Abstract
Self-reported short sleep duration is linked to higher blood pressure and incident hypertension in adults. Few studies have examined sleep and blood pressure in younger samples. We evaluated the associations between actigraphy-assessed time spent asleep and ambulatory blood pressure in adolescents. Participants were 246 black and white adolescents (mean age = 15.7) who were free from cardiovascular or kidney disease and were not taking sleep, cardiovascular, or psychiatric medications. Sleep duration and efficiency were assessed with in-home wrist actigraphy and sleep diaries across one week; ambulatory blood pressure monitoring was used to obtain 24-hour, sleep, wake blood pressure, and sleep-wake blood pressure ratios across two full days and nights. Results showed that shorter actigraphy-assessed sleep across one week was related to higher 48-hour blood pressure and higher nighttime blood pressure. Shorter sleep was also related to a higher systolic blood pressure sleep-wake ratio. These results were independent of age, race, sex, and body mass index. Follow-up analyses by race revealed that associations between sleep duration and blood pressure were largely present in white, but not black, adolescents. These data are consistent with the hypothesis that the cardiovascular consequences of short sleep may begin as early as adolescence.
Keywords: ambulatory blood pressure, sleep duration, actigraphy, adolescent, race
Reports of usual sleep duration are inversely linked to resting blood pressure (BP) and hypertension risk in adults (1-4). For instance, in the First National Health and Nutrition Examination Survey, persons reporting usual sleep duration of ≤ 5 hours per night were at increased risk of hypertension over the 8- to 10-year follow-up period, after adjusting for a variety of sociodemographic and health factors (3). Correlations between self-reports of typical sleep duration and behavioral measures of sleep are modest (5). Thus, actigraphy that infers sleep duration based on nocturnal movement is a useful complement to subjective reports. Of the two studies that have examined actigraphy-assessed sleep and BP in adults, one reported that short sleep was related to higher BP and increased hypertensive risk in middle-aged adults(6), while the other failed to detect an association between sleep duration and hypertension in the elderly (7).
Few studies have examined whether the link between sleep and BP is present in childhood or adolescence. BP levels in early life track into adulthood (8) and may predict metabolic syndrome and carotid intima media thickness decades later (9-13). Developmental changes in a variety of biological, social, and environmental factors in adolescence result in a large number being exposed to a continual state of insufficient sleep (14). Thus, adolescence provides a critical period for studying the physiological consequences of chronic sleep deprivation, as well as a potential opportunity for prevention or early intervention for hypertension risk. The one study of actigraphy and BP in adolescents measured sleep across at least 3 week nights and classified participants as prehypertensive based on an average of clinic BP measurements on a single day (15). In that study, low sleep efficiency (percentage of time in bed spent asleep) was associated with a 3.5-fold increase in the odds of prehypertension after adjustment for body mass index (BMI) and other covariates (15). Relationships of short sleep duration with prehypertension and systolic BP were reduced to statistical trends after adjustment for BMI and other covariates. With regard to younger samples, no association was observed between actigraphy-measured sleep over 6 nights and 24-hour ambulatory BP levels in Finnish 8-year-olds (16), and the link between short sleep, as assessed by waist actigraphy for 24 hours, and higher resting BP in New Zealand 7-year-olds was attenuated after adjustment for BMI (17).
In this study, we examine actigraphy measures of sleep duration and efficiency across one week in relation to 48-hours of ambulatory BP in a sample of black and white adolescents. There are several benefits of examining ambulatory BP relative to clinic BP, including increased reliability and a stronger approximation of cardiovascular risk (18). Use of ambulatory monitoring also permits measures of circadian BP pattern via separate day and night estimates, as well as the sleep-wake BP ratio, which predicts cardiovascular outcomes independent of daytime BP (19-21). In adult samples, higher sleep-wake BP ratios, or nondipping, are associated with sleep fragmentation and sleep disturbance (22-24). Our secondary aim is to investigate whether the links between sleep duration or efficiency and BP are similar in black and white adolescents, given that blacks are at increased risk for hypertension and have higher sleep-wake BP ratios and shorter sleep than whites in adult samples (25;26). We hypothesized that short, inefficient sleep would be related to higher 24-hour ambulatory BP and higher sleep-wake BP ratios, and we examined whether these associations varied by race.
Methods
Participants
We recruited 250 adolescents between the ages of 14 and 19 from a local high school within 15 miles of downtown Pittsburgh, PA. The high school served a diverse population, both in terms of ethnicity and socioeconomic status. Approval of the research project was obtained from the University of Pittsburgh Institutional Review Board. Participants, and in the case of students under the age of 18, a parent or legal guardian provided written informed consent prior to any research procedures. Exclusionary criteria included cardiovascular or kidney disease, medication use for emotional or psychological disorders, use of diabetes or BP medication, use of any medication known to affect the cardiovascular system or sleep, and inability to obtain BP readings (usually due to extreme obesity). Sixteen students who were screened were ineligible to participate due to taking medication that could affect study variables, and 7 students who signed consent did not begin the study.
Procedure
The study protocol lasted one week. Participants wore the ambulatory monitor for the 2 school days and nights and wore an actigraph on their non-dominant wrist continuously over the 7 days and nights of the study. They answered several questions about their sleep in a handheld computer each morning and evening. Additional study procedures included assessment of anthropometric measures, fasting venous blood draw, and psychosocial questionnaires and interview. After completion of the protocol, participants were compensated $100, and a follow-up report of the student’s BP, sleep, anthropometric, and lipid levels was sent to the student and his or her parent/guardian.
Measures
Ambulatory Blood Pressure
Ambulatory BP was measured using the Spacelabs model 90217 ambulatory BP monitor (Issaquah, WA), with pediatric, adult, or thigh cuff used depending on body size. The BP monitors were worn for at least 2 school days and nights and were programmed to take BP readings every 30 minutes during the day (from 7am to 10pm) and hourly during the night (from 10pm to 7am). Participants were told to wear the monitor at all times except when bathing. BP that fell within the participant’s self-reported sleep window were used to calculate average nighttime BP based on a minimum of 5 readings across the night. BP readings that were ± 3 standard deviations from a participant’s individual mean were excluded. Outcome variables were: 24-hour BP, defined as the average of all valid BP readings obtained across the 2-3 day period; nighttime BP, defined as the average of all valid BP readings obtained within the nighttime hours that the participant reported s/he was attempting to sleep; daytime BP, defined as the average of all valid BP readings during reported waking hours; and the sleep-wake BP ratio, defined as the ratio of average nighttime BP to average daytime BP. To examine effects of potential clinical relevance, we also included 24-hour prehypertension, defined as an average systolic BP (SBP) > 120 or diastolic BP (DBP) > 80 over the entire study (n=109) (27). Using the recommended adult cutoff of daytime SBP >135 or DBP >85 captured a small subset of the sample (n = 20) (18), so we reduced the cutoff for elevated daytime BP to include those with average daytime SBP >130 or DBP > 80 (N=57). BP nondipping was defined as <10% decline in sleep SBP relative to wake SBP or <10% decline in sleep DBP relative to wake DBP (n=68) (21).
Sleep
The Mini-mitter actiwatch model AW-16 (Phillips Respironics, Bend, OR) collected actigraphy data continuously over 7 days and nights. Actigraphs were configured to collect data over a 1-minute epoch. Stored data were downloaded into the Actiware software program (version 5.57) for processing and analysis. The medium threshold (default) was selected to detect nocturnal sleep periods of at least 3 hours in duration based upon sleep onset and offset using the 10-minute criteria. Each night’s data were reviewed individually and revealed that many sleep periods occurred before or after the recorded diary bedtimes and awake times. Thus, sleep periods occurring within 30 minutes of the major nocturnal sleep interval (either 30 minutes prior to sleeping or after waking) that were at least 15 minutes in duration were combined with the major sleep interval (i.e., if a 6-hour sleep interval was detected from 12am–6am, and a 20-minute sleep interval was detected beginning at 11:30pm, the 20-minute interval was combined with the major sleep interval. The new major rest interval would become 11:30pm–6am). All subsequent sleep variables were then calculated from data within these set rest periods. Total sleep time was calculated as the time spent asleep between initial sleep onset and final sleep offset, excluding periods of wakefulness throughout the night. Sleep efficiency was calculated as the percentage of time attempting to sleep that was actually spent asleep. The actiwatch has been widely used in research studies and has been validated against polysomnography measures in clinic (28;29).
Diary total sleep time was calculated as the difference between the time that one reported trying to fall asleep and the time that he or she reported finally waking, minus the self-reported number of minutes it took to fall asleep and the number of minutes spent awake after sleep onset. All sleep measures were averaged across the 7 nights of the study.
Covariates
Age, sex, and race/ethnicity were determined by self-report. Height was measured using a stadiometer, and weight was measured on a Tanita digital scale to the 1/10 of a pound. Body mass index (BMI) was calculated using the National Heart, Lung, and Blood Institute on-line calculator.
Analysis
Data were checked for normality. Two participants had BMI values that fell over 4 standard deviations from the mean and were excluded from analyses. Two participants were excluded due to equipment failure or misuse. Therefore, analyses examining daytime and 24-hour ambulatory BP were based on a total of 246 participants. An additional 2 participants had incomplete nocturnal BP data due to taking off the cuff on at least one night; therefore, analyses examining nocturnal BP or sleep-wake BP ratios were based on a total N=244. We examined the associations by linear regression between total sleep time and sleep efficiency with continuous ambulatory BP outcomes, including 24-hour BP, daytime BP, nighttime BP, and sleep-wake BP ratios. Standardized betas are reported, with coefficients signifying the standard deviation (SD) change in BP as a result of a 1 SD increase in sleep duration in hours (1 SD = .79 hours or 47.4 minutes). Associations with categorical cutoffs of elevated BP were tested in logistic regression models in one hour unit of sleep. Finally, we included a race X total sleep time interaction term in all of the above models in order to test for differences in the links between sleep duration and efficiency with BP between white and black adolescents. All base models were adjusted for age, sex, and race. BMI was added in the final step to examine whether it attenuated associations between sleep and BP. Results were also adjusted for BMI percentile and revealed identical results.
Results
Slightly more than half of the sample was female (53.3%), and black (56.5%) (Table 1). Average time spent asleep across the 7 nights of the study by actigraphy was 6.45 hours (SD = .79, range = 4.27 – 9.22) and average sleep efficiency was 82.66% (SD = 4.87, range = 67.78 – 94.11). On average, ambulatory nighttime BP declined by about 13% for SBP and by 20% for DBP. After adjustment for age, sex, and BMI, black adolescents had higher 24-hour DBP, nighttime SBP, and sleep-wake SBP ratios.
Table 1.
Sample Characteristics by Race
| Variable | Black (n = 139) | White (n = 107) | ||||
|---|---|---|---|---|---|---|
| No (%) | M (SD) | Range | No (%) | M (SD) | Range | |
| Male | 49 (45.8) | 66 (47.5) | ||||
| Age | 15.7 (1.3) | 14 - 19 | 15. 7 (1.3) | 14 - 19 | ||
| Body Mass Index | 26.1 (5.5) | 17.8 – 43.5 | 26.2 (6.5) | 16.4 – 46.9 | ||
| Body Mass Index Percentile | 80.7 (19.5) | 10 – 100 | 76.3 (25.9) | 3 - 100 | ||
| Total Sleep Time (hrs) | 6.2 (.69)* | 4.3 – 7.7 | 6.7 (.83)* | 4.9 – 9.2 | ||
| ≤ 5 hours total sleep time | 6 (4.3) | 1 (.9) | ||||
| ≤ 6 hours total sleep time | 43 (30.9) | 17 (15.9) | ||||
| ≤ 7 hours total sleep time | 70 (50.4) | 53 (49.5) | ||||
| ≤ 8 hours total sleep time | 20 (14.4) | 28 (26.2) | ||||
| ≤ 10 hours total sleep time | 0 (0) | 8 (7.5) | ||||
| 24-Hour Ambulatory SBP | 119.7 (8.7) ‡ | 97.0 – 144.3 | 117.7 (9.0) ‡ | 97.3 – 148.9 | ||
| 24-Hour Ambulatory DBP | 69.7 (6.0) † | 54.2 – 82.2 | 68.3 (5.3) † | 58.0 – 79.1 | ||
| Daytime SBP | 122.6 (9.2) ‡ | 93.5 – 149.5 | 121.0 (9.1) ‡ | 101.7 – 154.2 | ||
| Daytime DBP | 72.6 (6.3) | 53.8 – 86.2 | 71.4 (5.6) | 58.9 – 82.8 | ||
| Nighttime SBP | 107.1 (8.3) † | 87.0 – 137.2 | 104.2 (9.3) † | 86.2 – 128.8 | ||
| Nighttime DBP | 57.4 (5.6) ‡ | 43.6 – 72.3 | 56.3 (5.4) ‡ | 45.2 – 70.6 | ||
| Sleep-Wake SBP Ratio | .88 (.06) † | .75 – 1.10 | .86 (.05) † | .70 - .99 | ||
| Sleep-Wake DBP Ratio | .79 (.07) | .59 – 1.04 | .79 (.06) | .59 - .98 | ||
| 24-Hour Prehypertension (24-Hr SBP > 120 or DBP > 80) | 65 (46.8) | 44 (41.1) | ||||
| Elevated Daytime BP (Day SBP > 130 or DBP > 80) | 34 (24.5) | 23 (21.5) | ||||
| Nondipping (SBP or DBP sleep-wake ratio >.90) | 46 (33.3) † | 22 (20.8) † | ||||
p < .01,
p < .05,
p < .10, indicate differences by race after adjusting for age, sex, and body mass index.
Actigraphy- measured Sleep and Blood Pressure
Shorter sleep duration across the study was associated with higher 24-hour SBP and DBP across the 2 days of ambulatory monitoring (Table 2; Figure 1). The relationship between sleep and 24-hour BP levels was driven largely by nocturnal BP; shorter sleep was related to higher nighttime SBP and DBP. Sleep duration tended to be associated with daytime BP in the hypothesized direction, but these relationships were not statistically significant. Finally, there was an inverse association between sleep duration and sleep-wake SBP ratios, such that shorter sleep was related to a smaller nocturnal decline in BP. All associations between sleep duration and BP remained after adjusting for BMI. Sleep duration was not related to sleep-wake DBP ratios.
Table 2.
Results from Linear Regression Models of Total Sleep Time and Ambulatory BP
| Variable | 24-Hour | Daytime | Nighttime | Sleep-Wake Ratio | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | |||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Total Sleep Time | -.17 | .01 | -.17 | .01 | -.11 | .10 | -.11 | .09 | -.21 | .002 | -.13 | .05 | -.16 | .02 | -.04 | .54 |
| Race X Total Sleep Time | .97 | .05 | .82 | .10 | .87 | .09 | .69 | .18 | 1.06 | .04 | .88 | .08 | .42 | .41 | .38 | .47 |
| Total Sleep Time: Blacks | -.02 | .80 | -.04 | .60 | .03 | .73 | -.01 | .94 | -.01 | .95 | -.06 | .51 | .01 | .99 | -.01 | .99 |
| Total Sleep Time: Whites | -.34 | .001 | -.32 | .02 | -.27 | .005 | -.24 | .02 | -.36 | .001 | -.25 | .02 | -.20 | .05 | -.06 | .55 |
Covariates for full sample analyses include age, sex, race, and BMI; covariates for race-stratified analyses include age, sex, and BMI. Standardized beta coefficients signify the standard deviation (SD) change in BP as a result of a 1 SD increase in total sleep time (1 SD = .79 hours or 47.4 minutes).
Figure 1.
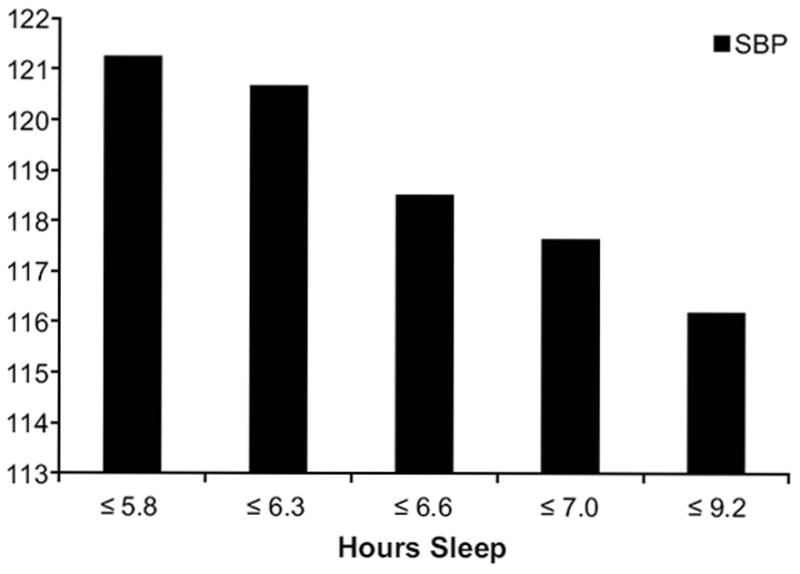
24-hour ambulatory systolic blood pressure by quintiles of sleep duration; categories are as follows: ≤5.8 (n = 49); ≤6.3 (n = 49); ≤6.6 (n = 49); ≤7.0 (n = 46); ≤9.2 (n = 53)
Adolescents sleeping for a shorter duration were more likely to be classified as prehypertensive based on 24-hour BP (average 24-hour SBP > 120 or DBP > 80; Table 3). In a separate analysis using the cut-off of daytime SBP >130 or DBP >80, shorter sleepers were also more likely to be classified as having elevated daytime BP. Using the criterion of <10% decline in nocturnal SBP or DBP relative to daytime levels, adolescents with shorter sleep were more likely to be categorized as nondippers. All associations between sleep duration and BP cutoffs remained after adjusting for BMI. Sleep efficiency was not related to any of the continuous or categorical ambulatory BP outcomes.
Table 3.
Results from Logistic Regression Models of Total Sleep Time and Ambulatory BP
| Variable | 24-Hour Prehypertension (SBP >120 or DBP >80) | Elevated Daytime BP (SBP >130 or DBP > 80) | BP Nondipping (SBP or DBP sleep-wake ratio >.90) | |||
|---|---|---|---|---|---|---|
| N = 109 | N = 57 | N =68 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Total Sleep Time | .66 | .46 - .97 | .65 | .42 - .98 | .66 | .44 - .99 |
| Total Sleep Time: Blacks | 1.04 | .63 – 1.73 | 1.03 | .59 – 1.82 | .83 | .48 – 1.45 |
| Total Sleep Time: Whites | .36 | .19 - .68 | .27 | .12 - .61 | .56 | .29 – 1.06 |
Covariates for full sample analyses include age, sex, race, and BMI; covariates for race-stratified analyses include age, sex, and BMI. OR - odds ratio for meeting the outcome criterion, given a 1-hour increase in total sleep time.
Race Interactions
Results from models testing the interactive effects of race and sleep duration in relation to continuous BP are displayed in Table 2. Many of the race X total sleep time interaction terms approached or reached statistical significance; therefore, we stratified the sample by race to examine sleep duration and BP in whites and Blacks separately. Relationships between sleep duration and BP were present only in white adolescents. All associations between sleep and BP were non-significant in black adolescents. When examining categorical BP outcomes, the race X total sleep time interaction term was significant for 24-hour prehypertension (p = .02) and elevated daytime BP (p = .01), but not for nondipping status (p = .15). Associations by race are shown in Table 3. None of the interactions with sleep efficiency were significant (ps > .10).
Diary total sleep time was not related to any of the BP outcomes (ps > .20), nor did it interact with race.
Discussion
Our primary finding was that actigraphy-assessed sleep duration across one week was inversely related to 24-hour ambulatory SBP and DBP in adolescents. This association was primarily driven by higher nighttime BP among shorter sleepers, rather than higher daytime BP. Those getting less sleep also showed a smaller decline in nocturnal SBP relative to daytime. Short sleep was associated with increased odds of meeting prehypertensive levels across a 24-hour period and average daytime SBP > 130 or DBP > 80 across two school days. Shorter sleepers were also more likely to be classified as nondippers relative to those getting more sleep. Whereas previous data have suggested a potential mediating role of BMI in the link between sleep and BP (17), all of the associations in the current sample were independent of BMI.
Actigraphy-assessed sleep efficiency, an index of sleep continuity, was unrelated to ambulatory BP in this sample. This is contrast to Javaheri et al.’s finding that in-clinic BP was more strongly and consistently linked with sleep efficiency than with sleep duration in the Cleveland Children’s Sleep and Health Study (15). One reason for this discrepancy may be sample differences in sleep characteristics. Mean sleep duration and efficiency in the current study were relatively low compared to the Cleveland sample, which included a similar racial/ethnic composition but was slightly younger. Whereas only 25% of adolescents in the Cleveland study were classified as having a sleep efficiency ≤ 85%, over 2/3 of adolescents in the current sample met that criterion. Among adults in the Coronary Artery Risk Development in Young Adults (CARDIA), both actigraphy-assessed short sleep duration and low sleep maintenance (an index similar to sleep efficiency) were associated with increased BP; however, only short sleep duration predicted increased risk of incident hypertension (6).
Our results were by and large present in white, but not black, adolescents. It is possible that a restricted range of sleep duration among black adolescents inhibited our ability to detect an association with BP (only 14% exceeded 7 hours of sleep measured by actigraphy across the week compared to 30% of whites). The Cleveland Sleep and Health Study did not test for race differences in their findings, and in CARDIA, race did not modify associations between sleep duration or sleep maintenance and BP (6). With regard to other cardiovascular risk factors, some studies have reported that short sleep is more closely linked to inflammatory biomarkers in black adults than in whites (30), while others have failed to detect a difference in links between sleep duration and traditional cardiovascular risk factors or coronary calcification by race (31;32). Although the interaction analyses in this sample were exploratory and did not reach statistical significance in all cases, the overall pattern of effects suggest that ethnic variation in sleep and BP may warrant further study.
Several physiological mechanisms could link shorter sleep to higher BP. Acute total sleep deprivation studies in healthy young adults produces an increase in DBP along with decreased muscle sympathetic nerve activity, consistent with a shift in the arterial baroreflex toward a higher BP (33). Experimental sleep loss also can cause increases in vascular endothelial adhesion markers, followed by elevated BP (34). Partial sleep deprivation over several nights has been linked to changes in sympathovagal activity, as evidenced by decreased heart rate variability and increased catecholamine release (35-37). Thus, small amounts of sleep loss accrued over time could potentially result in similar pathophysiological effects, leading to increased BP. The association between sleep duration and 24-hour BP was largely due to higher nocturnal BP. Prior work has shown that parasympathetic activity increases across the night, reaching a peak in the fourth non-rapid eye movement sleep period (38). It is possible that those with curtailed sleep do not reach this sleep stage and the concurrent increase in parasympathetic activity, and, thus, exhibit increased nocturnal pressure. An additional possibility is that higher nocturnal BP and nondipping reflect elevated nocturnal activity among shorter sleepers. This is less likely, given that nocturnal BP measurements were based on reported sleep hours, which capture individuals’ sleep more accurately than an arbitrary definition of nighttime (39), and sleep efficiency was unrelated to nighttime BP. However, variation in sleep architecture not captured by actigraphy, such as less time spent in deep sleep, could contribute to higher nocturnal BP (22;23).
A limitation of this study was our inability to assess sleep-disordered breathing. Obstructive sleep apnea is associated with increased daytime and nighttime ambulatory BP in children and adolescents in a number of studies, although the overall literature is mixed (40), and actigraphy may misestimate total sleep time among those with sleep-disordered breathing (41). Thus, sleep apnea may be a confounder of the observed relationships. The cross-sectional design precludes implications about causation, and it is possible that underlying autonomic dysfunction resulted in both shorter sleep and elevated BP. The substantial number of participants had ambulatory BP during the day at pre-hypertensive levels, in part due to the high BMI of the participants. Thus the findings would not generalize to samples with fewer obese individuals. Finally, the reasons for individual amounts of sleep duration are unknown, and voluntary (i.e., time demands) versus involuntary (i.e., inability to fall asleep) sleep curtailment may have different physiological correlates. Future work examining the factors that influence sleep duration and the potentially modifying role of such variables on the sleep-BP relationship may be worthwhile.
Strengths of this study include the use of ambulatory BP monitoring for at least 48 hours and actigraphic assessment of sleep. Ambulatory monitoring captures BP in one’s typical environment, provides more reliable estimates than clinic BP (18), reduces the likelihood of phenomena such as white-coat or masked hypertension (42), and allows for the examination of circadian BP levels. Assessing sleep duration with actigraphy provided a more objective estimate than self-reports and allowed us to measure nocturnal movement over an extended period of time outside the laboratory. We were able to include a nearly equal number of black and white adolescents in this study, which revealed that similar to adult samples (26), black adolescents are exposed to higher total BP than are white adolescents, independent of body size. Thus, blacks have an earlier exposure to their well-established risk for hypertension and its complications in adulthood.
Perspectives
The transition from childhood to adolescence is typically accompanied by shifts in the circadian phase and increasing psychosocial demands, leading to later sleep times and overall shorter sleep for many adolescents. The result is a chronic state of insufficient sleep, accompanied by a variety of cognitive, affective, and behavioral consequences (43;44). Findings from the current study show that elevated 24-hour ambulatory BP and higher night-to-day BP ratios are additional correlates of short sleep in teenagers, especially whites in this sample, and complement the literature linking sleep disturbance to BP in adults (1-4;6). Studies aiming to track adolescents’ sleep over time and designs aimed to enhance sleep duration in younger populations will be critical in elucidating the causal relationship between sleep and BP. If short sleep leads to increased BP, adolescence may provide a critical window for intervention.
Acknowledgments
None
Funding Source: Supported by HL025767.
Footnotes
Conflict of Interest/Disclosure: None.
Reference List
- 1.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, Ferrie JE, Shipley MJ, Brunner EJ, Marmot MG. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 3.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Jo I. Age-dependent association between sleep duration and hypertension in the adult Korean population. Am J Hypertens. 2010;23(12):1286–1291. doi: 10.1038/ajh.2010.166. [DOI] [PubMed] [Google Scholar]
- 5.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson KL, Van CE, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169(11):1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg JF, Tulen JH, Neven AK, Hofman A, Miedema HM, Witteman JC, Tiemeier H. Sleep duration and hypertension are not associated in the elderly. Hypertension. 2007;50(3):585–589. doi: 10.1161/HYPERTENSIONAHA.107.092585. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48(1):40–44. doi: 10.1161/01.HYP.0000227029.10536.e8. [DOI] [PubMed] [Google Scholar]
- 10.Litwin M, Niemirska A, Sladowska J, Antoniewicz J, Daszkowska J, Wierzbicka A, Wawer ZT, Grenda R. Left ventricular hypertrophy and arterial wall thickening in children with essential hypertension. Pediatr Nephrol. 2006;21(6):811–819. doi: 10.1007/s00467-006-0068-8. [DOI] [PubMed] [Google Scholar]
- 11.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 12.Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–246. doi: 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- 13.Vos LE, Oren A, Uiterwaal C, Gorissen WH, Grobbee DE, Bots ML. Adolescent blood pressure and blood pressure tracking into young adulthood are related to subclinical atherosclerosis: the Atherosclerosis Risk in Young Adults (ARYA) study. Am J Hypertens. 2003;16(7):549–555. doi: 10.1016/s0895-7061(03)00857-4. [DOI] [PubMed] [Google Scholar]
- 14.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118(10):1034–1040. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martikainen S, Pesonen AK, Feldt K, Jones A, Lahti J, Pyhala R, Heinonen K, Kajantie E, Eriksson J, Raikkonen K. Poor sleep and cardiovascular function in children. Hypertension. 2011;58(1):16–21. doi: 10.1161/HYPERTENSIONAHA.111.172395. [DOI] [PubMed] [Google Scholar]
- 17.Nixon GM, Thompson JM, Han DY, Becroft DM, Clark PM, Robinson E, Waldie KE, Wild CJ, Black PN, Mitchell EA. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31(1):71–78. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering TG, White WB. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2008;2(3):119–124. doi: 10.1016/j.jash.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166(8):846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 20.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51(1):55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 21.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Satoh H, Hisamichi S. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10(11):1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 22.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27(6):1097–1103. doi: 10.1093/sleep/27.6.1097. [DOI] [PubMed] [Google Scholar]
- 23.Matthews KA, Kamarck TW, Hall H, Strollo PJ, Owens JF, Buysse DJ, Lee L, Reis SE. Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women. Am J Hypertens. 2008;21(7):826–831. doi: 10.1038/ajh.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–988. doi: 10.1038/ajh.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr, Buysse DJ, Kamarck TW, Owens JF, Reis SE. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med. 2008;70(4):410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33(5):1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 27.National High Blood Pressure Treatment Program. The Seventh Report of the Joint National Committee on Prevention, Detections, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Heart, Lung, and Blood Institute; 2004. 04-5230. [PubMed] [Google Scholar]
- 28.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 29.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27(1):158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 30.Matthews KA, Zheng H, Kravitz HM, Sowers M, Bromberger JT, Buysse DJ, Owens JF, Sanders M, Hall M. Are inflammatory and coagulation biomarkers related to sleep characteristics in mid-life women?: Study of Women’s Health across the Nation sleep study. Sleep. 2010;33(12):1649–1655. doi: 10.1093/sleep/33.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews KA, Strollo PJ, Hall M, Mezick EJ, Kamarck TW, Owens JF, Buysse DJ, Reis SE. Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle-aged men and women: Pittsburgh SleepSCORE study. Sleep. doi: 10.5665/SLEEP.1032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26(8):986–989. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 34.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van BP, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108(1):68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 35.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84(6):1979–1985. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 36.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12(1 Pt 1):63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel K, Leproult R, Van CE. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 38.Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, Kupfer D, Thayer JF. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66(1):56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 39.Jones HE, Sinha MD. The definition of daytime and nighttime influences the interpretation of ABPM in children. Pediatr Nephrol. 2011;26(5):775–781. doi: 10.1007/s00467-011-1791-3. [DOI] [PubMed] [Google Scholar]
- 40.Zintzaras E, Kaditis AG. Sleep-disordered breathing and blood pressure in children: a meta-analysis. Arch Pediatr Adolesc Med. 2007;161(2):172–178. doi: 10.1001/archpedi.161.2.172. [DOI] [PubMed] [Google Scholar]
- 41.Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, Emancipator JL, Kibler AM, Redline S. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep. 2007;30(7):899–905. doi: 10.1093/sleep/30.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40(6):795–796. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 43.Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31(6 Suppl):175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 44.Carskadon MA. Sleep’s effects on cognition and learning in adolescence. Prog Brain Res. 2011;190:137–143. doi: 10.1016/B978-0-444-53817-8.00008-6. [DOI] [PubMed] [Google Scholar]


