Abstract
Previously, we reported the characterization of two novel antibodies that react with tau nitrated at tyrosine 197 (Tau-nY197) and tyrosine 394 (Tau-nY394) in Alzheimer’s disease (AD). In this report, we examined whether tau nitration at these sites also occurs in corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Pick’s disease (PiD), three neurodegenerative tauopathies that contain abundant tau deposits within glial and neuronal cell types but lack amyloid deposition. The reactivity of these antibodies was also compared to two previously characterized antibodies Tau-nY18 and Tau-nY29, specific for tau nitrated at tyrosine 18 and tyrosine 29, respectively. In the present experiments, Tau-nY18 did not label the classical pathological lesions of CBD or PSP but did label the neuronal lesions associated with PiD to a limited extent. In contrast, Tau-nY29 revealed some, but not all classes of tau inclusions associated with both CBD and PSP but did label numerous Pick body inclusions in PiD. Tau-nY197 was restricted to the neuropil threads in both CBD and PSP; however, similar to Tau-nY29, extensive Pick body pathology was clearly labeled. Tau-nY394 did not detect any of the lesions associated with these disorders. In contrast, extensive neuronal and glial tau pathology within these diseases was labeled by Tau-Y197, a monoclonal antibody that reacts within the Y-197-containing proline-rich region of the molecule. Based on our Western and IHC experiments, it appears that nitration of tau at tyrosine 29 is a pathological modification that might be associated with neurodegeneration. Collectively, our data suggest that site-specific tau tyrosine nitration events occur in a disease and lesion-specific manner, indicating that nitration appears to be a highly controlled modification in AD and non-AD tauopathies.
Keywords: Tau, Tyrosine nitration, Alzheimer’s disease, Monoclonal antibody, Tauopathies
Introduction
Alzheimer’s disease (AD), corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Pick’s disease (PiD) are a diverse group of neurodegenerative tauopathies that share several pathological similarities, notably progressive accumulation of modified tau proteins in selective brain regions [29]. Non-AD tauopathies, however, differ significantly from AD in several ways. First, non-AD tauopathies are rare disorders that exhibit a variety of clinical features including cognitive and motor deficits [30]. Second, non-AD tauopathies are uniquely characterized by the intracellular aggregation of the tau protein within both glial and neuronal cell types, affecting mostly the frontal neocortex, basal ganglia, deep cerebellar nuclei as well as certain elements of the limbic system [8, 29]. Third, unlike AD which involves the self-aggregation of all six tau isoforms [14, 15], non-AD tauopathies exhibit remarkable selectivity in tau isoform aggregation (for review, see [21]). For instance, tau isoforms containing four microtubule binding repeats (4R) compose the major tau inclusions identified within the glial and neuronal cell types in both CBD and PSP [9, 28]. In contrast, aggregates formed in PiD are largely composed of tau isoforms containing three microtubule binding repeats (3R) [6]. Furthermore, the formation of amyloid plaques, a well-known pathological hallmark in AD, is not considered to be a pathological marker in these rare tauopathies, indicating that tau may serve as the primary agent of neurodegeneration. Support for this contention is provided by the discovery of mutations within the tau gene associated with frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), leaving little doubt that the altered tau protein alone is sufficient to cause neurodegeneration [10, 23, 34].
In AD, the temporal and spatial progression of tau inclusion formation correlates well with neurodegeneration and cognitive decline [1, 3]. Although relatively little is known about this process in non-AD tauopathies, recent findings indicate that formation of tau aggregates in these diseases is similar but not identical to those found in AD [17]. For instance, several modifications associated with tau aggregation identified during early stages of tangle formation in AD have also been documented in non-AD tauopathies, including the Alz-50 conformation [4] and several phosphorylation events within tau [2]. However, as tau inclusions mature, post-translational modifications known to occur during the intermediate (Tau-C3, Tau-66) or late (MN423) stages of tangle formation in AD are absent in these rare tauopathies [4, 17]. These observations suggest that tau inclusions in non-AD tauopathies are likely processed differently by the cells, indicating potential mechanistic divergence between the pathogenesis leading to AD versus non-AD tauopathies.
In a previous report, our laboratory characterized two nitration-specific monoclonal antibodies termed Tau-nY18 and Tau-nY29 which react with tau nitrated at tyrosine 18 and tyrosine 29, respectively [36, 39]. In AD, Tau-nY18 localized largely to reactive glia cell types, whereas Tau-nY29 recognized the classic tau pathology in tissue sections [36, 39]. In non-AD tauopathies, Tau-nY29 revealed several pathological features associated with CBD, PSP and PiD suggesting that tau at tyrosine 29 was susceptible to nitration in all tauopathies including AD [39]. Intriguingly, Tau-nY18 did not detect either the neuronal or the glial tau lesions characteristic of CBD or PSP, indicating that tau nitration within the glial pathology in AD may be different from the glial pathology in these rare tauopathies [36]. In the present report, we compared the nitration of tau at tyrosine 18 and tyrosine 29 with nitration of tau at tyrosine 197 and tyrosine 394 using antibodies selective for tau nitrated at each of these residues [35]. Collectively, our data signify that site-specific nitration events occur within the tau molecule in a disease- and lesion-specific manner, indicating that nitration is likely a highly controlled modification in neurodegenerative tauopathies.
Materials and methods
Sarkosyl-soluble and sarkosyl-insoluble tau preparations
Frozen blocks from the frontal cortex were obtained from the Northwestern University Cognitive Neurology and Alzheimer’s Disease Center (CNADC) Brain Bank (Table 1). Sarkosyl-soluble and sarkosyl-insoluble tau fractions were prepared as previously reported [20]. Briefly, 1 g of frozen tissue was homogenized in solution A (10 mM Tris (pH 7.5), 10 mM EGTA, 1 mM dithiothreitol, 10% sucrose) at 4°C and centrifuged at 23,000×g for 15 min. The supernatant from this centrifugation containing the bulk of the soluble tau was discarded. The pellets were then extracted in solution A containing 1% Triton X-100 and centrifuged at 386,000×g for 20 min also at 4°C. The resultant pellets were subsequently homogenized in solution A containing 1% sarkosyl, incubated at 37°C for 1 h and centrifuged at 386,000×g for 20 min at 4°C. The supernatant from this spin was used as the sarkosyl-soluble tau fraction. The sarkosyl-insoluble pellets were then solubilized in 7 M guanidine and dialyzed in Tris–EDTA at 4°C overnight using a Slide-A-Lyzer dialysis cassette (Pierce, Rockford IL) and stored at −80°C until further use as the sarkosyl-insoluble tau fractions.
Table 1.
Human tauopathy cases used for IHC and Western analysis
| CBD | Sex | Age | PMI |
|---|---|---|---|
| IHC | |||
| 1 | F | 60 | 0 |
| 2 | M | 61 | 0 |
| 3 | F | 56 | 0 |
| 4 | M | 57 | 2 |
| Western | |||
| 5 | F | 64 | 13 |
| 6 | M | 45 | 20 |
| 7 | M | 67 | 12 |
|
| |||
| PSP | Sex | Age | PMI |
|
| |||
| IHC | |||
| 8 | M | 62 | 3 |
| 9 | M | 54 | 3 |
| 10 | F | 62 | 2 |
| 11 | M | 57 | 0 |
| Western | |||
| 12 | F | 84 | 6 |
| 13 | F | 75 | 18 |
| 14 | M | 68 | 14 |
|
| |||
| PiD | Sex | Age | PMI |
|
| |||
| IHC | |||
| 15 | M | 66 | 16 |
| 16 | M | 75 | 0 |
| 17 | F | 74 | 4 |
| 18 | M | 55 | 4 |
| Western | |||
| 19 | M | 36 | 19 |
| 20 | M | 55 | 9 |
Cases pathologically diagnosed with corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Pick’s disease (PiD) were used to analyze site-specific tau tyrosine nitration. Note that different cases were used for immunohistochemistry (IHC) and Western analysis
PMI postmortem interval (hours), M male, F female
Tau nitration in vitro
Wild type (ht40) and mutant tau proteins (on the ht40 background) were expressed using a pT7C-tau plasmid which drives the expression of the full-length human tau (441 amino acids) fused to an amino terminal 6X His affinity tag [4]. Four tau mutants, each containing a single tyrosine (Y) residue at position Y18, Y29, Y197, or Y394 were generated by mutating all of the other native tyrosine residues to phenylalanine (F) as previously described [37]. Mutant and wild type proteins were treated with peroxynitrite to induce tyrosine nitration [41]. Briefly, purified proteins were buffer exchanged into nitration buffer (100 mM potassium phosphate, 25 mM sodium bicarbonate and 0.1 mM diethylenetriaminepentaacetic acid) [25], then treated with 100-fold molar excess of peroxynitrite (ONOO−) (Cayman Co, Arbor, MI) at room temperature with constant stirring; samples were then stored at −80°C until further use. The peroxynitrite concentration was measured spectrophotometrically at 302 nm in 0.3 M NaOH (ε302 = 1,670 M −1 cm−1) prior to each experiment [41]. Peroxynitrite treatment resulted in proteins nitrated at tyrosines in wild type tau (nht40), and in mutant proteins at Y18 (nY18), Y29 (nY29), Y197 (nY197), and Y394 (nY394). Tau harboring only a single tyrosine residue at tyrosine 310 was not used in this study because previous work demonstrated that tau is seldom nitrated at this site in vitro [37].
Western blot analysis
Human tau samples (Table 1) were separated on 10% SDS PAGE and transferred to nitrocellulose membranes for immunoblot analysis (Trans-Blot, Bio-Rad, Hercules, CA). Where noted, membranes were treated with calf intestinal phosphatase (CIP, 10 U/ml in Tris buffer saline) overnight at 4°C to remove phosphate groups (New England Biolabs, Ipswich, MA). Membranes were then blocked with 5% non-fat dry milk in Tris buffered saline and incubated with primary antibodies at 4°C overnight. After rinsing, membranes were incubated in peroxidase-conjugated goat anti-mouse IgG H + L secondary antibodies for an hour at room temperature (Vector Laboratories, Burlingame, CA), and then washed and incubated with ECL substrate (Pierce, Rockford, IL). Signal was visualized on Kodak Bio-max XAR film (VWR International, Batavia, IL).
Immunohistochemistry
Human postmortem brain tissue fixed in 4% paraformaldehyde was obtained as free-floating 40-μm thick sections from the CNADC Brain Bank (Table 1). Tissue sections from frontal cortex and hippocampus from pathologically diagnosed cases with CBD, PSP and PiD were processed for immunohistochemistry as described previously [12, 13]. Where noted, CIP treatment was performed to remove phosphate groups from proteins (as described above) prior to processing sections for immunohistochemistry. Sections were then incubated with Tau-nY18 (1 μg/ml), Tau-nY29 (1 μg/ml), Tau-nY197 (40 ng/ml), Tau-Y197 (1 μg/ml), or Tau-nY394 (cell culture medium) at 4°C overnight (Table 2). After rinsing in phosphate buffered saline (pH 7.4), sections were incubated with biotinylated goat anti-mouse antibodies (μ-chain or γ-chain specific) according to the manufacturer’s instructions (Jackson Immunoresearch, West Grove, PA), followed by an hour incubation in avidin–biotin complex solution (Vector Laboratories, Burlingame, CA). Sections were then developed with metal enhanced 3,3′-diaminobenzine (Pierce, Rockford, IL) and mounted onto glass slides, air dried overnight, dehydrated through graded alcohols, cleared with xylenes, and coverslipped with Permaslip (Alban Scientific Inc., St. Louis, MO). Primary antibody dilutions employed were predetermined by testing serial dilutions on adjacent tissue sections to assure the use of subsaturating primary antibody concentrations.
Table 2.
Summary of immunohistochemical observations
| Tau-nY18 | Tau-nY29 | Tau-nY197 | Tau-nY394 | Tau-Y197 | |
|---|---|---|---|---|---|
| CBD | |||||
| Neuropil threads | ± | + | + | − | + |
| Coiled bodies | − | + | − | − | + |
| Perinuclear inclusions | − | + | − | − | + |
| Globose tangles | − | + | − | − | + |
| Astrocytic plaques | − | − | − | − | + |
| PSP | |||||
| Globose tangles | − | + | − | − | + |
| Neuropil threads | − | + | + | − | + |
| Perinuclear inclusions | − | + | − | − | + |
| Coiled bodies | − | + | ± | − | + |
| Thorny and tufted astrocytes | − | + | − | − | + |
| PiD | |||||
| Pick bodies | ± | + | + | − | + |
| Coiled bodies | − | ± | − | − | + |
| Neuropil threads | − | + | + | − | + |
Immunohistochemistry (IHC) was performed on tissue sections from corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Pick’s disease (PiD) using site-specific nitro-tau antibodies and the qualitative observations are presented above
+, pathology observed; −, pathology not observed; ±, limited pathology observed
Immunofluorescence
Double-label immunofluorescence was performed as previously described [12] and tissue sections were incubated with Tau-nY18 (1 μg/ml), Tau-nY29 (1 μg/ml), Tau-nY197 (20 ng/ml), Tau-Y197 (1 μg/ml) or Alz-50 (0.2 μg/ml) antibodies overnight at 4°C (Table 2). Sections were then incubated with either a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse γ-chain specific or a Texas-red-conjugated goat anti-mouse μ chain-specific secondary antibody (Jackson Immunoresearch, West Grove, PA). Autofluorescence due to lipofuscin was blocked with Sudan Black (0.05%) in all sections analyzed [12, 24]. All images were captured as z-stacks of single optical sections using a Zeiss 510 laser scanning confocal microscope.
Results
Site-specific antibodies to nitrated tau
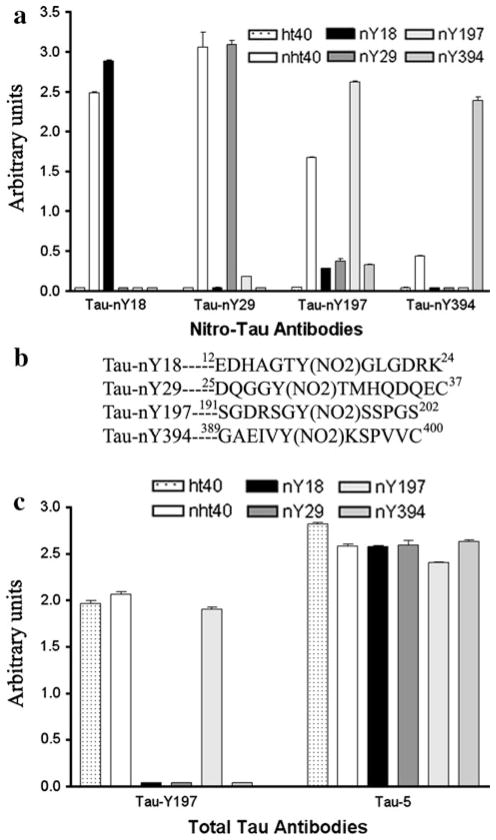
To demonstrate that each nitro-specific tau antibody (Tau-nY18, Tau-nY29, Tau-nY197, and Tau-nY394) reacts exclusively with the correct nitro-tyrosine residue within the tau molecule, wild type recombinant (ht40) and mutant tau proteins containing a single tyrosine residue at position Y18, Y29, Y197 or Y394 were nitrated in vitro using ONOO- and then tested by ELISA (Fig. 1). As expected, each monoclonal antibody selectively bound to nitrated wild type tau (nht40) and mutant proteins containing a nitro-tyrosine corresponding to their specific epitope (Fig. 1a; Table 3). The high selectivity of these antibodies with their respective immunogen is due to their recognition of the nitro-group (NO2) in the context of the surrounding amino acids comprising each epitope, as the peptides used to generate these antibodies were unique (Fig. 1b). It is worth mentioning that Tau-nY18 and Tau-nY29 appear to have a greater affinity to nitrated wild type tau at Y18 and Y29 compared to Tau-nY197 and Tau-nY394, likely due to the in vitro selectivity of the nitrating agent (ONOO−) for tau’s N-terminal tyrosine residues as previously reported [37]. The presence of total tau within these samples was confirmed using Tau-Y197 and Tau-5 (Fig. 1c), pan tau antibodies that react with all tau proteins. Note, however, that Tau-Y197 fails to react with mutant proteins singly nitrated at Y18, Y29 or Y394 since part of this antibody’s epitope requires a tyrosine residue that has been mutated to phenylalanine within these constructs [35]. Although nitration of the phenol group does not affect Tau-Y197’s reactivity, the presence of the tyrosine hydroxyl group appears to be obligatory [35].
Fig. 1.
Determination of relative antibody affinities to non-nitrated and nitrated tau proteins by ELISA. a Tau-nY18, Tau-nY29, Tau-nY197, and Tau-nY394 were incubated with wild type tau (ht40), wild type nitrated tau (nht40) and nitrated mutant tau proteins containing a single tyrosine residue at position Y18, Y29, Y197 or Y394. Note that each antibody selectively binds to wild type nitrated tau and mutant proteins that correspond to its epitope. b The selectivity of these antibodies is due to both the nitro-tyrosine group and the amino acids surrounding it. c Total tau within these samples was labeled using Tau-Y197 and Tau-5, antibodies that bind to both 3R and 4R tau. Tau-Y197 did not react with mutant proteins nitrated at Y18, Y29 and Y394 as these proteins have a Y → F substitution at position 197. Note that all peptides contain an additional cysteine residue at the carboxy end of the sequence to facilitate binding to maleimide-activated KLH for immunizations
Table 3.
Antibodies used within this study
| Antibody | Epitope | Class | Specifications | Reference |
|---|---|---|---|---|
| Alz-50 | 5–15, 312–322 | IgM | Conformation specific | [26] |
| Tau-5 | 210–230 | IgG | Pan tau | [16] |
| Tau-12 | 9–18 | IgG | Pan tau | [25, 32] |
| Tau-nY18 | nY18 | IgG | Nitration specific | [19] |
| Tau-nY29 | nY29 | IgG | Nitration specific | [18] |
| Tau-nY197 | nY197 | IgG | Nitration specific | [20] |
| Tau-nY394 | nY394 | IgG | Nitration specific | [20] |
| Tau-Y197 | Y197 | IgM | Pan tau | [20] |
| RD3 | khqpgggkvqivykpv | IgG | 3R tau specific | [31] |
| RD4 | vqiinkkldlsnvqskc | IgG | 4R tau specific | [31] |
Nitro-specific antibodies were used to determine the presence or absence of nitrated tau at specific tyrosine residues in conjunction with isoform specific or pan tau antibodies
Selective tau tyrosine nitration in corticobasal degeneration (CBD)
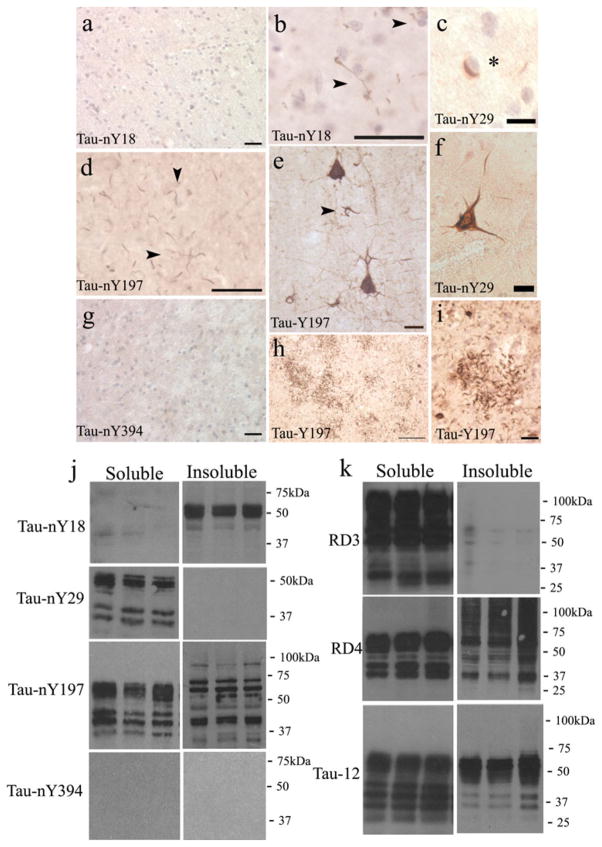
In order to investigate whether the hallmark pathological lesions of CBD contained nitrated tau, immunohisto-chemical analysis (IHC) was performed on tissue sections from the frontal cortex using Tau-nY18, Tau-nY29, Tau-nY197, and Tau-nY394. In cases pathologically diagnosed with CBD, Tau-nY18 did not label the lesions characteristic of the disease, even when CIP treatments or epitope retrieval methods were applied (Fig. 2a), a result that was similar to that previously reported [36]. However, in contrast to the earlier results, one case did show a limited number of Tau-nY18-stained neuropil threads that were present within the gray matter of the frontal cortex following citric acid retrieval methods (Fig. 2b, arrowheads) [33], suggesting that a minor degree of nitration at this site is possible within some aggregates in CBD. In contrast, Tau-nY29 labeled a subset of CBD-associated pathology that included the perinuclear inclusions (Fig. 2c, asterisk), neuropil threads as well as a limited number of ballooned neurons (Fig. 2f). Similar to Tau-nY18, the glial pathology associated with this disorder was not labeled using the Tau-nY29, even when sections were subjected to several epitope retrieval methods (data not shown). Tau-nY197 only labeled a subset of neuropil threads that were present mostly within the gray matter of the frontal cortex (Fig. 2d, arrowheads). However, unlike Tau-nY18 or Tau-nY29, CIP treatment of tissue sections was required to unveil this epitope, suggesting that phosphorylation within the Tau-nY197 epitope prevents antibody binding as shown previously in tissue sections from brains pathologically diagnosed with AD [35]. As expected using the pan tau antibody Tau-Y197, numerous ballooned neurons with extensive processes protruding from the cell bodies (Fig. 2e), as well as the coiled bodies (Fig. 2e, arrowhead), and the distal processes of astrocytes commonly known as astrocytic plaques (Fig. 2h, i) were clearly labeled following CIP treatment, confirming that phosphorylation within the Y197 or nY197 epitopes inhibit antibody binding in CBD [35]. Finally, as was found in AD cases [35], Tau-nY394 did not detect any tau inclusions associated with the disorder even after several epitope retrieval methods were performed (Fig. 2g).
Fig. 2.
Limited tau tyrosine nitration in CBD. a In most cases pathologically diagnosed with CBD, Tau-nY18 did not stain the characteristic lesions of the disease, although one case b did show some neuropil thread staining following citric acid treatment. c Tau-nY29 reacted with the perinuclear inclusions as well as some f globose tangles. d Tau-nY197 only labeled the neuropil treads following CIP treatment. g Tau-nY394 did not label any inclusions. e Numerous globose tangles, coiled bodies (arrow), h, i as well as the astrocytic plaques were labeled using Tau-Y197. j Western blot analysis of soluble and insoluble tau extracts demonstrates that Tau-nY18 did not label soluble tau but did label the insoluble fractions. Conversely, Tau-nY29 labeled soluble but not insoluble tau. Tau-nY197 labeled both, the soluble and insoluble fractions, while Tau-nY394 did not label either of the fractions analyzed. k Abundant soluble tau was detected with RD3, RD4 and Tau-12. The insoluble fractions, however, were only labeled with RD4 and Tau-12 antibodies. In all panels, calibration bars represent 20 μm
To determine whether the tau inclusions observed in CBD contain nitrated sarkosyl-soluble or sarkosyl-insoluble tau, frozen tissue blocks from cases pathologically diagnosed with CBD were fractionated as outlined in “Methods” and as reported previously [20], processed for Western blot analysis, then blotted with Tau-nY18, Tau-nY29, Tau-nY197, and Tau-nY394 (Fig. 2j). Tau-nY18 did not react with the sarkosyl-soluble tau fractions but did label the sarkosyl-insoluble tau proteins in all CBD cases analyzed. Tau-nY29 labeled the sarkosyl-soluble tau but did not react with any of the sarkosyl-insoluble fractions, a result that is strikingly opposite to that observed using Tau-nY18. Tau-nY197 reactivity was shown within both the soluble and insoluble tau fractions, a result that is similar to the results shown from non-demented controls as well as AD brains [35], suggesting that nitration at this site may be a normal event within tau as previously suggested [31]. It is worth noting that within the sarkosyl-insoluble tau fractions, Tau-nY197 appears to label more proteolytic fragments than Tau-nY18. This difference could be due to truncation events at the N-terminus hypothesized to occur at D25 that would eliminate nitration at tyrosine 18 in these proteolytic fragments [22, 40]. Finally, similar to our IHC results, Tau-nY394 did not react with any of the CBD samples analyzed, a result that somewhat mimics those in cases pathologically diagnosed with AD [35]. To confirm the presence of 3R and 4R tau isoforms within these samples, the RD3 and RD4 tau antibodies were used (Fig. 2k). These antibodies selectively label tau proteins containing either 3R or 4R tau isoforms, respectively [5]. Note that both RD3 and RD4 antibodies label the sarkosyl-soluble fractions however, the RD3 antibody reacted poorly with the insoluble fractions, indicating that within the CBD cases analyzed, the majority of tau in the insoluble state is composed mainly of 4R tau isoforms. Total tau within these samples was labeled with Tau-12 [22], an antibody that binds to the N-terminal region of the molecule and does not discriminate between 3R or 4R tau isoforms (Fig. 2k).
Tau tyrosine nitration in PSP
We then stained brain sections from pathologically con-firmed PSP cases with each of the nitro-specific antibodies. As expected, Tau-nY18 did not label the tau inclusions characteristic of this disorder as previously reported [36]. Unlike CBD, epitope retrieval methods had no effect on any of the tissue sections analyzed (Fig. 3a). Tau-nY29 clearly labeled the globose tangles (Fig. 3b) as well as a few neuropil threads [39]. However, the thorny and tufted astrocytic lesions associated with this disorder were not detected. Tau-nY197 reacted poorly with the globose tangles but did label the coiled bodies (Fig. 3c, asterisk) and the neuropil threads to a limited extent following CIP treatment (Fig. 3d, e, arrowheads). In contrast, Tau-Y197 labeled a large number of neuropil threads (Fig. 3g), globose tangles (Fig. 3h, asterisks) as well as the thorny and tufted astrocytes (Fig. 3i, asterisk and arrow, respectively). Similar to Tau-nY197, CIP treatment was required to reveal the tau aggregates in PSP using Tau-Y197, indicating that both CBD and PSP may exhibit similar phosphorylation events that target the proline rich region of the tau protein. Once again, Tau-nY394 did not label any tau inclusions in PSP tissue sections even after epitope retrieval or CIP treatments were performed (Fig. 3f).
Fig. 3.
Selective tau tyrosine nitration in PSP. a Tau-nY18 did not label the pathological inclusions associated with PSP. b Tau-nY29 reacted with the globose tangles and with c the perinuclear inclusions but d, e Tau-nY197 localized to the neuropil threads following CIP treatment. f Tau-nY394 did not label any of the pathological lesions associated with PSP. g Tau-Y197 reacted with numerous neuropil threads and h globose tangles (asterisk) as well as i the thorny (asterisk) and tufted astrocytes associated with PSP. j By Western blot analysis Tau-nY18 did not react with the soluble tau but did label the insoluble fractions whereas Tau-nY29 labeled soluble tau but not insoluble fractions. Tau-nY197 labeled both the soluble and insoluble fractions, while Tau-nY394 did not react with any fractions analyzed. k Abundant soluble tau was detected with RD3, RD4 and Tau-12. The insoluble fractions, however, were only labeled with the Tau-12 antibody. In all panels, calibration bars represent 20 μm
To determine whether tau nitration was present in the sarkosyl-soluble and/or the sarkosyl-insoluble state, Western analysis was performed as above (Fig. 3j). Tau-nY18 did not label the sarkosyl-soluble tau fractions but did label the sarkosyl-insoluble tau isolated from PSP frontal cortex (Fig. 3j). Tau-nY29 labeled the sarkosyl-soluble fractions but failed to react with the sarkosyl-insoluble tau within the PSP cases analyzed. Tau-nY197 labeled both the soluble and insoluble fractions but Tau-nY394 failed to show any reactivity, suggesting nitration at tyrosine 394 does not occur in either CBD or PSP.
As shown for CBD, the RD3 and RD4 antibodies robustly labeled the soluble tau fractions in PSP, suggesting that our soluble tau preparations from CBD and PSP contain a mixture of both 3R and 4R tau isoforms. As expected, the RD3 antibody did not label the sarkosyl-insoluble fractions in PSP, as the tau inclusions associated with this disorder are mainly composed of tau containing the 4R tau isoforms. Note, however, that the RD4 antibody also failed to react with these fractions, a result similar to that previously reported [20]. The lack of reactivity of the RD4 antibody with insoluble PSP tau is surprising, since this antibody clearly labels recombinant tau proteins containing 4R isoforms [5]. The presence of total tau within these samples was shown by its reactivity with Tau-12 (Fig. 3k), rendering this finding even more enigmatic (see “Discussion”).
Abundant nitrated tau is present in Pick’s disease (PiD)
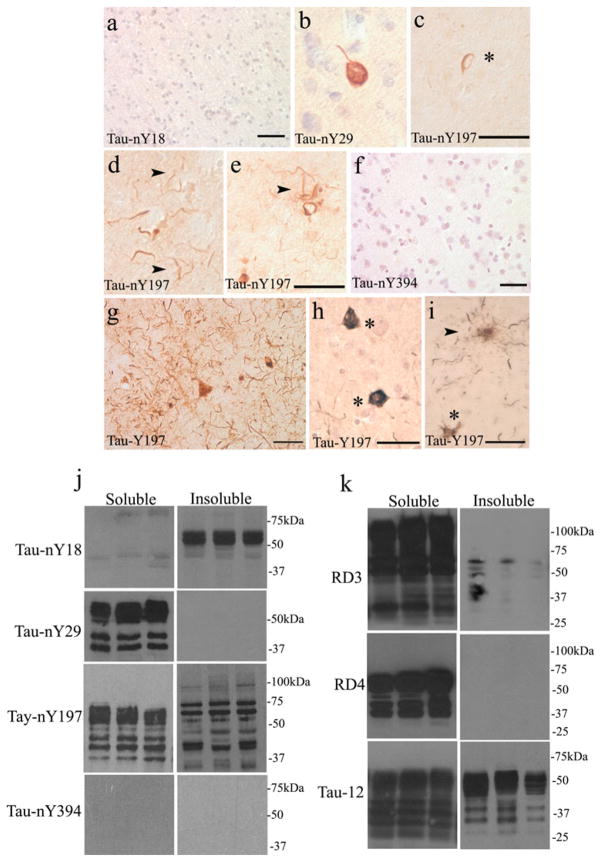
Finally, to determine whether the pathological lesions commonly known as Pick bodies contain nitrated tau, tissue sections from PiD cases were processed for IHC and incubated with all nitro-specific tau antibodies. Unlike CBD or PSP, Tau-nY18 did label a few Pick body inclusions, although a citric acid retrieval method was required to obtain reactivity (Fig. 4a). In contrast, extensive Pick body labeling was observed using Tau-nY29 and Tau-nY197 (Fig. 4b–e, asterisks). Furthermore, the ramified astrocytic pathology characteristic of this disorder was also labeled using both antibodies (Fig. 4c, d, arrowheads). Similarly, Tau-Y197 labeled abundant neuropil threads (Fig. 4h), neuronal Pick body pathology (Fig. 4g, h), coiled bodies (Fig. 4i, arrowheads) and the ramified astrocytic pathology (Fig. 4j, arrowheads) following CIP treatment. As was the case for CBD and PSP, Tau-nY394 did not label the pathology in any of the PiD cases analyzed (Fig. 4f).
Fig. 4.
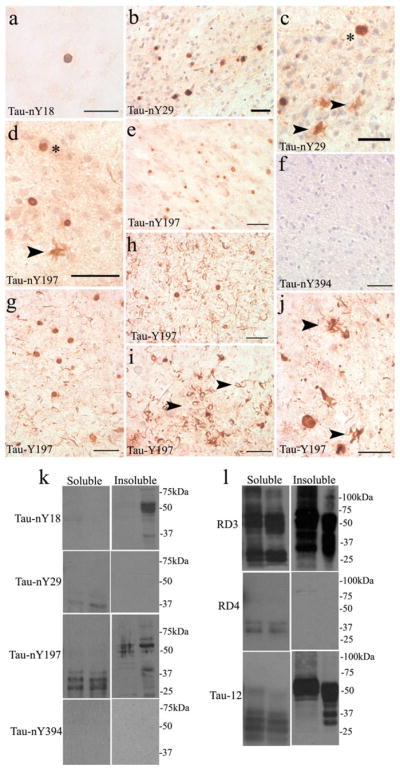
Tau nitration in PiD. a Only a limited number of Pick body aggregates were labeled with Tau-nY18. In contrast, numerous Pick body inclusions reacted with Tau-nY29 (b, c arrowheads) and Tau-nY197 (d, e arrowhead). f Tau-nY394 did not react with any of the lesions of PiD, g, h but were clearly labeled using Tau-Y197. The coiled bodies (i arrowheads), and the ramified glial pathology were also labeled using Tau-Y197 (j arrowheads). k Western blot analysis indicated that Tau-nY18 did not label soluble tau but did label the insoluble fractions. No reactivity for soluble or insoluble tau was observed using Tau-nY29. Tau-nY197 sparsely labeled tau within the soluble and insoluble fractions. Tau-nY394 did not label either of the fractions analyzed. l However, the soluble fractions were clearly labeled using RD3, and to a limited extent using the Tau-12 antibody. The insoluble fractions were only labeled with RD3 and Tau-12 but did not react with the RD4 antibody. In all panels, calibration bars represent 20 μm
We then investigated whether the Pick brain homogenates also contain nitrated soluble or insoluble tau using Western blot analysis. As was the case in CBD and PSP, Tau-nY18 did not label the sarkosyl-soluble fractions and only one PiD case appears to show reactivity in the sarkosyl-insoluble tau fractions. Tau-nY29 demonstrated limited reactivity in the sarkosyl-soluble fraction and did not label the sarkosyl-insoluble fraction, even though abundant Pick body pathology was observed by IHC (Fig. 4b, c). However, it is important to note that in the cases used for Western blotting there was very little Tau-12 immunoreactivity in the sarkosyl-soluble fraction (Fig. 4l), therefore one might expect less labeling with Tau-nY29 because the proportion of nitrated tau is certainly less than the proportion of total tau. In contrast to CBD and PSP, Tau-nY197 poorly labeled both the sarkosyl-soluble and sarkosyl-insoluble fractions (Fig. 4k), whereas Tau-nY394 showed no reactivity in either fraction.
Abundant sarkosyl-soluble and sarkosyl-insoluble tau was clearly labeled using the RD3 antibody (Fig. 4l). Note that the RD4 antibody did not react with the insoluble fractions, indicating that most of tau within this fraction is mainly composed of the 3R variety. The sarkosyl-soluble tau fractions displayed only labeling of a few proteolytic fragments with RD4 indicating that most of tau within the sarkosyl-soluble fractions is of the 3R variety. Tau-12 robustly labeled the sarkosyl-insoluble fractions, but as shown with Tau-nY197 and RD4 antibodies, it reacted poorly with the sarkosyl-soluble fractions, largely recognizing proteolytic fragments. However, the uncleaved tau was clearly labeled using the RD3 antibody again suggesting that 4R tau is largely absent from both sarkosyl-soluble and sarkosyl-insoluble fractions.
Tau tyrosine nitration co-localizes with the Alz-50 antibody
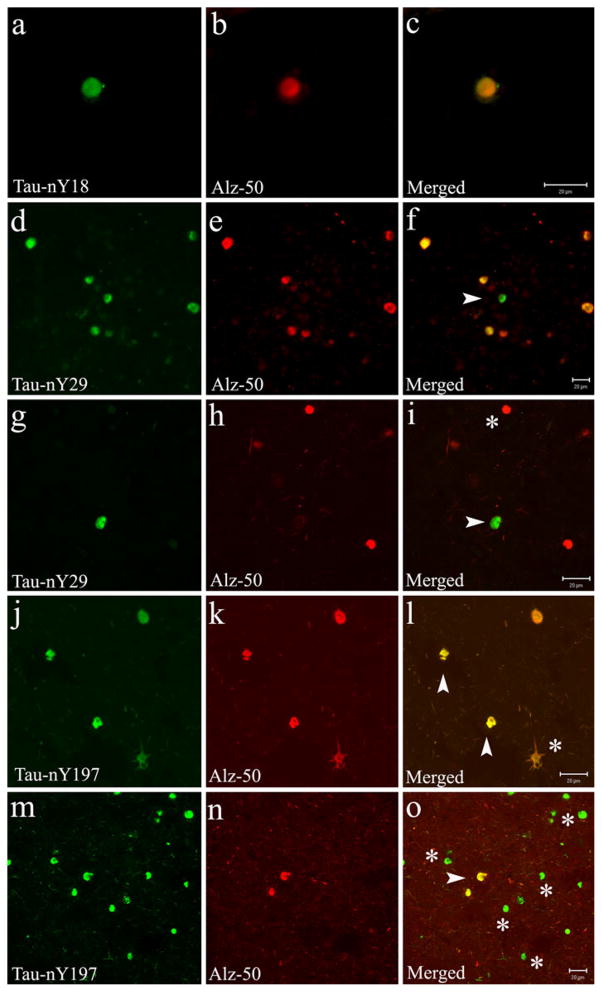
Due to the widespread labeling of nitrated tau within the Pick body pathology using Tau-nY18, Tau-nY29, and Tau-nY197, we investigated whether these nitration events are associated with tau modifications that occur early in AD [16]. Double-label immunofluorescence was performed using a monoclonal antibody known to recognize tau in a conformation where the N terminus interacts with the microtubule binding region of the molecule (Alz-50), an event thought to facilitate tau aggregation during the initial stages of tangle formation in AD [4]. Notably, our qualitative observations revealed that while most Pick body inclusions labeled with Tau-nY18 and Tau-nY29 appear to co-localize with the Alz-50 antibody (Fig. 5a–i), some Tau-nY29-positive Pick bodies were found that lacked Alz-50 reactivity (Fig. 5d–i, arrowheads). Similarly, some Alz-50-positive inclusions were identified that lacked Tau-nY29 labeling (Fig. 5g–i, asterisk). In contrast, while some Tau-nY197-positive Pick body inclusions and some ramified astrocytes co-localized with tau in the Alz-50 conformation (Fig. 5j–o, arrowheads), most Pick body inclusions positive for Tau-nY197 did not (Fig. 5m–o, asterisks), suggesting that nitration at Y197 might precede the formation of the Alz-50 conformation in PiD as it does in AD. However, although unlikely, it is also possible that nitration at this site occurs after the Alz-50 conformation vanishes as the inclusions mature; additional cases and stereological techniques may be necessary to further validate this hypothesis.
Fig. 5.
Nitration of tau at Y18, Y29 and Y197 co-localizes with the Alz-50 antibody in PiD. Double-label immunofluorescence was performed on tissue sections from areas of the frontal cortex using Tau-nY18, Tau-nY29, Tau-nY197, and the Alz-50 antibody. a–c Pick body inclusions positive for Tau-nY18 clearly co-localized with the Alz-50 antibody. d–i (arrowheads) Only a fraction of Tau-nY29-positive inclusions co-localized with Alz-50 epitope. Limited co-localization was also observed when Tau-nY197 was paired with Alz-50, both Pick body inclusions (j–o), and some ramified glial pathology (j–l asterisk) showed some co-localization however, most Tau-nY197 reactive Pick bodies were not labeled by the Alz-50 antibody (m–o asterisk). In all panels, calibration bars represent 20 μm
Discussion
In the present study, we investigated the presence or absence of nitrated tau within CBD, PSP and PiD using two novel antibodies Tau-nY197 and Tau-nY394 [35] and compared the reactivity to two previously characterized antibodies termed Tau-nY18 and Tau-nY29 [36, 39]. As previously reported, all but one antibody identified nitrated tau within the pathological inclusions associated with AD [5, 22]. Herein, we consider the differences between the nitration state of tau within the fibrillar tau aggregates in non-AD tauopathies and tau’s nitration state in AD. Our findings suggest that while nitration at specific tyrosine sites may be indicative of a pathological event associated with neurodegeneration, nitration at other residues is likely a normal tau modification within the human brain (Table 4).
Table 4.
Summary of Western blot data
| Tau-nY18
|
Tau-nY29
|
Tau-nY197
|
Tau-nY394
|
|||||
|---|---|---|---|---|---|---|---|---|
| Sol | Insol | Sol | Insol | Sol | Insol | Sol | Insol | |
| AD | + | + | + | + | + | + | − | ± |
| CBD | − | + | + | − | + | + | − | − |
| PSP | − | + | + | − | + | + | − | − |
| PiD | − | ± | − | − | ± | ± | − | − |
| Normal | + | − | − | − | + | − | − | − |
Sarkosyl soluble (Sol) and insoluble (Insol) tau proteins were isolated from frozen tissue section from cases pathologically diagnosed with corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Pick’s disease (PiD) and the reactivity of the nitro-specific antibodies is presented above
+, presence; −, absence; ±, minimal reactivity
Limited Tau-nY18 reactivity in AD and non-AD tauopathies
Although several attempts were made to reveal the pathological tau inclusions reacting with Tau-nY18 by performing numerous epitope retrieval methods in non-AD tauopathies, very few were observed by IHC (Table 2). These findings are consistent with our previous results which indicated that Tau-nY18 does not label the tau inclusions associated with either CBD or PSP by IHC [36]. However, following citric acid retrieval methods, one CBD case did appear to have a limited number of neuropil threads that were clearly positive for Tau-nY18. Similarly, a select number of Pick body inclusions were also positive for Tau-nY18 in PiD; however, the vast majority were unlabeled.
In addition to the few neuronal tau inclusions detected by Tau-nY18 in AD, this antibody mainly labeled the reactive glial cells that were associated with amyloid plaques [36]. While glial tau inclusions are part of the hallmark tau pathology associated with CBD, PSP and PiD, Tau-nY18 did not label any of the glial inclusions in these disorders, suggesting that nitration of tau at tyrosine 18 may be associated with the generation of amyloid deposits which are largely absent in the non-AD tauopathies [36].
That said, Western analysis revealed that Tau-nY18 reactivity was readily detected within the sarkosyl-soluble and sarkosyl-insoluble tau fractions isolated from severe AD cases [36]. However, it was also found within the sarkosyl-soluble tau fractions isolated from non-cognitively impaired controls [36]. By contrast, Tau-nY18 labeled the sarkosyl-insoluble fractions in CBD, PSP and PiD (Table 4), but did not label the sarkosyl-soluble fractions even though the samples were isolated from the same brain region (frontal cortex) as that assayed in non-demented controls [36]. This indicates that nitrated sarkosyl-soluble tau may be selectively garnered by the insoluble fraction as part of the disease processes active in these non-AD tauopathies.
Although Tau-nY18 reacted with insoluble tau by Western analysis, this antibody seldom labeled the tau inclusions in non-AD tauopathies (Table 4). This result may be due to the sensitivity of the Western technique compared to IHC. Alternatively, it is also possible that Tau-nY18 is labeling a tau aggregate that has yet to become a part of a readily identifiable pathological inclusion. In one specific PiD case, Tau-nY18 did not react with sarkosyl-insoluble tau even though abundant insoluble tau was detected using Tau-12 and RD3 antibodies (Table 4). These findings suggest that some tauopathy cases may be susceptible to nitration at Y18 while others are not. Clearly, nitration of tau at tyrosine 18 is variable in PiD cases and is not required for tau inclusion formation.
Perhaps, our most interesting finding regarding nitration of tau at tyrosine 18 is that it is readily detectable in sarkosyl-soluble tau within the frontal cortex of normal controls and within cases pathologically diagnosed with AD. All of these suggest that nitration of tau at this site may be a post-translational modification involved in a normal biological function. However, additional studies are necessary to validate this hypothesis.
Tau-nY29 reactivity in AD and non-AD tauopathies
Unlike Tau-nY18, Tau-nY29 clearly labeled a select number of neuronal tau lesions associated with CBD and PSP. However, similar to Tau-nY18, the hallmark glial lesions associated with these disorders were never labeled, even after numerous epitope retrieval methods were applied to tissue sections [33]. These findings indicate that the glial cell types in CBD or PSP are not susceptible to nitration at tyrosine 18 or 29. In contrast, numerous neuronal and glial tau inclusions were clearly labeled in PiD using Tau-nY29, suggesting that nitration of tau at tyrosine 29 is a more frequent event in this tauopathy (Table 2).
Western blot analysis indicated that Tau-nY29 reacted with the sarkosyl-soluble fractions in both CBD and PSP but did not react with any of the sarkosyl-insoluble fractions analyzed (Table 4). This is exactly the opposite result to that obtained using Tau-nY18. Although the meaning of these findings remains unclear, it does serve to differentiate between CBD and PSP cases based on the aggregation state and the selective nitration of two closely spaced tyrosine residues within the tau molecule.
In contrast to our IHC observations in PiD, Tau-nY29 did not react with either the sarkosyl-soluble or sarkosyl-insoluble tau fractions in any of the cases analyzed by Western analysis (Table 4). These results may be explained by a potential proteolytic event within tau at D25 [40], which would certainly eliminate Y18 and perhaps render Y29 non-nitratable. This hypothesis is supported by the lack of Tau-12 reactivity, a pan-tau antibody whose epitope is also within the N-terminal region of the molecule and would be eliminated by a truncation at D25. Alternatively, it is also possible that the lack of Tau-nY29 reactivity is not due to the cleavage of proteins but rather to a post-translational modification or a conformation within the molecule that prevents antibody binding. Some credence is lent to this hypothesis by the binding of the RD3 antibody to tau polypeptides migrating at the normal apparent molecular weight. However, even though Tau-nY29 did not bind tau in PiD via Western analysis, it reacted well with Pick bodies via IHC. Unfortunately, the cases used in IHC experiments were different from those analyzed by Western blots (Table 1). Hence, one could posit many reasons for the differences that might include the IHC-staining structures being so insoluble that they do not enter even a stacking gel. It should be noted that tau in most NFTs in AD is truncated on its carboxy end and yet evidence for such truncation is never seen in sarkosyl-insoluble biochemical preparations from human AD brains [11, 19, 32].
In AD, Tau-nY29 labeled the sarkosyl-soluble and sarkosyl-insoluble tau fractions by Western analysis (Braak stage V–VI; Table 4). However, in contrast to Tau-nY18, Tau-nY29 did not react with the sarkosyl-soluble tau fractions isolated from non-cognitively impaired controls [39]. These findings suggest that nitration at this site may be strictly a pathological modification because (1) it occurs within some of the hallmark tau pathologies in both AD and non-AD tauopathies and (2) its presence in sarkosyl-soluble tau appears to be disease-dependent (PSP and CBD).
Tau-nY197 reactivity in AD and non-AD tauopathies
Similar to Tau-nY18, Tau-nY197 reacted with a limited number of tau inclusions associated with CBD and PSP by IHC (Table 2). In PiD however, a number of Pick body inclusions were clearly labeled using Tau-nY197. Similarly, in AD, Tau-nY197 selectively localized to the pathological inclusions associated with the disease, including the NFT, neuropil threads and neuritic plaques [35].
Western analysis further revealed abundant nitrated tau at tyrosine 197 within the sarkosyl-soluble and sarkosyl-insoluble fractions in CBD and PSP and to a limited extent in PiD, a result that does not match the limited nitration events observed using IHC in CBD and PSP or the abundant labeling in PiD (Tables 2, 4). In AD, Tau-nY197 reactivity was present not only within the sarkosyl-soluble and sarkosyl-insoluble tau fractions but also within sarkosyl-soluble tau isolated from non-cognitively impaired controls [35]. These findings suggest that nitration at tyrosine 197 like nitration at tyrosine 18 may be a normal modification within tau [36, 39].
Overall, the reactivity of these monoclonal antibodies (Tau-nY18, Tau-nY29, and Tau-nY197) with non-AD tauopathies suggests that tau aggregates composed mainly of 3R tau isoforms may be more susceptible to nitration than tau inclusions composed mainly of 4R tau isoforms (Table 2). However, relatively little is known about tau inclusion progression and its association with cognitive decline in these rare tauopathies (Table 1).
Lack of Tau-nY394 reactivity in AD and non-AD tauopathies
In contrast to the nitro-tau antibodies shown above, nitration of tau at tyrosine 394 was not detected in any of the disease states analyzed (Tables 2, 4). The lack of nitration at tyrosine 394 in these rare diseases was somewhat expected based on previous results that indicated nitration at this site occured to only a limited extent within sarkosyl-insoluble tau aggregates isolated from severe AD cases [35]. However, tyrosine 394 was shown to be nitrated in vitro [37, 38]; therefore, it may be that a truncation event at the C terminus of tau can remove this residue eliminating nitration at this site [27]. Another possibility is that nitration at this site in AD and these rare disease states may be masked by the presence of a phosphorylation at tyrosine 394 [7, 27, 42]. Indeed, previous work by Hanger and colleagues [18] identified phosphorylation of tyrosine 394 in AD using Edman degradation and mass spectrometry analysis. Phosphorylation at tyrosine 394 would likely preclude nitration on the same tyrosine residue.
Nitration and Tau solubility
It is worth mentioning that in PiD, no full-length sarkosyl-soluble tau was identified with any of the nitro-specific antibodies used, and only a limited amount was detected with the Tau-12 antibody (Fig. 4), an extremely high titer tau antibody. The RD3 result, however, clearly demonstrates the presence of 3R tau in these fractions and Tau-12 certainly binds to 3R tau proteins (unpublished observations). It is possible that nitrated sarkosyl-soluble tau may be a subset of total soluble tau and not sediment during the isolation of the sarkosyl-soluble and sarkosyl-insoluble tau fractions, or be so insoluble as to not enter even the stacking gels during Western analysis. Alternatively, the PMI from these diseases may have negatively contributed to these observations (Table 1). It must also be noted that different cases were used for IHC and Western analysis and therefore these results must be interpreted with caution. In addition, one of the PiD cases analyzed by Western analysis was diagnosed with a familial mutation of PiD (Table 2) which may also contribute to the discrepancy of our results. Certainly, more PiD cases of soluble and insoluble tau fractions must be analyzed to confirm these findings.
Overall, the findings presented herein and those published previously [35, 36, 39] indicate that tyrosine nitration occurs on tau with site-selective specificity in normal and diseased brain states and suggests that nitration at specific tyrosine sites (Y18 and Y197) may subserve a normal biological function that remains to be elucidated. The shear specificity of nitration events at specific tyrosine residues among non-demented control subjects and those with different disease states suggests a potential enzymatic event or events that may be responsible for nitration [26]. Support for this contention is further obviated by the report of nitration at Y197 in the brains of young wild type mice [31]. Indeed, nitrating enzymes would add another level of cellular control to events dependent on tyrosine phos-phorylation and signal transduction.
Acknowledgments
The authors would like thank Katherine Gasho and Dr. Eileen Bigio from the CNADC of Northwestern University for kindly providing the tissue necessary to complete this study. We are grateful to Dr. Rohan de Silva for kindly providing us with the RD3 and RD4 antibodies and Dr. Sarah Ward for critical readings of the manuscript. This work is supported by NIH awards AG 14449 (L.I.B.), AG 21184 (L.I.B.), AG 032091 and AG 013854 to the Northwestern University Alzheimer’s Disease Center (C.G.).
Contributor Information
Juan F. Reyes, Email: juan-reyes@northwestern.edu, Juan_F.Reyes@med.lu.se, Department of Cell and Molecular Biology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA, Tarry Bldg. 8-754, 300 E. Superior St., Chicago, IL 60611, USA
Changiz Geula, Cognitive Neurology and Alzheimer’s Disease Center, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA.
Laurel Vana, Department of Cell and Molecular Biology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA.
Lester I. Binder, Department of Cell and Molecular Biology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA, Cognitive Neurology and Alzheimer’s Disease Center, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA
References
- 1.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Berry RW, Sweet AP, Clark FA, Lagalwar S, Lapin BR, Wang T, Topgi S, Guillozet-Bongaarts AL, Cochran EJ, Bigio EH, Binder LI. Tau epitope display in progressive supranuclear palsy and corticobasal degeneration. J Neurocytol. 2004;33(3):287–295. doi: 10.1023/B:NEUR.0000044190.96426.b9. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 4.Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271(51):32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 5.de Silva R, Lashley T, Strand C, Shiarli AM, Shi J, Tian J, Bailey KL, Davies P, Bigio EH, Arima K, Iseki E, Murayama S, Kretzschmar H, Neumann M, Lippa C, Halliday G, MacKenzie J, Ravid R, Dickson D, Wszolek Z, Iwatsubo T, Pickering-Brown SM, Holton J, Lees A, Revesz T, Mann DM. An immunohistochemical study of cases of sporadic and inherited frontotemporal lobar degeneration using 3R- and 4R-specific tau monoclonal antibodies. Acta Neuropathol. 2006;111(4):329–340. doi: 10.1007/s00401-006-0048-x. [DOI] [PubMed] [Google Scholar]
- 6.Delacourte A, Robitaille Y, Sergeant N, Buee L, Hof PR, Wattez A, Laroche-Cholette A, Mathieu J, Chagnon P, Gauvreau D. Specific pathological Tau protein variants characterize Pick’s disease. J Neuropathol Exp Neurol. 1996;55(2):159–168. doi: 10.1097/00005072-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Derkinderen P, Scales TM, Hanger DP, Leung KY, Byers HL, Ward MA, Lenz C, Price C, Bird IN, Perera T, Kellie S, Williamson R, Noble W, Van Etten RA, Leroy K, Brion JP, Reynolds CH, Anderton BH. Tyrosine 394 is phosphorylated in Alzheimer’s paired helical filament tau and in fetal tau with c-Abl as the candidate tyrosine kinase. J Neurosci. 2005;25(28):6584–6593. doi: 10.1523/JNEUROSCI.1487-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40(2):139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- 9.Flament S, Delacourte A, Verny M, Hauw JJ, Javoy-Agid F. Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol. 1991;81(6):591–596. doi: 10.1007/BF00296367. [DOI] [PubMed] [Google Scholar]
- 10.Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D’Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference participants. Ann Neurol. 1997;41(6):706–715. doi: 10.1002/ana.410410606. [DOI] [PubMed] [Google Scholar]
- 11.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100(17):10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Sierra F, Ghoshal N, Quinn B, Berry RW, Binder LI. Conformational changes and truncation of tau protein during tangle evolution in Alzheimer’s disease. J Alzheimers Dis. 2003;5(2):65–77. doi: 10.3233/jad-2003-5201. [DOI] [PubMed] [Google Scholar]
- 13.Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp Neurol. 2002;177(2):475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- 14.Goedert M, Cohen ES, Jakes R, Cohen P. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer’s disease [corrected] FEBS Lett. 1992;312(1):95–99. doi: 10.1016/0014-5793(92)81418-l. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1990;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging. 2005;26(7):1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Guillozet-Bongaarts AL, Glajch KE, Libson EG, Cahill ME, Bigio E, Berry RW, Binder LI. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta Neuropathol. 2007;113(5):513–520. doi: 10.1007/s00401-007-0209-6. [DOI] [PubMed] [Google Scholar]
- 18.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem. 2007;282(32):23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 19.Harrington CR, Mukaetova-Ladinska EB, Hills R, Edwards PC, Montejo de Garcini E, Novak M, Wischik CM. Measurement of distinct immunochemical presentations of tau protein in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88(13):5842–5846. doi: 10.1073/pnas.88.13.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa M. Biochemistry and molecular biology of tauopathies. Neuropathology. 2006;26(5):484–490. doi: 10.1111/j.1440-1789.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64(17):2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci. 2004;24(36):7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 24.Ikonomovic MD, Mufson EJ, Wuu J, Bennett DA, DeKosky ST. Reduction of choline acetyltransferase activity in primary visual cortex in mild to moderate Alzheimer’s disease. Arch Neurol. 2005;62(3):425–430. doi: 10.1001/archneur.62.3.425. [DOI] [PubMed] [Google Scholar]
- 25.Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364(3):279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- 26.Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, Behbod F, Lee YC, Murad F. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci USA. 1998;95(20):11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khuebachova M, Verzillo V, Skrabana R, Ovecka M, Vaccaro P, Panni S, Bradbury A, Novak M. Mapping the C terminal epitope of the Alzheimer’s disease specific antibody MN423. J Immunol Methods. 2002;262(1–2):205–215. doi: 10.1016/s0022-1759(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 28.Ksiezak-Reding H, Morgan K, Mattiace LA, Davies P, Liu WK, Yen SH, Weidenheim K, Dickson DW. Ultrastructure and biochemical composition of paired helical filaments in cortico-basal degeneration. Am J Pathol. 1994;145(6):1496–1508. [PMC free article] [PubMed] [Google Scholar]
- 29.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 31.Nonnis S, Cappelletti G, Taverna F, Ronchi C, Ronchi S, Negri A, Grassi E, Tedeschi G. Tau is endogenously nitrated in mouse brain: identification of a tyrosine residue modified in vivo by NO. Neurochem Res. 2008;33(3):518–525. doi: 10.1007/s11064-007-9467-x. [DOI] [PubMed] [Google Scholar]
- 32.Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM. Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci USA. 1991;88(13):5837–5841. doi: 10.1073/pnas.88.13.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellicer EM, Sundblad A. Antigen retrieval by microwave oven with buffer of citric acid. Medicina (B Aires) 1994;54(2):129–132. [PubMed] [Google Scholar]
- 34.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43(6):815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 35.Reyes JF, Fu Y, Vana L, Kanaan NM, Binder LI. Tyrosine nitration within the proline-rich region of Tau in Alzheimer’s disease. Am J Pathol. 2011;178(5):2275–2285. doi: 10.1016/j.ajpath.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyes JF, Reynolds MR, Horowitz PM, Fu Y, Guillozet-Bongaarts AL, Berry R, Binder LI. A possible link between astrocyte activation and tau nitration in Alzheimer’s disease. Neurobiol Dis. 2008;31(2):198–208. doi: 10.1016/j.nbd.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds MR, Berry RW, Binder LI. Site-specific nitration and oxidative dityrosine bridging of the tau protein by peroxynitrite: implications for Alzheimer’s disease. Biochemistry. 2005;44(5):1690–1700. doi: 10.1021/bi047982v. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds MR, Berry RW, Binder LI. Site-specific nitration differentially influences tau assembly in vitro. Biochemistry. 2005;44(42):13997–14009. doi: 10.1021/bi051028w. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds MR, Reyes JF, Fu Y, Bigio EH, Guillozet-Bongaarts AL, Berry RW, Binder LI. Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer’s disease and other tau-opathies. J Neurosci. 2006;26(42):10636–10645. doi: 10.1523/JNEUROSCI.2143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11(2):341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 41.Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys. 1999;371(2):169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay MA, Acker CM, Davies P. Tau phosphorylated at tyrosine 394 is found in Alzheimer’s disease tangles and can be a product of the Abl-related kinase, Arg. J Alzheimers Dis. 2010;19(2):721–733. doi: 10.3233/JAD-2010-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]