Abstract
Selection of dairy cattle for increased milk production with little or no emphasis on health traits leads to an increased prevalence of disease. A possible genetic solution to this problem is to combine production and immune response traits in a weighted selection index. In the current study, leukocyte populations in heifers identified as having a high antibody-mediated immune response (HiAMIR) or high cell-mediated immune response (HiCMIR) phenotype were compared before and after immunization in order to identify leukocyte population profiles associated with these phenotypes. The results demonstrated that the HiCMIR-phenotype animals had a higher baseline proportion of gamma-delta T-cells in peripheral blood. Also, the observed increase in the proportion of B-cells in peripheral blood in response to immunization was greater in the HiAMIR-phenotype animals. It is expected that identifying leukocyte population profiles associated with immune response phenotypes will improve our ability to identify animals with enhanced overall immune responsiveness.
Résumé
La sélection des bovins laitiers pour une production laitière augmentée avec peu ou pas d’emphase sur les caractéristiques de santé peut mener à une augmentation de la prévalence de maladies. Une solution génétique possible à ce problème est de combiner les caractéristiques de production et de réponse immune dans un indice de sélection pondéré. Au cours de la présente étude, on a comparé les populations de leucocytes retrouvées chez les taures identifiées comme ayant un phénotype pour une forte réponse immunitaire à médiation par les anticorps (HiAMIR) ou une forte réponse immunitaire à médiation cellulaire (HiCMIR) avant et après immunisation afin d’identifier les profils de populations leucocytaires associées avec ces phénotypes. Les résultats ont montré que les animaux avec un phénotype HiCMIR avaient une proportion plus élevée au départ de lymphocytes T gamma-delta dans le sang périphérique. Également, l’augmentation observée de la proportion de lymphocytes B dans le sang périphérique en réponse à une immunisation était plus grande chez les animaux de phénotype HiAMIR. Il est attendu qu’en identifiant les profils des populations leucocytaires associées avec les phénotypes des réponses immunitaires améliorera notre capacité à identifier les animaux avec une capacité de réponse immunitaire globale supérieure.
(Traduit par Docteur Serge Messier)
Selecting dairy cattle for increased milk production leads to an increase in the prevalence of disease (1). Positive genetic correlations have been reported between milk production and the prevalence of many common diseases of dairy cattle (2). For example, it has been estimated that the genetic correlation between milk production and the incidence of mastitis is from 0.15 to 0.37 (2,3). A possible genetic solution to this problem is to combine production and immune response (IR) traits in a weighted selection index with the goal of breeding high-producing animals with enhanced overall immune responsiveness, thereby improving immune defence against infectious disease (4).
As immune response is a complex polygenic trait, it may not be feasible to identify genes of major effect that could be used to select for enhanced overall immune responsiveness (4). A more appropriate strategy is to consider IR as a quantitative trait with a characteristic phenotype that can be objectively measured (4). Adaptive cell-mediated immune responses (CMIR) and antibody-mediated immune responses (AMIR) tend to predominate in the control of intra- and extracellular pathogens, respectively (5). Therefore, a protocol was previously developed and tested to estimate the breeding value of the adaptive immune responsiveness of individual animals as measured by their ability to mount both CMIR and AMIR (5,6).
Extensive field testing conducted by this laboratory has revealed that CMIR and AMIR, as measured using the IR testing protocol described here, are negatively genetically correlated in dairy cattle (7). This suggests that selecting animals based on their resistance to extracellular pathogens may increase their susceptibility to intracellular pathogens and vice versa. It has therefore been proposed that selecting animals with enhanced overall immune responsiveness (an above-average ability to mount both CMIR and AMIR) will improve broad-based disease resistance and overall animal welfare, and reduce reliance on the use of antibiotics and other therapeutic agents to control infectious disease in the dairy industry (4). It has been previously shown that dairy cows with a high-AMIR and high-CMIR phenotype have a lower occurrence of diseases such as mastitis (8).
The relationship between the number and/or proportion of cells in various blood leukocyte subsets on one hand and immune competence on the other has been investigated in various livestock species (9,10). Samma et al (10) investigated the genetic variation in the proportion of cells in various lymphocyte subsets in dairy cows before and after treatment with dexamethasone, a glucocorticoid hormone used to mimic the immunosuppressive effects of parturition. Baseline measurements of all lymphocyte traits showed significant genetic variation with moderate to high heritability estimates ranging from 0.21 to 0.60. Furthermore, basal values were strongly genetically correlated with the linear recovery of both CD4− and CD8− T-cell proportions after treatment. Based on these results, it was concluded that the proportions of lymphocyte subsets are potentially important indicators of immune competence in dairy cattle.
The objective of the current study was to compare peripheral blood leukocyte populations in heifers identified as having high-AMIR/low-CMIR (HiAMIR) or low-AMIR/high-CMIR (HiCMIR) phenotypes before and after immunization in order to identify leukocyte population profiles associated with these phenotypes. This profile information could then be used, in conjunction with our current IR testing protocol, to further improve our ability to identify animals with enhanced overall immune responsiveness.
The heifers used in the current study were previously classified as having a HiAMIR- or HiCMIR-phenotype (11). Briefly, primary IR testing was conducted on 128 Canadian Holstein replacement heifers. After primary IR testing, heifers were ranked based on their ability to mount CMIR and AMIR. A subset of 40 heifers, identified as having a HiAMIR (n = 20) or HiCMIR (n = 20) phenotype, were selected to undergo secondary IR testing with the same antigens (11). The IR-testing procedure used was developed to assess overall immune responsiveness in dairy cattle. It involves immunizing animals with Candida albicans and hen egg white lysozyme (HEWL) to induce predominantly type-1 (cell-mediated) and type-2 (antibody-mediated) immune responses, respectively, and then assessing immune responses to these antigens. The current study describes the leukocyte profiles of these HiAMIR and HiCMIR heifers before and after secondary IR testing.
At the start of secondary IR testing, heifers ranged in age from 5 to 26 mo. Of the heifers tested, 19 were non-pregnant (NP) (11 HiAMIR, 8 HiCMIR), 8 were in early pregnancy (EP, gestation < 100 d) (3 HiAMIR, 5 HiCMIR), and 13 were in mid-pregnancy (MP, gestation 100 to 250 d) (6 HiAMIR, 7 HiCMIR). Heifers in late pregnancy (gestation > 250 d) were not tested, as the effects of approaching parturition on immune response are already well-described (12). Heifers were obtained from the Elora Dairy research herd of the University of Guelph. During the IR challenge, heifers were housed indoors in group pens and were fed a standard dry cow ration. All experimental procedures were approved by the Animal Care Committee of the University of Guelph under the guidelines of the Canadian Council on Animal Care.
Blood was collected in Vacutainers containing ethylenediamine tetra-acetic acid (EDTA) (Becton Dickinson, Franklin Lakes, New Jersey, USA) by caudal venipuncture both before (day 0) and after (day 7) secondary immunization and briefly stored at room temperature for flow cytometry staining procedures and automated total and differential leukocyte counting. To identify blood leukocytes expressing specific cell surface markers, a 2-color staining procedure for whole blood was used as described previously (13) with the following minor modifications. Cells were washed with 2 mL of phosphate buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide and, after incubation with secondary antibody, red blood cells were lysed by adding 2 mL of FACSLyse (Becton Dickinson) and vortexing cells.
Primary antibodies used in surface antigen labelling procedures were mouse anti-bovine CD45 (pan leukocyte marker) (VMRD, Pullman, Washington, USA; clone: CACTB51A), mouse anti-sheep CD45 (pan leukocyte marker) (AbD Serotec, Kidlington, United Kingdom; clone: 1.11.32), mouse anti-bovine WC1 [gamma-delta (γδ) T-cell marker] (AbD Serotec; clone: CC15), mouse anti-bovine CD4 (T-helper cell marker) (AbD Serotec; clone: CC8), mouse anti-bovine CD8 (T-cytotoxic cell marker) (AbD Serotec; clone: CC63), mouse anti-bovine CD5 (T-cell and a subset of B-cells marker) (VMRD; clone: B29A), mouse anti-bovine IgM (B-cell marker) (AbD Serotec; clone: BM-23), mouse anti-bovine CD14 (monocyte marker) (VMRD; clone: MM61A), mouse IgG1 isotype control (AbD Serotec; clone: MCA928), and mouse IgG2a isotype control (AbD Serotec; clone: MCA929). Secondary antibodies used to label cells were goat anti-mouse IgG1 PE-TR (Invitrogen, Carlsbad, California, USA; product: M32017) and goat anti-mouse IgG2a FITC (Invitrogen; product: M32301).
Unstained samples, concentration matched isotype control samples, and samples receiving secondary antibody only were included in all cell-labelling procedures to assist in defining positive cell populations. When staining for cell surface expression of IgM, blood (100 μL) was washed twice with 2 mL of PBS containing 0.5% BSA and 0.1% sodium azide to remove soluble IgM before adding primary antibody. A subset of samples was also stained with a combination of CD45/CD14 to assist in setting gates around the lymphocyte population at the exclusion of monocytes. Flow cytometry data were collected using a FACScan flow cytometer (Becton Dickinson) by gating on the lymphocyte population as defined by forward versus side scatter. A total of 10 000 events (within the lymphocyte gate) were collected for each sample. For analysis, quadrants were set based on control samples and results analyzed using Cell Quest software (Becton Dickinson). Total and differential blood leukocyte counts were determined at the Animal Health Laboratory (Guelph, Ontario) using an Advia-120 hematology analyzer (Siemens Diagnostics, Deerfield, Illinois, USA). Differential cell counts were conducted to determine both the proportion and absolute numbers of lymphocytes, monocytes, neutrophils, eosinophils, and basophils (not reported here) in blood.
Flow cytometry and hematology data were analyzed independently with a linear mixed model using Proc MIXED (Version 9.1.3, SAS Institute, Cary, North Carolina, USA). For lymphocyte subsets, the absolute number of cells was obtained by multiplying the percentage positive cells within the lymphocyte gate (determined by flow cytometry) by the absolute number of lymphocytes (determined by differential cell count). The effects of phenotype classification (HiAMIR or HiCMIR), age (linear and quadratic, defined as age in months at day 0), pregnancy status (NP, EP, or MP), and their 2-way interactions were fitted to all models. Previous studies have shown that age and pregnancy status influence type-1 and type-2 immune responses in dairy heifers (11). The random effect of sampling group (groups 1 to 4) was also fitted to all models. For analysis of flow cytometry and hematology data from samples collected at day 7, pre-immunization (day 0) values were fitted as covariates in the model so that responses to immunization at day 7 could be assessed while accounting for the variability observed between individual animals in their baseline (day 0) values.
The effect of phenotype classification was retained in models regardless of P-value. However, models were reduced by removing main effects (age and pregnancy status) if P > 0.25 and removing interaction terms if P > 0.1. When removing nonsignificant interaction terms, hierarchy was preserved at all times and those effects involved in significant interactions were retained regardless of their P-values. To test the assumptions of the analysis of variance (ANOVA), comprehensive residual analyses were conducted. Proc UNIVARIATE (Version 9.1.3, SAS) was used to formally test the residuals for normality (Anderson-Darling, Cramérvon Mises, Kolmogorov-Smirnov, and Shapiro-Wilk tests). The residuals were plotted against predicted and explanatory variable values to reveal potential outliers or other problems in the data and to assess the need for data transformation. When transformation improved the normality of residuals, absolute cell count data (cells/milliliter) and cell proportion data (%) were log (base e) or logit (base e) transformed, respectively. When the effect of phenotype on cell populations varied with pregnancy status (as indicated by a significant interaction between phenotype and pregnancy status), least squares means (LSMs) for each phenotype were estimated at each stage of pregnancy for reporting purposes. Similarly, when the effect of phenotype on cell populations varied with age (as indicated by a significant interaction between phenotype and age), LSMs for each phenotype were estimated at 9, 15, and 21 mo of age for reporting purposes.
After selection in the previous study, it was confirmed that primary AMIR were higher (P < 0.001) in HiAMIR- than in HiCMIR-phenotype heifers and that primary CMIR were higher (P < 0.001) in HiCMIR- than in HiAMIR-phenotype heifers (11). Furthermore, when selected heifers underwent secondary IR testing, CMIR were higher in HiCMIR than in HiAMIR heifers (0.922 ± 0.061 versus 0.791 ± 0.068), although the difference only approached significance (P = 0.075). Previous studies have demonstrated that primary and secondary antibody responses to the type-2 antigen used in IR testing are highly genetically correlated (r = 0.985) (7), which suggests that the immune factors defining the AMIR phenotype may be common to both primary and secondary antibody responses. In contrast to CMIR, secondary AMIR were higher in HiAMIR-phenotype than in HiCMIR-phenotype heifers at day 7 (LSM difference 0.389 ± 0.102, P= 0.003). The mean increase in AMIR from day 0 to day 7, however, was similar for both phenotypes (P = 0.261). As the type-1 antigen used in the IR testing protocol can be found ubiquitously in some environments, CMIR measured during primary and secondary IR testing could both be considered as memory responses.
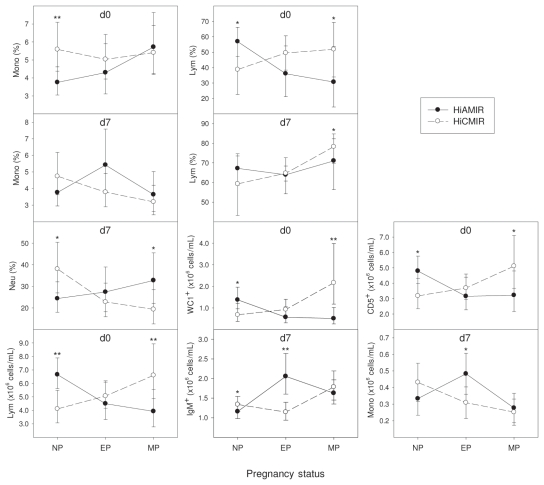
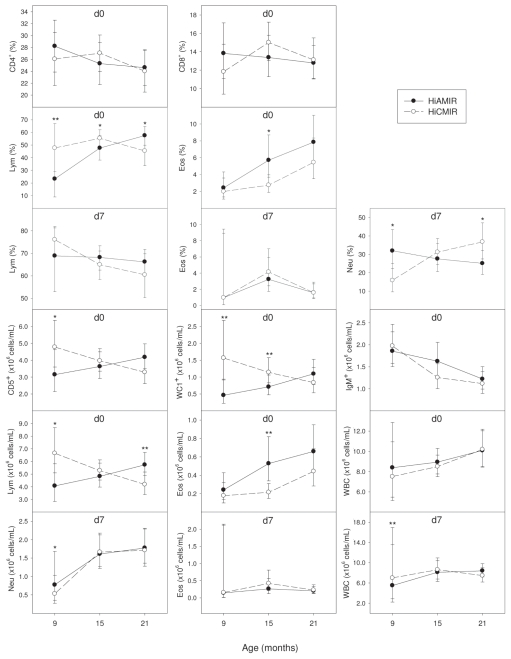
Cell profiling results, based on the proportion or absolute numbers of peripheral blood cell subsets in HiAMIR- and HiCMIR-phenotype heifers before (day 0) and after (day 7) secondary immunization, are summarized in Tables 1 and 2, respectively. Phenotype least squares means (LSMs) (estimated at each stage of pregnancy), when the effect of phenotype on cell populations varied with pregnancy status, are presented in Figure 1. Similarly, when the effect of phenotype on cell populations was influenced by age, phenotype LSMs (estimated at 9, 15, and 21 mo of age) are presented in Figure 2.
Table I.
Statistical probabilities supporting differences in the proportions of peripheral blood cell subsets in heifers classified as having a high antibody-mediated/low cell-mediated (HiAMIR) or low antibody-mediated/high cell-mediated (HiCMIR) immune response phenotype before (day 0) and after (day 7) secondary immunization
| Factor1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ptype | Ptype*Age | Ptype*Age*Age | Ptype*Status | |||||
| Cell phenotype2 | Time3 | HiAMIR4 | HiCMIR4 | Ratio/Difference5 | P-value | P-value6 | P-value6 | P-value6 |
| % of CD45+ lymphocytes | ||||||||
| aWC1+ | day 0 | 17.6 | 22.2 | 0.750 (0.578 to 0.972) | 0.031 | NS | NS | NS |
| aWC1+ | day 7 | 22.4 | 20.2 | 1.139 (1.008 to 1.287) | 0.038 | NS | NS | NS |
| bCD4+ | day 0 | 26.2c | 25.0c | 1.221 (−1.310 to 3.752) | 0.332 | 0.042 | 0.044 | NS |
| bCD4+ | day 7 | 25.9 | 27.1 | 1.263 (−0.582 to 3.108) | 0.172 | NS | NS | NS |
| aCD8+ | day 0 | 13.3c | 12.6c | 1.061 (0.905 to 1.244) | 0.452 | 0.024 | 0.033 | NS |
| aCD8+ | day 7 | 11.3 | 12.1 | 0.927 (0.843 to 1.020) | 0.116 | NS | NS | NS |
| aIgM+ | day 0 | 26.3 | 26.1 | 1.008 (0.825 to 1.231) | 0.938 | NS | NS | NS |
| aIgM+ | day 7 | 28.6 | 26.7 | 1.102 (1.003 to 1.211) | 0.044 | NS | NS | NS |
| aCD5+ | day 0 | 73.0 | 73.5 | 0.972 (0.803 to 1.176) | 0.764 | NS | NS | NS |
| aCD5+ | day 7 | 73.1d | 73.3d | 0.986 (0.897 to 1.085) | 0.769 | NS | NS | NS |
| % of total leukocytes | ||||||||
| aMonocyte | day 0 | 4.5 | 5.3 | 0.840 (0.692 to 1.019) | 0.075 | NS | NS | 0.066 |
| aMonocyte | day 7 | 4.2 | 3.9 | 1.094 (0.881 to 1.358) | 0.405 | NS | NS | 0.050 |
| aLymphocyte | day 0 | 40.8c | 46.6c | 0.790 (0.618 to 1.009) | 0.058 | 0.006 | NS | 0.030 |
| aLymphocyte | day 7 | 67.4c | 68.0c | 0.976 (0.836 to 1.139) | 0.748 | 0.062 | 0.065 | 0.051 |
| aNeutrophil | day 0 | 26.6 | 25.7 | 1.047 (0.877 to 1.251) | 0.598 | NS | NS | NS |
| aNeutrophil | day 7 | 28.0c | 26.0c | 1.108 (0.855 to 1.437) | 0.423 | 0.005 | 0.025 | 0.056 |
| aEosinophil | day 0 | 4.7c | 3.4c | 1.376 (0.947 to 1.999) | 0.091 | 0.040 | 0.041 | NS |
| aEosinophil | day 7 | 1.8c | 1.8c | 0.993 (0.788 to 1.252) | 0.952 | 0.091 | 0.080 | NS |
Factor: Ptype — phenotype classification; Status — pregnancy status; A*B — interaction between factor A and B.
Data logit transformed a or did not require transformation b for analysis.
Time: day 0 — before immunization; day 7 — 7 d after immunization.
Values represent least squares means (LSMs) or back-transformed geometric LSMs (transformed variables); Where interactions of Ptype and age c or Ptype and day 0 covariate d were included in the model, Ptype LSMs were estimated at the mean age of heifers or mean day 0 value respectively.
Values represent the difference between Ptype LSMs or the back-transformed ratio of Ptype LSMs (transformed variables); Numbers in brackets indicate 95% confidence interval.
NS — factors removed from model statement (P > 0.1).
Table II.
Statistical probabilities supporting differences in the numbers of peripheral blood cell subsets in heifers classified as having a high antibody-mediated/low cell-mediated (HiAMIR) or low antibody-mediated/high cell-mediated (HiCMIR) immune response phenotype before (day 0) and after (day 7) secondary immunization
| Factor1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ptype | ||||||||
| HiAMIR4 | HiCMIR4 | Ptype*Age | Ptype*Age*Age | Ptype*Status | ||||
| Cell phenotype2 | Time3 | (×106 cells/mL) | Ratio/Difference5 | P-value | P-value6 | P-value6 | P-value6 | |
| aWC1+ | day 0 | 0.74d | 1.11d | 0.665 (0.515 to 0.859) | 0.003 | 0.008 | NS | 0.003 |
| aWC1+ | day 7 | 1.08 | 1.07 | 1.010 (0.914 to 1.116) | 0.840 | NS | NS | NS |
| cCD4+ | day 0 | 1.51 | 1.40 | 0.112 (−0.083 to 0.307) | 0.252 | NS | NS | NS |
| aCD4+ | day 7 | 1.38 | 1.46 | 0.941 (0.840 to 1.054) | 0.281 | NS | NS | NS |
| aCD8+ | day 0 | 0.86 | 0.79 | 1.092 (0.920 to 1.297) | 0.303 | NS | NS | NS |
| aCD8+ | day 7 | 0.47 | 0.55 | 0.859 (0.723 to 1.020) | 0.080 | NS | NS | NS |
| aIgM+ | day 0 | 1.50d | 1.47d | 1.016 (0.839 to 1.229) | 0.867 | 0.031 | 0.038 | NS |
| aIgM+ | day 7 | 1.57 | 1.40 | 1.123 (0.981 to 1.286) | 0.088 | NS | NS | 0.003 |
| aCD5+ | day 0 | 3.66d | 3.92d | 0.934 (0.809 to 1.078) | 0.336 | 0.028 | NS | 0.033 |
| aCD5+ | day 7 | 3.22 | 3.46 | 0.929 (0.836 to 1.033) | 0.166 | NS | NS | NS |
| bCD4+:CD8+ | day 0 | 1.97 | 1.95 | 1.010 (0.877 to 1.163) | 0.888 | NS | NS | NS |
| bCD4+:CD8+ | day 7 | 4.23 | 3.89 | 1.088 (0.960 to 1.233) | 0.180 | NS | NS | NS |
| cMonocyte | day 0 | 0.42 | 0.50 | 0.079 (−0.001 to 0.159) | 0.054 | NS | NS | NS |
| cMonocyte | day 7 | 0.36 | 0.33 | 0.034 (−0.037 to 0.104) | 0.338 | NS | NS | 0.023 |
| aLymphocyte | day 0 | 4.90d | 5.16d | 0.949 (0.833 to 1.082) | 0.422 | 0.006 | NS | 0.007 |
| aLymphocyte | day 7 | 5.31 | 5.43 | 0.979 (0.884 to 1.083) | 0.669 | NS | NS | NS |
| aNeutrophil | day 0 | 2.21 | 2.24 | 0.986 (0.900 to 1.080) | 0.757 | NS | NS | NS |
| aNeutrophil | day 7 | 1.21d,e | 1.29d,e | 0.936 (0.808 to 1.085) | 0.364 | 0.020 | 0.028 | NS |
| aEosinophil | day 0 | 0.42d | 0.29d | 1.450 (1.010 to 2.082) | 0.044 | 0.016 | 0.014 | NS |
| aEosinophil | day 7 | 0.18d | 0.19d | 0.901 (0.675 to 1.203) | 0.465 | 0.035 | 0.034 | NS |
| aLeukocyte | day 0 | 9.26d | 8.85d | 1.046 (0.971 to 1.127) | 0.226 | 0.085 | NS | NS |
| aLeukocyte | day 7 | 6.95d,e | 7.27d,e | 0.956 (0.874 to 1.046) | 0.310 | 0.007 | NS | NS |
Factor: Ptype — phenotype classification; Status — pregnancy status; A*B — interaction between factor A and B.
Data log (ln) transformeda, ratio of logit transformed datab or did not require transformationc for analysis.
Time: day 0 — before immunization; day 7 — 7 d after immunization.
Values represent least squares means (LSMs) or back-transformed geometric LSMs (transformed variables); Where interactions of Ptype and aged or Ptype and day 0 covariatee were included in the model, Ptype LSMs were estimated at the mean age of heifers or mean day 0 value respectively.
Values represent the difference between Ptype LSMs or the back-transformed ratio of Ptype LSMs (transformed variables); Numbers in brackets indicate 95% confidence interval.
NS — factors removed from model statement (P > 0.1).
Figure 1.
Effect of immune response phenotype on blood cell populations in heifers before (day 0) and after (day 7) secondary immunization. Values represent least squares means (LSMs) for high antibody-mediated/low cell-mediated (HiAMIR) and low antibody-mediated/high cell-mediated (HiCMIR) phenotype animals. Error bars represent 95% confidence intervals (CIs). When data were transformed for analysis, values represent back-transformed LSMs and CIs. Cell populations were plotted only if the effect of phenotype on the cell population at day 0 or day 7 varied significantly with pregnancy status.
NP — non-pregnant; EP — early pregnancy (gestation < 100 d); MP — mid-pregnancy (gestation 100 to 250 d).
*P < 0.05
**P < 0.01
Figure 2.
Effect of immune response phenotype on blood cell populations in heifers before (day 0) and after (day 7) secondary immunization. Values represent least squares means (LSMs) for high antibody-mediated/ low cell-mediated (HiAMIR) and low antibody-mediated/high cell-mediated (HiCMIR) phenotype animals. Error bars represent 95% confidence intervals (CIs). When data were transformed for analysis, values represent back-transformed LSMs and CIs. Cell populations were plotted only if the effect of phenotype on the cell population at day 0 or day 7 varied significantly with age.
*P < 0.05
**P < 0.01
It is well known that T-cells, a type of lymphocyte, play a major role in steering the polarization of immune responses and as such have a central role in CMIR and AMIR. Results from the current study suggest that, although HiAMIR- and HiCMIR-phenotype animals produce greater antibody and DTH responses, respectively, the proportion and absolute numbers of CD4+ and CD8+ T-cells along with the CD4:CD8 T-cell ratio are similar in both HiAMIR- and HiCMIR-phenotype animals, both before (day 0) and after (day 7) secondary immunization. An exception is the proportion of CD4+ and CD8+ T-cells at day 0, which was influenced by phenotype. Although phenotype influenced the proportion of CD4+ and CD8+ T-cells at day 0, this effect was dependent on age and it was found that the proportion of CD4+ and CD8+ T-cells did not differ between phenotypes at 9, 15, or 21 mo of age. The number of CD5+ lymphocytes at day 0 was also influenced by phenotype, but this effect was again dependent on age and pregnancy status and no consistent trend in the effect of phenotype was evident.
Although it was found that populations of CD4+ and CD8+ T-cells were similar in both phenotypes before (day 0) and after (day 7) secondary immunization, significant differences in the population of WC1+ T-cells were observed between phenotypes. Unlike the lymphoid systems of humans and mice, the lymphoid system of ruminants contains large numbers of γδ T-cells (14). This is especially the case in neonates in which γδ T-cells are the predominant T-cell fraction found in blood, representing up to 60% of the T-cell pool. Following the neonatal period, however, the relative proportion of γδ to alpha-beta (αβ) T-cells declines continually with age to the point that γδ T-cells represent less than 10% of peripheral blood mononuclear cells (PBMCs) in adult ruminants (14). It is thought that ruminant γδ T-cells play an important role in protecting epithelial surfaces, linking the innate and adaptive immune systems and regulating immune responses (14,15). Regulatory T-cells (Tregs) play a critical role in maintaining immune homeostasis. In humans and mice, natural Tregs (CD4+/CD25high) expressing Foxp3 act as important regulators of immune reactivity (15). In ruminants, however, CD4+/CD25high/Foxp3+ T-cells do not exhibit regulatory activity, which is a function that appears to reside with the γδ T-cell population (15).
In the current study, HiCMIR animals had a higher proportion of γδ T-cells before immunization (day 0) than their HiAMIR counterparts. The effect of phenotype on absolute numbers of γδ T-cells was dependent on both age and pregnancy status. Absolute numbers of γδ T-cells were higher in HiCMIR animals at 9 and 15 mo of age but did not differ at 21 mo of age. Furthermore, γδ T-cell numbers were higher in HiCMIR animals during mid-pregnancy. Although numbers of γδ T-cells were similar in both phenotypes after immunization (day 7), γδ T-cells made up a higher proportion of total lymphocytes in HiAMIR animals than in HiCMIR animals. In vivo depletion of the γδ T-cell population in calves using monoclonal antibodies enhances the production of antibodies against the type-2 antigen, ovalbumin, which suggests that γδ T-cells exhibit suppressor activity on B-cell responses either directly or through effects on helper T-cell populations (16). In contrast, during delayed-type hypersensitivity (DTH) skin-test reactions to the type-1 antigen, purified protein derivative (PPD), it was found that depletion of the γδ T-cell population did not adversely affect the development of DTH responses in calves, even though γδ T-cells were found to infiltrate the test site in response to antigen (17).
B-cells are lymphocytes that have the unique ability to produce antibody in response to antigen stimulation. These cells also play an important role in antigen presentation and as such are a critical component of the adaptive immune system. Results indicated that the proportion and absolute numbers of B-cells (IgM+) were higher in HiAMIR animals at day 7. Although phenotype did influence the number (but not the proportion) of B-cells at day 0, this effect was dependent on age and the number of B-cells was similar in both phenotypes at 9, 15, or 21 mo of age. When combined, these results suggest that proliferation of the B-cell population is greater in HiAMIR than in HiCMIR animals in response to immunization with the specific test antigens.
An inverse relationship between antibody production and macrophage function has previously been reported in cattle selected for resistance or susceptibility to Brucella abortus (18). A similar relationship has also been reported in mice selected for high and low antibody production (19), whereas in pigs selected for overall immune responsiveness, monocyte function was similar in high IR (HiAMIR/ HiCMIR) and low IR (LoAMIR/LoCMIR) lines (20). Although monocyte function was not investigated here, the proportion and numbers of blood monocytes were found to vary between phenotypes. HiCMIR-phenotype animals tended to have a higher number of monocytes at day 0, although such a difference only approached significance (P = 0.054). Although the proportion of monocytes at day 0 was influenced by phenotype, the effect was dependent on pregnancy status, with non-pregnant HiCMIR-phenotype animals having a higher number of monocytes than non-pregnant HiAMIR-phenotype animals. After immunization, the effect of phenotype classification on the monocyte population also varied with pregnancy status. Although differences were observed between phenotypes in lymphocyte, neutrophil, eosinophil, and total leukocyte populations at day 0 and/or day 7, a consistent trend in the effect of phenotype was not evident.
In summary, differences in leukocyte populations were observed between HiAMIR- and HiCMIR-phenotypes both before and in response to immunization. Results suggested that both the baseline proportion and the number of γδ T-cells observed in an animal before immunization (day 0) and changes in the proportion and number of B-cells observed in an animal in response to immunization (day 7) may be associated with an animal’s ability to mount a CMIR and AMIR, respectively. Further studies will be required to confirm these findings in a large cohort of animals, to investigate the biological basis of such relationships, and to assess whether or not combining measures of leukocyte population traits with measures of CMIR and AMIR to define IR phenotypes can further improve our ability to assess overall immune responsiveness.
Acknowledgments
The authors gratefully acknowledge the Canadian Bovine Mastitis Research Network (CBMRN) and the Natural Science and Research Council of Canada (NSERC) for financial support to Dr. Bonnie A. Mallard. Special thanks to Dr. Laura Wright and the staff at the University of Guelph, Elora Dairy research facility for their cooperation and assistance; Kathryn Hopperton, Amanda Murdoch, Jacqueline Gallienne, and Lonnie Pyne for technical assistance; William Sears for statistical advice; and Dr. Bruce Wilkie for valuable discussions and intellectual input.
References
- 1.Simianer H, Solbu H, Schaeffer LR. Estimated genetic correlations between disease and yield traits in dairy cattle. J Dairy Sci. 1991;74:4358–4365. doi: 10.3168/jds.S0022-0302(91)78632-3. [DOI] [PubMed] [Google Scholar]
- 2.Van Dorp TE, Dekkers JC, Martin SW, Noordhuizen JP. Genetic parameters of health disorders, and relationships with 305-day milk yield and conformation traits of registered Holstein cows. J Dairy Sci. 1998;81:2264–2270. doi: 10.3168/jds.S0022-0302(98)75806-0. [DOI] [PubMed] [Google Scholar]
- 3.Uribe HA, Kennedy BW, Martin SW, Kelton DF. Genetic parameters for common health disorders of Holstein cows. J Dairy Sci. 1995;78:421–430. doi: 10.3168/jds.S0022-0302(95)76651-6. [DOI] [PubMed] [Google Scholar]
- 4.Wilkie B, Mallard B. Selection for high immune response: An alternative approach to animal health maintenance? Vet Immunol Immunopathol. 1999;72:231–235. doi: 10.1016/s0165-2427(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 5.Mallard BA, Wilkie BN, Kennedy BW, Gibson J, Quinton M. Immune responsiveness in swine: Eight generations of selection for high and low immune response in Yorkshire pigs. Proc 6th World Congr Genet Appl Livest Prod; 1998. pp. 257–264. [Google Scholar]
- 6.Heriazon A, Yager JA, Sears W, Mallard BA. Induction of delayed-type hypersensitivity and interferon-gamma to Candida albicans and anti-hen-egg white lysozyme antibody as phenotypic markers of enhanced bovine immune response. Vet Immunol Immunopathol. 2009;129:93–100. doi: 10.1016/j.vetimm.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez A, Quinton VM, Miglior F, Mallard BA. Genetic parameters of dairy cattle immune response traits. Proc 8th World Congr Genet Appl Livest Prod; 2006. [Google Scholar]
- 8.Wagter LC, Mallard BA, Wilkie BN, Leslie KE, Boettcher PJ, Dekkers JC. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J Dairy Sci. 2000;83:488–498. doi: 10.3168/jds.S0022-0302(00)74908-3. [DOI] [PubMed] [Google Scholar]
- 9.Clapperton M, Diack AB, Matika O, et al. Traits associated with innate and adaptive immunity in pigs: Heritability and associations with performance under different health status conditions. Genet Sel Evol. 2009;41:54. doi: 10.1186/1297-9686-41-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saama PM, Jacob JB, Kehrli ME, Jr, et al. Genetic variation in bovine mononuclear leukocyte responses to dexamethasone. J Dairy Sci. 2004;87:3928–3937. doi: 10.3168/jds.S0022-0302(04)73532-8. [DOI] [PubMed] [Google Scholar]
- 11.Hine BC, Cartwright SL, Mallard BA. Effect of age and pregnancy status on adaptive immune responses of Canadian Holstein replacement heifers. J Dairy Sci. 2011;94:981–991. doi: 10.3168/jds.2010-3329. [DOI] [PubMed] [Google Scholar]
- 12.Saad AM, Concha C, Astrom G. Alterations in neutrophil phagocytosis and lymphocyte blastogenesis in dairy cows around parturition. Zentralbl Veterinarmed B. 1989;36:337–345. doi: 10.1111/j.1439-0450.1989.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson BH, Watson DL, Colditz IG. The effect of dexamethasone on some immunological parameters in cattle. Vet Res Commun. 1999;23:399–413. doi: 10.1023/a:1006365324335. [DOI] [PubMed] [Google Scholar]
- 14.Hein WR, Mackay CR. Prominence of gamma-delta T-cells in the ruminant immune system. Immunol Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- 15.Hoek A, Rutten VP, Kool J, et al. Subpopulations of bovine WC1(+) gammadelta T cells rather than CD4(+)CD25(high) Foxp3(+) T cells act as immune regulatory cells ex vivo. Vet Res. 2009;40:6. doi: 10.1051/vetres:2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard CJ, Sopp P, Parsons KR, Finch J. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur J Immunol. 1989;19:757–764. doi: 10.1002/eji.1830190428. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy HE, Welsh MD, Cassidy JP, et al. The role of WC1(+) gamma-delta T-cells in the delayed-type hypersensitivity (DTH) skin-test reaction of Mycobacterium bovis-infected cattle. Vet Immunol Immunopathol. 2003;93:169–176. doi: 10.1016/s0165-2427(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 18.Price RE, Templeton JW, Smith R, 3rd, Adams LG. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect Immun. 1990;58:879–886. doi: 10.1128/iai.58.4.879-886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hale C, Howard JG. Immunological regulation of experimental cutaneous leishmaniasis. 2. Studies with Biozzi high and low responder lines of mice. Parasite Immunol. 1981;3:45–55. doi: 10.1111/j.1365-3024.1981.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 20.Groves TC, Wilkie BN, Kennedy BW, Mallard BA. Effect of selection of swine for high and low immune responsiveness on monocyte superoxide anion production and class II MHC antigen expression. Vet Immunol Immunopathol. 1993;36:347–358. doi: 10.1016/0165-2427(93)90030-8. [DOI] [PubMed] [Google Scholar]