Abstract
The objective of this study was to determine the effects of the administration of a high volume of isotonic crystalloid at a rapid rate on cardiovascular function in normovolemic, isoflurane-anesthetized dogs during induced hypotension.
Using a prospective study, 6 adult dogs were induced to general anesthesia and cardiovascular and hematological values were measured while the dogs were maintained at 3 hemodynamic states: first during light anesthesia with 1.3% end-tidal isoflurane (ETI); then during a hypotensive state induced by deep anesthesia with 3% ETI for 45 min while administered 1 mL/kg body weight (BW) per minute of isotonic fluids; and then decreased to 1.6% ETI while receiving 1 mL/kg BW per minute of fluids for 15 min. End-tidal isoflurane (ETI) at 3.0 ± 0.2% decreased arterial blood pressure (ABP), cardiac index (CI), and stroke volume index (SVI), and increased stroke volume variation (SVV) and central venous pressure (CVP). Fluid administration during 3% ETI decreased only SVV and systemic vascular resistance index (SVRI), while CVP increased progressively. Decreasing ETI to 1.6 ± 0.1% returned ABP and SVI to baseline (ETI 1.3 ± 0.1%), while CI and heart rate increased and SVV decreased. There was significant progressive clinical hemodilution of hemoglobin (Hb), packed cell volume (PCV), total protein (TP), colloid osmotic pressure (COP), arterial oxygen content (CaO2), and central-venous oxygen content (CcvO2).
High-volume, rapid-rate administration of an isotonic crystalloid was ineffective in counteracting isoflurane-induced hypotension in normovolemic dogs at a deep plane of anesthesia. Cardiovascular function improved only when anesthetic depth was reduced. Excessive hemodilution and its adverse consequences should be considered when a high volume of crystalloid is administered at a rapid rate.
Résumé
Cette étude visait à déterminer les effets de l’administration d’un grand volume de cristalloïde isotonique à un débit rapide sur la fonction cardio-vasculaire de chiens normo-volémique, anesthésiés à l’isoflurane et en hypotension induite.
Dans une étude prospective, 6 chiens adultes ont été soumis à une anesthésie générale et des données cardio-vasculaires et hématologiques ont été prises pendant que les chiens étaient maintenus à 3 états hémodynamiques : premièrement durant l’anesthésie légère avec 1,3 % d’isoflurane en phase télo-expiratoire (ETI); par la suite durant un état d’hypotension induit par une anesthésie profonde avec une ETI de 3 % pour 45 min alors que l’on administrait des fluides isotoniques à raison de 1 mL/kg de poids corporel (BW) par minute; et finalement après réduction de l’ETI à 1,6 % tout en recevant 1 mL/kg de BW par minute de fluide pendant 15 min. L’isoflurane à 3,0 ± 0,2 % lors de l’ETI a entraîné une diminution de la pression sanguine artérielle (ABP), de l’index cardiaque (CI), et de l’index du débit systolique (SVI), et une augmentation de la variation du volume systolique (SVV) et de la pression veineuse centrale (CVP). L’administration de fluide durant l’ETI de 3 % a diminué uniquement la SVV et l’index de résistance vasculaire systémique (SVRI), alors que la CVP augmentait progressivement. La diminution de l’ETI à 1,6 ± 0,1 % a ramené l’ABP et le SVI aux valeurs de base (ETI 1,3 ± 0,1 %), alors que le CI et le rythme cardiaque augmentèrent et la SVV diminua. Il y eu une hémodilution clinique progressive significative de l’hémoglobine (Hb), de l’hématocrite (PCV), des protéines totales (TP), de la pression osmotique colloïdale (COP), du contenu artériel en oxygène (CaO2), et du contenu veineux central en oxygène (CcvO2).
L’administration rapide d’un grand volume d’un cristalloïde isotonique était inefficace pour contrecarrer une hypotension induite par l’isoflurane chez des chiens normo-volémique dans un stade profond d’anesthésie. La fonction cardio-vasculaire ne s’est améliorée uniquement que lorsque la profondeur de l’anesthésie fut réduite. Une hémodilution excessive et ses conséquences néfastes devraient être considérées lorsqu’un grand volume de cristalloïde est administré à un débit rapide.
(Traduit par Docteur Serge Messier)
Introduction
Intravenous fluid therapy is commonly administered during general anesthesia to replace ongoing losses related to both the anesthetic and surgical procedures (blood loss, use of dry anesthetic gases, exposure of cavities, and evaporation). Alterations in neurohumoral mechanisms are responsible for regulating the balance between perfusion pressure and blood flow to organs in order to maintain homeostasis. During general anesthesia, however, this balance can be altered by drugs that induce changes in vascular resistance and myocardial contractility.
In dogs, volatile anesthetics decrease myocardial contractility and cardiac output (CO), which results in dose-dependent hypotension (1,2). It has been shown that this hypotension is not counteracted by administering intravenous isotonic fluids in dogs anesthetized at a surgical depth of anesthesia [1 to 2 minimum alveolar concentration (MAC)], even under conditions of surgical stimulation (3–5). Despite this lack of effect, intravenous fluid therapy is often recommended in anesthetized hypotensive patients to increase blood pressure since hypovolemia may be the cause of the hypotension and volume replacement will therefore benefit the patient. The adverse effects of this practice in normovolemic patients, however, are not always considered.
Unlike arterial blood pressure (ABP), CO can be positively influenced by administering intravenous fluid. By increasing circulating volume and therefore venous return (preload), it is possible to increase stroke volume, which contributes significantly to changes in CO (3,6,7). The effects of fluid therapy in treating hypotension at deep levels of isoflurane anesthesia (> 2.0 MAC), while cardiac contractility is impaired and without concurrent surgical stimulation to drive the sympathetic system, have not been determined.
The objective of this study was to determine the effects of intravenous administration of a high volume of an isotonic crystalloid at a rapid rate to normovolemic dogs under deep isoflurane anesthesia on cardiovascular parameters, changes in blood and plasma volume, and blood values for electrolytes, total protein (TP), packed cell volume (PCV), hemoglobin (Hb), and colloid osmotic pressure (COP). Our hypothesis was that intravenous administration of a high volume of an isotonic crystalloid at a rapid rate would increase CO, but not ABP under conditions of isoflurane-induced hypotension in normovolemic dogs. It was further hypothesized that this type of therapy would negatively affect TP, Hb, and COP values.
Materials and methods
Animals
The study used 6 mixed-breed dogs (3 males and 3 females) between 1 and 4 y of age [2.2 ± 1.3; mean ± standard deviation (s)] and weighing 19.8 to 27.0 kg (22.1 ± 2.8). Findings of physical examinations were normal for all dogs and their health status was further confirmed by determining complete blood (cell) count (CBC) and serum biochemical analysis before they were included in the study. The Institutional Animal Care and Use Committee of the University of Guelph approved animal usage protocol for this study.
Anesthesia and instrumentation
These dogs had been previously used in another study conducted and completed the same day before the study described here. For the earlier study, dogs were induced to general anesthesia by delivering isoflurane (AErrane; Baxter Corporation, Mississauga, Ontario) in oxygen via face mask. The trachea was intubated and the dogs were maintained with isoflurane in oxygen using an F-coaxial circuit with an oxygen flow of 100 mL/kg body weight (BW) per minute for 2 to 3 h at an anesthetic plane close to reported MAC values. Dogs were positioned in right lateral recumbency and administered ketamine (1 mg/kg BW) intravenously (IV) as initial loading dose and a constant rate infusion of 40 μg/kg BW per minute for 60 to 76 min and then a washout period of 80 to 110 min to eliminate ketamine before beginning the present study.
End-tidal isoflurane (ETI) and carbon dioxide concentrations were monitored using an infrared gas analyzer (Datex-Ohmeda S/5 Anesthesia Monitor; GE Healthcare Finland, Helsinki, Finland) connected to the endotracheal tube. The monitor was calibrated before each experiment with a standardized calibration gas mixture designed for the analyzer. Intermittent positive pressure ventilation at a rate of 8 to 10 breaths/min, a tidal volume of 10 to 15 mL/kg BW, and zero positive end-expiratory pressure was used to maintain the end-tidal carbon dioxide concentration between 30 and 40 mmHg. Rectal temperature was monitored by use of a thermometer and maintained between 37°C and 38°C by use of a fan heater. In the earlier study, dogs were instrumented during an initial equilibration period of at least 30 min.
A 20-SWG, 1.88-in catheter (Insyte-W; Becton Dickinson Infusion Therapy Systems, Sandy, Utah, USA) was inserted into the dorsal pedal artery and connected to an electronic pressure transducer (Flotrac Sensor; Edwards Lifesciences, Irvine, California, USA), which interfaced the anesthesia monitor with a pulse pressure waveform analysis system (Vigileo; Edwards Lifesciences). The transducer was positioned and zeroed at the level of the sternal manubrium to directly derive systolic, diastolic, and mean arterial pressure and to collect arterial blood gases and electrolytes (pH, PaCO2, PaO2, HCO3−, base deficit, SaO2, Na+, Cl−, K+, Ca2+, lactate, and Hb) (CCX; Nova Biomedical, Waltham, Massachusetts, USA) into blood gas syringes (Gastlyte; Marquest Medical Products, Englewood, Colorado, USA). Colloid osmotic pressure was also measured from the arterial samples (4420 Colloid Osmometer; Wescor, Logan, Utah, USA) and the remaining blood was centrifuged (IEC MB Centrifuges; Damon/IEC Division, Needham Heights, Massachusetts, USA) in capillary tubes at 14 000 rpm (12 700 × g) for 3 min to determine PCV, and the plasma fraction was placed on a refractometer (RHC-200; Westover Scientific, Mill Creek, Washington, USA) for determination of TP. A lithium chloride sensor (LiDCO Sensor; LiDCO, London, UK) was also attached to the arterial catheter for determination of cardiac output (CO). A second 20-SWG, 1.88-in catheter was placed in the cephalic vein and an isotonic solution (Plasmalyte-A; Baxter Corporation) was administered at a rate of 3 mL/kg BW per h. A 19-SWG, 12-in central venous catheter (Intracath; Becton Dickinson Infusion Therapy Systems) was placed in the jugular vein close to the right atrium based on pressure waves generated by connecting the catheter to a second electronic transducer and also zeroed at the level of the sternal manubrium and attached to the anesthetic monitor to measure central venous pressure (CVP) and collect central venous blood gas tensions and electrolytes. Arterial blood pressures, electrocardiogram (ECG), and heart rate were monitored continuously and results displayed throughout the study. A urinary catheter for urine collection was placed with a syringe at the pre-determined time intervals for measuring all variables.
Measurements of CO were determined by lithium dilution using a LiDCO computer (LiDCO Plus; LiDCO). The lithium chloride sensor was attached with a 3-way valve to the arterial catheter and to tubing compressed by a peristaltic pump (LiDCO Flow Regulator; LiDCO) that bled the vessel into a disposable blood collection bag (Disposable blood collection bag and tube; LiDCO) at a flow of 4 mL/min across the sensor. The hemoglobin (Hb) and sodium ion (Na+) concentration required by the LiDCO computer were determined immediately before obtaining CO measurement. The dose (0.006 mmol/kg BW) of lithium chloride (LiDCO chloride injection; LiDCO) was parked into an extension set attached to the catheter in the cephalic vein and flushed with 8 mL of heparinized saline solution [0.9% sodium chloride (NaCl)], 8 s after starting the injection phase on the LiDCO computer. At least 2 measurements of CO were obtained to verify <20% variation between values and then averaged.
Experimental design
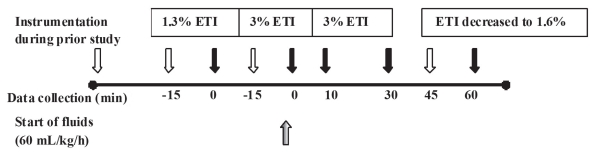
At the conclusion of the previous experiment, dogs were maintained at 1.3% ETI (1.0 MAC) (8) for at least 15 min until a steady hemodynamic state was reached, based on constant values for arterial blood pressure (ABP). At this point, baseline cardiovascular variables, including ABP, heart rate, CVP, CO, SVV (Vigileo; Edwards Lifesciences), end-tidal carbon dioxide, temperature, urine output, arterial and central venous blood gas tensions, and electrolytes were collected (Figure 1). A hypotensive steady state was then maintained at deep anesthesia for at least 15 min at 3% ETI (2.3 MAC) (8) and cardiovascular variables were collected again at the end of this period. Isotonic fluids (Plasmalyte-A solution; Baxter Corporation) were then administered into the cephalic catheter at 1 mL/kg BW per min for 1 h using a volumetric infusion pump (Flogard 6201; Baxter Healthcare Corporation, Deerfield, Illinois, USA), while the ETI was maintained at 3% for 45 min before dialing 1% isoflurane on the vaporizer. This gradually reduced the ETI to approximately 1.6% (1.2 MAC) by the end of the last 15 min of fluid therapy. Cardiovascular variables were collected again at 10, 30, and 60 min and blood gases at 30 and 60 min of fluid therapy.
Figure 1.
Timeline in 6 dogs administered end-tidal isoflurane (ETI) fluids at 1 mL/kg body weight per minute and collection of cardiovascular parameters and blood values for electrolytes, total protein (TP), packed cell volume (PCV), hemoglobin (Hb), and colloid osmotic pressure (COP).
(
 = Start of the event;
= Start of the event;
 = Collection of parameters and blood values; =
= Collection of parameters and blood values; =
 Start of fluid therapy).
Start of fluid therapy).
With these data, the following variables were derived: cardiac index (CI), stroke volume, stroke volume index (SVI), systemic vascular resistance (SVR), systemic vascular resistance index (SVRI), arterial oxygen content (CaO2), central venous oxygen content (CcvO2), oxygen consumption (VO2), oxygen delivery (DO2), and oxygen extraction ratio (ERO2) The formulae for calculating all variables are provided in the Appendix. The changes (%) in plasma and blood volume from baseline were calculated for each respective time interval and the degree of change for SVI and SVV were calculated for the 10, 30, and 60 min time points during fluid therapy. Plasma dilution at a specific time (t) from initiation of fluid administration was calculated as {[(Hb0 − Hbt)/Hbt/(1 − PCV0)] × 100} where “0” indicates baseline. Blood dilution was calculated as {[(PCV0 − PCVt)/PCVt] × 100} (9).
Appendix.
Formulas Used to Calculate Variables
| Variable | Formula | |
|---|---|---|
| CI (mL/kg per minute) | ||
| SV (mL/beat) | ||
| SVI (mL/beat per kilogram) | ||
| SVV (%) | ||
| SVR (dynes · s/cm5) | ||
| SVRI (dynes · s/cm5 per kilogram) | ||
| CaO2 (mL/dL) | ||
| CcvO2 (mL/dL) | ||
| VO2 (mL O2/kg per minute) | ||
| DO2 (mL O2/kg per minute) | ||
| ERO2 (%) |
CI — Cardiac index; CO — Cardiac output; BW — Bodyweight; SV — Stroke volume; HR — Heart rate; SVI — Stroke volume index; SVV — Stroke volume variation; SVR — Systemic vascular resistance; CVP — Central-venous pressure; SVRI — Systemic vascular resistance index; MAP — Mean arterial pressure; CaO2 — Arterial oxygen content; Hb — Hemoglobin; SaO2 — Saturation of hemoglobin in arterial blood; PaO2 — Arterial partial pressure of oxygen; CcvO2 — Central-venous oxygen content; PcvO2 — Central-venous partial pressure of oxygen; ScvO2 — Saturation of hemoglobin in central-venous blood; VO2 — oxygen consumption; DO2 — oxygen delivery; ERO2 — oxygen extraction ratio.
Recovery from anesthesia
Dogs were administered meloxicam (Metacam; Boehringer Ingelheim, Burlington, Ontario), 0.1 mg/kg BW, IV at the end of the study and all instrumentation was removed. Dogs were observed throughout the recovery period for adverse effects related to the treatment.
Data analysis
Data were tested for normality using Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises, and Anderson-Darlin procedures; UNIVARIATE and PLOT procedures were used to detect unequal variances, outliers, and other non-random patterns within the data (Version 9.1; SAS Institute, Cary, North Carolina, USA). The effects of treatment and time on cardiovascular variables and blood values were analyzed with a generalized mixed linear model allowing correlations and non-constant variability for normal distributions (proc MIXED) and NPARIWAY if the assumptions of a normal distribution were not met. The error structure over time was selected based on the Akaike Information Criteria (AIC) among structures offered by SAS: ar(1), arh(1), toep(2–4), toeph(2–4), un(2–4), using for treatment a random effect and a 2-factor (time and treatment)-factorial design with repeated measurements over time. When appropriate, multiple pairwise comparisons were done by use of the Holm-Sidak test. Regression analyses (Version 11.2.1; MedCalc Software, Mariakerke, Belgium) were used to compare the change in SVI during IV administration of fluid with SVV and with the change in SVV. Results were considered statistically significant if the value of P was < 0.05.
Results
Deep anesthesia without fluids
Baseline values at 1.0 MAC (ETI of 1.3 ± 0.1%) were considered appropriate for the depth of anesthesia. Deep anesthesia at 2.3 MAC (ETI of 3.0 ± 0.2%) decreased ABP, CI, SVI, CaO2, CcvO2, ScvO2, DO2, Hb, PCV, TP, COP, Ca2+, Na+, arterial bicarbonate ion (HCO3−), and urine output, whereas SVV, CVP, ERO2, change in plasma and blood volume, and K+ increased. No statistically significant changes were detected for arterial and central venous base deficit, lactate, pH, and oxygen and carbon dioxide tensions, and for end-tidal carbon dioxide, SaO2, venous HCO3−, Cl−, heart rate, SVRI, and temperature, with respect to baseline values (Tables I and II).
Table I.
Mean ± standard deviation cardiovascular variables from 6 anesthetized dogs with 3% end-tidal isoflurane (ETI) to decrease arterial blood pressure (ABP) and cardiac index (CI) and treated for 1 h with 1 mL/kg body weight per min of an isotonic solution while at 3% ETI for the first 45 min and then lowered towards 1.6% ETI for the last 15 min of treatment
| Variables | Baseline (1.0 MAC) | Deep anesthesia (2.3 MAC) | Fluid therapy | ||
|---|---|---|---|---|---|
| 10 min (2.3 MAC) | 30 min (2.3 MAC) | 60 min (1.2 MAC) | |||
| ETI (%) | 1.3 ± 0.1a | 3.0 ± 0.2b | 3.0 ± 0.2b | 3.0 ± 0.2b | 1.6 ± 0.1c |
| Temperature (°C) | 37.4 ± 0.6a,b | 37.5 ± 0.4a | 37.4 ± 0.6a,b | 37.1 ± 0.5b,c | 36.9 ± 0.3c |
| Heart rate (beats/min) | 98 ± 17a | 103 ± 12a,c | 110 ± 8a | 111 ± 9a | 122 ± 16b,c |
| CI (mL/kg per minute) | 159 ± 34.6a | 96 ± 31.2b | 106 ± 23.6b | 123 ± 46.5b | 239 ± 73.8c |
| SVI (mL/beat per kilogram) | 1.6 ± 0.4a | 0.9 ± 0.2b | 1.0 ± 0.2b | 1.1 ± 0.4b | 1.9 ± 0.4a |
| Δ SVI (%) | ND | ND | 11a | 22b | 111c |
| SVV (%) | 9.3 ± 2.2a | 17.5 ± 3.4b | 11.8 ± 2.8a,d | 12.8 ± 4.1a,d | 5.7 ± 1.2c |
| Δ SVV (%) | ND | ND | 33a | 27a | 67b |
| Systolic ABP (mmHg) | 125 ± 18a | 71 ± 13b | 74 ± 2b | 70 ± 15b | 105 ± 30a |
| Diastolic ABP (mmHg) | 64 ± 16a | 39 ± 6b | 41 ± 9b,c | 37 ± 5b | 52 ± 13a,c |
| Mean ABP (mmHg) | 79 ± 15a | 47 ± 6b | 49 ± 11b | 46 ± 7b | 69 ± 15a |
| SVRI (dynes · s/cm5 per kilogram) | 76 ± 25a | 69 ± 32a | 58 ± 25a,b | 47 ± 26b | 42 ± 28b |
| CVP (mmHg) | 9.0 ± 2a | 12 ± 3b | 14 ± 3c | 16 ± 2d | 15 ± 3c,d |
| Urine output (mL/kg) | 1.3 ± 0.06a | 0.06 ± 0.09b | 0.05 ± 0.05b | 0.27 ± 0.22b | 2.1 ± 0.7c |
Different superscript letters within a row indicate significant (P < 0.05) differences between values in that row.
MAC — Minimum alveolar concentration; ETI — End-tidal isoflurane; CI — Cardiac index; SVI — Stroke volume index; Δ SVI — Change in stroke volume index; ND — Not determined; SVV — Stroke volume variation; Δ SVV — Change in stroke volume variation; ABP — Arterial blood pressure; SVRI — Systemic vascular resistance index; CVP — Central-venous pressure.
Table II.
Mean ± standard deviation respiratory and metabolic variables and change in plasma and blood volume in 6 anesthetized dogs with 3% end-tidal isoflurane (ETI) to decrease arterial blood pressure (ABP) and cardiac index (CI) and treated for 1 h with 1 mL/kg bodyweight per min of an isotonic solution while at 3% ETI for the first 45 min and then lowered towards 1.6% ETI for the last 15 min of treatment
| Variables | Baseline (1.0 MAC) | Deep anesthesia (2.3 MAC) | Fluid therapy | |
|---|---|---|---|---|
| 30 min (2.3 MAC) | 60 min (1.2 MAC) | |||
| End-tidal CO2 (mmHg) | 38 ± 2a | 36 ± 3a | 37 ± 3a | 43 ± 4b |
| Arterial pH | 7.395 ± 0.033a | 7.403 ± 0.034a | 7.379 ± 0.035a | 7.345 ± 0.043b |
| PacO2 (mmHg) | 38 ± 3a | 36 ± 2a | 39 ± 4a | 44 ± 5b |
| PaO2 (mmHg) | 583 ± 41a | 584 ± 29a | 620 ± 16b | 627 ± 17b |
| Arterial HCO−3 (mEq/L) | 24 ± 1.5a,c | 23 ± 1.8b | 23 ± 1.9a,b | 24 ± 1.9c |
| Arterial base deficit (mEq/L) | −1.8 ± 1.8a | −2.4 ± 2.1a | −2.5 ± 1.9a | −1.7 ± 2.1a |
| Arterial lactate (mmol/L) | 1.3 ± 0.6a | 1.5 ± 0.7a | 1.5 ± 0.7a | 1.5 ± 0.7a |
| SaO2 (%) | 99.1 ± 0.1a | 99.1 ± 0.1a | 99.2 ± 0.1a | 99.1 ± 0.1a |
| Central venous pH | 7.355 ± 0.027a,b | 7.352 ± 0.034a,b | 7.332 ± 0.031a | 7.318 ± 0.036b |
| Central venous CO2 (mmHg) | 45 ± 4a | 45 ± 3a | 47 ± 5a | 49 ± 4b |
| Central venous O2 (mmHg) | 78 ± 16a,b | 53 ± 8a | 64 ± 31a | 109 ± 68b |
| Central venous HCO−3 (mEq/L) | 25 ± 2.1a,b | 25 ± 1.1a,b | 25 ± 1.7a | 26 ± 1.2b |
| Central venous base deficit (mEq/L) | −0.9 ± 2.1a | −0.8 ± 1.6a | −1.2 ± 1.5a | −1.0 ± 1.5a |
| Central venous lactate (mmol/L) | 1.5 ± 0.7a | 1.4 ± 0.7a | 1.5 ± 0.7a | 1.5 ± 0.7a |
| ScvO2 (%) | 93.8 ± 5.1a | 81.6 ± 9.3b | 80.3 ± 18.0b | 94.7 ± 5.5a |
| VO2 (mL O2/kg per minute) | 4.1 ± 1.5a | 4.6 ± 1.2a | 4.8 ± 2.1a | 5.0 ± 1.3a |
| DO2 (mL O2/kg per minute) | 33.1 ± 7.6a | 19.0 ± 6.3b | 20.0 ± 7.9b | 37.5 ± 11.9a |
| ERO2 (%) | 12 ± 4.7a | 25 ± 7.4b | 27 ± 15.9b | 14 ± 5.1a |
| CaO2 (mL/L) | 20.8 ± 1.4a | 19.8 ± 1.4b | 16.3 ± 1.2c | 15.7 ± 1.5d |
| CcvO2 (mL/L) | 18.2 ± 2.0a | 14.9 ± 1.7b | 11.7 ± 2.5c | 13.5 ± 1.2c |
| Hb (g/dL) | 14.3 ± 1.1a | 13.6 ± 1.0b | 10.8 ± 0.9c | 10.4 ± 1.2d |
| PCV (%) | 40 ± 4a | 37 ± 2b | 31 ± 3c | 28 ± 3d |
| Change in plasma volume (%) | ND | 13*,a | 41§,b | 49§,c |
| Change in blood volume (%) | ND | 8*,a | 19§,b | 32§,c |
| TP (g/dL) | 5.3 ± 0.4a | 5.0 ± 0.3b | 3.8 ± 0.3c | 3.3 ± 0.4d |
| COP (mmHg) | 19.5 ± 2.2a | 16.4 ± 1.7b | 10.7 ± 0.7c | 9.8 ± 0.9d |
| Na+ (mEq/L) | 144 ± 1.0a | 143 ± 1.0b | 141 ± 0.5c | 141 ± 1.0c |
| K+ (mEq/L) | 3.8 ± 0.1a | 4.2 ± 0.1b,c | 4.2 ± 0.3b | 4.4 ± 0.4c |
| Cl− (mEq/L) | 116 ± 2a | 116 ± 2a | 115 ± 1a | 113 ± 2b |
| Ca2+ (mEq/L) | 1.29 ± 0.03a | 1.25 ± 0.03b | 1.12 ± 0.02c | 1.10 ± 0.03d |
Compared to baseline at 1.0 MAC.
Compared to 2.3 MAC without fluids.
Different superscript letters within a row indicate significant (P < 0.05) differences between values in that row.
MAC — Minimum alveolar concentration; CO2 — Carbon dioxide; mm/Hg — Millimeters of mercury; PaCO2 — Arterial partial pressure of carbon dioxide; PaO2 — Arterial partial pressure of oxygen; SaO2 — Saturation of hemoglobin in arterial blood; O2 — oxygen; HCO−3 — Bicarbonate ion; ScvO2 — Saturation of hemoglobin in central-venous blood; VO2 — oxygen consumption; DO2 — oxygen delivery; ERO2 — oxygen extraction ratio; CaO2 — Arterial oxygen content; CcvO2 — Central-venous oxygen content; Hb — Hemoglobin; PCV — Packed cell volume; TP — Total protein; COP — Colloid osmotic pressure; Na+ — Sodium ion; K+ — Potassium ion; CI− — Cardiac index; Ca2+ — Calcium ion.
Deep anesthesia with fluid administration
Administration of fluids at 1 mL/kg BW per minute for 45 min at an ETI of 3% decreased SVV towards baseline 1.0 MAC values (1.3%), whereas SVRI, temperature, CaO2, CcvO2, Hb, PCV, TP, COP, Ca2+, and Na+ decreased and CVP increased from the ETI values at 3% without fluids. Arterial oxygen tension and the change in plasma and blood volume increased from both 1.3% and 3% ETI without fluids. Other variables remained unchanged (Tables I and II).
Lighter anesthesia with fluid administration
Decreasing ETI toward 1.2 MAC (1.6 ± 0.1%) in the last 15 min of fluid administration was associated with a return of SVI, ABP, arterial HCO3−, ScvO2, DO2, and ERO2 toward values obtained at 1.0 MAC. Heart rate, CI, CVP, urine output, end-tidal carbon dioxide, arterial and venous CO2 tensions, and K+ all increased beyond 1.0 MAC values during this period, while temperature, SVV, SVRI, arterial pH, CaO2, CcvO2, Hb, PCV, TP, COP, Na+, Cl−, and Ca2+ all decreased beyond the 1.0 MAC values (Tables I and II).
With respect to values obtained at 3% ETI, the reduction towards 1.2 MAC increased heart rate, CI, SVI, change in SVI, change in SVV, ABP, urine output, end-tidal carbon dioxide, arterial and venous carbon dioxide tensions, arterial and venous HCO3−, CcvO2, ScvO2, DO2, ERO2, change in plasma volume, change in blood volume, and K+. Conversely, SVV, arterial and central venous pH, CaO2, Hb, PCV, TP, COP, Cl−, and Ca2 all decreased from values obtained at 3% ETI (Tables I and II).
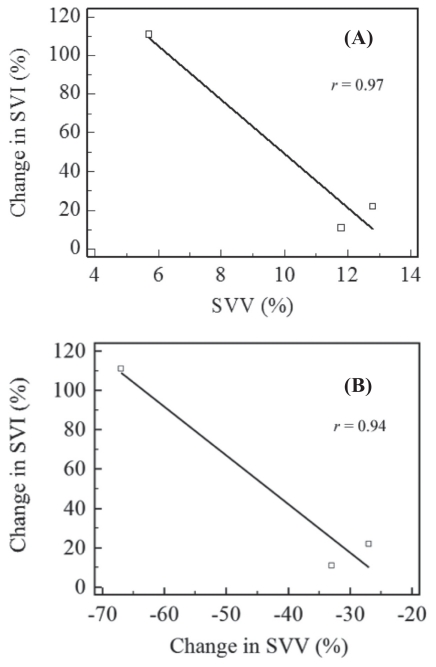
The correlation coefficient (r) between the change in stroke volume index (Δ SVI) with stroke volume variation (SVV) was 0.97 and between the change in SVI with the change in SVV (Δ SVV) was 0.94 (Figure 2 A and B).
Figure 2.
The correlation coefficient (r) between the change in stroke volume index (Δ SVI) with stroke volume variation (SVV) (A) and between the Δ SVI with the change in stroke volume variation (Δ SVV) (B) determined at 10, 30, and 60 min of fluid therapy in dogs administered 1 mL/kg body weight per minute of isotonic fluids.
All 6 dogs recovered uneventfully from anesthesia. During the recovery phase, 2 dogs vomited and 2 other dogs had evident chemosis and lip edema. One of the latter dogs also had aqueous nasal discharge. All dogs exhibited normal behavior and were fully awake within 20 min.
Discussion
This study corroborated findings from other studies in which IV administration of isotonic fluid at volumes of 15 to 80 mL/kg BW per hour during normotensive and hypotensive states did not increase ABP in anesthetized normovolemic patients (3–5). It was also demonstrated that, if hypotension is the result of a deep plane of anesthesia, administering isotonic fluids at 1 mL/kg BW per minute did not increase CO or stroke volume. We did not have a control group to determine if the cardiodepressive effects of an ETI of 3% are progressive over time. Our values obtained for heart rate, CI, SVI, SVRI, and ABP, however, are similar to those of other studies using similar MAC levels in dogs (1,2). It is possible that the volume of fluid administered during deep anesthesia contributed to the higher heart rate and CI and the lower SVRI obtained when the anesthetic plane was lightened to 1.6% ETI.
Similarly in another study (3), CO and stroke volume increased with 80 mL/kg BW per hour in dogs maintained at an ETI of 3.2% to 3.4% with a lesser degree of hypotension. This improvement occurred, however, at the expense of hemodilution-lowered PCV, COP, and TP (3,10–12), all of which may result in adverse side effects. We observed vomiting, facial pitting edema, and nasal discharge in some of the dogs in the recovery period as a result of the high volume of crystalloid administered. Other studies with similar rates (80 mL/kg BW per hour) given during anesthesia (3) and higher rates (225 and 360 mL/kg BW per hour) in conscious, mildly dehydrated dogs have also reported diarrhea, respiratory distress, and protrusion of the ocular globe, with the severity of these signs increasing with increased fluid rate (13).
It has been shown that anesthesia decreases COP to the same extent (3.5 to 5 mmHg) in dogs receiving 0 to 10 mL/kg BW per hour of isotonic fluids during surgery (10,12). This effect is due to the vasodilatory effects of isoflurane and the subsequent increase in blood volume (2,14). Normal COP values of 21 to 24 mmHg have been reported in healthy conscious dogs and slightly lower values have been measured under anesthesia (10,12). Our initial values (19.5 mmHg) decreased dramatically and progressively (9.8 mmHg) over the 60-minute period of fluid administration. Although edema formation is relatively independent of COP, this only applies if volume overload is avoided since increasing transvascular fluid filtration can be accompanied by protein extravasation, which aggravates edema formation (15). A useful way of determining how changes in CVP and COP influence edema formation is to use the COP-to-CVP gradient, which decreases as filtration pressure increases (15). Our baseline normotensive COP-to-CVP gradient was 10.5 mmHg and this had already decreased to 4.4 mmHg under baseline hypotension, and later decreased further to −5.3 and −5.2 mmHg at 30 and 60 min of fluid therapy, respectively. CVP increased during deep anesthesia and continued to increase with fluid therapy to values above normal, which reflects decreased cardiac contractility and efficiency at mobilizing the increased circulating volume secondary to fluid administration.
The amount of fluids administered to the dogs in this study can be transformed to the corresponding plasma volume to account for the actual volume that equilibrates with other fluid spaces in the body, including urine production. Plasma dilution during the administration of a high volume of a fluid at a rapid rate as in this study reflected an expansion of plasma volume of 41% at 30 min and 49% at 60 min. As normal plasma volume in dogs is estimated at 49 mL/kg (16), plasma volume at 30 and 60 min corresponded to 69 mL/kg and 73 mL/kg, respectively. For blood volume, the 16% decrease in PCV at 30 min was the result of expansion of the blood volume by 19%, and the 24% decrease in PCV at 60 min was the result of expansion of the blood volume by 32%. As normal blood volume in dogs is estimated at 81.5 mL/kg (16), blood volume at 30 and 60 min corresponded to 97 mL/kg and 108 mL/kg, respectively. The total amount of fluids administered at 30 and 60 min corresponded to 30 mL/kg BW and 60 mL/kg BW, respectively.
The volume of fluids infused that are actually retained in the vascular space can be estimated from {[(BVt–BV0)/Vi] × 100}, where Vi is volume infused. Therefore, at 30 min, 52% {[(97–81.5)/30] × 100} of the infused volume was in the vascular space and only 44% {[(108–81.5)/60] × 100} at 60 min. Despite the reduction in retention, however, total volume expansion was greater at 60 min than at 30 min (32% versus 19%) since twice the volume had been administered at this time.
In general, it has been stated that an isotonic crystalloid solution can increase blood volume by 20% to 25% of the administered volume (17). In another study, dogs under anesthesia were administered 80 mL/kg BW in less than 15 min and had a 76% retention of the infused volume immediately after infusion, which progressively decreased to approximately 35%, 25%, and 18% at 30, 60, and 240 min, respectively (18). The lower retention in the latter study than in this study is because the infused volume was administered as a bolus rather than as a constant infusion until the end of the measurements. Theoretically, to maintain the same desired degree of volume expansion as obtained initially, it is only necessary to follow the initial higher rate with a lower infusion steady-state rate using nomograms described for humans (19). Such data have not been determined for dogs, however, and data from humans should not be extrapolated since there are important differences related to body weight and percentage of water in the extracellular space even between male and female volunteers (19).
Despite the increase in circulating volume caused by the high volume and rapid rate, urine output was less than 0.3 mL/kg BW under deep anesthesia (during the first 45 min) and did not increase until the anesthetic plane was lightened. Based on the above calculations for fluid retention at 60 min, 44% retention frees 33.6 mL/kg (56%) of the administered volume, but total urine output for the hour of high-volume, rapid-rate fluid administration was only 2.5 mL/kg BW per h (∑ 10, 30, and 60 min). Possible explanations for this discrepancy include the distribution of fluids to the interstitial space while urinary excretion decreases, which has been demonstrated to be exacerbated by inhalant anesthetics (4,20). Isoflurane increases secretion of vasopressin and favors fluid retention (21). Urine output in sheep anesthetized with isoflurane was only 3.6% of the infused volume (25 mL/kg BW in 20 min) 60 min after the start of the infusion compared to 32% for conscious sheep (20). In our study, urine output was 4% (2.5/60) of the total infused volume at the end of the 60-min infusion period. This represents a net gain in interstitial fluid of 31 mL/kg (52% = 56% of administered volume minus 4% urine output), thereby predisposing to edema.
The increase in CVP and the reduced COP-to-CVP gradient predispose to edema formation, since both changes have been correlated with increased pulmonary blood volume and pulmonary edema (15). Administering colloid fluids instead of crystalloids may decrease the predisposition to edema because colloids can increase COP as long as capillary permeability is not increased (15).
Deep isoflurane anesthesia before the high-volume, rapid-rate administration of fluids also resulted in lower Hb, CaO2, CcvO2, ScvO2, and DO2, as well as increased ERO2 in the present study. Oxygen delivery (DO2), ERO2, and ScvO2 improved to baseline values (1.3% ETI) when the anesthetic plane was lightened. This was probably due in part to the earlier administration of fluid and the volume expansion achieved, which increased CI beyond baseline values. Adequate fluid resuscitation should aim at restoring the balance between demand and delivery of oxygen. Even though demand and delivery are not uniform in all vascular beds (22), DO2/VO2 parameters may have advantages over driving pressures such as ABP and filling pressures such as right atrial pressure or flow (CO) when assessing the effectiveness of fluid therapy. Fluid therapy can predictably increase CO and CVP, but the overall DO2 may be simultaneously unaffected or decreased due to hemodilution (23). Despite this discrepancy, the tendency has always been to clinically monitor hemodynamic variables rather than oxygen transport variables (24,25).
Stroke volume variation (SVV) was the only cardiovascular parameter that showed an early change in response to a deeper plane of anesthesia, to fluid therapy, and to a lighter plane of anesthesia. The sensitivity of SVV for assessing responsiveness and improvement in venous return during fluid resuscitation has been well documented (26–29) and provides information on the patient’s dynamic response to fluid therapy while under intermittent positive pressure ventilation, in contrast to static variables such as right-atrial pressure or CVP that do not reflect cardiac filling pressures or changes in preload. Stroke volume variation (SVV) relies on the beat-to-beat variation in left ventricular stroke volume during a single respiratory cycle on intermittent positive pressure ventilation and quantifies the variations or undulations in the arterial pressure curve trace by analysis of the pulse contour, which can be used to estimate CO (26,27,29–33). The increase in airway pressure and lung volume caused by intermittent positive pressure ventilation does not affect systemic vascular resistance but can negatively affect CO and ABP. These changes are more prominent in the hypovolemic patient (32). The mechanism responsible involves an increase in right atrial pressure that decreases the pressure gradient towards the heart from the peripheral circulation (venous return) and consequently decreases left ventricular preload and hence stroke volume, CO, and systolic ABP (33–35).
Administration of IV fluids to hypovolemic patients with a high SVV helps to normalize the circulating volume and increases venous return. The resultant change in stroke volume index (Δ SVI) from baseline (hypotensive/hypovolemic state) as a result of volume administration has been significantly correlated with CO (6,7). The Δ SVI inversely correlates to SVV and to Δ SVV values. Therefore, Δ SVI increases as SVV or Δ SVV decreases (28). Our results indicate that these correlations were also very high (r > 0.97 for SVV and r > 0.94 for Δ SVV) even in the normovolemic dogs and that there were significant changes in Δ SVI and Δ SVV due to fluid therapy before any of the other cardiovascular parameters changed.
In our study, SVV was measured with the FloTrac Vigileo method as the interface cable required for the LiDCO method for SVV measurement was not available. A recent study indicates that readings from these 2 methods are not interchangeable, but the changes in SVV reflected by both techniques are in agreement with the expected clinical results in response to fluid therapy (36). Although the FloTrac/Vigileo method determines CO based on algorithms that are specific for humans, the determination of SVV by the FloTrac/Vigileo is independent of arterial tone factor, age, and body surface area and should be accurate in both animals and humans.
In this and another study (37), hypotension was induced by deep levels of isoflurane anesthesia while the animals were normovolemic. Average mean ABP readings as low as 24 mmHg in foals (37) and 46 mm Hg in dogs from this study were not associated with deleterious effects on organ function in the recovery period, although they were short-lived and always normalized before the end of both studies. In both studies, CO values were acceptable despite the degree of hypotension. In fact, a poor correlation between CO and ABP has been demonstrated in several studies (37–42). In addition, lactate, venous and arterial pH, venous and arterial HCO−3, and base deficit did not indicate compromised tissue perfusion by the low ABP induced in this study. It is often recommended that a mean ABP greater than 60 mmHg is needed to maintain adequate cerebral, renal, and coronary blood flow, especially in critical patients (43). Interestingly, increasing mean ABP from 65 to 85 mmHg in critical patients does not provide additional improvements in VO2, blood lactate concentrations, and renal function than a mean ABP of 65 mmHg (44).
Electrolytes measured in this study were maintained within reported reference intervals because the crystalloid solution (Plasmalyte-A; Baxter Corporation) has a similar composition to plasma. While potassium ion (K+) values did increase slightly, they remained within the reference interval and K+ levels have been shown to increase during anesthesia (11,45). Conversely, calcium ions (Ca2+) decreased due to the absence of this ion in the crystalloid solution.
The distribution of fluids in the different compartments, including the intravascular and extravascular spaces, of dogs in this study was determined under conditions that are probably different in a critically ill and sympathetically stimulated patient. As this study was conducted on healthy, normovolemic, and non-sympathetically stimulated (anesthetized) dogs, the results must be interpreted cautiously when applying them to critical patients. Hypoproteinemia and blood loss are common occurrences in critical patients, however, and aggressive crystalloid fluid therapy will have a greater negative impact on COP despite the need for volume expansion in a hypovolemic animal. The model of hypotension used in this study does not reflect conditions of hypovolemia, which was beyond the objectives of this study. It is common practice, however, to administer “crystalloid” fluids to counteract hypotension in anesthetized patients despite unreliable results. The results of this and other studies (3–5) should caution against this practice unless hypovolemia is implicated. Furthermore, addressing the anesthetic plane and avoiding excessive depression associated with volatile anesthesia are more effective ways of preventing cardiovascular impairment than fluid administration in normovolemic animals.
In conclusion, this study determined that the rapid administration of high volumes of fluid to normovolemic animals with isoflurane-induced hypotension had minimal benefits to cardiovascular variables unless the anesthetic plane was concurrently lightened. Furthermore, such benefits are accompanied by transient detrimental effects on hematological values that control fluid distribution in the body.
Acknowledgments
This study was funded in part by a grant from the Ontario Veterinary College Pet Trust Fund. The authors especially thank Anne Valliant for statistical advice.
Footnotes
This paper was presented at the 16th International Veterinary Emergency and Critical Care Symposium and Annual Conference of the American College of Veterinary Anesthesiologists, San Antonio, Texas, 2010.
References
- 1.Kazama T, Ikeda K. The comparative cardiovascular effects of sevoflurane with halothane and isoflurane. J Anesth. 1988;2:63–68. doi: 10.1007/s0054080020063. [DOI] [PubMed] [Google Scholar]
- 2.Bernard JM, Wouters PF, Doursout MF, Florence B, Chelly JE, Merin RG. Effects of sevoflurane and isoflurane on cardiac and coronary dynamics in chronically instrumented dogs. Anesthesiology. 1990;72:659–662. doi: 10.1097/00000542-199004000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Aarnes TK, Bednarski RM, Lerche P, Hubbell JA, Muir WW., 3rd Effect of intravenous administration of lactated Ringer’s solution or hetastarch for the treatment of isoflurane-induced hypotension in dogs. Am J Vet Res. 2009;70:1345–1353. doi: 10.2460/ajvr.70.11.1345. [DOI] [PubMed] [Google Scholar]
- 4.Hauptman JG, Richter MA, Wood SL, Nachreiner RF. Effects of anesthesia, surgery, and intravenous administration of fluids on plasma antidiuretic hormone concentrations in healthy dogs. Am J Vet Res. 2000;61:1273–1276. doi: 10.2460/ajvr.2000.61.1273. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor JS, Wertz EM, Kesel LM, et al. Effects of intravenous administration of fluids on packed cell volume, blood pressure, and total protein and blood glucose concentrations in healthy halothane-anesthetized dogs. J Am Vet Med Assoc. 1996;208:2013–2015. [PubMed] [Google Scholar]
- 6.Reuter DA, Felbinger TW, Schmidt C, et al. Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med. 2002;28:392–398. doi: 10.1007/s00134-002-1211-z. [DOI] [PubMed] [Google Scholar]
- 7.Berkenstadt H, Margalit N, Hadani M, et al. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg. 2001;92:984–989. doi: 10.1097/00000539-200104000-00034. [DOI] [PubMed] [Google Scholar]
- 8.Valverde A, Morey TE, Hernández J, Davies W. Validation of several types of noxious stimuli for use in determining the minimum alveolar concentration for inhalation anesthetics in dogs and rabbits. Am J Vet Res. 2003;64:957–962. doi: 10.2460/ajvr.2003.64.957. [DOI] [PubMed] [Google Scholar]
- 9.Svensén CH, Rodhe PM, Prough DS. Pharmacokinetic aspects of fluid therapy. Best Pract Res Clin Anaesthesiol. 2009;23:213–224. doi: 10.1016/j.bpa.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Wright BD, Hopkins A. Changes in colloid osmotic pressure as a function of anesthesia and surgery in the presence and absence of isotonic fluid administration in dogs. Vet Anaesth Analg. 2008;35:282–288. doi: 10.1111/j.1467-2995.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 11.Valverde A, Hatcher E, Stämpfli HR. Effects of fluid therapy on total protein and its influence on calculated unmeasured anions in the anesthetized dog. J Vet Emerg Crit Care. 2008;18:480–487. [Google Scholar]
- 12.Dismukes DI, Thomosvky EJ, Mann FA, Middleton JR. Effects of general anesthesia on plasma colloid oncotic pressure in dogs. J Am Vet Med Assoc. 2010;236:309–311. doi: 10.2460/javma.236.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Cornelius LM, Finco DR, Culver DH. Physiologic effects of rapid infusion of Ringer’s lactate solution into dogs. Am J Vet Res. 1978;39:1185–1190. [PubMed] [Google Scholar]
- 14.Sano Y, Sakamoto A, Oi Y, Ogawa R. Anaesthesia and circulating blood volume. Eur J Anaesthesiol. 2005;22:258–262. doi: 10.1017/s0265021505000438. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijden M, Verheij J, van Nieuw Amerongen GP, Groeneveld AB. Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and non-septic critically ill patients with hypovolemia. Crit Care Med. 2009;37:1275–1281. doi: 10.1097/CCM.0b013e31819cedfd. [DOI] [PubMed] [Google Scholar]
- 16.Wamberg S, Sandgaard NCV, Bie P. Simultaneous determination of total body water and plasma volume in conscious dogs by the indicator dilution principle. J Nutr. 2002;132:1711S–1713S. doi: 10.1093/jn/132.6.1711S. [DOI] [PubMed] [Google Scholar]
- 17.Svensén C, Hahn RG. Volume kinetics of Ringer solution, dextran 70, and hypertonic saline in male volunteers. Anesthesiology. 1997;87:204–212. doi: 10.1097/00000542-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Silverstein DC, Aldrich J, Haskins SC, Drobatz KJ, Cowgill LD. Assessment of changes in blood volume in response to resuscitative fluid administration in dogs. J Vet Emerg Crit Care. 2005;15:185–192. [Google Scholar]
- 19.Hahn RG, Svensén C. Plasma dilution and rate of infusion of Ringer’s solution. Br J Anaesth. 1997;79:64–67. doi: 10.1093/bja/79.1.64. [DOI] [PubMed] [Google Scholar]
- 20.Connolly CM, Kramer GC, Hahn RG, et al. Isoflurane but not mechanical ventilation promotes extravascular fluid accumulation during crystalloid volume loading. Anesthesiology. 2003;98:670–681. doi: 10.1097/00000542-200303000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Adams HA, Schmitz CS, Baltes-Gotz B. Endocrine stress reaction, hemodynamics and recovery in total intravenous and inhalation anesthesia: Propofol versus isoflurane. Anaesthetist. 1994;43:730–737. doi: 10.1007/s001010050115. [DOI] [PubMed] [Google Scholar]
- 22.Marik PE. Gastric intramucosal pH: A better predictor of multi-organ dysfunction syndrome and death than oxygen-derived variables in patients with sepsis. Chest. 1993;104:225–229. doi: 10.1378/chest.104.1.225. [DOI] [PubMed] [Google Scholar]
- 23.Wan L, Bellomo R, May CN. The effects of normal and hyper-tonic saline on regional blood flow and oxygen delivery. Anesth Analg. 2007;105:141–147. doi: 10.1213/01.ane.0000266438.90360.62. [DOI] [PubMed] [Google Scholar]
- 24.Bauer M, Korgten A, Hartog C, Riedemann N, Reinhart K. Isotonic and hypertonic crystalloid solutions in the critically ill. Best Pract Res Clin Anaesthesiol. 2009;23:173–181. doi: 10.1016/j.bpa.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Scheeren TWL, Schwarte LA, Arndt JO. Metabolic regulation of cardiac output during inhalation anesthesia in dogs. Acta Anaesthesiol Scand. 1999;43:421–430. doi: 10.1034/j.1399-6576.1999.430410.x. [DOI] [PubMed] [Google Scholar]
- 26.Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F. Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 2008;101:761–768. doi: 10.1093/bja/aen277. [DOI] [PubMed] [Google Scholar]
- 27.Hofer CK, Senn A, Weibel L, Zollinger A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Critical Care. 2008;12:R82. doi: 10.1186/cc6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31:1399–1404. doi: 10.1097/01.CCM.0000059442.37548.E1. [DOI] [PubMed] [Google Scholar]
- 29.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients. A critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann A, Kufner C, Hofbauer S, et al. The accuracy of the Vigileo/FloTrac continuous cardiac output monitor. J Cardiothorac Vasc Anesth. 2008;22:388–393. doi: 10.1053/j.jvca.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 32.Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67:498–502. doi: 10.1097/00000542-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Renner J, Scholz J, Bein B. Monitoring fluid therapy. Best Pract Res Clin Anaesthesiol. 2009;23:159–171. doi: 10.1016/j.bpa.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Dhainaut JF, Devaux JY, Monsallier JF, Brunet F, Villemant D, Huyghebaert MF. Mechanisms of decreased left ventricular preload during continuous positive pressure ventilation in ARDS. Chest. 1986;90:74–80. doi: 10.1378/chest.90.1.74. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg PCM, Jansen JRC, Pinsky MR. Effect of positive pressure on venous return in volume-loaded cardiac surgical patients. J Appl Physiol. 2002;92:1223–1231. doi: 10.1152/japplphysiol.00487.2001. [DOI] [PubMed] [Google Scholar]
- 36.de Wilde RB, Geerts BF, van den Berg PC, Jansen JR. A comparison of stroke volume variation measured by LiDCOplus and FloTrac-Vigileo system. Anaesthesia. 2009;64:1004–1009. doi: 10.1111/j.1365-2044.2009.06009.x. [DOI] [PubMed] [Google Scholar]
- 37.Valverde A, Giguère S, Sánchez LC, Shih A, Ryan C. Effects of dobutamine, norepinephrine, and vasopressin on cardiovascular function in anesthetized neonatal foals with induced hypotension. Am J Vet Res. 2006;67:1730–1737. doi: 10.2460/ajvr.67.10.1730. [DOI] [PubMed] [Google Scholar]
- 38.Valverde A, Giguère S, Morey TE, Sanchez LC, Shih A. Comparison of noninvasive cardiac output measured by use of partial carbon dioxide rebreathing or the lithium dilution method in anesthetized foals. Am J Vet Res. 2007;68:141–147. doi: 10.2460/ajvr.68.2.141. [DOI] [PubMed] [Google Scholar]
- 39.Giguère S, Knowles HA, Valverde A, Bucki E, Young L. Accuracy of indirect measurement of blood pressure in neonatal foals. J Vet Intern Med. 2005;19:571–576. [PubMed] [Google Scholar]
- 40.Raisis AL, Blissitt KJ, Henley W, Rogers K, Adams V, Young LE. The effects of halothane and isoflurane on cardiovascular function in laterally recumbent horses. Br J Anaesth. 2005;95:317–325. doi: 10.1093/bja/aei180. [DOI] [PubMed] [Google Scholar]
- 41.Gunkel CI, Valverde A, Morey TE, Hernández J, Robertson SA. Comparison of non-invasive cardiac output measurement by partial carbon dioxide rebreathing with the lithium dilution method in anesthetized dogs. J Vet Emerg Crit Care. 2004;14:187–195. [Google Scholar]
- 42.Wagner AE, Dunlop CI, Wertz EM, Chapman PL. Evaluation of five common induction protocols by comparison of hemodynamic responses to surgical manipulation in halothane-anesthetized horses. J Am Vet Med Assoc. 1996;208:252–257. [PubMed] [Google Scholar]
- 43.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Bourgoin A, Leone M, Delmas A, Garnier F, Albanèse J, Martin C. Increasing mean arterial pressure in patients with septic shock: Effects on oxygen variables and renal function. Crit Care Med. 2005;33:780–786. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 45.Steffey EP, Farver T, Zinkl J, Wheat JD, Meagher DM, Brown MP. Alterations in horse blood cell count and biochemical values after halothane anesthesia. Am J Vet Res. 1980;41:934–939. [PubMed] [Google Scholar]