Abstract
The objective of this study was to evaluate the bactericidal effect of shock waves (SWs) on gram-negative or gram-positive monocultured biofilms grown on an orthopedic implant in vitro. Cortical bone screws were individually cultured with Escherichia coli or Staphylococcus epidermidis to produce a biofilm. In each run of 8 screws, 6 screws were treated with shock waves and then sonicated to disrupt the biofilm. One screw was sonicated only and one was not shock waved or sonicated before sampling for plate count dilutions. Post-treatment serial dilutions and plate counts were done on an aliquot from the vial containing each screw to obtain the number of colony-forming units (CFUs). Shock waves were at a constant energy of 0.15 mJ/mm2. Pulse number and screw orientation were varied. A linear mixed-effects model was used with “treatment” as a fixed effect and “run” as a random effect. Pairwise comparisons of treatments were performed with Tukey-Cramer’s adjustment for P-values. Sonicated plate counts were greater than nonsonicated counts for each run. When all sonicated screws were compared to all nonsonicated screws, the counts were significantly increased (P = 0.0091). For each paired comparison between sonicated and shock wave treatment, the only significant difference was in the S. epidermidis biofilm treated at 2000 pulses in a horizontal position, which increased the post-treatment count (P = 0.0445). No bactericidal effects were seen on monocultured biofilms on cortical bone screws treated with shock waves.
Résumé
L’objectif de la présente étude était d’évaluer l’effet bactéricide d’ondes de choc (SW) sur des biofilms de monoculture de bactéries à Gram négatif ou Gram positif cultivées in vitro sur des implants orthopédiques. Des vis pour os cortical ont été mises en présence de culture individuelle d’Escherichia coli ou Staphylococcus epidermidis afin de produire un biofilm. Lors de chaque essai avec 8 vis, 6 vis ont été traitées par SW et par la suite aux ultrasons afin de déloger le biofilm. Une vis a été traitée aux ultrasons seulement et une ne fut soumise à aucun traitement avant un dénombrement sur gélose. Des dilutions sériées post-traitement et des dénombrements sur gélose ont été effectués sur une aliquote provenant du tube contenant chaque vis afin d’obtenir le nombre d’unités formatrices de colonies (CFU). Les ondes de choc étaient d’énergie constante de 0,15 mJ/mm2. Le nombre de pulsations et l’orientation des vis ont été variés. Un modèle linéaire à effets mixtes a été utilisé avec le «traitement» comme effet fixe et «l’essai» comme l’effet aléatoire. Des comparaisons pairées des traitements ont été effectuées avec l’ajustement de Tukey-Cramer pour les valeurs de P. Lors de chaque essai, les dénombrements des vis soumis aux ultrasons étaient supérieurs à ceux des vis non-traitées aux ultrasons. Lorsque tous les résultats des vis traitées aux ultrasons ont été comparés à ceux des vis non-traitées aux ultrasons, les dénombrements étaient significativement augmentés (P = 0,0091). Pour chaque paire de comparaison entre le traitement aux ultrasons et le traitement par SW, la seule différence significative notée était pour le biofilm de S. epidermidis traité à 2000 pulsations dans une position horizontale, qui augmenta le dénombrement post-traitement (P = 0,0445). Aucun effet bactéricide n’a été observé sur les biofilms de monoculture sur des vis pour os cortical traitées avec des ondes de choc.
(Traduit par Docteur Serge Messier)
Introduction
Shock wave (SW) therapy has become commonplace for the disruption of calculi in multiple organ systems (1,2). While the ability to fragment calculi has been realized for years, more recent information has shown that SWs have a positive effect when treating musculoskeletal conditions. Enthesiopathies including tennis elbow and plantar fasciitis (3,4) are being successfully treated with SWs to avoid surgical procedures that are known to have notable morbidity rates and cost. Delayed unions and nonunions are being successfully treated with SWs (5) and SWs can also enhance the regeneration of alveolar bone that was lost due to periodontal disease (6).
Shock waves are pressure waves that can reach 1000 times atmospheric pressure in a few nanoseconds (7,8). They return to atmospheric pressure or have a short period of negative pressure within milliseconds. Shock waves can travel through fluid and soft tissues with minimal loss of energy. When a shock wave is reflected by hitting a surface with a much different acoustic impedance than fluid, such as air or metal, a strong tensile wave is produced (7,8). Tensile waves can cause cavitational effects and have strong disruptive potential at a fluid-solid interface, which increases the possibility of cellular damage (7–9).
Investigations of shock waves (SWs) on a cellular level have shown a cytocidal effect on neoplastic cells (10,11). In addition, a synergism has been recognized between SWs and chemotherapy for enhanced destruction of tumor cells (12). Different cells have been found to respond differently to SWs. Bone marrow stromal cells have shown an increase in proliferation, while chondrocytes have decreased in proliferation (13).
While the literature contains many positive results for shock waves (SWs) applied to eukaryotes, less is known about the effects of SWs on prokaryotes. Current work with SWs and prokaryotes is aimed not at the healing properties of SWs, but rather at their bactericidal effects (14–17). Several studies have shown that shock waves can be bactericidal to organisms in suspension with energy flux densities (EFDs) and pulse numbers tolerable to surrounding tissues (14,15,17). One study treated Staphylococcus aureus organisms in suspension with SWs and found that 1000 pulses at an EFD of 0.96 mJ/mm2 significantly decreased bacterial counts (14). Another study reported that a minimum of 350 pulses at 20 kV (approximate EFD of 0.5 mJ/mm2) also decreased bacterial counts (17). These results were encouraging for the continued study of SWs as a novel therapeutic treatment for bacterial infections. While in-vitro suspensions of bacteria have been susceptible to SWs, there are additional considerations when applying SWs to biofilms.
Biofilms are a structured consortium of 1 or several species of bacteria embedded in a self-produced matrix of polysaccharides, proteins, and deoxyribonucleic acid (DNA) (18). Biofilms can cause chronic infections despite aggressive antibiotic treatment. Postoperative implant infections with biofilm formation represent a devastating complication with high morbidity and substantial cost (19). A number of surfaces support the growth of biofilms including heart valves (20), intravenous catheters (21), and orthopedic implants (22). Biofilms can be difficult to destroy as they show an increased tolerance to antibiotics and disinfectants and resist phagocytosis and other immune system defenses. Furthermore, organisms in a mature biofilm may be able to increase their antimicrobial resistance (23).
It is not known how shock waves (SWs) and bacteria interact in a biofilm on a metallic surface. With the ultimate goal of using the bactericidal effect of SWs to treat in-vivo bacterial infections, research that transitions from bacterial suspensions to infected implants is warranted. The purpose of this study was to evaluate the effects of focused shock waves (SWs) on gram-negative and gram-positive biofilms grown on an orthopedic implant in vitro. Our hypothesis was that SWs are bactericidal to organisms in a biofilm.
Materials and methods
Bacterial preparation
Two organisms that are known to produce biofilms, Staphylococcus epidermidis [ATCC #35984] and Escherichia coli [ATCC #10798], were selected for the study (24,25). Each organism was evaluated separately in monoculture. Bacteria were streaked onto trypticase soy agar and incubated for 24 h at 37°C. After incubation, 5 to 10 isolated colonies were removed with a sterile cotton swab and placed into 10 mL of trypticase soy broth. The broth was then incubated at 37°C and agitated at 200 rpm for 1 h. A 2-mL aliquot of the broth was diluted with fresh medium to an absorbance of 0.35 at a wavelength of 595 nm in a spectrophotometer. This absorbance approximates a bacterial concentration of 1 to 5 × 108/mL. The diluted bacterial suspension was then used to inoculate the implants.
Biofilm growth
A 4.5-mm × 30-mm stainless steel cortical screw (Synthes, Paoli, Pennsylvania, USA) was selected as the orthopedic implant to support biofilm growth. Sterile screws were placed individually in a 15-mL graduated conical bottom centrifuge tube (Catalog number 14-959-53A; Fisher Scientific, Pittsburgh, Pennsylvania, USA). The head of the screw was oriented towards the top of the tube and the screw rested in a vertical position. Trypticase soy broth (4 mL) and 2 mL of the inoculum were added to the tube, which completely immersed the screw. The screw was then incubated under static conditions for 18 h at 37°C. After incubation, it was observed that a thick biofilm covered the screw. To confirm the presence of the biofilm in this model, 2 screws cultured with the E. coli and 2 screws cultured with the S. epidermidis, along with 1 control screw, were imaged with scanning laser confocal microscopy (Leica Confocal SP5X; Leica Microsystems, Exton, Pennsylvania, USA) by bright field and ultraviolet fluorescent techniques with a 10× objective. No fluorescent probes were used; there was autofluorescence with ultraviolet illumination.
Screw preparation for shock wave treatment
After 18 h of incubation, each screw was removed from the culture broth. Each screw was rinsed by grasping the head of the screw with sterile forceps and placing it in a 50-mL conical bottom centrifuge tube containing 20 mL of phosphate buffered saline (PBS) for 3 s. This was repeated in 20 mL of fresh PBS at room temperature for a total of 2 rinses. After the second rinse, each screw was placed in a sterile 2-mL polypropylene conical bottom microcentrifuge tube with a snap cap (Catalog number 02-681-260; Fisher Scientific) that contained 1.7 mL of trypticase soy broth. The cap of each tube was closed and the interface between the cap and the broth was evaluated to ensure there were no air bubbles. The caps were then sealed with paraffin wax that was evaluated after treatment to ensure that it remained intact. In a preliminary evaluation of the methods, a microcentrifuge tube containing methylene blue dye was sealed with paraffin wax and treated with 2000 pulses at an EFD of 0.15 mJ/mm2 and the seal was found to be secure.
Treatment chamber
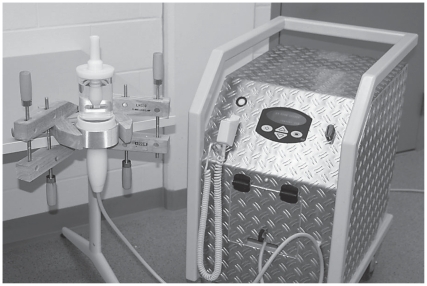
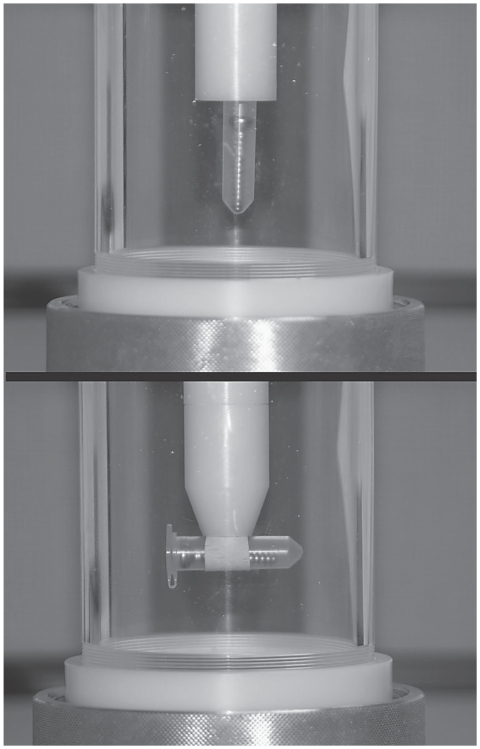
A treatment chamber containing 750 mL of degassed water was used (Figure 1). The water was maintained at 37°C and replaced at every third treatment. The bottom of the chamber was connected to the shock wave probe (Equitron; Pulse Veterinary Technologies, Alpharetta, Georgia, USA). The opposite end of the chamber contained a cap with a polyvinyl chloride rod that passed through the center of the cap. The end of the rod contained a fastener to accommodate the microcentrifuge tube in either a vertical or horizontal position in the center of the focal point (Figure 2). The shock wave generator had an elliptical focal point in the longitudinal axis of the probe. In the vertical position, the distal end of the screw was at the bottom of the elliptical focal point and in the horizontal position, the screw was centered in the widest portion of the elliptical focal point. The SW generator was set at the maximum energy of 0.15 mJ/mm2 with a pulse frequency of 4 Hz. At this energy, the focal region has a diameter of 6.9 mm and an ellipsoid focal point of 40.5 mm long.
Figure 1.
Shock wave generator (right) connected to a 35-mm treatment probe shown entering the bottom of a treatment chamber (upper left). The treatment chamber is secured to a table with wooden clamps.
Figure 2.
A treatment chamber demonstrating the different treatment orientations. The probe enters the chamber from the bottom. A threaded lid at the top of the chamber can be removed to exchange screws. The chamber contains degassed water which surrounds the microcentrifuge tube that holds the screw.
Treatment groups
In each treatment group of 8 inoculated screws, 6 screws were treated with shock waves and then sonicated, 1 screw served as a sonicated control, and the remaining screw was used as an untreated control. This provided an unsonicated control and a sonicated control to verify that the bacterial count increased after sonication and to ensure that the bacteria were dislodged from the biofilm. The sonicated screw that was not treated with shock waves (SWs) served as the control with which to compare the sonicated SW-treated screw in each run. Each treatment group consisted of 6 screws with either E. coli or S. epidermidis that were treated with the assigned number of SW pulses. A total of 7 groups was evaluated. The number of SW pulses administered to E. coli. biofilms was 250, 500, 1000, and 2000. Preliminary evaluation of the E. coli data showed that no differences were detected. The 2000 horizontal group was then added to determine if orientation was a factor which resulted in 5 E. coli groups. The S. epidermidis was evaluated with 2000 pulses in vertical and horizontal positions.
Sonication
Sonication was performed to release bacteria in the biofilm for plate counting (26–28). The sonication procedure was similar to that used in previous studies, but was optimized for this study by sonicating screws with E. coli and S. epidermidis biofilms at various amplitudes in preliminary studies to maximize the number of bacteria on plate counts. The sonicator used produces a constant frequency of 20 kHz and was fitted with a microtip probe with a diameter of 1.6 mm (Sonicator 4000; Misonix Sonicators, Qsonica, Newtown, Connecticut, USA). The time of sonication was held constant at 30 s and the amplitudes were varied to determine the optimum amplitude for releasing bacteria from each different bio-film. The final selection of parameters for sonication of the E. coli was an amplitude of 40, which translates to 9 W of power and 260 J of energy over 30 s. Staphylococcus epidermidis was sonicated at an amplitude of 90, which translates to 30 W of power and 900 J of energy over 30 s. For sonication, the microcentrifuge tube with the biofilm-coated screw was placed in a beaker containing 80 mL of sterile water and sonicated at the predetermined amplitude in a horizontal position for 30 s.
Serial dilutions and plate counts
After sonication, the paraffin seal of the microcentrifuge tube was broken, the screw was removed with sterile forceps, and 0.5 mL of broth was removed. This volume was used to carry out 7 serial 10-fold dilutions. A spread plate method was used, with 0.1 mL of broth from each of the 7 dilutions on trypticase soy agar plates. Plates were incubated at 37°C for 24 h and isolated colonies were counted and recorded. The dilution with a count of 20 to 200 colonies was used for statistical analysis.
Data analysis
All data were entered into an Excel spreadsheet (Microsoft Office; Microsoft, Redmond, Washington, USA) and imported into a statistical software package (Version 9.2, SAS System for Windows; SAS Institute, Cary, North Carolina, USA) for analysis. Data were loge transformed to remove skewness. A linear mixed-effects model was used to analyze data with ‘treatment’ as a fixed effect and ‘run’ as a random effect. Pairwise comparisons of treatments were performed with Tukey-Cramer’s adjustment for P-values. P-values of ≤ 0.05 were considered to be significant.
Results
A biofilm was imaged on the surface of the screws cultured with each organism (Figure 3). When all sonicated screws (mean ± SE; loge 16.8758 ± 0.3438) were compared to all nonsonicated screws (loge 15.4494 ± 0.3438), the group means were significantly different (P = 0.0091). In every run, the sonicated plate count was greater than the nonsonicated plate count, which indicated that the bacteria were released from the biofilm and were available for counting.
Figure 3.
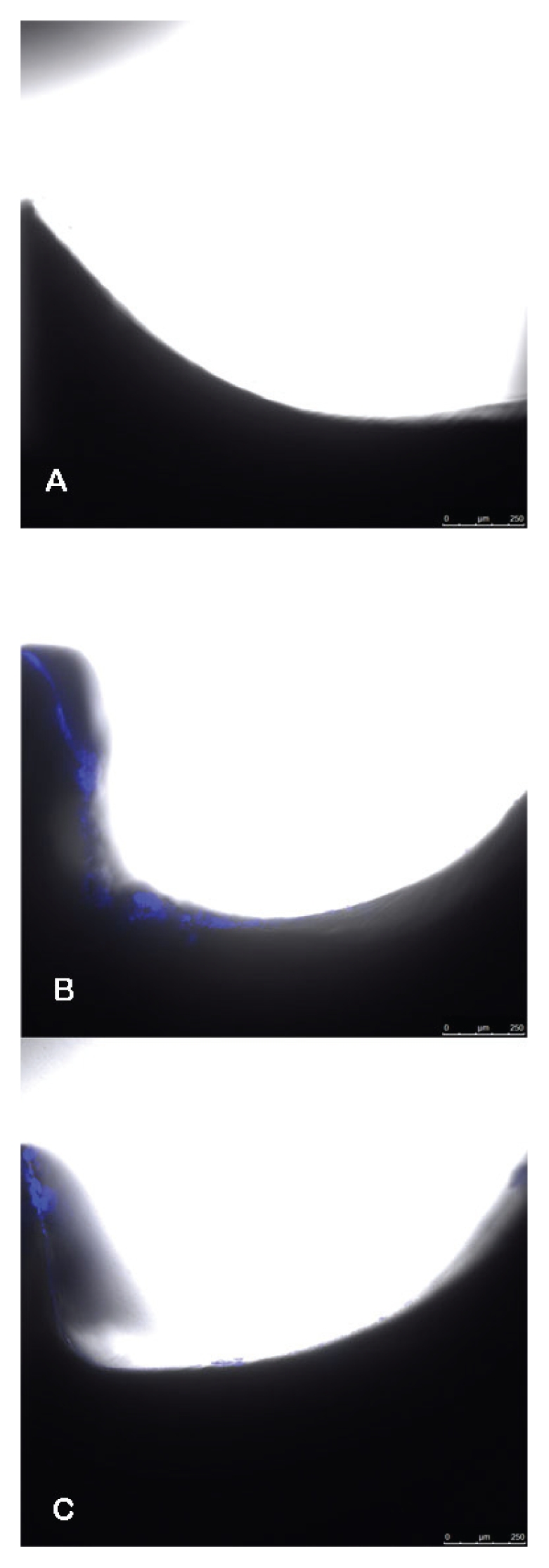
Overlaid bright field and ultraviolet fluorescence images. The control screw (A) shows a smooth surface of the screw with no adherent material for fluorescence. The S. epidermidis (B) and E. coli (C) have an adherent layer with areas of fluorescence.
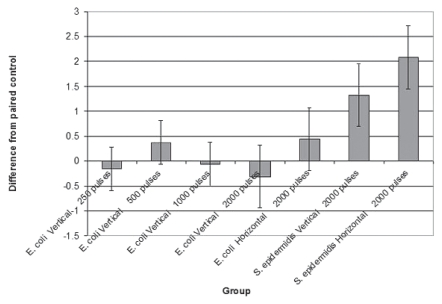
For each individual paired comparison of sonicated and shock wave (SW) treatment, the only significant difference was in the S. epidermidis biofilm treated at 2000 pulses in a horizontal position (loge 18.9623 ± 0.6200), at which the post-treatment count was increased (P = 0.0445) over the sonicated control (loge 16.8758 ± 0.3438) (Figure 4).
Figure 4.
The treatment effect was determined as the difference of log transformed count between each treatment and the paired sonicated control that removed any variation between runs. A negative number indicates fewer organisms and a positive number indicates a higher plate count. Standard error bars are included. All of the E. coli groups are centered around 0, which means there were no differences. There were more organisms in the S. epidermidis groups treated with shock waves, with the group treated with 2000 pulses in the horizontal position being significantly higher (P = 0.0445).
Discussion
In this study using E. coli or S. epidermidis cultured on a cortical bone screw to form a biofilm, no bactericidal effect of shock wave treatment was observed. There are a number of possible explanations for not seeing an effect, including the energy flux density (EFD), pulse number, and model of generator chosen. The maximum energy setting of the generator used herein was 0.15 mJ/mm2. The maximum number of pulses was stopped at 2000 per screw because we felt that 2000 pulses was the maximum amount for a reasonable treatment protocol in a clinical case of implant infection.
Bactericidal effects in suspension have been shown with as few as 100 pulses at an EFD of 0.3 mJ/mm2 with Streptococcus mutans and Porphyromonas gingivalis (16). A threshold of 2000 pulses at an EFD of 0.96 mJ/mm2 or 0.59 mJ/mm2 at 4000 pulses was required to be bactericidal to S. aureus in suspension (14). A number of gram-positive and gram-negative organisms in suspension was killed at a set treatment of 4000 pulses at an EFD of 0.96 mJ/mm2 (15).
The energy flux density (EFD) may not be as important as the environment. Most of the previous studies on the effect of shock waves on bacteria were conducted on bacterial suspensions (14–16). The media in which the bacteria are located can change the effect of the shock waves. Proteus mirabilis organisms in agar were reduced by 55% with 1000 pulses with an estimated EFD of > 0.5 mJ/mm2 (29). When the bacteria were encompassed in agar beads under the same testing parameters, there was no reduction in organisms. When calcium carbonate crystals were added to the beads to simulate struvite stones, however, viable organisms were reduced by 82% (26). The addition of the solid surface (crystals) to the media increased the bactericidal effect.
This outcome can be explained by the change in acoustic impedance at the interface of the liquid media and the solid. The reflection of the wave at the interface creates large tensile stresses and cavitation which are capable of cellular disruption (7–9). Organisms in the biofilm on the surface of the screw would be subjected to these forces. It was a feasible hypothesis that there would be a bactericidal effect in the biofilm at the pulse numbers and EFD used in this study.
The biofilms in this study were maintained in broth at 37°C for treatment with shock waves. In vivo, the organisms in a biofilm would not be devoid of nutrients as they would be if treatments were done in a saline solution. The results of studies of bacteria in saline suspension at 20°C with limited metabolic activity are different than those in broth (29). When known bactericidal SW treatments of 4000 pulses at an EFD of 0.59 mJ/mm2 were administered to S. aureus suspended in broth at 36°C, the number of colony-forming units (CFUs) increased significantly compared to a significant decrease in CFUs with the same treatment parameters in saline suspension at 20°C. In this study, an untreated control was run in each group to remove any differences in time on bacterial proliferation. A number of environmental variables including temperature and the presence of biofilms require further in-vitro investigation before any in-vivo studies are carried out.
The 4.5-mm × 30-mm cortical bone screw provided a clinically relevant, consistently sized structure on which to grow the biofilm. Shock waves (SWs) are entirely reflected or refracted by the metal. This could be both beneficial and detrimental. The maximum physical effect of the SWs would occur at the interface of the biofilm and screw on the surface of the screw. The threads of the screw create an uneven surface so that the entire surface area is not directly exposed to the SWs, which could protect the bacteria in the biofilm. To see if the threads provided some protective mechanism, we added the horizontal treatment group. This would expose the area between the threads on the side of the screw to the generator. When the screws were treated with the shock waves (SWs), the screws vibrated so they rotated and exposed the circumference of the screw to the generator. While no attempt was made to quantify this rotation, it was clear from observation of the hexagonal head that the circumference of the screw was exposed to SWs during the 2000 pulses. The surface of the screw does add a variable when compared to using a simple flat metallic object. We elected to use a screw, however, because of its clinical relevance.
For this in-vitro model, the screws had to be placed within a container to perform the study. Polypropylene vials are routinely used for studies, but the curvature of the vial may affect the SW (30). We recognized this potential effect and selected tubes with conical bottoms to provide a surface as perpendicular as possible to the converging shock waves. Furthermore, we also evaluated the effect with the tube turned perpendicular to the SWs. It may be possible to perform a similar study with the media contained in a latex-type balloon, which would decrease the potential effect of the polypropylene vial. The polypropylene vials have the advantage of making it easier to remove the visible air bubbles from the container. Air bubbles within the test system can increase nuclei for cavitation and increase cell death (31).
In this in-vitro study, we used a fairly simplistic, static biofilm model that has been used in a number of previous studies (32–35). This model used biofilms with a single organism, whereas naturally occurring biofilms often contain multiple species acting in synergism. A monoculture, as used in this study, provides an environment with a high degree of reproducibility. It has been stated that data generated from pure cultures are accurate and useful, but must be considered in relation to the entire system (27). Biofilms that form on solid-liquid interfaces may be exposed to shear forces, which could affect the composition of the biofilms (28,34). Devices that rotate or utilize flow of media over colonized surfaces can create more complex biofilms (27,34). The biofilms in this study were grown in nutrient-rich broth that was devoid of inflammatory cells, which can result in protein deposition within the biofilm as can occur in vivo (19,36). If SWs can be used to destroy bacteria in a simple in-vitro model, more complex models will be needed before the outcome can be considered for in-vivo use.
We chose a biofilm model utilizing both a gram-negative (E. coli) and a gram-positive (S. epidermidis) organism known to produce bio-films that occur with implant infection. Not all bacteria are equally susceptible to shock waves (SWs). Streptococcus mutans were killed by SWs with as little as 100 pulses at an EFD of 0.3 mJ/mm2 but there was no effect on S. aureus at 500 pulses (16). It has been suggested that gram-negative bacteria are more susceptible because of the thin peptidoglycan layer, which is more sensitive to disruption (16). Not all gram-positive organisms are equally sensitive. Staphylococcus aureus and S. epidermidis were more sensitive to 4000 pulses at an EFD of 0.96 mJ/mm2 than Entercoccus faecium (15). Staphylococcus aureus is an organism that is more resistant to killing by ultrasound energy than other organisms, so it may be less likely to be damaged by SWs (37).
Shock waves (SWs) and ultrasound can both lead to disaggregation of bacterial agglomerates (16,38). There was a similar finding in this study when the number of S. epidermidis organisms increased after 2000 pulses in the horizontal position. We assume that the SWs created further disruption of the biofilm, which exposed more bacteria that was then available for plate counting. The preliminary work to identify the sonication parameters required to release the organisms from the biofilm to be available for plate counting worked well for the E. coli. There were differences in the sonication parameters for both organisms, with the S. epidermidis requiring greater sonication amplitude. Data would indicate that there is a potential for improving the technique of sonication for the S. epidermidis.
In our study we chose to use 1 sonicated control within each run. The largest variable in the methods was the initial inoculation based on spectrophotometry. Potential differences in incubation time, temperature, or initial inoculations were removed by running a group of 8 screws at 1 time and comparing the treated screws to each group’s sonicated control. Only the maximum number of pulses was done for the S. epidermidis in the vertical and horizontal positions. The hypothesis that shock waves (SWs) were bactericidal to these organisms in biofilms was rejected when the bacterial counts did not decrease at the maximum treatment dose of 2000 pulses at 0.15 mJ/mm2.
In a study using uroliths, it was found that the biofilm may dampen the bactericidal effect of SWs in some way (26). While the results of this study indicate no direct bactericidal effect, they may indicate a potential for SWs to disrupt biofilms. In studies with ultrasound, it has been demonstrated that there are 2 phases, first disruption of aggregates followed by decreasing bacterial cell counts (38). As indicated by the S. epidermidis group with the increased plate counts, the physical disruption of the biofilm with SWs could be clinically useful by exposing the bacteria within the biofilm to antimicrobial therapy. It could also be detrimental by releasing a number of sequestered organisms that lead to septicemia or endotoxemia.
While disrupting the biofilm to expose organisms may be useful, shock waves (SWs) did not increase the susceptibility of S. aureus to gentamicin (39). Potential mechanisms for the bactericidal effect in previous studies are unknown. When SWs, ultrasound, pneumatic, and laser-induced lithotripsy were evaluated on infected struvite stones, the Holmium:YAG laser was the only mechanism that decreased Proteus organisms (40). The bactericidal effect likely came from thermal mechanisms. There are minimal thermal effects with shock waves. Alternative mechanisms of bacterial death have been investigated. Disruption of cell wall permeability does not appear to be the sole mechanism of bactericidal effects (39). Other effects may occur, including intracellular formation of free radicals and disruption of organelles. Despite the lack of knowledge about the mechanism, the bactericidal effect on suspensions treated with shock waves is irreversible (39). In the study reported here, no bactericidal effect of shock waves was seen.
References
- 1.Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2:1265–1268. doi: 10.1016/s0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- 2.Iro H, Schneider H, Födra C, et al. Shockwave lithotripsy of salivary duct stones. Lancet. 1992;339:1333–1336. doi: 10.1016/0140-6736(92)91968-e. [DOI] [PubMed] [Google Scholar]
- 3.Rompe J, Hope C, Küllmer K, Heine J, Bürger R. Analgesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg Br. 1996;78:233–237. [PubMed] [Google Scholar]
- 4.Kudo P, Dainty K, Clarfield M, Coughlin L, Lavoie P, Lebrun C. Randomized, placebo-controlled, double-blind clinical trial evaluating the treatment of plantar fasciitis with extracorporeal shockwave therapy (ESWT) device: A North American confirmatory study. J Orthop Res. 2006;24:115–123. doi: 10.1002/jor.20008. [DOI] [PubMed] [Google Scholar]
- 5.Haupt G. Use of extracorporeal shock waves in the treatment of pseudarthrosis, endinopathy and other orthopedic diseases. J Urol. 1997;158:4–11. doi: 10.1097/00005392-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Sathishkumar S, Meka A, Dawson D, et al. Extracorporeal shock wave therapy induces alveolar bone regeneration. J Dent Res. 2008;87:687–691. doi: 10.1177/154405910808700703. [DOI] [PubMed] [Google Scholar]
- 7.McClure S, Dorfmüller C. Extracorporeal shockwave therapy: Theory and equipment. Clin Tech Eq Pract. 2003;2:348–357. [Google Scholar]
- 8.Ogden JA, Kischkat-Togh A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Rel Res. 2001;387:8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Howard D, Sturtevant B. In vitro study of the mechanical effects of shock-wave lithotripsy. Ultrasound Med Biol. 1997;23:1107–1122. doi: 10.1016/s0301-5629(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 10.Diehl P, Schmitt M, Blümelhuber G, et al. Induction of tumor cell death by high hydrostatic pressure as a novel supporting technique in orthopedic surgery. Oncol Rep. 2003;10:1851–1855. doi: 10.3892/or.10.6.1851. [DOI] [PubMed] [Google Scholar]
- 11.Yao C, Ishizuka J, Bold R, Townsend C, Jr, Thompson J. Cytocidal effect of high energy shock wave on tumour cells enhanced with larger dose and multiple exposures. Surg Oncol. 1994;3:229–235. doi: 10.1016/0960-7404(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 12.Kambe M, Ioritani N, Kanamaru R. Enhancement of chemotherapeutic effects with focused shock waves: Extracorporeal shock wave chemotherapy (ESWC) Hum Cell. 1997;10:87–94. [PubMed] [Google Scholar]
- 13.Dorotka R, Kubista B, Schatz K, Trieb K. Effects of extracorporeal shock waves on human articular chondrocytes and ovine bone marrow stromal cells in vitro. Arch Orthop Trauma Surg. 2003;123:345–348. doi: 10.1007/s00402-003-0551-7. [DOI] [PubMed] [Google Scholar]
- 14.Gerdesmeyer L, von Eiff C, Horn C, et al. Antibacterial effects of extracorporeal shock waves. Ultrasound Med Biol. 2005;31:115–119. doi: 10.1016/j.ultrasmedbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Gollwitzer H, Horn C, von Eiff C, Henne M, Gerdesmeyer L. Antibacterial effectiveness of high-energetic extracorporeal shock waves: An in vitro verification [Article in German] Z Orthop Ihre Grenzgeb. 2004;142:462–466. doi: 10.1055/s-2004-822825. [DOI] [PubMed] [Google Scholar]
- 16.Novak K, Govindaswami M, Ebersole J, Schaden W, House N, Novak M. Effects of low-energy shock waves on oral bacteria. J Dent Res. 2008;87:928–931. doi: 10.1177/154405910808701009. [DOI] [PubMed] [Google Scholar]
- 17.von Eiff C, Overbeck J, Haupt G, et al. Bactericidal effect of extra-corporeal shock waves on Staphylococcus aureus. J Med Microbiol. 2000;49:709–712. doi: 10.1099/0022-1317-49-8-709. [DOI] [PubMed] [Google Scholar]
- 18.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Khardori N, Yassien M. Biofilms in device-related infections. J Ind Micro. 1995;15:141–147. doi: 10.1007/BF01569817. [DOI] [PubMed] [Google Scholar]
- 20.Høiby N, Doring G, Schiotz P. The role of immune complexes in the pathogenesis of bacterial infections. Annu Rev Microbiol. 1986;40:29–53. doi: 10.1146/annurev.mi.40.100186.000333. [DOI] [PubMed] [Google Scholar]
- 21.Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: Literature- and registry-based estimates. J Hosp Infect. 2009;72:97–103. doi: 10.1016/j.jhin.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Del Pozo J, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito A, Taniuchi A, May T, Kawata K, Okabe S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl Environ Microbiol. 2009;75:4093–4100. doi: 10.1128/AEM.02949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitao T, Ishimaru M, Nishihara S. Detection of biofilm-producing and methicillin resistance genes in Staphylococcus epidermidis isolated from healthy humans and in blood culture tests. J Infect Chemother. 2010;16:170–173. doi: 10.1007/s10156-010-0037-9. [DOI] [PubMed] [Google Scholar]
- 25.Sendi P, Frei R, Maurer T, Trampuz A, Zimmerli W, Graber P. Escherichia coli variants in periprosthetic joint infection: Diagnostic challenges with sessile bacteria and sonication. J Clin Microbiol. 2010;48:1720–1725. doi: 10.1128/JCM.01562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid G, Jewett M, Nickel J, McLean R, Bruce A. Effect of extracorporeal shock wave lithotripsy on bacterial viability. Relationship to the treatment of struvite stones. Urol Res. 1990;18:425–427. doi: 10.1007/BF00297377. [DOI] [PubMed] [Google Scholar]
- 27.Costerson J, Nickel J, Ladd T. Bacteria in Nature: Suitable Methods for the Comparative Study of Free-living and Surface Associated Bacterial Populations. New York: Plenum; 1986. [Google Scholar]
- 28.Rochex A, Godon J, Bernet N, Escudié R. Role of shear stress on composition, diversity and dynamics of biofilm bacterial communities. Water Res. 2008;42:4915–4922. doi: 10.1016/j.watres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Horn C, Gerdesmeyer L, von Eiff C, Gradinger R, Gollwitzer H. Energy-dependent stimulatory and inhibitory effects of extra-corporeal shock waves on bacteria and on gentamicin activity. Med Sci Monit. 2009;15:MT77–83. [PubMed] [Google Scholar]
- 30.Cleveland R, McAteer J, Andreoli S, Crum L. The effect of polypropylene vials on lithotripter shock waves. Ultrasound Med Biol. 1997;23:939–952. doi: 10.1016/s0301-5629(97)00026-4. [DOI] [PubMed] [Google Scholar]
- 31.Williams J, Stonehill M, Colmenares K, et al. Effect of macroscopic air bubbles on cell lysis by shock wave lithotrips in vitro. Ultrasound Med Biol. 1999;25:473–479. doi: 10.1016/s0301-5629(98)00149-5. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi N, Bauer T, Tuohy M, Fujishiro T, Procop G. Brief ultrasonication improves detection of biofilm-formative bacteria around a metal implant. Clin Orthop Relat Res. 2007;457:210–213. doi: 10.1097/BLO.0b013e3180312042. [DOI] [PubMed] [Google Scholar]
- 33.Bigelow T, Northagen T, Hill T, Sailer F. The destruction of Escherichia coli biofilms using high-intensity focused ultrasound. Ultrasound Med Biol. 2009;35:1026–1031. doi: 10.1016/j.ultrasmedbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 34.McBain A. In vitro biofilm models: An overview. Adv Appl Microbiol. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 35.Moussa F, Gainor B, Anglen J, Christensen G, Simpson W. Disinfecting agents for removing adherent bacteria from orthopaedic hardware. Clin Orthop Relat Res. 1996;329:255–262. doi: 10.1097/00003086-199608000-00032. [DOI] [PubMed] [Google Scholar]
- 36.Buret A, Ward K, Olson M, Costerton J. An in vivo model to study the pathobiology of infectious biofilms on biomaterial surfaces. J Biomed Mater Res. 1991;25:865–874. doi: 10.1002/jbm.820250706. [DOI] [PubMed] [Google Scholar]
- 37.Allinger H. Ultrasonic disruption. American Laboratory. 1975;10:75–85. [Google Scholar]
- 38.Joyce E, Phull S, Lorimer J, Mason T. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason Sonochem. 2003;10:315–318. doi: 10.1016/S1350-4177(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 39.Horn C, Mengele K, Gerdesmeyer L, Gradinger R, Gollwitzer H. The effect of antibacterial acting extracorporeal shockwaves on bacterial cell integrity. Med Sci Monit. 2009;15:BR364–369. [PubMed] [Google Scholar]
- 40.Prabakharan S, Teichman J, Spore S, Sabanegh E, Glickman R, McLean R. Proteus mirabilis viability after lithotripsy of struvite calculi. J Urol. 1999;162:1666–1669. [PubMed] [Google Scholar]