Abstract
The B cell–depleting IgG1 monoclonal antibody rituximab can persistently suppress disease progression in some patients with autoimmune diseases. However, the mechanism underlying these long-term beneficial effects has remained unclear. Here, we evaluated Ig gene usage in patients with anti–myelin-associated glycoprotein (anti-MAG) neuropathy, an autoimmune disease of the peripheral nervous system that is mediated by IgM autoantibodies binding to MAG antigen. Patients with anti-MAG neuropathy showed substantial clonal expansions of blood IgM memory B cells that recognized MAG antigen. The group of patients showing no clinical improvement after rituximab therapy were distinguished from clinical responders by a higher load of clonal IgM memory B cell expansions before and after therapy, by persistence of clonal expansions despite efficient peripheral B cell depletion, and by a lack of substantial changes in somatic hypermutation frequencies of IgM memory B cells. We infer from these data that the effectiveness of rituximab therapy depends on efficient depletion of noncirculating B cells and is associated with qualitative immunological changes that indicate reconfiguration of B cell memory through sustained reduction of autoreactive clonal expansions. These findings support the continued development of B cell–depleting therapies for autoimmune diseases.
Introduction
Rituximab is a chimeric mouse-human IgG1 monoclonal antibody that targets the CD20 antigen, which is expressed on immature and mature B lymphocytes and lost upon plasma cell differentiation (1). The primary mechanism of action of rituximab, at least early in therapy, is a complete but transient depletion of B cells through a combination of antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and direct triggering of apoptosis (2–4). A single course of rituximab leads to depletion of B cells from peripheral blood for 6–12 months (1). The FDA has previously approved rituximab for the therapy of B cell lymphomas, chronic lymphocytic leukemia, and rheumatoid arthritis. More recently, rituximab received FDA approval for the treatment of patients with granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis. Its off-label use extends to a broad spectrum of autoimmune diseases, including systemic lupus erythematosus, idiopathic thrombocytopenic purpura, myasthenia gravis, inflammatory neuropathies, and multiple sclerosis (1).
The rationale for B cell–depleting therapies in autoimmune diseases has been that immune depletion could eliminate autoreactive B lymphocytes and that de novo regeneration of B cell memory from pro–B cell precursors — which do not express CD20 — could reestablish tolerance. However, to our knowledge, no study to date has demonstrated that B cell–depleting therapies can actually reconfigure B cell memory through detection of phenotypic or functional renewal of the B cell repertoire. Thus, despite its clinical efficacy and widespread use, the mechanisms whereby rituximab treatment confers its long-term clinical efficacy in patients with autoimmune diseases are unclear (5).
Anti–myelin-associated glycoprotein (anti-MAG) neuropathy is a well-defined antibody-mediated disease of the peripheral nervous system that develops in individuals with an IgM monoclonal gammopathy of unknown significance (MGUS) and is characterized by autoreactivity toward MAG, a protein expressed in the peripheral myelin sheath. IgM anti-MAG antibodies, which are consistently detectable in these patients, are very likely pathogenic, since their adoptive transfer to susceptible host animals induces peripheral demyelination and symptoms resembling those observed in patients with anti-MAG neuropathy (6–9). Thus, anti-MAG neuropathy stands out among other human autoimmune diseases due to the known identity of the target antigen and a clear disease association with IgM autoantibodies. Most available immunomodulatory treatments offer only transient benefits to some patients with anti-MAG neuropathy, whereas most remain treatment resistant (10). A recent randomized controlled clinical trial demonstrated that rituximab is, so far, the most effective therapeutic agent, providing long-term benefits to a subset of these patients (11). To understand whether these beneficial effects are mediated by lymphodepletion alone or are sustained by a newly developed peripheral B cell compartment, we examined the Ig gene repertoire in patients with anti-MAG neuropathy during rituximab therapy.
Results
Ig gene repertoire analysis during therapeutic B cell depletion.
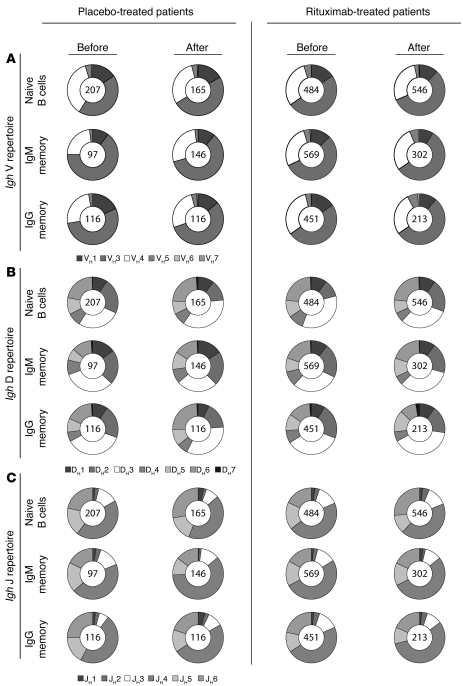
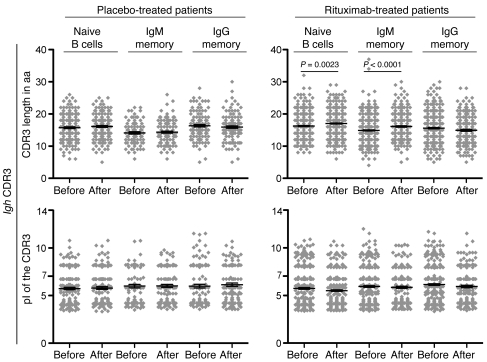
To determine whether rituximab-mediated B cell depletion leads to substantial changes in the peripheral Ig gene repertoire, we amplified and sequenced Ig heavy chain (Igh) genes of single sorted naive B cells (CD19+CD27–IgM+), IgM memory B cells (CD19+CD27+IgM+), and IgG memory B cells (CD19+CD27+IgG+) before and 12 months after therapy. Samples were obtained during a placebo-controlled clinical trial of rituximab in patients with anti-MAG neuropathy, during which patients were randomized to 4 weekly infusions of 375 mg/m2 rituximab or normal saline solution as placebo (11). The VH, DH, and JH gene family usage before treatment initiation did not differ between the 2 subgroups (Figure 1) and was similar to demographically matched healthy blood donors (data not shown). VH3, the largest variable region family, was found most frequently in any of the B cell subsets, followed by VH4 and VH1. B cell reconstitution 12 months after rituximab treatment did not significantly alter the VH, DH, and JH gene repertoire in circulating naive, IgM memory, and IgG memory B cells (Figure 1 and Supplemental Figures 2–4; supplemental material available online with this article; doi: 10.1172/JCI58743DS1). Next, we examined Igh characteristics such as the length, charge, and hydrophobicity of the Igh complementarity-determining region 3 (CDR3), which have previously been associated with antibody-mediated autoreactivity (12, 13). Analysis of a large number of sequences enabled us to detect small differences such as a modest increase in Igh CDR3 lengths in naive and IgM memory B cells from rituximab-treated patients, whereas isoelectric points (pIs) remained unchanged after peripheral B cell reconstitution (Figure 2). We conclude that there are no consistent abnormalities in the Igh repertoires in patients with anti-MAG neuropathy and that rituximab-mediated peripheral B cell depletion does not markedly change the VH, DH, and JH gene family usage of naive and memory B cells.
Figure 1. Ig gene repertoire analysis in patients with anti-MAG neuropathy before and 12 months after rituximab-mediated B cell depletion.
Igh gene variable (V) region (A), diversity (D) region (B), and joining (J) region (C) repertoires in single cell–sorted naive B cells (CD19+CD27–IgM+), IgM memory B cells (CD19+CD27+IgM+), and IgG memory B cells (CD19+CD27+IgG+). Samples were analyzed before and 12 months after therapy with rituximab and placebo (normal saline solution). Clonally expanded sequences were counted as 1 sequence. Numbers within circles indicate the number of individual sequences analyzed per patient cohort and time point. Data for each individual donor are provided in Supplemental Figures 2–4. The 2-tailed Fisher exact test was used to evaluate VH, DH, and JH repertoires.
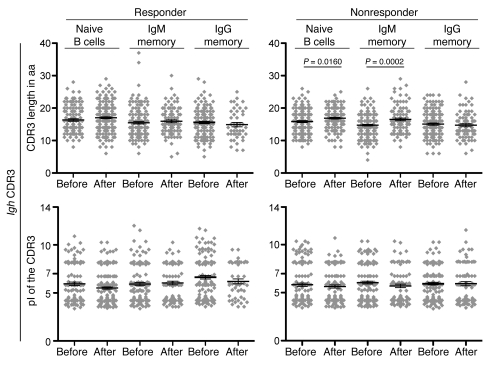
Figure 2. CDR3 length and CDR3 pI before and after rituximab therapy.
The same number of individual Igh gene sequences as in Figure 1 were analyzed, and results are displayed as data derived from individual sequences. Data points represent individual sequences; horizontal bars and error bars indicate mean ± SEM. The unpaired Student’s t test was used to evaluate CDR3 lengths and pIs.
Size and antigen specificities of IgM memory B cell expansions in anti-MAG neuropathy.
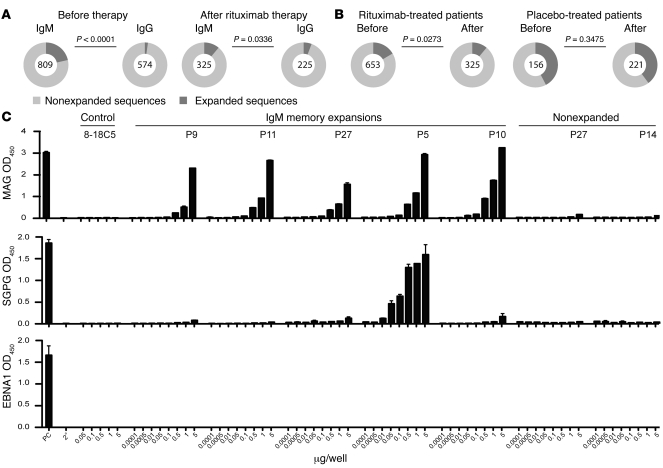
Patients with anti-MAG neuropathy showed substantial clonal and oligoclonal expansions within the IgM memory B cell compartment before treatment initiation: 21.5% of 809 analyzed single sorted cells had identical Igh CDR3 gene sequences, compared with 2.3% of 574 sequences derived from IgG memory B cells (P < 0.0001; Figure 3A). The size of clonal IgM memory B cell expansions was markedly reduced after rituximab therapy, but not in placebo-treated patients (Figure 3B). We detected a few expanded clones in the naive compartment: placebo-treated patients showed 0% of a total of 207 sequences before and 3% of 170 sequences after therapy, compared with 0.3% of 539 sequences before and 1.4% of 353 sequences after therapy in rituximab-treated patients. These expansions — but not nonexpanded sequences — were somatically hypermutated, which suggests that these clones represent post–germinal center B cells. In our estimation, the most likely explanation for these few expanded and somatically hypermutated sequences is the presence of CD27– memory B cells, which occur at low frequencies in the blood of healthy individuals and patients with autoimmune diseases (14). The overall size of these expansions was minimal compared with the size of expanded sequences within the IgM memory B cell compartment. We observed that the frequency of expanded IgG memory B cells increased over time in placebo-treated patients. However, rituximab therapy had no substantial effect on the size of clonally related IgG memory B cells (data not shown).
Figure 3. Size and antigen specificities of clonal IgM memory B cell expansions.
(A) Frequency of clonally expanded IgM memory B cells in patients with anti-MAG neuropathy before and after rituximab therapy compared with the IgG memory compartment. Before therapy, data from all study subjects were included. Post-treatment data refer to those patients that received rituximab therapy. Numbers within circles indicate the number of individual sequences analyzed per patient cohort and time point. (B) IgM memory B cell clonal expansions before and after therapy in patients treated with placebo versus rituximab. Results are based on the analysis of individual sequences in both clinical subgroups. The 2-tailed Fisher exact test was used to compare proportions of clonal expansions. (C) Specificity of IgM memory B cell expansions, as assessed by ELISA. Recombinant antibodies expressed from highly expanded sequences (see Figure 5B) were tested for binding to purified MAG protein, SGPG, and EBNA1. Shown are OD values from the positive control (PC) provided by the manufacturer, a secondary antibody control (2°), an antibody (8-18C5) that recognizes myelin oligodendrocyte glycoprotein (MOG) to control for antigen specificity, and recombinantly expressed antibodies from patients (P) with anti-MAG neuropathy.
To determine whether expanded IgM memory B cell clones recognize MAG, we re-sorted IgM memory B cells isolated before treatment initiation and amplified full-length Igh and Ig light chain variable region gene transcripts derived from highly expanded sequences by RT-PCR. Amplified products were cloned into eukaryotic expression vectors to produce monoclonal IgG antibodies with antigen specificities identical to the initial B cell antigen receptors (15). The antigenic determinant of MAG resides in the carbohydrate component of the molecule, as demonstrated by the loss of reactivity following deglycosylation of purified human MAG (16). A similar carbohydrate structure is found in the ganglioside fraction of the human peripheral nerve and has been identified as sulfoglucuronyl glycosphingolipid (SGPG), an acidic glycolipid exclusively expressed in the peripheral nervous system (17). Pathogenic IgM antibodies in patients with MAG neuropathy may recognize both MAG and SGPG, although the avidity for SGPG is reported to be 10- to 100-fold lower than that for MAG (18). Therefore, we tested both specificities in comparison to an irrelevant control protein, EBV nuclear antigen 1 (EBNA1) a prominent B cell antigen of the ubiquitous γ-herpesvirus EBV.
All of the 5 clones resurrected from blood IgM memory B cell expansions before therapy recognized MAG purified from human myelin in a concentration-dependent manner (Figure 3C). Of these 5 clones, 1 showed strong cross-reactivity toward SGPG. Nonexpanded IgM memory clones recognized neither MAG nor SGPG. Moreover, we did not observe any binding to EBNA1 (Figure 3C), which indicates that IgM memory B cell expansions in patients with anti-MAG neuropathy recognize the target antigen of the disease.
Persistence of clonally expanded IgM memory B cells is associated with a poor clinical response to rituximab.
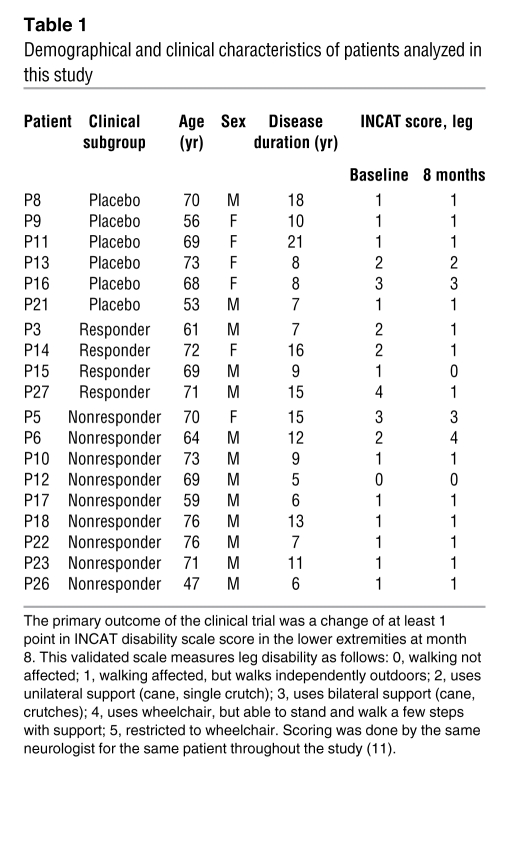
Next, we determined whether patients showing a clinical improvement after rituximab treatment (referred to herein as clinical responders; n = 4) could be distinguished from patients with stable or worsened disease (clinical nonresponders; n = 9) through Igh gene repertoire changes, CDR3 characteristics, and the kinetics of clonal expansions during rituximab treatment. The clinical response was quantified using a standardized clinical disability scale, and details were previously reported in the publication of the clinical trial (Table 1 and ref. 11). Data obtained in clinical responders and nonresponders were compared with those in placebo-treated patients (n = 6).
Table 1 .
Demographical and clinical characteristics of patients analyzed in this study
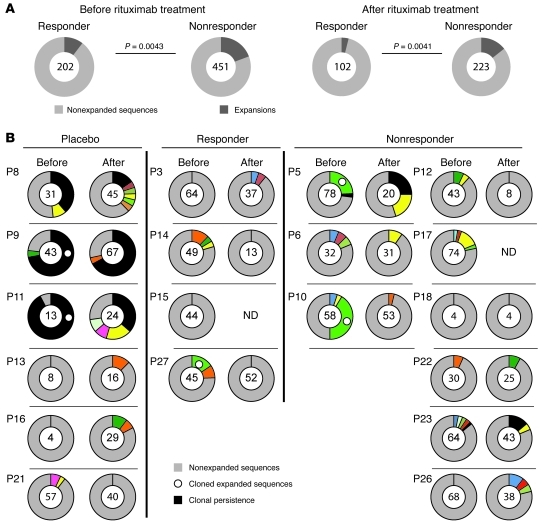
VH and DH gene family usage in circulating naive, IgM memory, and IgG memory B cells before treatment initiation did not differ between the clinical subgroups, and peripheral B cell reconstitution did not significantly alter the VH and DH gene repertoire 1 year after treatment (Supplemental Figures 2 and 3). Before therapy, JH5 usage was more frequently found in the group of clinical responders compared with nonresponders, but these differences lost statistical significance 12 months after rituximab therapy (Supplemental Figure 4). These data indicate that the clinical response to rituximab therapy is not associated with substantial changes in VH, DH, and JH gene family usage of naive and memory B cells. The modest increase in CDR3 length after peripheral B cell reconstitution (Figure 2) could be attributed to clinical nonresponders (Figure 4). We observed that the group of clinical nonresponders started with a higher load of IgM memory B cell expansions compared with responders (P = 0.0043; Figure 5A). Moreover, the reduction of IgM memory B cell expansions after B cell depletion, as determined by Igh gene sequence analysis, was less strong in the group of clinical nonresponders compared with responders (expansions reduced by factors of 1.3 and 2.7, respectively). Consequently, the amount of IgM memory B cell expansions remained increased in clinical nonresponders compared with responders after peripheral B cell reconstitution (P = 0.0041; Figure 5A). Notably, clinical responders that were included in our study showed a higher then 2-fold decrease in MAG-specific IgM antibody titers (11), which indicates that inhibition of MAG-specific B cell expansions is associated with inhibition of pathogenic MAG-specific antibody secretion. However, we did not detect a significant correlation between MAG titers and the size of clonal IgM memory expansions in individual patients.
Figure 4. CDR3 length and pI before and after rituximab therapy in clinical responders and nonresponders.
Patients showing a clinical improvement after rituximab therapy were classified as clinical responders; patients with stable or worsened disease severity were classified as clinical nonresponders. The same number of individual Igh gene sequences derived from rituximab-treated patients as in Figure 2 were analyzed; results are displayed as pooled data derived from individual sequences. Data points represent individual sequences; horizontal bars and error bars indicate mean ± SEM. The unpaired Student’s t test was used to evaluate Igh characteristics.
Figure 5. Higher load of IgM memory B cell expansions and persistence of expanded IgM memory B cells is associated with a poor clinical response to rituximab.
(A) IgM memory expansions before and after rituximab therapy in pooled samples from clinical responders and nonresponders. Results are based on the analysis of individual sequences in both clinical subgroups. Numbers within circles indicate the number of individual sequences analyzed per patient cohort and time point. The 2-tailed Fisher exact test was used to compare proportions of clonal expansions. (B) IgM memory B cell expansions in individual clinical responders or nonresponders compared with placebo-treated patients. Gray shading, nonexpanded sequences; differently colored shading, clonally expanded CDR3 sequences; black shading, sequences detected before and after therapy in individual patients are highlighted in black. Circles denote expanded sequences that were resurrected as IgG monoclonal antibodies (see Figure 3B). Due to the low frequency of memory B cells in 2 post-treatment samples, we were not able to amplify PCR products (ND, not determined).
Since the Ig gene repertoire analysis was based on pooled individual sequences, we additionally analyzed the proportions of clonal expansions per individual patient and compared the mean proportions between 4 clinical responders and 9 nonresponders. In line with the analysis based on sequences, clinical nonresponders tended to start with a higher load of IgM memory B cell expansions compared with responders, and the amount of IgM memory B cell expansions tended be higher in clinical nonresponders than in responders after peripheral B cell reconstitution. However, the statistical significance was lost, possibly as a result of the small sample size (data not shown). Analysis both of pooled sequences and of sequences per individual patient did not detect any changes in the Ig gene family usage during rituximab therapy (data not shown). Continuous as well as categorical outcomes per individual patient are shown in Supplemental Table 1 and Supplemental Figures 2–4.
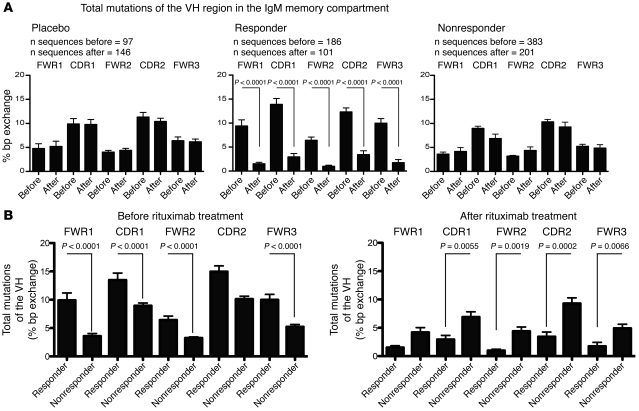
We identified several identical, expanded IgM memory B cell clones, which persisted for 12 months in individual placebo-treated patients as well as in clinical nonresponders (Figure 5B). Persisting clonal expansions were not observed in clinical responders (Figure 5B), which suggests that these persisting expansions may have contributed to the progression of the disease; however, we cannot exclude the possibility that persisting, low abundant clonal expansions would have been detected if a larger number of samples had been analyzed. Frequencies of expanded sequences per individual patient are listed in Supplemental Table 1. To further address whether IgM memory B cells in rituximab-treated patients developed anew or persisted over time, we compared the frequency of somatic hypermutations (SHMs) in IgM memory B cells before and after therapy. The degree of SHM was stable in placebo-treated patients (Figure 6A). Likewise, and despite efficient depletion of circulating B cells, the overall SHM frequency in circulating IgM memory B cells remained unchanged in the group of clinical nonresponders (Figure 6A). In contrast, clinically effective rituximab treatment was associated with a significant reduction in the SHM frequency in VH of IgM memory B cells (P = 0.0001; Figure 6A). Notably, the frequency of SHMs in pooled sequences derived from clinical responders was higher before rituximab therapy, but lower 12 months after, compared with nonresponders (Figure 6B). Mean SHM frequencies per individual patient also tended to be higher before but lower 12 months after rituximab therapy in clinical responders compared with nonresponders; however, statistical significance was lost, possibly due to the small sample size (data not shown). The reduction after therapy was strongest in patient P27, who showed the strongest clinical improvement in response to rituximab of all clinical responders (Table 1 and Supplemental Tables 2 and 3). We conclude that clinical nonresponders can be distinguished from responders with respect to clonal IgM memory B cell expansion size before and after therapy, persistence of clonal expansions despite efficient peripheral B cell depletion, and stable SHM frequencies within the IgM memory compartment.
Figure 6. Lack of change in SHM frequencies in IgM memory B cells is associated with a poor clinical response to rituximab.
(A) Mean SHM frequencies of IgM memory B cells in framework and CDR regions (FWR1, CDR1, FWR2, CDR2, and FWR3) in placebo-treated patients and in clinical responders and nonresponders before and after rituximab therapy. (B) Mean SHM frequencies before and after rituximab therapy in clinical responders versus nonresponders. Expanded sequences were taken into account only once in order to analyze SHM frequencies in IgM memory B cells independent from clonal expansions. Results are based on the analysis of individual sequences in clinical subgroups. The unpaired Student’s t test was used to compare SHM frequencies.
Discussion
Our study demonstrated that the long-term immunomodulatory effects of rituximab in anti-MAG neuropathy are mediated by the sustained reduction of expanded autoreactive IgM memory B cells. In patients with peripheral nervous system autoimmunity associated with IgM MGUS, which is believed to arise from somatically hypermutated but non–class-switched B cells that acquire IgM secretory capacity (19, 20), we showed that IgM memory B cells contained an important reservoir of autoreactive species, and that sustained elimination of these cells was associated with clinical disease remission. Conversely, high load and clonal persistence of expanded autoreactive IgM memory B cell clones despite efficient depletion of circulating B cells were associated with a poor clinical response to rituximab.
Previous studies demonstrated expansion and persistence of foreign antigen-specific memory B cells following infection and vaccination (21–23) and of candidate pathogenic T cell clones in human autoimmune diseases, such as multiple sclerosis and type 1 diabetes (24–26). The known identity of the target antigen allowed us to address the dynamic behavior of expanded autoreactive B cells in patients with anti-MAG neuropathy. The presence of oligoclonal expansions within the IgM memory B cell compartment before treatment initiation, their ability to recognize MAG protein, and their persistence in patients with stable or worsened disease suggest that these persisting expansions may have contributed to disease progression and argue for an important role of MAG-specific IgM memory B cells in the pathogenesis of this inflammatory neuropathy.
Clinical responders showed qualitative immunological changes that indicated reconfiguration of B cell memory through sustained reduction of autoreactive expansions and changes in SHM frequencies. While these changes might be driven by different cellular subsets that contributed to the CD27+IgM+ B cell pool in both clinical subgroups, they indicate that the IgM memory compartment in clinical responders was repopulated by newly developed B cells. In contrast, based on the lack of significant changes in SHM frequencies, we found no evidence for a substantial de novo generation of IgM memory B cells in clinical nonresponders, which suggests that at least a fraction of disease-relevant B cells are not accessible for or susceptible to CD20 immunotherapy. In patients with rheumatoid arthritis, the depleting effect of rituximab was reported to be more profound in the peripheral blood than in inflamed joints or bone marrow (27–29). Although the clinical response status was not addressed in the prior studies, the results obtained here suggest that targeting of B cell–depleting antibodies to professional lymphoid tissues and sites of autoimmune inflammation for the sustained elimination of autoreactive B cells might increase the clinical efficacy of CD20 immunotherapy.
Rituximab was evaluated for its clinical efficacy in anti-MAG neuropathy based on its potential to reduce pathogenic IgM antibodies by depleting B cells. Initial uncontrolled phase II studies reported that some patients show clinical improvement following rituximab therapy (30–32). Patients who initially responded well to rituximab in one of the aforementioned clinical trials were prospectively observed without treatment for another 36 months. Despite the reappearance of circulating B cells, a sustained clinical benefit after a single rituximab course was noted that lasted up to 24 months in 80% of patients and up to 36 months in 60% of patients (33). Disease recurrence was best correlated with high baseline anti-MAG titers and increasing anti-MAG antibodies at follow-up, and the authors suggested that patients with high anti-MAG titers may require additional courses with rituximab (33). In a small series of 8 patients, administration of a double dose of rituximab (750 mg/mβ every week for 1 month) 17–27 months after standard dose treatment (375 mg/mβ) improved the clinical outcome. High-dose treatment was also efficient in inducing a first clinical response associated with reduced anti-MAG antibody titers in patients that were refractory to the standard treatment (31). We found that a high load of oligoclonal B cell expansions before therapy and low efficiency to reduce B cell expansions with rituximab was associated with a poor clinical response. The small sample size of our study and the pooled analysis did not allow us to address whether the size or reduction of oligoclonal expansions is a biomarker for the clinical response to rituximab in individual patients. Such analyses require larger study populations and confirmation in independent cohorts. Our data, however, suggest that patients who show no clinical improvement after a first round of rituximab therapy could benefit from additional treatment rounds to deplete or further reduce the frequency of clonally related autoreactive B cells.
In conclusion, our data indicate that the therapeutic efficacy of rituximab in anti-MAG neuropathy depends on efficient depletion of noncirculating B cells and is associated with qualitative immunological changes that indicate reconfiguration of B cell memory. These findings provide a mechanistic basis for the differential clinical response to rituximab in anti-MAG neuropathy and support the therapeutic concept of ongoing and future clinical trials of B cell–depleting therapies in autoimmune diseases.
Methods
Patients.
We analyzed specimens from patients that were included in a double-blind, placebo-controlled study of rituximab in patients with anti-MAG demyelinating polyneuropathy that was carried out at the NIH (Table 1 and ref. 11). Patients were selected if they had clinical and electrophysiological evidence of a demyelinating neuropathy, a benign IgM monoclonal spike, and anti-MAG antibodies. Patients did not receive any immunosuppressive therapy for at least 6 months before enrollment. At entry, all patients (except 1) had impaired function, as evidenced by affected balance and/or coordination, frequent falls, or muscle weakness, reflected in an Inflammatory Neuropathy Course and Treatment (INCAT) disability score of at least 1 (see Table 1). The study was conducted at the NIH under a Cooperative Research and Development Agreement between National Institute of Neurological Disorders and Stroke and Genetech. The trial was registered at ClinicalTrials.gov (NCT00050245) and was conducted under a protocol approved by the National Institute of Neurological Disorders and Stroke institutional review board. After randomization, 4 weekly intravenous cycles of 375 mg/m2 rituximab or placebo (consisting of a normal saline solution) were administered. Patients were premedicated with acetaminophen (650 mg) and diphenhydramine (25 mg) before infusions. The primary outcome was a change of at least 1 point in INCAT disability scale score in the lower extremities at month 8. This is a validated scale previously used in inflammatory demyelinating neuropathies, including the IgM anti-MAG neuropathy (11, 34, 35). The 4 patients that improved by at least 1 INCAT point after 8 months were classified as clinical responders; the other patients with stable or worsened disease were classified as clinical nonresponders. Individual demographical and clinical profiles of all patients included in this study are given in Supplemental Table 1.
Flow cytometry and single cell analysis.
Frozen PBMCs were thawed in FACS buffer (2 mM EDTA, 5% FCS, 20 μg/ml DNAse [Roche] in PBS). After thawing, the PBMCs were strained (0.22-μm cell strainer; BD) and centrifuged for 15 minutes at 150 g and 4°C. The cells were incubated with PE-conjugated anti-CD27 clone M-T271, PE-Cy7–conjugated anti-CD19 clone SJ25C1, allophycocyanin-conjugated anti-IgM clone G20-127, biotin-conjugated anti-IgG clone G18-145, and FITC-conjugated streptavidin (all BD) and with component H (Invitrogen) for dead cell discrimination. Single CD19+CD27–IgM+ naive B cells, CD19+CD27+IgM+ memory B cells, and CD19+CD27+IgG+ memory B cells were purified by flow cytometric cell sorting using a Facs Aria machine and Diva software (both BD). The gating strategy is depicted in Supplemental Figure 1. Single cell sorting was directly into a 96-well plate (thermoquick PCR-Plate; Greiner bioOne) with 20 μl reverse transcription buffer or Qiagen OneStep RT-PCR reaction mix (Qiagen) supplemented with 0.5 μM each of Igh variable region–specific forward and IgG/IgM reverse primers per well (15). Directly after sorting, buffer plates were frozen on dry ice, and Qiagen OneStep RT-PCR plates were incubated for 60 minutes at 50°C for reverse transcription. After the reverse transcription step, DNA polymerase was activated with an initial step of 15 minutes at 95°C. Amplification of the resulting cDNA of Igh was performed at an annealing temperature of 58°C for 45 seconds, an elongation temperature of 72°C for 1 minute, and a denaturation temperature of 94°C for 30 seconds for 48 cycles. Nontemplate controls were included to exclude contaminations in the master mix.
To reduce PCR error rates, we used the HotStar Taq DNA polymerase (Qiagen) with an error rate of 2 × 10–5 per nucleotide and cycle. PCR amplicons were separated by electrophoresis on a 1.2% agarose gel. To avoid cross contaminations on the gel, PCR products were physically separated from each other. Bands with the correct mass (approximately 500 bp) were excised using individual sterile scalpels; DNA was extracted (Qiagen MiniElute Gel Extraction Kit) and sequenced. The sequenced variable region of the amplified Igh was corrected with CLC Main Workbench and analyzed on the international immunogenetic information system (36, 37). The variable region was analyzed for VH, DH, and JH usage; SHMs; CDR3 length; pI; and clonal expansion.
Sequence analysis.
After blasting, the sequences’ clonal expansions were determined by similar CDR3 aa sequences and VH, DH, and JH usage. The length of the CDR3 was defined as the number of aas from the third position after the cysteine motive of the CDR3 to the tryptophan aa. For pI analysis, we used the entire aa sequence of the CDR3 and calculated it with the tool on ExPASy proteomics server (ref. 38 and Figures 2 and 4). The VH, DH, and JH gene usage was weight balanced by the number of sequences derived from each donor to avoid tempering the result by different amount of sequences due to clonal expansion or PCR efficacy. For the SHM analysis, sequences were first blasted against the germline sequences in the database of IMGT. The blast result of unique IgM memory B cells in framework and CDR regions was used to calculate percent bp exchange by dividing the number of mutations by the length (in bp) of the individual variable region elements (FWR1, CDR1, FWR2, CDR2, and FWR3).
Expression vector cloning.
Expanded sequences were reconfirmed via an additional PCR from the presorted 20-μl reverse transcription buffer plate. The corresponding Ig light chain was used to rule out contaminations and the cloning of the antibodies. For this purpose, RNA of the single frozen cell was divided into 3 RT-PCRs: 1 for Igh and 2 for Ig light chain (λ and κ; Igl and Igk, respectively). The recovered Igh sequences were then compared with the previous sequences. The corresponding Igh and Igk or Igl genes were cloned into expression vectors as previously described (15). The recombinant monoclonal antibodies were expressed in 293T cells after calcium phosphate–mediated transfection with 22.5 μg of each vector DNA (Igh and Igl). After 3 days, antibodies were purified from cell culture supernatants over a protein G column. The elution was performed with 0.1 M glycine (pH 2) and dialyzed overnight in PBS (pH 7.4).
ELISA.
Specificities of recombinant antibodies were evaluated at different concentrations (0.001, 0.005, 0.01, 1, 10, and 50 μg/ml) using 3 commercially available ELISA kits (anti-SPGP ELISA kit, anti-MAG ELISA kit, both Bühlmann Laboratories; EBNA1 IgG ELISA, Bio-Rad). The ELISAs were performed according to the manufacturers’ recommendations. The OD signal obtained with the negative control provided by the manufacturer was subtracted from the OD signals of experimental samples. To detect the recombinantly expressed IgG molecules, a goat anti-human IgG secondary antibody coupled to HRP was used in the MAG and SGPG ELISA (Sigma-Aldrich). The detection was done with TMB substrate that was stopped with 1M H2SO4, and absorbance was measured at 450 nm.
Statistics.
Unpaired Student’s t test (2-tailed) was used to compare CDR3 length, pI, length of DH and N elements, and SHM frequencies before and after rituximab treatment. The 2-tailed Fisher exact test was used to compare frequencies of VH gene family usage and the size of clonal expansions. All analyses are based on individual sequences, and data in figures in which error bars are shown are presented as mean ± SEM. Since we analyzed pooled sequence data, a repeated-observation analysis was not applied. A P value less than 0.05 was considered significant.
Study approval.
The study was conducted under a protocol approved by the National Institute of Neurological Disorders and Stroke institutional review board. With informed consent from all patients included in the study, peripheral blood mononuclear cells were cryopreserved and used for immunological experiments.
Supplementary Material
Acknowledgments
The study was funded, in part, by the Betty and David Koetser Foundation for Brain Research. M.A. Maurer was supported by the Neuroscience Center Zürich. N. Goebels received funding from the Swiss Multiple Sclerosis Society, the Swiss National Science Foundation (310030-116838), and the Forschungskommission of the Heinrich-Heine-University, Düsseldorf. C. Münz is supported by the National Cancer Institute (R01CA108609), Cancer Research Switzerland (KFS-02652-08-2010), the Sassella Foundation (10/02), the Vontobel Foundation, the Association for International Cancer Research, Novartis, and the Swiss National Science Foundation (310030_126995). J.D. Lünemann receives grant support from the Swiss National Science Foundation (310030_130524), the Gemeinnutzige Hertie Foundation, the Swiss Multiple Sclerosis Foundation, the Betty and David Koetser Foundation, the Ernst Schering Foundation, and the Baxter Research Grant Program.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(4):1393–1402. doi:10.1172/JCI58743.
References
- 1.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6(5):394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 2.Reff ME, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. [PubMed] [Google Scholar]
- 3.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 4.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44(16):3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 5.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5(8):433–441. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 6.Willison HJ, Trapp BD, Bacher JD, Dalakas MC, Griffin JW, Quarles RH. Demyelination induced by intraneural injection of human antimyelin-associated glycoprotein antibodies. Muscle Nerve. 1988;11(11):1169–1176. doi: 10.1002/mus.880111111. [DOI] [PubMed] [Google Scholar]
- 7.Tatum AH. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti-myelin-associated glycoprotein. Ann Neurol. 1993;33(5):502–506. doi: 10.1002/ana.410330514. [DOI] [PubMed] [Google Scholar]
- 8.Ilyas AA, Gu Y, Dalakas MC, Quarles RH, Bhatt S. Induction of experimental ataxic sensory neuronopathy in cats by immunization with purified SGPG. J Neuroimmunol. 2008;193(1–2):87–93. doi: 10.1016/j.jneuroim.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latov N. Pathogenesis and therapy of neuropathies associated with monoclonal gammopathies. Ann Neurol. 1995;37 suppl 1:S32–S42. doi: 10.1002/ana.410370705. [DOI] [PubMed] [Google Scholar]
- 10.Steck AJ, Stalder AK, Renaud S. Anti-myelin-associated glycoprotein neuropathy. Curr Opin Neurol. 2006;19(5):458–463. doi: 10.1097/01.wco.0000245368.36576.0d. [DOI] [PubMed] [Google Scholar]
- 11.Dalakas MC, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65(3):286–293. doi: 10.1002/ana.21577. [DOI] [PubMed] [Google Scholar]
- 12.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 13.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei C, et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178(10):6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 15.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329(1–2):112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilyas AA, et al. IgM in a human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin-associated glycoprotein and to a ganglioside. Proc Natl Acad Sci U S A. 1984;81(4):1225–1229. doi: 10.1073/pnas.81.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendell JR, et al. Polyneuropathy and IgM monoclonal gammopathy: studies on the pathogenetic role of anti-myelin-associated glycoprotein antibody. Ann Neurol. 1985;17(3):243–254. doi: 10.1002/ana.410170305. [DOI] [PubMed] [Google Scholar]
- 18.Dalakas MC. Pathogenesis and treatment of anti-MAG neuropathy. Curr Treat Options Neurol. 2010;12(2):71–83. doi: 10.1007/s11940-010-0065-x. [DOI] [PubMed] [Google Scholar]
- 19.Kriangkum J, Taylor BJ, Treon SP, Mant MJ, Belch AR, Pilarski LM. Clonotypic IgM V/D/J sequence analysis in Waldenstrom macroglobulinemia suggests an unusual B-cell origin and an expansion of polyclonal B cells in peripheral blood. Blood. 2004;104(7):2134–2142. doi: 10.1182/blood-2003-11-4024. [DOI] [PubMed] [Google Scholar]
- 20.Chng WJ, et al. Gene-expression profiling of Waldenstrom macroglobulinemia reveals a phenotype more similar to chronic lymphocytic leukemia than multiple myeloma. Blood. 2006;108(8):2755–2763. doi: 10.1182/blood-2006-02-005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamani-Matsuda M, et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood. 2008;111(9):4653–4659. doi: 10.1182/blood-2007-11-123844. [DOI] [PubMed] [Google Scholar]
- 22.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009;39(5):1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosado MM, et al. Switched memory B cells maintain specific memory independently of serum antibodies: the hepatitis B example. Eur J Immunol. 2011;41(6):1800–1808. doi: 10.1002/eji.201041187. [DOI] [PubMed] [Google Scholar]
- 24.Bielekova B, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6(10):1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 25.Kent SC, et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature. 2005;435(7039):224–228. doi: 10.1038/nature03625. [DOI] [PubMed] [Google Scholar]
- 26.Skulina C, et al. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101(8):2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leandro MJ, Cooper N, Cambridge G, Ehrenstein MR, Edwards JC. Bone marrow B-lineage cells in patients with rheumatoid arthritis following rituximab therapy. Rheumatology (Oxford). 2007. 46 1 29 36 . 10.1093/rheumatology/kel148 [DOI] [PubMed] [Google Scholar]
- 28.Teng YK, et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum. 2007;56(12):3909–3918. doi: 10.1002/art.22967. [DOI] [PubMed] [Google Scholar]
- 29.Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67(7):917–925. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine TD, Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using Rituximab. Neurology. 1999;52(8):1701–1704. doi: 10.1212/wnl.52.8.1701. [DOI] [PubMed] [Google Scholar]
- 31.Renaud S, et al. High-dose rituximab and anti-MAG-associated polyneuropathy. Neurology. 2006;66(5):742–744. doi: 10.1212/01.wnl.0000201193.00382.b3. [DOI] [PubMed] [Google Scholar]
- 32.Benedetti L, et al. Predictors of response to rituximab in patients with neuropathy and anti-myelin associated glycoprotein immunoglobulin M. J Peripher Nerv Syst. 2007;12(2):102–107. doi: 10.1111/j.1529-8027.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 33.Benedetti L, et al. Long-term effect of rituximab in anti-mag polyneuropathy. Neurology. 2008;71(21):1742–1744. doi: 10.1212/01.wnl.0000335268.70325.33. [DOI] [PubMed] [Google Scholar]
- 34.Hughes R, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 2001;50(2):195–201. doi: 10.1002/ana.1088. [DOI] [PubMed] [Google Scholar]
- 35.Hughes RA, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–144. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- 36.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36(Web Server issue):W503–W508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giudicelli V, Brochet X, Lefranc MP. IMGT/V-QUEST: IMGT standardized analysis of the immunoglobulin (IG) and T cell receptor (TR) nucleotide sequences. Cold Spring Harb Protoc. 2011;2011(6):695–715. doi: 10.1101/pdb.prot5633. [DOI] [PubMed] [Google Scholar]
- 38.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31(13):3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.