Abstract
Genetic mutations that give rise to active mutant forms of Ras are oncogenic and found in several types of tumor. However, such mutations are not clear biomarkers for disease, since they are frequently detected in healthy individuals. Instead, it has become clear that elevated levels of Ras activity are critical for Ras-induced tumorigenesis. However, the mechanisms underlying the production of pathological levels of Ras activity are unclear. Here, we show that in the presence of oncogenic Ras, inflammatory stimuli initiate a positive feedback loop involving NF-κB that further amplifies Ras activity to pathological levels. Stimulation of Ras signaling by typical inflammatory stimuli was transient and had no long-term sequelae in wild-type mice. In contrast, these stimuli generated prolonged Ras signaling and led to chronic inflammation and precancerous pancreatic lesions (PanINs) in mice expressing physiological levels of oncogenic K-Ras. These effects of inflammatory stimuli were disrupted by deletion of inhibitor of NF-κB kinase 2 (IKK2) or inhibition of Cox-2. Likewise, expression of active IKK2 or Cox-2 or treatment with LPS generated chronic inflammation and PanINs only in mice expressing oncogenic K-Ras. The data support the hypothesis that in the presence of oncogenic Ras, inflammatory stimuli trigger an NF-κB–mediated positive feedback mechanism involving Cox-2 that amplifies Ras activity to pathological levels. Because a large proportion of the adult human population possesses Ras mutations in tissues including colon, pancreas, and lung, disruption of this positive feedback loop may be an important strategy for cancer prevention.
Introduction
Constitutively active mutants of Ras are oncogenic and are found in several tumor types (1). For this reason, K-Ras mutations have been extensively examined as a potential cancer biomarker. However, mutant K-Ras has not proved to be a useful biomarker due to the consistent observation that Ras mutations occur frequently in normal individuals, especially as they age. For example, studies in pancreatic samples also indicate the prevalence of K-Ras mutations in normal individuals (2–4). K-Ras mutations were also reported to be present in 20% of stool samples from normal healthy adults (3). In another study, it was reported that K-Ras codon 12 mutations were identified in 10% of lung tissues obtained from patients with no lung diseases and 12.5% of patients diagnosed with non-oncological lung diseases (5). The prevalence of Ras mutations in normal healthy individuals suggest that activating Ras mutations per se are not efficient for disease initiation. This conclusion has been confirmed in genetic mouse models. Expression of oncogenic K-Ras in multiple tissues during development leads to neoplasias at an extremely low efficiency (6). Recently, increasing evidence has suggested that not only the presence of mutated Ras but the actual activity of this molecule is important in determining pathological outcomes. For example, in the mammary gland, chronic low-level Ras induction resulted in tumor formation only after the spontaneous upregulation of activated Ras (7). Ras mutations have also been reported to initiate hematopoietic malignancies in a dose-dependent manner (8). Similarly, physiological levels of oncogenic K-Ras expressed in adult pancreatic cells were without pathologic effect (9, 10). In contrast, high levels of Ras activity very efficiently caused severe inflammation and a rapid progression to cancer (10). Therefore, it seems clear that activating Ras mutations simply do not by themselves induce sufficient Ras activity to pose a large risk for development of disease.
However, it has also been observed that the Ras activity levels in tumor cells are significantly higher than those in non-tumor cells in mice expressing mutant K-Ras from its endogenous promoter (11). Therefore, while the expression of oncogenic Ras at physiological levels generally does not directly cause pathological outcomes, the presence of oncogenic Ras appears to predispose to further elevations in Ras activity (7, 10) through unknown mechanisms. We hypothesized that inflammatory stimulation could accelerate oncogenic Ras-induced pathologies through potentiating its signaling. This hypothesis was supported by observations that inflammatory stimuli could accelerate pathological responses in animals expressing physiological levels of mutant Ras (9, 12).
In the current study, we investigated the interactions between oncogenic Ras and inflammatory mechanisms. We found that inflammatory stimuli induce transient Ras signaling in cells expressing wild-type Ras, but induce greater and more prolonged Ras, NF-κB, and Cox-2 activity in mice expressing oncogenic K-Ras. We propose that in the presence, but not the absence, of oncogenic Ras, inflammatory stimuli initiate a positive feedback loop involving NF-κB that generates inflammatory mediators including PGE2 that further amplify Ras activity to pathological levels. At these higher levels of Ras activity, chronic inflammation and precancerous lesions result. Because Ras mutations are widespread in the adult human population, disruption of this positive feedback loop may be an important strategy for cancer prevention.
Results
In cells bearing oncogenic Ras, inflammatory stimuli greatly increase active Ras.
Inflammation is a risk factor for cancer and has been found to accelerate the development of cancer in the presence of oncogenic Ras. At the same time, Ras activity levels are key to the efficiency of Ras-induced transformation. Therefore, we hypothesized that inflammatory stimuli would generate abnormal Ras activity in cells with oncogenic Ras. To test this hypothesis, we isolated cells from mice bearing a loxP-stop-loxP mutant K-Ras driven by its endogenous promoter (LSL-K-RasG12D mice) (13). We focused on pancreas-specific expression of experimental genes because the pancreas is a common site of Ras-induced cancer. Therefore, the LSL-K-RasG12D mice were crossed with mice in which a full-length elastase promoter drives the expression of CreErT (14). After treatments with tamoxifen to fully activate the Cre, the resulting double-transgenic mice expressed physiological levels of mutant K-Ras specifically in adult pancreatic acinar cells and are referred to throughout this article as acinar-Ras mice.
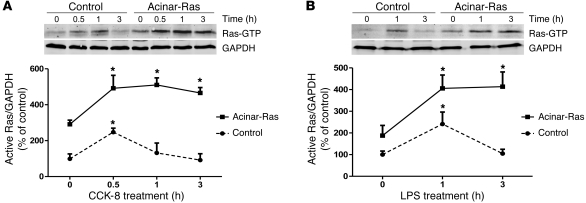
Examination of levels of active Ras indicated that basal activity was elevated in cells from acinar-Ras mice compared with control animals, as expected (Figure 1). We then treated acinar-Ras and control cells with inflammatory stimuli, including cholecystokinin (CCK) (Figure 1A) and LPS (Figure 1B). These stimulants induced a transient increase in active Ras that returned to baseline in control cells at 1–3 hours, as has been previously reported (15–17). In contrast, in cells from acinar-Ras mice, the effects of the stimulants were prolonged and levels remained greatly elevated for at least 3 hours (Figure 1).
Figure 1. Stimuli induced prolonged increases in Ras activity in cells prepared from acinar-Ras but not control mice.
Freshly isolated acini from control or acinar-Ras mice were stimulated with 10 nM CCK-8 (A) or 1 μg/ml LPS (B) for different time periods. The GTP-bound active Ras was measured by a Raf pull-down assay. In each case, a representative Western blot is shown with quantitative data from n = 3–4 independent experiments (*P < 0.05 versus time 0).
The presence of oncogenic Ras is required for the induction of chronic pathologies after transient inflammatory stimulation.
Inflammatory stimuli induced prolonged elevation of active Ras in the presence of oncogenic Ras (current study), and high sustained Ras activity generates inflammatory mediators (10, 18). Therefore, we hypothesized that a positive feedback loop might form in cells expressing oncogenic Ras, but not in control mice, such that chronic diseases would develop after a transient inflammatory insult in acinar-Ras animals.
To test this hypothesis, we treated acinar-Ras and control mice with cerulein, a CCK analog often used to generate a transient inflammatory response in the pancreas of normal mice in order to model acute pancreatitis (19). We performed a series of injections typically used to generate acute pancreatitis (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI59743DS1). As previously reported, in the absence of treatment the pancreata of acinar-Ras mice could not be distinguished from those of control mice, except for the rare appearance of low-grade preneoplastic pancreatic intraepithelial neoplasms (PanINs) (10). We observed that cerulein treatments caused a similar level of rapid acute inflammation in both acinar-Ras and control mice as assessed by histology, serum amylase, and inflammatory cell infiltration (Supplemental Figure 1, B–E). However, the pancreata of control mice fully recovered within a few days and were completely normal in appearance within 1 week (Figure 2A). In contrast, acinar-Ras mice developed profound histological changes (Figure 2B) including widespread loss of acinar cells, persistent occupation with inflammatory cells, and widespread replacement of parenchyma with stromal collagen depositions (Supplemental Figure 2, A–C). These features resembled the histological characteristics of human chronic pancreatitis. Stromal development was accompanied by high levels of activated stellate cells (Supplemental Figure 2D). Within 4 weeks after the transient insult, acinar-Ras mice developed multiple PanINs (Figure 2C). After 8 months, pancreatic ductal adenocarcinoma was identified in some cerulein-treated acinar-Ras animals (Figure 2D). In contrast, none of the cerulein-treated control mice or nontreated acinar-Ras mice developed tumors. The pathological changes observed in the acinar-Ras mice after cerulein treatments appeared identical to those observed in mice engineered to express high levels of oncogenic K-Ras in the pancreas (10). This indicated that Ras activity itself is capable of generating all of the observed responses.
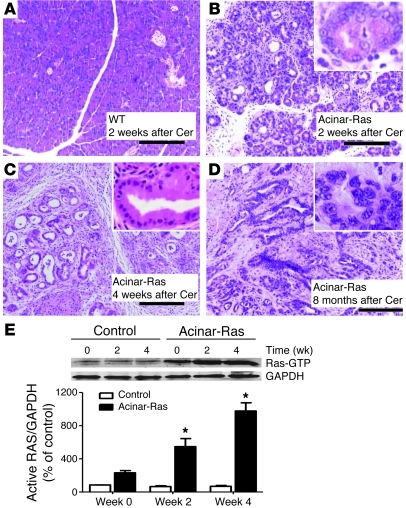
Figure 2. Cerulein administration led to the development of chronic pancreatitis and PanINs and prolonged elevated Ras activity only in the presence of mutant K-Ras.
The CCK analog cerulein was injected using a scheme known to induce acute inflammation in the pancreas (Supplemental Figure 1). Pancreata from control littermates recovered from the acute effects of these injections and were histologically normal by day 14 (A, original magnification, ×100). In contrast, acinar-Ras animals showed a depletion of acinar cells, sustained edema, and inflammation 2 weeks after the first series of cerulein (Cer) treatments (B, original magnification, ×100; inset, ×400). By day 28, the pancreata from acinar-Ras mice showed abundant fibrosis and PanINs (C, original magnification, ×100; inset, ×400). Pancreatic ductal adenocarcinoma developed in acinar-Ras mice 8 months after cerulein treatments (D, original magnification, ×100; inset, ×400). For Ras activity assays, animals were sacrificed without treatment (week 0), 2 weeks after one series of cerulein injections (week 2), and 2 weeks after a second series of cerulein injections (week 4). Cerulein treatments caused a transient increase in signaling in littermate controls but a sustained and increasing level of Ras activity in acinar-Ras mice (E) (*P < 0.05 compared with controls; n = 8 animals).
Active Ras levels in samples taken at various times from these in vivo models indicated early elevation of Ras in both control and acinar-Ras mice (data not shown). However, prolonged elevation of active Ras levels was evident only in acinar-Ras animals (Figure 2E). Surprisingly, active Ras levels were elevated even 2 weeks after the transient cerulein treatments in acinar-Ras mice and were further elevated at 4 weeks (Figure 2E). At 4 week, we also observed a small increase in total Ras protein (Supplemental Figure 2E). The increase in Ras activity was due to direct effects on acinar cells and not to infiltration of inflammatory cells, as p-Erk, a downstream target of Ras activity, was localized to acinar cells, metaplastic ductal cells, and PanIN lesions in acinar-Ras mice (Supplemental Figure 3A) but not control mice (Supplemental Figure 3B). Total levels of p-Erk were also elevated in the pancreata of acinar-Ras mice compared with controls (Supplemental Figure 3C). These data support that the increased Ras activity in the pancreas of acinar-Ras mice was primarily in acinar and acinar-derived cells.
Non-pathological levels of endogenous CCK lead to chronic inflammation and precancerous lesions in the presence of oncogenic Ras.
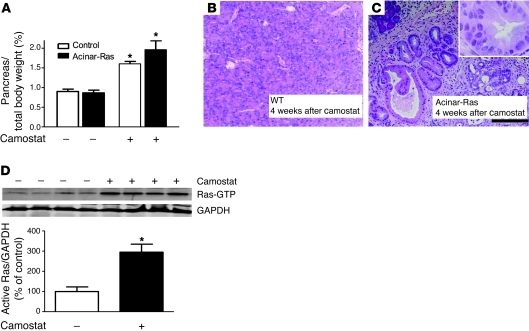
Supraphysiologic cerulein is an artificial inflammatory stimulus used in models of experimental acute pancreatitis. Its endogenous counterpart, CCK, is elevated in the blood after a meal high in fats or proteins (20). Inhibition of gut trypsin activity also increases CCK release from intestinal endocrine cells by activating trypsin feedback regulation of CCK release (21–23). Camostat is a common trypsin inhibitor that has been widely used experimentally to increase circulating endogenous CCK. Increased levels of CCK generated by camostat feeding have trophic actions in rodents leading to increased pancreatic mass (24). However, no pathologies have been reported after camostat feeding. Because CCK activates Ras, we hypothesized that camostat feeding would induce chronic inflammation in animals bearing oncogenic Ras. Therefore, we fed wild-type or acinar-Ras mice camostat for 4 weeks. Camostat feeding increased size (Figure 3A) but caused no histological changes in the pancreata of control mice (Figure 3B), confirming previous reports (24). In contrast, acinar-Ras animals fed camostat developed severe chronic pancreatitis and formed PanINs (Figure 3C). Analysis of levels of active Ras indicated that camostat feeding resulted in a sustained elevation of active Ras levels in acinar-Ras (Figure 3D) but not control mice (data not shown). These data further support that various stimuli that increase Ras activity can generate pathologies in animals bearing oncogenic Ras. This latter observation may be of clinical significance, as camostat is widely used to treat or prevent chronic pancreatitis in some countries (25).
Figure 3. Camostat feeding caused chronic pancreatitis and PanINs in acinar-Ras but not control mice.
Acinar-Ras or control mice were fed a diet containing 0.1% camostat for 4 weeks to raise endogenous levels of CCK. Pancreas growth was measured as a fraction of body weight (A) (*P < 0.05; n = 4 animals). Camostat feeding of control mice did not lead to noticeable histologic changes in the pancreas (B, original magnification, ×100). In contrast, camostat feeding of acinar-Ras mice led to the development of chronic pancreatitis and PanINs (C, original magnification, ×100; inset, ×400). Camostat feeding also caused a sustained elevation of Ras activity in the pancreata of acinar-Ras mice (D) (*P < 0.05; n = 4 animals).
Infectious stimuli lead to chronic inflammation and precancerous lesions in acinar-Ras but not control mice.
Infection is a common risk factor for chronic inflammation and cancers. Therefore, we next examined the effects of LPS, a clinically relevant inflammatory inducer associated with Gram-negative bacteria. LPS exacerbates acute pancreatitis in mouse models of the disease (26, 27). However, LPS alone does not induce pancreatitis in normal mice (26). We hypothesized that the presence of oncogenic Ras would lead to more severe and prolonged effects of LPS treatments. Therefore, acinar-Ras and control mice were treated with LPS (10 mg/kg, i.p.) by weekly injection for 4 weeks (Supplemental Figure 4A). As expected, LPS treatments had no observed effects in control mice (Supplemental Figure 4B). In contrast, LPS treatments caused severe chronic pancreatitis and PanIN lesions in acinar-Ras mice (Supplemental Figure 4C). As predicted by our model, these pathological changes were accompanied by sustained elevated Ras activity (Supplemental Figure 4D).
The NF-κB pathway plays a key role in the stimuli-induced positive feedback loop.
The transcription factor NF-κB is activated downstream of Ras (28). NF-κB has many roles and influences many facets of cell biology, including inflammation and survival (29). We hypothesized that NF-κB and its target genes would be involved in the perpetuation of the positive feedback loop that amplifies Ras activity in cells with oncogenic Ras. In control mice, inflammatory stimuli cause a transient increase in NF-κB activation (30). However, we observed persistent nuclear localization of the active p65 NF-κB subunit, an indication of NF-κB activity, even 4 weeks after cerulein treatment in acinar-Ras mice (Figure 4A) but not in control mice (Figure 4B).
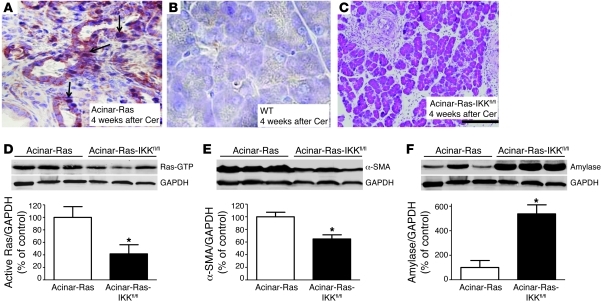
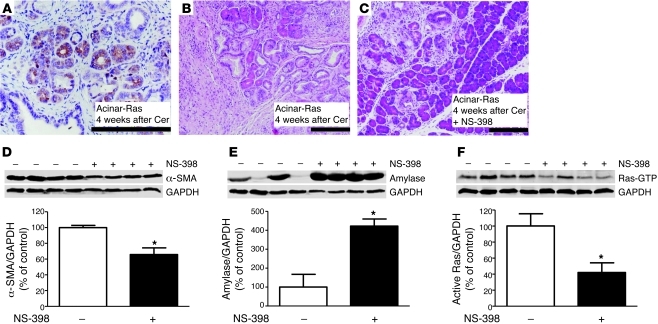
Figure 4. NF-κB activation was essential for cerulein to induce chronic inflammation and precancerous lesions in acinar-Ras mice.
After 2 series of cerulein treatments (week 4), pancreata of acinar-Ras mice showed increased NF-κB subunit p65 nucleus translocation (A, arrows; original magnification, ×400) as compared with controls (B, ×400). Triple transgenic mice (acinar-Ras-IKKfl/fl), which express mutant K-RasG12D with IKK2 deletion, displayed a significant reduction of fibrosis and inflammation (C, original magnification, ×100) and Ras activity (D) after 2 series of cerulein treatments. The degree of stellate cell activation and retention of pancreas parenchyma in acinar-Ras-IKKfl/fl mice was measured by quantification of α-SMA for stellate cells (E) and amylase for acinar cells (F) (*P < 0.05 compared with acinar-Ras mice; n = 4 animals). GAPDH was probed for relative protein loading control (note: GAPDH in D and F was from the same gel).
Positive staining of p–IκB-α, a direct target of IKK kinase, further supported the presence of high IKK kinase activity in the acinar-Ras mice after cerulein treatment (Supplemental Figure 5). To directly examine the role of NF-κB in the positive-feedback loop, we utilized mice expressing oncogenic Ras but lacking inhibitor of NF-κB kinase 2 (IKK2) in adult pancreatic acinar cells (acinar-Ras-IKKfl/fl). IKK2 is the NF-κB pathway upstream regulator that primarily controls the phosphorylation and subsequent degradation of the NF-κB inhibitory subunit IKBs, resulting in the release of the active p65 subunit to the nucleus. The acinar-Ras and acinar-Ras-IKKfl/fl mice were then treated transiently with cerulein as described above. Pancreatic fibrosis, inflammation, the extent of PanIN formation (Figure 4C), and active Ras levels (Figure 4D) were all dramatically reduced in the mice after IKK2 deletion. Using α-SMA as an indicator of active stromal stellate cell mass and amylase as a marker for acinar cells, we observed reduced α-SMA (Figure 4E) and increased amylase (Figure 4F) levels in animals in which IKK2 was deleted. Therefore, a reduction in NF-κB activation diminished the increase in Ras activity and also prevented its prolonged consequences.
In a complementary experiment, we investigated the influence of increasing NF-κB activity in cells with wild-type or oncogenic Ras. We hypothesized that active NF-κB would accelerate inflammatory progression and cancer in the presence of oncogenic K-Ras. For this experiment, acinar-Ras mice were crossed with mice with loxP-restricted conditional expression of constitutively active IKK2. In the resulting acinar-Ras-IKKTg animals, Cre recombination led to the expression of both K-RasG12D and constitutively active IKK2. Expression of K-RasG12D alone had no obvious histological effect on the pancreas (Figure 5A). As has previously been reported (31), expression of IKK2 generated a low level of inflammation resembling mild acute pancreatitis (Figure 5B). However, as predicted by our model, expression of both K-RasG12D and IKK2 led to dramatic fibrosis of the pancreas and the rapid development of multiple PanINs (Figure 5C). As an indication of increased Ras activity, p-Erk was dramatically elevated in the pancreas of acinar-Ras-IKKTg mice (Figure 5D) but not in mice expressing K-RasG12D (Figure 5E) or IKK2 (Figure 5F) alone. Taken together, these data support a model in which NF-κB plays an important role in the positive feedback loop between inflammation and oncogenic Ras signaling.
Figure 5. NF-κB activation accelerated oncogenic Ras–induced pathologies.
Pancreata of acinar-Ras mice 2 weeks after induction of mutant K-RasG12D expression were histologically normal (A, original magnification, ×200). Pancreata of IKKTg mice showed limited inflammation (B, original magnification, ×200). In contrast, expression of both K-RasG12D and IKK2 led to dramatic fibrosis of the pancreas and the development of multiple PanINs (C, original magnification, ×200). The downstream Ras effector p-Erk was dramatically increased in the pancreas of acinar-Ras-IKKTg mice (D, original magnification, ×200) in comparison to mice expressing acinar-Ras (E, ×200) or IKK (F, ×200) alone.
The NF-κB target gene Cox-2 is a key mediator in the oncogenic Ras positive feedback loop.
Cox-2 is a target gene of NF-κB and is the enzyme responsible for the generation of several inflammatory mediators including PGE2 (32). Cox-2 expression is induced by high levels of Ras activity (10) and was dramatically elevated in acinar-Ras mice after cerulein treatment (Figure 6A). To examine the role of Cox-2 in the pathological response to cerulein treatments, we administered acinar-Ras or control mice cerulein in the presence or absence of a highly selective Cox-2 inhibitor, NS-398. Treatments with NS-398 dramatically reduced pancreatic inflammation and fibrosis in cerulein-treated acinar-Ras mice (Figure 6, B and C). Histological evaluation, as well as measurements of α-SMA and amylase, indicated that NS-398 reduced stromal expansion (Figure 6D) and preserved acinar cells (Figure 6E). Treatment with the Cox-2 inhibitor also decreased the level of active Ras observed after cerulein treatments (Figure 6F). Therefore, Cox-2 appears to play a role in the positive feedback pathway.
Figure 6. Cox-2 inhibition reduced the development of cerulein-induced chronic inflammation in acinar-Ras mice.
Cerulein treatment increased Cox-2 expression in the pancreas of acinar-Ras mice (week 4) (A, original magnification, ×200) and generated chronic inflammation and fibrosis (B, ×100). Simultaneous treatment with a selective Cox-2 inhibitor (NS-398, 5 mg/kg/d) dramatically reduced the severity of these effects (week 4) (C, original magnification, ×100). These data were confirmed by quantification of decreased α-SMA (D) and increased amylase expression (E) in acinar-Ras mice with NS-398 administration. Treatment with the selective Cox-2 inhibitor also significantly decreased Ras activity (F) (*P < 0.05 versus nontreated acinar-Ras mice; n = 4 animals).
To further examine the influence of Cox-2 on the positive feedback mechanism, we also examined mouse models in which acinar cells expressed oncogenic K-Ras or Cox-2 or both. For this purpose, we generated acinar-Ras-Cox-2 mice. We observed that Cox-2 expression alone for 6 months increased pancreas size (Supplemental Figure 6A) but did not affect histology (n = 20) (Figure 7A). However, at later times (>8 months), Cox-2–expressing mice developed chronic inflammation with numerous pancreatic cysts (ductal ectasia) (Supplemental Figure 6B). Nonetheless, no Cox-2 only–expressing mice developed PanINs or tumors within 1 year. Similarly, pancreata from mice expressing physiological levels of oncogenic K-Ras were for the most part histologically normal (Figure 7B). In contrast, acinar-Ras-Cox-2 mice rapidly developed dramatic inflammation resembling severe chronic pancreatitis and also developed abundant PanINs (Figure 7C). Carcinoma in situ was detected in one acinar-Ras-Cox-2 animal after 6 months (Figure 7D).
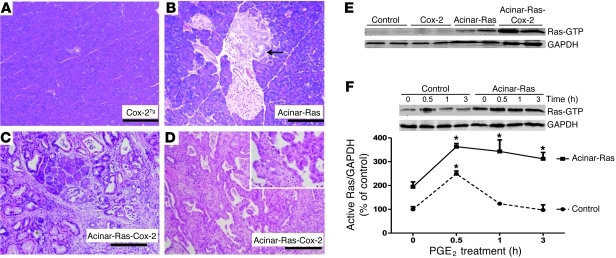
Figure 7. Cox-2 and oncogenic K-Ras synergized to promote the development of chronic inflammation and cancer.
Pancreata from mice expressing acinar cell Cox-2 were histologically normal at 2 months of age (A, original magnification, ×100). Similarly, pancreata from mice with expression of mutant K-Ras in acinar cells were mostly normal, although occasional low-grade PanIN lesions were observed at 2 months of age (B, arrow; original magnification, ×100). In contrast, mice expressing both Cox-2 and mutant K-Ras developed severe chronic pancreatitis at 2 months of age, with dramatic fibrosis, inflammation, destruction of acinar cells, and multiple PanINs (C, original magnification, ×100). Cancer in situ was observed (1 of 6) in K-Ras-Cox-2 mice within 6 months (D, original magnification, ×100; inset, ×400). Ras activity was greatly increased in the pancreata expressing both Cox-2 and oncogenic Ras (E). Stimulation of freshly isolated acini with 10 μM PGE2 caused a prolonged increase in Ras activity in acinar-Ras mice but not control mice (F) (*P < 0.05 versus time 0).
Levels of active Ras were significantly increased in acinar-Ras-Cox-2 mice (Figure 7E), as were levels p-Erk (Supplemental Figure 7, A–C). Consistent with these in vivo findings, treatment with PGE2, a major product of Cox-2, generated prolonged elevation of Ras activity in isolated cells from acinar-Ras but not control mice (Figure 7F). These results support a model in which Cox-2, a downstream inflammatory mediator regulated by NF-κB, could act to potentiate Ras signaling in the presence of oncogenic K-Ras.
Ras, NF-κB, and Cox-2 pathways were upregulated in human pancreatic cancer.
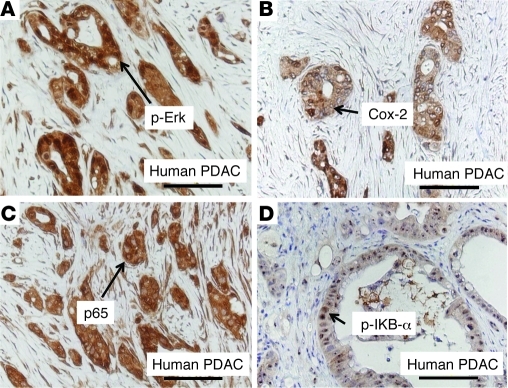
In order to examine whether increased Ras, NF-κB, and Cox-2 pathways are relevant to human pancreatic cancer, we evaluated these pathways in human pancreatic cancer tissues. Increased p-Erk (Figure 8A), an indicator of elevated Ras signaling, was observed in cancer tissues but not in normal pancreas (Supplemental Figure 8). Similarly, Cox-2 expression was highly upregulated in human pancreatic cancer cells (Figure 8B). p65 nuclear translocation in cancer cells indicted an increase in NF-κB signaling (Figure 8C). Positive staining of p–IκB-α (Figure 8D), a direct target of IKK kinase, suggested the presence of high IKK kinase activity in cancer cells. Taken together, the data suggested that these signaling pathways may also play an important role in the disease of humans.
Figure 8. Ras, Cox-2, and NF-κB pathways were upregulated in human pancreatic cancer.
Increased p-Erk, an indicator of elevated Ras signaling, was observed in human cancer tissues (A). Similarly, Cox-2 expression was highly upregulated in human pancreatic cancer cells (B). p65 nuclear translocation in cancer cells induced an increase in NF-κB signaling (C). Positive staining of p–IκB-α, a direct target of IKK kinase, suggested the presence of high IKK kinase activity (D). Negative staining of these signaling molecules in normal human pancreas is shown in Supplemental Figure 8. PDAC, pancreatic ductal adenocarcinoma.
Discussion
In the current study, we identified an important interaction between two cancer risk factors, mutations in K-Ras and inflammatory stimuli. While interactions between these two risk factors have long been well known, the mechanisms involved have not been clear. In several examples, we documented that stimuli that normally increase Ras activity only transiently in wild-type animals caused enhanced and prolonged stimulation in animals bearing oncogenic K-Ras. Importantly, this increased Ras signaling led to the development of chronic inflammatory disease and cancer. Our observations suggest that in the presence of oncogenic Ras, even mild inflammatory or physiologic stimuli may be able to trigger an NF-κB–mediated positive feedback mechanism involving Cox-2 that amplifies Ras activity to pathological levels. This positive feedback mechanism helps explain many of the observations previously made concerning Ras and inflammation as cancer risk factors. Furthermore, because a large portion of the adult human population possesses Ras mutations, disruption of this positive feedback loop may be an important strategy for cancer prevention.
In this study, both control and acinar-Ras animals developed acute inflammation after transient treatments with inflammatory stimuli, but only mice with oncogenic K-Ras developed chronic inflammation and cancer. Therefore, the presence of oncogenic Ras is necessary for transient inflammatory stimulation to induce chronic pathologies and ultimately tumorigenesis. This notion differs from the previous suggestion that chronic inflammation is essential for oncogenic Ras to induce tumorigenesis (9). Rather, at sufficient levels of activity, Ras is capable of generating both inflammation and tumorigenesis (7, 33). Therefore, mechanisms that increase the level of Ras activity are the key to the pathological consequences. The mechanisms responsible for elevating Ras to pathological levels were the subject of the current study, and inflammatory stimuli appear to be able to initiate a process that amplifies the Ras signaling to its pathological levels.
Inflammatory stimulants caused prolonged elevation of Ras signaling in cells with oncogenic Ras. K-Ras is a typical guanine nucleotide–binding protein that cycles between an active GTP-bound form and an inactive GDP-bound form. The GDP-bound form is converted to the active GTP-bound form through a GDP/GTP exchange reaction that is facilitated by guanine nucleotide exchange factors (GEFs) (34, 35). Similarly, the GTP-bound form is converted to the GDP-bound form by intrinsic GTPase activity, which is accelerated by GTPase-activating proteins (GAPs) (36). Oncogenic mutations at codon glycine 12 result in a loss of approximately 85% of the intrinsic GTPase activity of K-Ras and also greatly reduce the responsiveness of K-Ras to inactivation by GAPs (37). Therefore, active oncogenic Ras cannot be efficiently inactivated. However, mutant Ras is not active without GTP binding. The observation that mutant Ras can be activated by stimuli has been previously reported (38). It is likely that in the absence of stimulation over long time periods the GTPase activity of the mutant Ras is sufficient to reduce the level of bound GTP. GEF loading of mutant Ras is not impaired. Therefore, the activity of mutant Ras can be increased by stimuli under specific circumstances. These characteristics of oncogenic Ras likely explain the prolonged elevation of Ras signaling after stimulation.
Nonetheless, the ramifications of increased Ras signaling are very apparent in vivo. In cells with wild-type Ras, inflammatory stimuli caused only a transient increase in Ras activity and short-lived acute inflammation. Wild-type mice completely recovered within 2 weeks after the inflammatory insult. In contrast, in mice with oncogenic Ras, Ras activity remained elevated for at least 2 weeks after treatments with inflammatory stimuli. The explanation for this is that in the presence of oncogenic Ras, inflammatory stimuli increased Ras activity sufficiently to generate inflammatory mediators (14, 29), which further amplified Ras signaling in a positive-feedback manner. Therefore, increased Ras activity is both the source and the effect of inflammatory stimuli. Associated with the prolonged elevation of Ras activity in this model, non-reversible chronic inflammation with fibrosis and cancer developed. Interestingly, we also observed that at later times after stimulation, there was some increase in total Ras protein levels. The mechanisms responsible for this elevation are unknown and will be the subject of future investigations.
To further understand the mechanisms of the positive feedback loop, we evaluated the role of the NF-κB pathway. NF-κB is a transcription factor that regulates genes involved in inflammation and cell survival and is activated downstream of Ras (28). Orthodox NF-κB activation involves IKK2-mediated phosphorylation of the IKB inhibitory subunits that sequester the gene regulatory subunits in the cytoplasm, where they are inactive. Once phosphorylated, the IKBs are degraded through ubiquitination, and the freed active form of NF-κB, usually the p65-p50 herterodimer, is then transported to the nucleus. NF-κB regulates transcription of specific genes including proinflammatory mediators (29). In this study, high levels of nuclear p65 and expression of the NF-κB target gene Cox-2 remained evident 2 weeks after treatments with inflammatory stimuli in acinar-Ras mice. These observations support increased activity of this pathway in the presence of oncogenic Ras, as predicted. High levels of NF-κB, Cox-2, and Ras were also observed in samples of human pancreatic ductal adenocarcinoma, supporting the relevance of these studies to human disease.
To more strongly support our hypothesis, we disrupted the positive-feedback pathway by genetic deletion of IKK2 to inhibit NF-κB activity, or by pharmacologically inhibiting Cox-2. In cells depleted of IKK2, NF-κB activity was blocked, and this resulted in diminished Ras activity and chronic pathologies, as predicted. Likewise, inhibition of Cox-2 reduced Ras activity and diminished the chronic diseases. Taken together, these data support that activation of NF-κB and its target gene Cox-2 mediate amplification of Ras signaling when oncogenic Ras is present (Figure 8). Further support for a role for Cox-2 in Ras-mediated pathologies comes from previous studies that indicated high levels of Cox-2 expression in human PanIN lesions (39) and reported that Cox-2 inhibitors reduced the development of pancreatic cancer in oncogenic Ras–based mouse models (40). The current study indicates that targeting NF-κB and Cox-2 pathways would be likely to reduce Ras activity in cells that bear this mutation and therefore may be clinically useful to prevent oncogenic Ras-induced diseases. It was noted that IKK2 deletion did not completely diminish the inflammation, suggesting that additional members of the IKK family might also involve the activation of NF-κB pathway, as has been suggested by others (41).
A clinically significant aspect of the current study was the observation that physiologically relevant environmental factors that have little effect on control animals had profound effects in the presence of Ras mutation. For example, camostat feeding, which increases endogenous serum levels of CCK through a well-described trypsin feedback mechanism, leads to acinar cell hypertrophy and hyperplasia in normal animals (21, 24, 42). In contrast, camostat feeding amplified Ras activity sufficiently to generate chronic inflammation and PanIN formation in animals bearing oncogenic Ras. Thus, in the presence of oncogenic Ras, non-pathological stimuli can generate pathological results. Likewise, LPS treatments had no long-term effects in control animals (26). However, in the presence of oncogenic Ras, LPS treatments led to fibrosis and PanIN formation. These data suggest that LPS may have greater pathological effects in patients harboring cells expressing oncogenic Ras, which may explain individual differences in response to infection. These results may also be relevant to the association of chronic bacterial infectious diseases in colon and pancreatic cancers (43, 44).
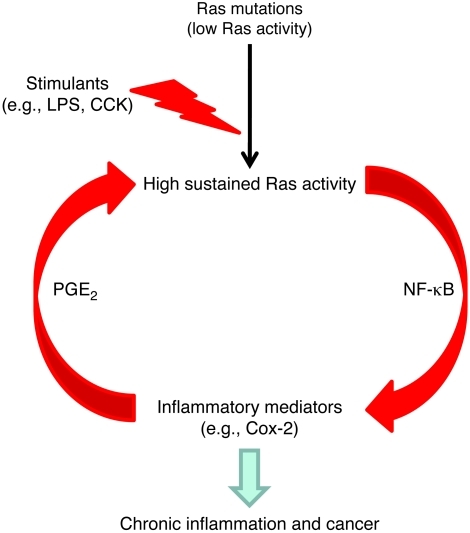
The evidence suggests that physiological levels of oncogenic Ras are of little consequence and generate levels of Ras activity that do not affect most cells. However, in the presence of oncogenic Ras, inflammatory, or even physiological, stimuli are able to amplify Ras activity to levels sufficient to induce NF-κB and generate inflammatory mediators such as Cox-2 that further enhance Ras signaling to pathological levels in a vicious circle. In the current model, the positive feedback loop was at least partially mediated by NF-κB and involved Cox-2 (Figure 9). Interruption of this self-perpetuating pathological mechanism may be useful for the prevention of oncogenic Ras–induced chronic inflammatory diseases and cancer.
Figure 9. An NF-κB pathway–mediated positive feedback loop amplifies Ras activity to pathological levels in cells expressing oncogenic Ras.
Physiological levels of oncogenic Ras generate increased Ras activity but rarely lead to pathologies. However, in the presence of oncogenic Ras, inflammatory stimuli induce high sustained levels of Ras activity, which in turn induce more inflammatory mediators. This positive feedback loop amplifies and prolongs Ras activity to pathological levels that cause chronic inflammation and cancer.
Methods
Genetically engineered mice.
LSL-K-RasG12D mice, which possess the conditional knock-in mutant K-RasG12D, were obtained from the Mouse Models for Human Cancer Consortium Repository (45). Ela-CreERT mice, which express tamoxifen-regulated CreERT specifically in pancreatic acinar cells under the control of a full-length elastase gene promoter, were developed in our laboratory as previously described (14). For targeted expression of K-RasG12D in pancreatic acinar cells, LSL-K-RasG12D mice were bred with Ela-CreERT mice to generate LSL-K-Ras/Ela-CreERT double-transgenic mice (acinar-Ras mice). To generate mice expressing Cox-2 in pancreatic acinar cells, Cox-2–floxed animals were obtained from Harvey R. Herschman, UCLA, Los Angeles, California, USA (32). Mice with conditional IKK2 deletion (IKK2fl/fl) were provided by Michael Karin, UCSD, San Diego, California, USA (46). To generate conditional expression of constitutive active IKK2, a fragment containing loxP-GFP-stop-loxP followed by constitutive active IKK2 (gift of Michael Karin) was cloned into a pCAGGS vector (gift from J. Miyazaki, Kumamoto University Medical School, Japan), which contains a CMV and chicken β-actin chimeric promoter. Transgenic mice were developed by pronuclear injection. All experiments were performed with tamoxifen-induced mice to activate Cre recombination in adult pancreatic acinar cells.
Preparation of acini.
Pancreatic acini were prepared as described previously (47) with modifications. Briefly, pancreata from mice were digested with purified collagenase (100 U/ml,Worthington Biochemicals) and incubated at 37°C for 50 minutes with shaking at 120 rpm. Digested tissue was then mechanically dispersed via pipette and passed through a 150-μm mesh nylon cloth. Acini were then purified 3 times with gradient separation in DMEM containing 4% bovine serum albumin. Purified acini were resuspended in DMEM containing 0.1 mg/ml soybean trypsin inhibitor and 1% bovine serum albumin for 1 hour in a tissue culture incubator. After stimulation with 10 nM CCK-8 (Sigma-Aldrich), 10 μM PGE2 (Cayman Chemicals), or 1 μg/ml LPS (Sigma-Aldrich) for different time periods, acini were assayed for Ras activity.
Ras activity assay.
Ras activity in this study refers to the level of GTP-bound Ras as measured using a Raf pull-down assay kit as recommended by the manufacturer (Millipore). Briefly, snap-frozen pancreatic samples were homogenized on ice in lysis buffer containing 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.5), 1% IGEPAL CA-630, 150 mM NaCl, 0.25% sodium deoxycholate, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM ethylenediaminetetraacetic acid, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM sodium orthovanadate. These samples were sonicated and centrifuged at 15,000 g for 10 minutes at 4°C to remove cellular debris. Aliquots of lysates were set aside to allow quantification of total Ras and protein concentrations. Equal amounts of lysate were incubated for 30 minutes at 4°C with agarose beads coated with Raf-Ras binding domain. The beads were then washed 3 times with ice-cold lysis buffer and boiled for 5 minutes at 95°C, and active Ras was analyzed by immunoblotting following standard Western blot analysis protocols.
Western blot analysis.
Cell or tissue lysates were prepared, separated by SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were blocked for 1 hour at room temperature with 3% nonfat milk in Tris-buffered saline containing 0.05% Tween 20 and incubated overnight at 4°C with one of the following primary antibodies: Ras (1:2,000; Millipore), GAPDH (1:10,000; Sigma-Aldrich), amylase (1:1,000; Sigma-Aldrich), p-Erk (1:1,000; Santa Cruz Biotechnology Inc.), total Erk (1:1,000; Cell Signaling Technology), or α-SMA (1:400; Abcam). Immunodetection was performed with the corresponding Alexa Fluor 680–conjugated secondary antibodies to allow detection with the Odyssey Infrared Imaging System (LI-COR Biosciences). All images were converted to grayscale.
Induction of experimental pancreatitis.
Adult (60- to 70-day-old) mice were treated with the appropriate stimuli as described below. Cerulein (Sigma-Aldrich) was administered via hourly injections over 5 hours by i.p. injection (50 μg/kg dissolved in 100 μl of sterile saline with 0.1% bovine serum albumin) on the first day and then one injection per day for 4 consecutive days. Two weeks after the initiation of treatment, the whole course of cerulein injections was repeated. LPS from Escherichia coli O111:B4 (Sigma-Aldrich) was administered via a single weekly i.p. injection (10 mg/kg dissolved in 100 μl sterile saline) for 4 consecutive weeks. Camostat mesylate (Santa Cruz Biotechnology Inc.) was added to a liquid Lieber-DeCarli diet (Bio-Serv) for 4 weeks at a dose 0.1% of the volume of solid food, as has been previously described (48). NS-398 (Cayman Chemical), a Cox-2 inhibitor, was administered once daily i.p. (5 mg/kg dissolved in 50 μl DMSO), 0.5 hours before cerulein treatment for 4 consecutive weeks. For experimental analyses, sex- and age-matched animals were sacrificed at specified times after the indicated treatments. Littermates that do not express genes of interest receiving the same treatments served as controls.
Histology.
At the end of the experiment, pancreata were removed, rinsed with phosphate-buffered saline, and fixed in 10% buffered formalin overnight or embedded in OCT medium and frozen. Formalin-fixed samples were then embedded in paraffin, serially sectioned (5 μm thick), and stained with standard hematoxylin and eosin.
Immunohistochemistry.
Immunohistochemical staining for cleaved p-ERK (1:200, Cell Signaling Technology), NF-κB p65 (1:50, Cell Signaling Technology), Cox-2 (1:300, Cayman Chemical), and anti–α-SMA (1:100, Abcam) was performed in pancreatic paraffin sections. Briefly, after deparaffinization and antigen retrieval, unspecific binding was blocked and primary antibodies were applied at 4°C overnight. After washing, sections were incubated with the appropriate horseradish peroxidase–labeled polymers (BioCare). Positive labeling was detected by exposing the samples to NovaRED substrate kit for peroxidase (Vector Laboratories). Samples were counterstained with Gill no. 3 hematoxylin solution. Immunohistochemical staining for leukocytes (CD45, 1:50, BD Biosciences — Pharmingen) and macrophages (F4/80, 1:20, eBioscience) was performed on frozen pancreatic sections. Briefly, after fixing in pure acetone at –20°C (10 minutes) and blocking with 10% fetal bovine serum/2% normal horse serum for 60 minutes at room temperature, primary antibodies were applied at 4°C overnight. After washing, incubation with biotinylated secondary antibodies and streptavidin-labeled horseradish peroxidase was performed. Positive labeling was detected using the NovaRED substrate kit for peroxidase. Counterstaining was performed with Gill no. 3 hematoxylin.
Collagen detection.
Trichrome staining (Sigma-Aldrich) was used to reveal collagen deposits in paraffin-embedded pancreatic tissue sections. Briefly, after deparaffinization sections were mordanted in preheated Bouin’s solution. After washing, slides were consecutively stained in Weigert’s iron hematoxylin solution, Biebrich scarlet-acid fuchsin, phosphotungstic/phosphomolybdic acid solution, and aniline blue solution and washed in 1% acetic acid. Samples were then briefly dried and dehydrated.
Amylase activity assay.
As a measure of acute pancreatitis, serum amylase activity was measured using the Phadebas test (Pharmacia Diagnostics). Results were expressed as the fold increase compared with control.
Statistics.
For statistical analysis, data were analyzed using a 2-sided unpaired Student’s t test. A P value less than 0.05 was considered to be statistically significant. Results are displayed as mean ± SEM.
Study approval.
All animal experiments were approved by The University of Texas MD Anderson Cancer Center’s IACUC. Usage of normal human pancreas and human pancreatic cancer tissues from the MD Anderson Cancer Center tissue bank for immunohistochemical studies was approved by the MD Anderson Cancer Center IRB.
Supplementary Material
Acknowledgments
This research was supported by funds from the NIH — DK052067 (to C.D. Logsdon), Cancer Center Support Core grant CA016672 (to the MD Anderson Cancer Center), and Pancreatic Specialized Programs of Research Excellence grant P20 CA101936 (to MD Anderson Cancer Center) — and by the Lockton Endowment (to C.D. Logsdon).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(4):1519–1528. doi:10.1172/JCI59743.
References
- 1.DeNicola GM, Tuveson DA. RAS in cellular transformation and senescence. Eur J Cancer. 2009;45(suppl 1):211–216. doi: 10.1016/S0959-8049(09)70036-X. [DOI] [PubMed] [Google Scholar]
- 2.Yan L, et al. Molecular analysis to detect pancreatic ductal adenocarcinoma in high-risk groups. Gastroenterology. 2005;128(7):2124–2130. doi: 10.1053/j.gastro.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Lu X, Xu T, Qian J, Wen X, Wu D. Detecting K-ras and p53 gene mutation from stool and pancreatic juice for diagnosis of early pancreatic cancer. Chin Med J (Engl). 2002;115(11):1632–1636. [PubMed] [Google Scholar]
- 4.Parsons BL, Meng F. K-RAS mutation in the screening, prognosis and treatment of cancer. Biomark Med. 2009;3(6):757–769. doi: 10.2217/bmm.09.95. [DOI] [PubMed] [Google Scholar]
- 5.Yakubovskaya MS, et al. High frequency of K-ras mutations in normal appearing lung tissues and sputum of patients with lung cancer. Int J Cancer. 1995;63(6):810–814. doi: 10.1002/ijc.2910630611. [DOI] [PubMed] [Google Scholar]
- 6.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4(2):111–120. doi: 10.1016/S1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 7.Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9(5):493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, et al. Endogenous oncogenic Nras mutation initiates hematopoietic malignancies in a dose- and cell type-dependent manner. Blood. 2011;118(2):368–379. doi: 10.1182/blood-2010-12-326058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Ji B, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137(3):1072–1082. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardeesy N, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103(15):5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382(3):561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 14.Ji B, et al. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis. 2008;46(8):390–395. doi: 10.1002/dvg.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan RD, Zheng CF, Guan KL, Williams JA. Activation of MAP kinase kinase (MEK) and Ras by cholecystokinin in rat pancreatic acini. Am J Physiol. 1995;268(6 pt 1):G1060–G1065. doi: 10.1152/ajpgi.1995.268.6.G1060. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65(5):1822–1829. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 17.An H, et al. Src homology 2 domain-containing inositol-5-phosphatase 1 (SHIP1) negatively regulates TLR4-mediated LPS response primarily through a phosphatase activity- and PI-3K-independent mechanism. Blood. 2005;105(12):4685–4692. doi: 10.1182/blood-2005-01-0191. [DOI] [PubMed] [Google Scholar]
- 18.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.al Mufti RA, Williamson RC. Experimental models of pancreatitis. Ann Acad Med Singapore. 1999;28(1):133–140. [PubMed] [Google Scholar]
- 20.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G784–G790. doi: 10.1152/ajpgi.00446.2003. [DOI] [PubMed] [Google Scholar]
- 22.Crozier SJ, Sans MD, Lang CH, D’Alecy LG, Ernst SA, Williams JA. CCK-induced pancreatic growth is not limited by mitogenic capacity in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1148–G1157. doi: 10.1152/ajpgi.00426.2007. [DOI] [PubMed] [Google Scholar]
- 23.Green GM, Levan VH, Liddle RA. Interaction of dietary protein and trypsin inhibitor on plasma cholecystokinin and pancreatic growth in rats. Adv Exp Med Biol. 1986;199:123–132. doi: 10.1007/978-1-4757-0022-0_7. [DOI] [PubMed] [Google Scholar]
- 24.Niederau C, Liddle RA, Williams JA, Grendell JH. Pancreatic growth: interaction of exogenous cholecystokinin, a protease inhibitor, and a cholecystokinin receptor antagonist in mice. Gut. 1987;28(suppl):63–69. doi: 10.1136/gut.28.suppl.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motoo Y. Antiproteases in the treatment of chronic pancreatitis. JOP. 2007;8(4 suppl):533–537. [PubMed] [Google Scholar]
- 26.Li YY, et al. Regulation of HSP60 and the role of MK2 in a new model of severe experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G981–G989. doi: 10.1152/ajpgi.00225.2009. [DOI] [PubMed] [Google Scholar]
- 27.Ding SP, Li JC, Jin C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J Gastroenterol. 2003;9(3):584–589. doi: 10.3748/wjg.v9.i3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh JJ, Der CJ. Targeting signal transduction in pancreatic cancer treatment. Expert Opin Ther Targets. 2007;11(5):673–694. doi: 10.1517/14728222.11.5.673. [DOI] [PubMed] [Google Scholar]
- 29.Buday L, Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786(2):178–187. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275(6 pt 1):G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 31.Baumann B, et al. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest. 2007;117(6):1502–1513. doi: 10.1172/JCI30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamei K, Ishikawa TO, Herschman HR. Transgenic mouse for conditional, tissue-specific Cox-2 overexpression. Genesis. 2006;44(4):177–182. doi: 10.1002/dvg.20199. [DOI] [PubMed] [Google Scholar]
- 33.Logsdon CD, Ji B. Ras activity in acinar cells links chronic pancreatitis and pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(11 suppl):S40–S43. doi: 10.1016/j.cgh.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal M, Willumsen BM, Levitzki A. Residues crucial for Ras interaction with GDP-GTP exchangers. Proc Natl Acad Sci U S A. 1993;90(12):5564–5568. doi: 10.1073/pnas.90.12.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overbeck AF, et al. Guanine nucleotide exchange factors: activators of Ras superfamily proteins. Mol Reprod Dev. 1995;42(4):468–476. doi: 10.1002/mrd.1080420415. [DOI] [PubMed] [Google Scholar]
- 36.Molloy DP, Owen D, Grand RJ. Ras binding to a C-terminal region of GAP. FEBS Lett. 1995;368(2):297–303. doi: 10.1016/0014-5793(95)00657-u. [DOI] [PubMed] [Google Scholar]
- 37.Adari H, Lowy DR, Willumsen BM, Der CJ, McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- 38.Braun BS, et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101(2):597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albazaz R, Verbeke CS, Rahman SH, McMahon MJ. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5(4–5):361–369. doi: 10.1159/000086536. [DOI] [PubMed] [Google Scholar]
- 40.Funahashi H, et al. Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras(G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res. 2007;67(15):7068–7071. doi: 10.1158/0008-5472.CAN-07-0970. [DOI] [PubMed] [Google Scholar]
- 41.Cheng A, Guo J, Henderson-Jackson E, Kim D, Malafa M, Coppola D. IkappaB kinase epsilon expression in pancreatic ductal adenocarcinoma. Am J Clin Pathol. 2011;136(1):60–66. doi: 10.1309/AJCP2JJGYNIUAS2V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tashiro M, Dabrowski A, Guo L, Sans MD, Williams JA. Calcineurin-dependent and calcineurin-independent signal transduction pathways activated as part of pancreatic growth. Pancreas. 2006;32(3):314–320. doi: 10.1097/01.mpa.0000218316.12577.c0. [DOI] [PubMed] [Google Scholar]
- 43.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66(2):828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manes G, Balzano A, Vaira D. Helicobacter pylori and pancreatic disease. JOP. 2003;4(3):111–116. [PubMed] [Google Scholar]
- 45.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen F, Lu Y, Castranova V, Li Z, Karin M. Loss of Ikkbeta promotes migration and proliferation of mouse embryo fibroblast cells. J Biol Chem. 2006;281(48):37142–37149. doi: 10.1074/jbc.M603631200. [DOI] [PubMed] [Google Scholar]
- 47.Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am J Physiol. 1978;235(5):517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- 48.Gurda GT, et al. Regulator of calcineurin 1 controls growth plasticity of adult pancreas. Gastroenterology. 2010;139(2):609–619. doi: 10.1053/j.gastro.2010.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.