with 25 μM BG and increasing doses of BCNU to establish the BCNU IC90 [Inhibitory concentration for 90% clonal lethality] in the presence of 25 μM BG. In K562 cells transduced with wt MGMT, the IC90 was ≈15 μM BCNU, whereas expression of the mutants enhanced K562 resistance 3- to 5-fold (Fig. 2A; P < 0.0001, Student's t test). When the cells were treated with increasing doses of BG plus a fixed dose of 20 μM BCNU, MGMT-2 and MGMT-5 transduction conferred the greatest drug resistance (IC90 was 410–500 μM BG; Fig. 2B) and MGMT-11 transduced cells were less drug resistant (IC90 of 160 μM BG; P < 0.05, Student's t test). Although concentrations of BG currently are limited to 25–40 μM in human clinical trials (15), improvements in dosing and the emerging use of new, more stable BG derivatives (29) may warrant gene transfer of strongly BG-resistant proteins to improve hematopoietic stem cell protection.
Figure 2.
K562 cell survival after BG and BCNU treatment. K562 cells retrovirally transduced with MGMT-2 (□), MGMT-3 (⋄), MGMT-5 (○), MGMT-11 (▵), or wt MGMT (▿) were treated with 25 μM BG plus 0–80 μM BCNU in A. Data represent the mean of four experiments. Retrovirally transduced K562 cells were treated with 0–800 μM BG plus 20 μM BCNU in B. There was 90–100% survival of transduced K562 cells at 0 μM BG plus 20 μM BCNU. Data represent the mean of four experiments (bars = SEM).
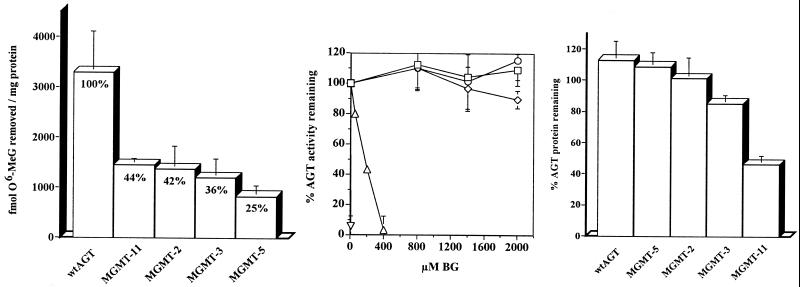
The mutants were characterized further by using extracts of K562 cells transduced with these four MGMT sequences. Assays measuring AGT activity, BG resistance, and protein stability were performed for each mutant in parallel with wt AGT. The activities of AGT-2, -3, -5, and -11 were lower than that of wt AGT by 56–75% (Fig. 3A). Relative to wt AGT, which has a BG ED50 of 0.2 μM (the dose of BG that causes a 50% reduction in AGT activity), AGT-11 was strongly BG-resistant, with a BG ED50 of 160 μM (P < 0.0001, Student's t test; Fig. 3B). AGT-2, -3, and -5 were even more BG resistant (P < 0.0001, Student's t test), retaining full AGT activity at up to 2 mM BG. Equivalent resistance has not been reported for any other MGMT mutant. Additionally, we measured AGT protein stability in K562 cells exposed to 50 μg/ml cycloheximide (Fig. 3C). Whereas AGT-2, -3, and -5 were approximately as stable as wt AGT, AGT-11 was significantly less stable (P < 0.005, Student's t test), with 60% degradation after 12 h in cycloheximide.
Figure 3.
Comparison of AGT activity (A), BG resistance (B), and protein stability (C) in the mutants AGT-2, -3, -5, and -11 and wt AGT. (A) AGT activity was determined in extracts from retrovirally transduced K562. Activity was normalized to protein expression. Percent values represent mutant AGT activity relative to wt AGT activity. Data represent the mean of five experiments. Bars = SD. (B) BG resistance was determined by preincubation of AGT-2 (□), AGT-3 (⋄), AGT-5 (○), AGT-11 (▵), and wt AGT (▿) transduced cell extracts with 0–2,000 μM BG before quantitation of activity. Data are presented as the percent activity remaining after exposure to BG relative to the activity in non-BG-treated controls. Data represent the mean of four experiments. (C) Protein stability was measured by Western blot comparisons of AGT expression levels in transduced K562 treated with 50 μg/ml cycloheximide for 12 h vs. steady-state AGT levels in untreated cells. Protein levels were determined by densitometric quantitation of signal intensity. Data represent the mean of four experiments.
Discussion
Gene transfer of BG-resistant MGMT into hematopoietic cells protects against myelosuppression induced by the combination of BG and O6-guanine-targeted alkylating agents (17, 19, 25, 26). We previously used applied molecular evolution to create and identify mutant human AGT proteins that are resistant to BG, yet retain the ability to protect bacteria against killing by alkylating agents. Here, we report a further improvement in the evolution of MGMT by directly assaying the competitive survival of human hematopoietic cells transduced with evolved mutant MGMT genes after treatment with high concentrations of BG plus BCNU. This represents a critical next step in the improvement of MGMT, as the eventual application of MGMT gene transfer is conferring BG and BCNU resistance in human hematopoietic cells.
Our approach used a two-step selection strategy to facilitate the analysis of a large library of randomized human AGTs. We first screened MGMT libraries, totaling 8 million randomized sequences, in bacteria by using genetic complementation and followed that with a competitive screen in human cells by using a pool of the 17 most favorable mutants. In this manner, we reduced 8 million random MGMT sequences to three strongly favorable proteins that are stable, functional, and highly resistant to BG and BCNU in human cells.
A comprehensive analysis of such large libraries would not be feasible without the bacterial selection, because the introduction of randomized sequence libraries into human cells is limited by poor transfection efficiency. In these studies a high percentage of transfected MGMTs is either inactive or unstable. By initially selecting the large library in E. coli, both nonfunctional proteins and active proteins lacking the desired phenotype are eliminated. This smaller pool of sequences is then amenable to selection in human cells.
The competitive selection not only facilitated the screen of the randomized MGMT pool but also distinguished small differences in drug resistance among the mutants. During competitive selection, cells harboring the most active mutants should exhibit an increased proliferation rate and expand preferentially in culture. Clonogenic assays lack competitive survival and, therefore, are less sensitive to subtle differences between mutants. The sensitivity of the competition assay is highlighted by the recovery of MGMT-2 three times more frequently than MGMT-5 during competitive selection, despite nearly equivalent survival of the two mutants in clonogenic assays.
The AGT protein tolerates multiple mutations while maintaining stability, retaining activity, and obtaining BG resistance. Strong selection seemed to favor proteins with fewer mutations: the average number of amino acid substitutions was 7.8 after randomization, 4.8 after bacterial selection, and 4.7 after high-stringency selection in human cells (P < 0.05, Student's t test). Greater numbers of mutations may destabilize the protein, unless the appropriate compensatory mutations are present.
The high-stringency selection protocol in human cells has identified specific hot spots. Frequently mutated codons included C150, A154, and A170. These codons have not been implicated previously in the protein's mechanism for resistance to BG. Codon Y158 was the most frequently substituted, with conversions to H (six of eight) or F (two of eight). A single Y158H mutation was shown recently to possess a BG ED50 of 600 μM, whereas the double mutant, P140K/Y158H, showed remarkable BG resistance, with an ED50 > 1.2 mM (24). The AGT proteins containing Y158 mutations obtained from our competition experiments contained 3–5 amino acid substitutions and had BG ED50 values of greater than 2 mM, establishing that these mutants are at least as BG-resistant as P140K, the most BG-resistant single mutant known previously (23). In comparison, mutants containing S152N, which were isolated frequently after bacterial selection, were selected against in human cells. One explanation for this may lie in the ability of the protein to fold in different cellular environments (bacteria vs. human). Regardless of mechanism, this finding suggests that bacterial selection alone does not necessarily provide what we eventually desire: mutants most efficacious in human hematopoietic cells.
Novel combinations of amino acid substitutions appear to be responsible for the observed extraordinary BG resistance, and, given our limited knowledge of AGT structure and function, these sequences could not have been rationally designed. In addition, the randomization of MGMT eliminated the structural constraints imposed by the wt neighboring amino acids in natural evolution, permitting the evaluation of sequences that could never evolve naturally.
Protein sequences optimized in human cells for stability and biological activity by applied molecular evolution may augment the success of human gene therapy. Randomization of gene sequences can generate enzymes with unique substrate specificities, increased activities, or resistance to specific inhibitors. Some potential gene therapy-relevant enzymes being explored for optimization include thymidine synthase, dihydrofolate reductase, glutathione S-transferase, 3-methyladenine-DNA glycosylase, aldehyde dehydrogenase, and ribonucleotide reductase (27). A comprehensive, competitive evaluation of libraries of bacterially selected mutants in human cells can identify altered genes not seen in nature that are potentially more efficacious for human gene therapy.
Acknowledgments
We thank Amy Hanson for technical assistance. This work was supported by Public Health Service Grants RO1CA73062, RO1ES06288, UO1CA75525, P30CA43703 (S.L.G.), and CA78885 (L.A.L.) and National Institute of Environmental Health Sciences Training Grant T32 ES07032 (L.P.E.).
Abbreviations
- AGT
O6-alkylguanine-DNA alkyltransferase protein
- MGMT
O6-methylguanine-DNA methyltransferase gene that encodes AGT
- BG
O6-benzylguanine
- BCNU
1,3-bis(2-chloroethyl)-1-nitrosourea
- wt
wild type
- MNNG
N-methyl-N′-nitro-N-nitrosoguanidine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Page, J., Giles, H. D., Phillips, W., Gerson, S. L., Smith, A. C. & Tomaszewski, J. E. (1994) Proc. Am. Assoc. Cancer Res. 35, 328 (abstr.).
References
- 1.Horwitz M, Loeb L A. Proc Natl Acad Sci USA. 1986;83:7405–7409. doi: 10.1073/pnas.83.19.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadwell R, Joyce G. PCR Methods Appl. 1994;3:S136–S140. doi: 10.1101/gr.3.6.s136. [DOI] [PubMed] [Google Scholar]
- 3.Stemmer W. Nature (London) 1994;370:389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Arnold F. Proc Natl Acad Sci USA. 1997;94:7997–8000. doi: 10.1073/pnas.94.15.7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black M, Newcomb T, Wilson H-M, Loeb L. Proc Natl Acad Sci USA. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulick A, Fahl W. Proc Natl Acad Sci USA. 1995;92:8140–8144. doi: 10.1073/pnas.92.18.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landis D, Loeb L A. J Biol Chem. 1998;273:11611–11618. doi: 10.1074/jbc.273.40.25809. [DOI] [PubMed] [Google Scholar]
- 8.Christians F, Loeb L A. Proc Natl Acad Sci USA. 1996;93:6124–6128. doi: 10.1073/pnas.93.12.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christians F, Dawson B, Coates M, Loeb L A. Cancer Res. 1997;57:2007–2012. [PubMed] [Google Scholar]
- 10.Encell L P, Coates M, Loeb L A. Cancer Res. 1998;58:1013–1020. [PubMed] [Google Scholar]
- 11.Encell L P, Loeb L A. Biochemistry. 1999;38:12097–12103. doi: 10.1021/bi9913606. [DOI] [PubMed] [Google Scholar]
- 12.Pegg A E, Roberfroid M, von Bahr C, Foote R S, Mitra S, Bresil H, Likhachev A, Montesano R. Proc Natl Acad Sci USA. 1982;79:5162–5165. doi: 10.1073/pnas.79.17.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sassanfar M, Dosanjh M K, Essigmann J M, Samson L. J Biol Chem. 1991;266:2767–2771. [PubMed] [Google Scholar]
- 14.Koike G, Maki H, Takeya H, Hayakawa H, Sekiguchi M. J Biol Chem. 1990;265:14754–14762. [PubMed] [Google Scholar]
- 15.Spiro T, Gerson S L, Liu L, Majka S, Haaga J, Hoppel C L, Ingalls S T, Pluda J M, Willson J K. Cancer Res. 1999;59:2402–2410. [PubMed] [Google Scholar]
- 16.Gerson S L, Phillips W, Kastan M, Dumenco L, Donovan C. Blood. 1996;88:1649–1655. [PubMed] [Google Scholar]
- 17.Koç O N, Reese J S, Davis B M, Liu L, Majczenko K J, Gerson S L. Hum Gene Ther. 1999;10:1021–1030. doi: 10.1089/10430349950018418. [DOI] [PubMed] [Google Scholar]
- 18.Dolan M, Roy S K, Fasanmade A A, Paras P R, Schilsky R L, Ratain M J. J Clin Oncol. 1998;16:1803–1810. doi: 10.1200/JCO.1998.16.5.1803. [DOI] [PubMed] [Google Scholar]
- 19.Davis B M, Koç O N, Gerson S L. Blood. 2000;95:3078–3084. [PubMed] [Google Scholar]
- 20.Crone T, Pegg A. Cancer Res. 1993;53:4750–4753. [PubMed] [Google Scholar]
- 21.Crone T, Goodtzova K, Edara S, Pegg A. Cancer Res. 1994;54:6221–6227. [PubMed] [Google Scholar]
- 22.Edara S, Kanugula S, Goodtzova K, Pegg A. Cancer Res. 1996;56:5571–5575. [PubMed] [Google Scholar]
- 23.Xu-Welliver M, Kanugula S, Pegg A. Cancer Res. 1998;58:1936–1945. [PubMed] [Google Scholar]
- 24.Xu-Welliver M, Leitao J, Kanugula S, Pegg A. Cancer Res. 1999;59:1514–1519. [PubMed] [Google Scholar]
- 25.Davis B M, Reese J S, Koç O N, Lee K, Schupp J E, Gerson S L. Cancer Res. 1997;57:5093–5099. [PubMed] [Google Scholar]
- 26.Chinnasamy N, Rafferty J A, Hickson I, Lashford L S, Longhurst S J, Thatcher N, Margison G P, Dexter T M, Fairbairn L J. Gene Ther. 1998;5:842–847. doi: 10.1038/sj.gt.3300699. [DOI] [PubMed] [Google Scholar]
- 27.Encell L P, Landis D, Loeb L A. Nat Biotechnol. 1999;17:143–147. doi: 10.1038/6142. [DOI] [PubMed] [Google Scholar]