Abstract
Although embryonic stem (ES) cell-derived hepatocytes have the capacity for liver engraftment and repopulation, their in vivo hepatic function has not been analyzed yet. We aimed to determine the metabolic function and therapeutic action of ES cell-derived hepatocytes after serial liver repopulations in fumaryl acetoacetate hydrolase knockout (Fah−/−) mice. Albumin expressing (Alb+) cells were obtained by hepatic differentiation of ES cells using two frequently reported methods. After transplantation, variable levels of liver repopulation were found in Fah−/− mice recipients. FAH expressing (FAH+) hepatocytes were found either as single cells or as nodules with multiple hepatocytes. After serial transplantation, the proportion of the liver that was repopulated by the re-transplanted FAH+ hepatocytes increased significantly. ES cell-derived FAH+ hepatocytes were found in homogenous nodules and corrected the liver metabolic disorder of Fah−/− recipients and rescued them from death. ES cell-derived hepatocytes had normal karyotype, hepatocytic morphology and metabolic function both in vitro and in vivo. In conclusion, ES cell-derived hepatocytes were capable of liver repopulation and correction of metabolic defects after serial transplantation. Our results are an important piece of evidence to support future clinical applications of ES cell-derived hepatocytes in treating liver diseases.
Keywords: Embryonic stem cell, Hepatocyte, Metabolic function, Liver repopulation, Cell transplantation
1. Introduction
Hepatocytes derived from pluripotent stem cells, both embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, may be used in the future for many applications including cell-based therapy for liver diseases and drug discovery (Murry and Keller, 2008; Si-Tayeb et al., 2010; Soto-Gutiérrez et al., 2006; Touboul et al., 2010). Previous studies have shown that ES cell-derived hepatic cells have a capacity for liver engraftment or repopulation (Basma et al., 2009; Cai et al., 2007; Chinzei et al., 2002; Choi et al., 2002; Duan et al., 2007; Gouon-Evans et al., 2006; Haridass et al., 2009; Heo et al., 2006; Ishii et al., 2007; Kumashiro et al., 2005; Li et al., 2010; Shafritz and Oertel, 2011; Sharma et al., 2008; Teratani et al., 2005; Yamada et al., 2002; Yamamoto et al., 2003; Yin et al., 2002). These ES cell-derived hepatic cells were obtained by either of two frequently used methods of hepatic induction from ES cells, referred to here as the embryoid body (EB) method (Chinzei et al., 2002; Duan et al., 2007; Haridass et al., 2009; Heo et al., 2006; Kumashiro et al., 2005; Sharma et al., 2008; Yamada et al., 2002; Yin et al., 2002) and the definitive endoderm (DE) method (Basma et al., 2009; Cai et al., 2007; Gouon-Evans et al., 2006; Li et al., 2010), which represent the state of the art in this field.
Generally, the EB method depends on spontaneous differentiation, including hepatic differentiation, of cells in embryoid bodies, while the DE method depends on a stepwise, controlled procedure of induction of definitive endoderm first, followed by induction of hepatic cells from DE cells. Previous studies examining the metabolic function of these cells were only conducted under in vitro culture conditions (Basma et al., 2009; Duan et al., 2010). After transplantation of ES cell-derived hepatic cells, a detectable level of liver engraftment or repopulation from ES cell-derived hepatic cells has been reported in recipients under various conditions, such as in normal mice (Yamada et al., 2002), partially hepatectomized mice (Yin et al., 2002), hepatectomized mice treated with 2-acetylaminofluorene (Chinzei et al., 2002; Kumashiro et al., 2005), the urokinase-type plasminogen activator (uPA) transgenic mice (Basma et al., 2009; Haridass et al., 2009; Heo et al., 2006), fumarylacetoacetate hydrolase-deficient (Fah−/−) mice (Gouon-Evans et al., 2006; Li et al., 2010; Sharma et al., 2008), and transgenic mice that expressed diphtheria toxin receptors under the control of an albumin (Alb) enhancer/promoter (Ishii et al., 2007). Significant levels of liver repopulation from ES cell-derived hepatic cells were found in some of the above models (Basma et al., 2009; Chinzei et al., 2002; Heo et al., 2006; Haumaitre et al., 2003; Ishii et al., 2007; Li et al., 2010; Sharma et al., 2008). Remarkably, the repopulation levels varied greatly among the different reports.
Until now, the in vivo metabolic function of ES cell-derived hepatocytes in recipients had not been characterized, and there was no report on the application of ES cell-derived hepatocytes in treating liver disease. Without proving the in vivo metabolic function and therapeutic action of ES cell-derived hepatocytes, it is difficult to conclude whether the induction of functional hepatocytes from ES cells has been successful. We chose Fah−/− mice as recipients to study ES cell-derived hepatocytes because of the unique features of this model of hereditary tyrosinaemia type I. Fah−/− mice have faulty metabolic function, and they depend on continuous medicinal treatment with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) (Overturf et al., 1996). After NTBC withdrawal, Fah−/− mice undergo liver failure and death. Fah−/− mice recipients of wild-type hepatocytes can be rescued from death by restoring metabolic function through liver repopulation (Overturf et al., 1996). In addition, the repopulating hepatocytes in primary Fah−/− recipients can be recollected and transplanted into the secondary recipients for serial liver repopulation, for continuous analysis of hepatocyte function over many cell divisions (He et al., 2010; Overturf et al., 1997). Furthermore, by quantifying the level of liver repopulation in Fah−/− mice (Wang et al., 2001; Wang et al., 2002), it is possible to directly compare the repopulation capacity between ES cell-derived hepatic cells that were derived using different methods of hepatic induction.
Here, we compare the capacity for liver engraftment and repopulation of hepatic cells that were derived from ES cells using either the DE or EB method. We use functional parameters to evaluate these cells and prove that they are functional hepatocytes capable of rescuing FAH−/− mice from death and restoring normal metabolic function in recipients with liver disease. To our knowledge, this is the first proof that ES cell-derived hepatocytes have a capacity for in vivo metabolic function and curative potential in treating liver diseases.
2. Materials and Methods
2.1. Establishment of ES cell line with Alb promoter/enhancer-controlled GFP expression
Maintenance of mouse ES Cells (E14 cells, ATCC, Manassas, VA) was performed as described previously (Li et al., 2010). Plasmid construction of pAlb-GFP and Establishment of E14 cell line with Alb promoter controlled GFP expression were summarized in Supplementary Materials and Methods. The pAlb-GFP positively integrated clone was selected and named as AG-ES cells.
2.2. Hepatic differentiation of AG-ES cells using EB method and DE method
Induction of hepatic differentiation of AG-ES cells using either the EB method or DE method was revised according to a procedure described previously (Heo et al., 2006; Gouon-Evans et al., 2006). The information of both methods was summarized in Supplementary Materials and Methods.
2.3. Fluorescence activated cell sorting of GFP positive cells
To isolate GFP positive cell populations (Alb-expressing cells), which were derived from two induction methods, cells were re-suspended in Iscove’s modified Dulbecco’s medium (IMDM, Gibco-BRL, Gaithersburg, MD) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT) and 2 mmol/L EDTA and sorted on FACSVantage (BD, Franklin Lakes, NJ). Cells were sorted for selection of GFP expression. Viability after sorting was > 90% as analyzed by Trypan blue dye exclusion.
2.4. RT-PCR and Q-PCR analysis for gene expression
Total RNA was extracted using the UNIQ-10 kit (Qiagen, Germany) and reverse-transcribed using the SuperScript Reverse Transcriptase (Invitrogen, CA) according to the manufacture’s guidelines. Ploymerase chain reactions (PCR) and Quantitative PCR (Q-PCR) were performed. The primers and conditions are listed in Supplementary Table 1 and 2.
2.5. Cell transplantation and serial transplantation
Fah−/− mice were maintained with drinking water containing NTBC at a concentration of 7.5 µg/ml. Animal care was in accordance with institutional guidelines. 1 × 105 GFP positive cells were injected into the spleens of mice recipients. Then, NTBC was totally discontinued after cell transplantation. NTBC was reinstituted for three days when the recipients lost over 20 percents of the original weight. The liver samples were harvested from Fah−/− recipients at 6 and 10 weeks after cell transplantation. At 6 weeks, liver samples were only harvested from the two front liver lobes of Fah−/− recipients. After biopsy, Fah−/− recipients were allowed to recover with NTBC water for three days. After fully recovery, NTBC was removed again from Fah−/− recipients for additional selection of liver repopulation for another 4 weeks.
For serial transplantation experiments, the engrafted GFP positive cells in chimeric liver of primary recipients were isolated after liver perfusion as described previously (Overturf et al., 1997). The recipients were first examined for liver repopulation by biopsy of liver tissue. Harvested liver sections were stained for FAH expression by immunohistochemical analysis. Recipients with the highest level of liver repopulation were selected for liver perfusion to collect donor cells for secondary transplantation.
2.6. Western-blot analysis
Western-blot analysis was performed as described in our previous publication (Wangensteen et al., 2008). The method information is summarized in Supplementary Materials and Methods. For detection of FAH, rabbit anti-mouse FAH (1:3000, Affinitein, CA) was used in combination with goat anti-rabbit IgG, HRP (1:10000, Sigma, St. Louis, MO).
2.7. Biochemical Analysis of Liver Metabolic Function
Blood was collected from the retro-orbital sinus of test animals. Plasma was prepared using Microtainer plasma separator tubes (BD) and stored at −80° C. Biochemical evaluation of liver function was performed as described in our previous publication (Wilber et al., 2007). Cytosolic fractions of total liver homogenates were assayed for FAH enzyme activity as previously described (Wang et al., 2001).
2.8. Immunohistochemistry
Two µm-thick sections were dewaxed with xylene, rehydrated, incubated with primary antibodies: Fah (1: 3000, Affinitein), GFP (1: 200, Abcam, Cambridge, UK), or Alb (1: 200, Dako, Denmark) and then HRP-conjugated IgG secondary antibody, followed by detection with DAB or AEC kit (Dako) and counterstaining with Mayer’s hematoxylin solution (Sigma). For immunofluorescence, fluorescence labeling secondary antibody (Donkey α Goat, 1: 800; Donkey α Rabbit, 1: 800; Jackson, West Grove, PA) was added, then mounted with DAPI mounting medium (SouthernBiotech, Birmingham, AL) and observed under a fluorescence microscope.
2.9. In vitro culture and cytogenetics analysis
GFP positive hepatocytes were sorted by FACS from surviving secondary Fah−/− recipients. The freshly isolated hepatocytes were plated on slides in hepatocytes medium. FAH staining was performed as described previously (Wang et al., 2003). Cytogenetics was conducted as described in a previous publication (Duncan et al., 2009; Sargent et al., 1996). Fifty metaphases from each of 3 mice were randomly selected and karyotype analysis was done. The method information was summarized in Supplementary Materials and Methods.
2.10. In vitro metabolic function assay
GFP positive hepatocytes were stained by Periodic-Acid-Schiff (PAS) Kit (Sigma) and DiI-ac-LDL (Invitrogen) following manufacturer’s instructions. ICG uptake assay and Albumin secretion assay were performed as described in our latest publication (Huang et al., 2011). To examine the activity of ammonia clearance, GFP positive hepatocytes, or control mouse embryonic fibroblast (MEF) cells and primary mouse hepatocytes were cultured in dulbecco's modified eagle medium (DMEM) containing 2.5 mmol/L NH4Cl and further incubated for 24 hours. Concentration of NH4Cl was measured in the culture supernatant at 0, 24, and 48 hours by Ammonia-Test (Wako, Japan).
2.11. Calculations of Liver Repopulation and Statistical Analysis
Calculations of liver sample size, cell numbers and percent of repopulation were performed as previously described (Wang et al., 2001; Wang et al., 2002). The statistical significance of differences between sample groups was calculated by Student's T test and ANOVA with Bonfferoni correction. Statistical calculation was performed using Statistical Program for Social Sciences software (SPSS, IBM). p values < 0.05 were regarded as statistically significant.
3. Results
Our experiments to derive hepatocytes from ES cells and to transplant them into FAH−/− mice were divided into two stages (see summary schematic, Supplementary Fig. 1). Briefly, in Stage I, we constructed plasmid pAlb-GFP in which the expression of GFP is controlled by Alb promoter/enhancer for tracking and sorting of ES cell-derived hepatic cells. After verifying the expression of our pAlb-GFP plasmid in Huh-7 hepatocarcinoma cells, we established a pAlb-GFP stable transfectant ES cell line, named AG-ES (Supplementary Fig. 2). Next, we used two methods of hepatic differentiation (DE vs EB) to generate AG-ES cells derived hepatic cells. In Stage II, we did parallel analyses to compare the capacities for liver repopulation of each kind of AG-ES cell-derived hepatic cells in primary recipients, and compared the capacity for liver repopulation and metabolic function for both cell types in secondary recipients after serial transplantation.
3.1. Differentiation and purification of ES cell-derived Alb+ hepatic cells using two induction methods
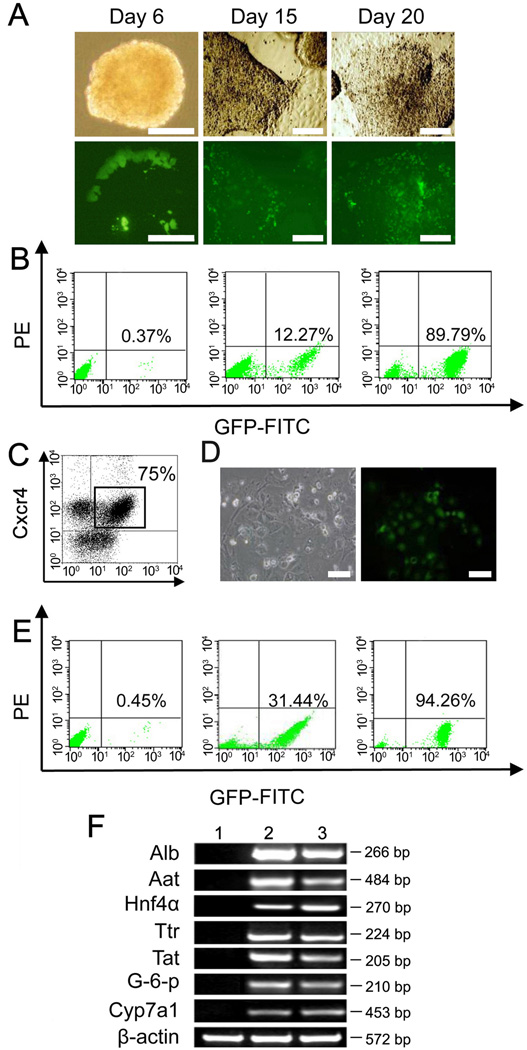
With the EB method, a few Alb+ cells with GFP fluorescent signal were found in spontaneous EBs at Day 6 of differentiation (Fig. 1A). The Alb+ cells inside EBs increased in numbers during the time course of induction from Day 15 to Day 20 (Fig. 1A). The number of Alb+ cells among total cells reached levels from ~10% to ~20% during EB differentiation (Fig. 1B). This result was similar to those in previous reports (Heo et al., 2006; Sharma et al., 2008). After differentiation was achieved, ES cell-derived Alb+ cells were harvested and sorted for GFP expression by FACS (Fig. 1B). With the DE method, the process was divided into two steps, DE differentiation and hepatic differentiation. During DE differentiation, DE cells were induced in stepwise fashion using specific growth factor cocktails and purified by cell sorting for co-expression of Cxcr4 and c-Kit in FACS as described previously (Fig. 1C) (Gouon-Evans et al., 2006; Iwamuro et al., 2010; Li et al., 2011). During hepatic differentiation, Cxcr4+c-Kit+ DE cells cultured at high density had induced alb-GFP expression (Fig. 1D). The level of Alb+ cells was from ~30% to ~40% (Fig. 1E). The expression of hepatic genes, including Alb, Aat, Hnf4α, Ttr, Tat, G-6-p and Cyp7a1, was examined by RT-PCR in the enriched ES cell-derived Alb+ cells using the EB and DE methods (Fig. 1F). As expected, hepatic genes were not expressed in negative controls without differentiation (Fig. 1F). Thus, ES cell-derived hepatic cells were successfully induced under the two methods and enriched by FACS sorting for GFP.
Figure 1. In vitro hepatic differentiation of AG-ES cells under EB and DE methods.
(A) Under EB method, Alb positive cells with GFP were first found inside EB structure at Day 6 of differentiation; Alb positive cells with GFP expanded after plating onto the collagen coated dish at Day 15; more Alb positive cells were found at Day 20. (B) FACS analysis of Alb positive cells harvested at Day 20. From left to right: AG-ES cells without differentiation, harvested Alb positive cells before and after sorting. (C) Under DE method, Cxcr4+c-kit+ cells as DE cells were induced to as high as 75% during the first stage and purified by FACS sorting. (D) During the second stage, Cxcr4+c-kit+ cells were induced to Alb positive cells after plating onto the collagen coated dish. (E) FACS analysis of Alb positive cells harvested at Day 12. From left to right: AG-ES cells without differentiation, harvested Alb positive cells before and after sorting. (F) RT-PCR analysis of hepatic marker genes expression. (lane 2, EB method; lane 3, DE method). RNA from AG-ES cells without differentiation was used as negative control (lane 1). Scale bars: 100 µm (A and D).
Remarkably, the DE method produced more Alb+ cells than EB method (~30% to ~40% versus ~10% to ~20%) for the same number of original ES cells (Fig. 1B, 1E), which suggested that DE method might have an advantage in capacity for generating ES cell-derived Alb+ cells.
3.2. Liver repopulation with ES cell-derived Alb+ cells in Fah−/− mice
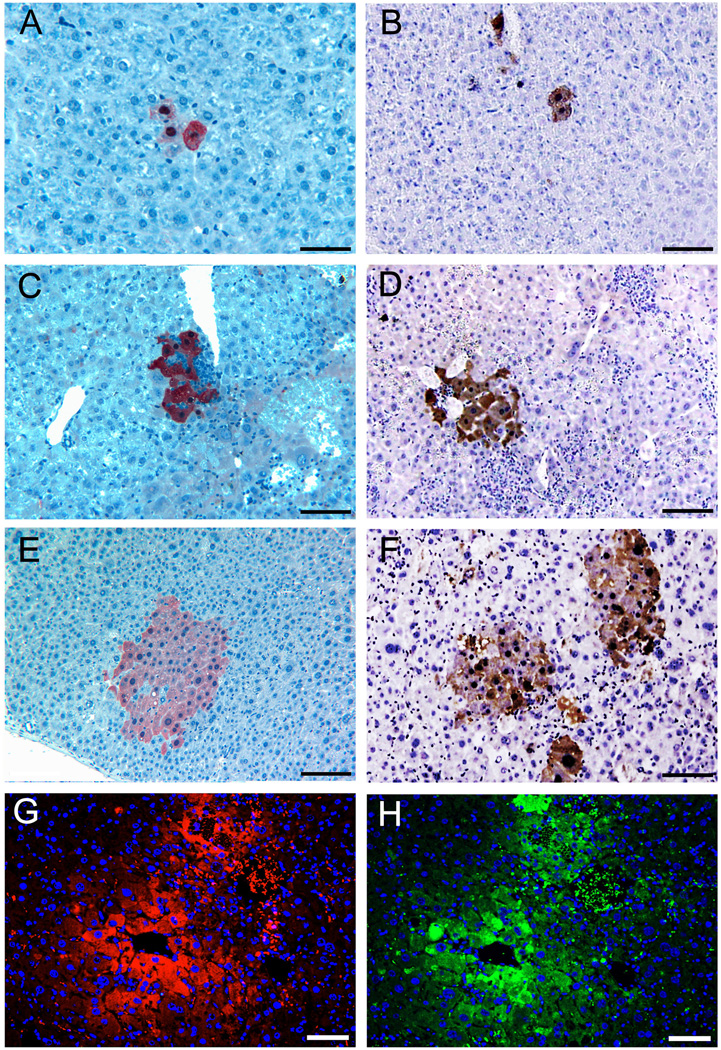
After FACS enrichment, two populations of ES cell-derived Alb+ cells obtained using the two different methods were compared for liver repopulation capacity under the same conditions. Boluses of 1 × 105 Alb+ cells were transplanted into the Fah−/− mouse recipients. After 6 and 10 weeks of selection by removal of NTBC from the drinking water, liver samples were harvested by biopsy of each Fah−/− recipient at two time points. Variable levels of 0.001% to 12.5% liver repopulation were found in Fah−/− mice recipients (Supplementary Table 3). Single FAH+ hepatocytes from donor Alb+ cells could be found in many recipients after 6 weeks (Fig. 2A, 2B). FAH+ nodules with up to 50 hepatocytes could be found in some recipients (Fig. 2C, 2D, 2E, 2F). FAH+ hepatocytes in the nodules also expressed GFP, proving that they originated from donor ES cells derived hepatocytes (Fig. 2G, H). Remarkably, the efficiency of liver repopulation was variable among the primary recipients, resulting in differences in prevalence of FAH-expressing single cells (1–4 cells), nodules with 5 to 9 cells, or 10 to 29 cells, or 30 to 50 cells (Fig.2, Supplementary Figure 3). In comparing the two populations of ES cell-derived Alb+ cells, we observed no significant difference in liver engraftment and repopulation (p > 0.05, Table 1 and Supplementary Table 3).
Figure 2. Liver engraftment after transplantation of enriched Alb positive cells.
(A, C, E) Liver engraftment of Alb positive cells differentiated under EB method. (B, D, F) Liver engraftment of Alb positive cells differentiated under DE method. (A, B) Single FAH positive hepatocytes were found in many liver samples of Fah−/− recipients; (C, D) In some liver samples of Fah−/− recipients, nodules with 5–15 FAH positive hepatocytes could be found; (E, F) Expansion of FAH positive hepatocytes in nodules of 30–50 cells in both methods. (G, H) Serial liver sections after immunofluorescence assay suggested that FAH positive hepatocytes (G) also expressed GFP (H), proving that they originated from donor ES cells derived hepatocytes. Scale bars: 100 µm.
Table 1.
Summary of liver engraftment after primary transplantation of AG-ES cell-derived Alb positive cells.
| Donor cells | Number of Fah−/− mice |
Number (percent) of Fah−/− mice with Fah+ hepatocytes at 6 weeks |
Number (percent) of Fah−/− mice with Fah+ hepatocytes at 10 weeks |
Liver repopulation by Fah+ hepatocytes at 6 weeks |
Liver repopulation by Fah+ hepatocytes at 10 weeks |
|---|---|---|---|---|---|
| Alb+ cells from EB method | 25 | 8 (32%) | 8 (32%) | 0.001% to 10.6% | 0.001% to 11.4% |
| Alb+ cells from DE method | 28 | 10 (35.7%) | 10 (35.7%) | 0.001% to 11. 2% | 0.001% to 12.5% |
| Hepatocytes from WT mice | 6 | 6 (100%) | 6 (100%) | 80% to 90% | 83% to 95% |
Compared to the samples harvested at 6 weeks, liver sections at 10 weeks showed similar frequencies of single FAH+ hepatocytes and nodules of multiple FAH+ hepatocytes in the same Fah−/− recipients (Table 1). Similarly, the recipients negative for engraftment or repopulation at 6 weeks also were negative at 10 weeks. Therefore, the two induction methods were equivalent in generating ES cell-derived hepatocytes with capacities for engraftment and repopulation.
We noted that FAH−/− primary recipients of ES cell-derived hepatocytes appeared less healthy upon removal on NTBC when compared to FAH−/− mice treated with wildtype hepatocytes or with serial repopulation. Primary recipients displayed signs of stress including hunched back and decreased vigor, and required frequent reinstitution of NTBC to maintain their weight (data not shown).
3.3. Serial transplantation of ES cell-derived FAH+ hepatocytes in Fah−/− mice
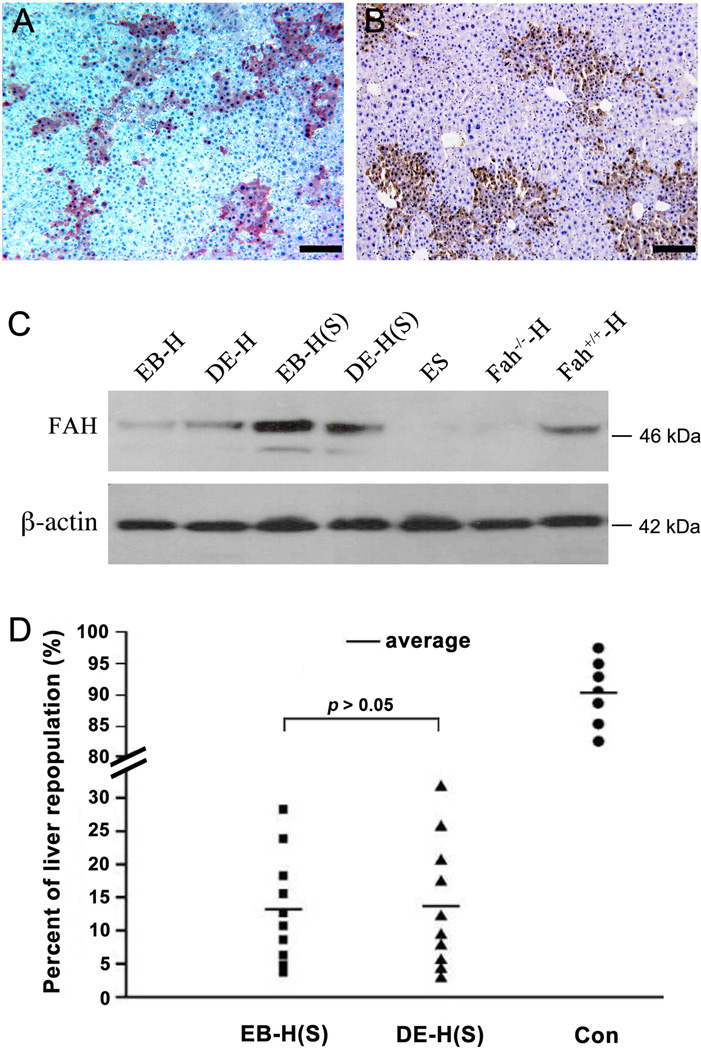
Fah−/− recipients with significant liver repopulation (> 10%) were selected for harvest of total hepatocytes. 1 × 106 isolated hepatocytes were transplanted into Fah−/− secondary recipients. For controls, 1 × 105 normal wild-type hepatocytes were mixed with 9 × 105 Fah−/− hepatocytes, in order to mimic the situation of about 10% ES cell-derived FAH+ hepatocytes existing within the total population of hepatocytes isolated from primary recipients. After selection for 8 weeks without NTBC in the drinking water, liver samples were harvested from all secondary Fah−/− recipients, as well as all control recipients. Both single FAH+ hepatocytes and nodules of multiple FAH+ hepatocytes were found in all 20 Fah−/− recipients. Unlike in primary recipients, most of FAH+ hepatocytes underwent proliferation to become nodules after engraftment in secondary recipients. Also different from the primary recipients, the nodules of FAH+ hepatocytes were homogeneous with 30 to 50 FAH+ hepatocytes in secondary recipients (Fig. 3A, 3B). The results of western blot for protein expression further proved that FAH protein had higher expression levels in secondary recipients than in primary recipients (Fig. 3C).
Figure 3. Serial transplantation of ES cells derived hepatocytes.
(A, B) FAH positive hepatocytic nodules in liver samples of secondary recipients after serial transplantation of ES cells derived hepatocytes under EB (A) or DE methods (B). (C) Western blot of liver samples showed the levels of FAH protein expressed in the repopulating hepatocytes after primary and secondary transplantations. (EB-H represents the repopulating hepatocytes derived under EB method; DE-H represents the repopulating hepatocytes derived under DE method; EB-H (S) or DE-H (S) represent either of the repopulating hepatocytes after serial transplantation; ES represents the liver sample of recipients with transplantation of ES cells without differentiation; Fah−/−-H represents liver sample of Fah−/− mice without cell transplantation; Fah+/+-H represents liver sample of 129S4 wild-type mice. (D) Comparison of liver repopulation levels of ES cells derived hepatocytes under EB or DE methods in serial transplantation (Liver repopulations transplanted with wild-type hepatocytes were used as control). Scale bars: 200 µm (A and B).
Remarkably, there was no significant difference between two populations of ES cell-derived hepatocytes for the levels of liver repopulation (3.7% to 28.2% vs. 2.8% to 31.6%, p > 0.05, Fig. 3D), suggesting that both populations of ES cell-derived FAH+ hepatocytes obtained from the two different induction methods had similar capacity for liver repopulation in Fah−/− secondary recipients. However, their capacities for liver repopulation were much lower than that of normal wild-type hepatocytes, which reached levels of repopulation greater than 80% (Fig. 3D, Fig. 4A, 4B).
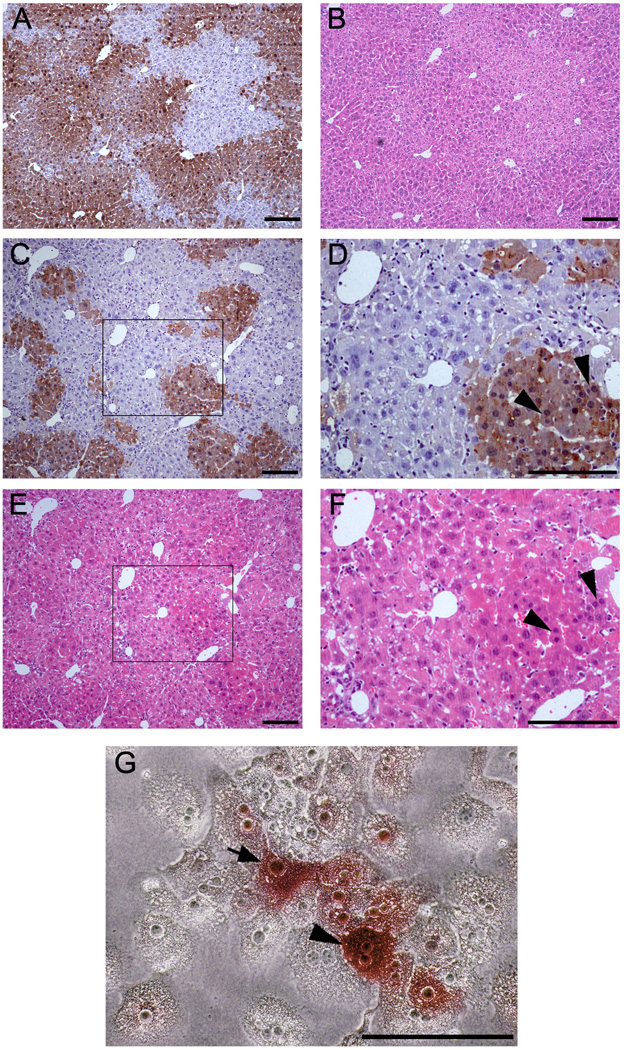
Figure 4. Morphological characters of ES cells derived hepatocytes engrafted in the liver structure after liver repopulation in secondary recipients and in culture after perfusion.
(A, B) Wild-type hepatocytes after liver repopulation in Fah−/− mouse recipient as control. A: anti-FAH immuno-staining; B: H&E staining. (C–F) Like with wild-type hepatocytes, ES cell-derived hepatocytes engrafted into liver sinusoids in the Fah−/− mouse recipients. C and D: anti-FAH immuno-staining; E and F: H&E staining. D and F highlighted the areas inside C and E. (G) Morphological characteristics of ES cell-derived hepatocytes in vitro. ES cell-derived hepatocytes were positive for FAH, showed the typical hepatocyte morphology in culture, shown with FAH-negative hepatocytes of Fah−/− mice after liver perfusion. (Arrowhead and arrow were used to indicate the FAH-positive hepatocyes with bi-nuclear or mono-nuclear, respectively). Scale bars: 200 µm (A–F), 100 µm (G).
We compared the gene expression profiles of the repopulating hepatic cells from primary recipients and secondary recipients by real-time quantitative PCR. ES cell-derived Alb+ cells and adult hepatocytes were used as controls. We found that there was down-regulation of the gene related to hepatic progenitor cells (Afp), and up-regulation of the genes related with mature hepatocytes (Foxa2, Alb, Tat, Hnf4α, Cps1, Trf, G-6-P, Cyp7a1), which suggested the gradual hepatic maturation for ES cell-derived Alb+ cells during serial transplantation. These data are shown in Supplementary Figure 4.
3.4. ES cell-derived hepatocytes showed normal morphology
In liver sections of secondary recipients, ES cell-derived hepatocytes were found to engraft into normal liver sinusoid structures (Fig. 4C–F). Some ES cell-derived hepatocytes had bi-nuclei (arrowhead, Fig. 4D, 4F), with morphology identical to wild-type hepatocytes (Fig. 4A, 4B).
ES cell-derived hepatocytes were positive for FAH (Fig. 4G) and also showed typical hepatocyte morphology in culture. Results of karyotype analysis on ES cell-derived FAH+ hepatocytes indicated that the ratios of diploidy, tetraploidy and octaploidy for ES cell-derived FAH+ hepatocytes were similar to those of wild-type hepatocytes (p > 0.05), and were significantly different from the hepatocytes from Fah−/− mice during tyrosinemia (p < 0.05, Supplementary Table 4). Hepatocytes with abnormal karyotype were only found in significant numbers in Fah−/− mice during tyrosinemia (p < 0.01, Supplementary Table 4).
Together, both morphological results of in vivo ES cell-derived hepatocytes engrafted in the liver parenchema and morphological results of in vitro ES cell-derived hepatocytes in culture suggested that ES cell-derived hepatocytes were the same as normal hepatocytes.
3.5. Metabolic function and therapeutic action of ES cell-derived hepatocytes in secondary recipients after serial transplantation
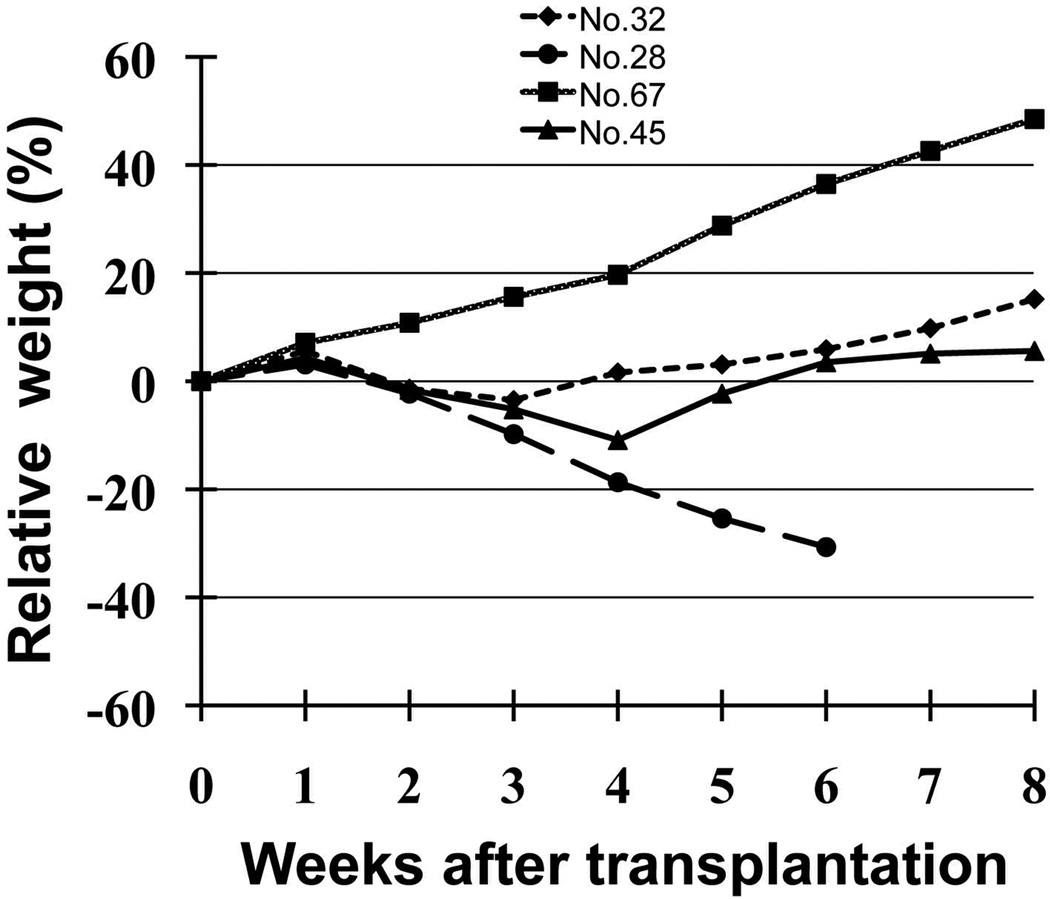
The effect of therapeutic liver repopulation was evaluated in secondary Fah−/− recipients of ES cell-derived hepatocytes. Eight of 20 secondary Fah−/− recipients survived and maintained their body weight after withdrawal of NTBC from the drinking water (Fig. 5). These rescued Fah−/− mice recipients were found to have obviously higher liver repopulation than Fah−/− mice recipients that lost significant weight or died (15.5% to 31.6% versus 2.8% to 12.6%, Fig. 3D).
Figure 5. Restoration of normal metabolic function in Fah−/− recipients transplanted with ES cell-derived hepatocytes after serial transplantation.
A total of eight secondary recipients Fah−/− mice survived without losing weight after ES cell-derived hepatocyte transplantation and withdrawal of NTBC treatment. Fah−/− mouse recipient (No.45) recovered to original weight at 5 weeks after serial transplantation; No.32 as positive control was the Fah−/− mouse after transplantation of wild-type hepatocytes; No.67 as negative control was Fah−/− mouse on NTBC and no hepatocyte transplantation; No.28 as negative control was Fah−/− mouse off NTBC and no hepatocyte transplantation, which died at six weeks.
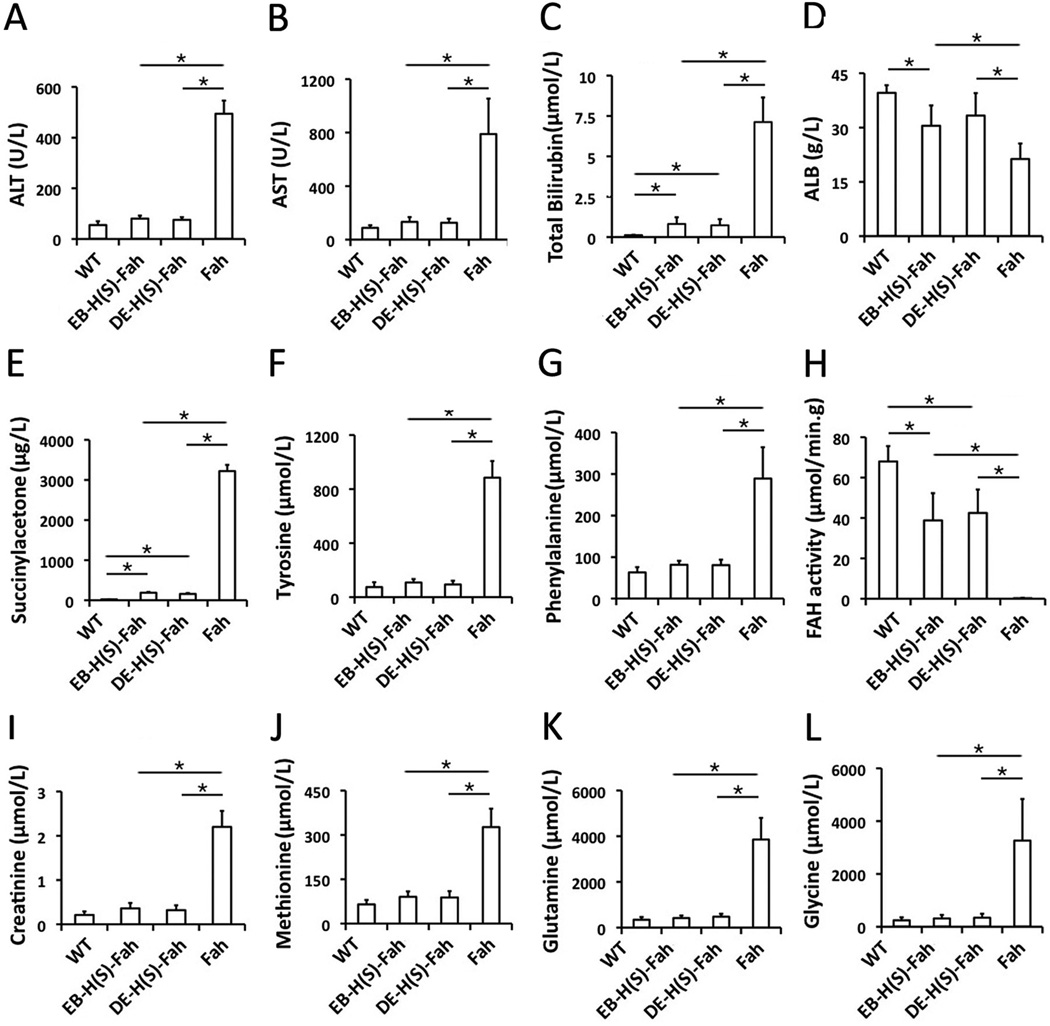
In order to investigate the metabolic function and therapeutic action of ES cell-derived hepatocytes in the surviving Fah−/− mice recipients, plasma was collected from these mice and analyzed. Liver function parameters of Fah−/− recipients, including the enzymes ALT, AST, total bilirubin and ALB, was close to the normal levels of wild-type mice, and significantly abnormal in Fah−/− mice without transplantation of ES cell-derived hepatocytes (Fig 6A–D). Remarkably, the plasma levels of succinylacetone, tyrosine and phenylalanine, which are regarded the clinical parameters of tyrosinemia, were improved to normal levels in secondary recipients (Fig 6E–G). By comparing to the results of FAH enzyme activity of surviving Fah−/− recipients with those of wild-type mice, we determined that about 30% of FAH enzyme activity was enough to rescue Fah−/− recipients of ES cell-derived hepatocytes from death (Fig 6H). In addition, the level of creatinine and the amino acids methionine, glutamine and glycine, were also improved to normal levels (Fig 6I–L).
Figure 6. Biochemical analysis of metabolic function of Fah−/− recipients with liver repopulation after serial transplantation.
The levels of ALT (A), AST (B), total bilirubin (C), ALB (D), succinylacetone (E), tyrosine (F), phenylalanine (G), FAH (H), creatinine (I), methionine (J), glutamine (K) and glycine (I) in WT mice (n=4), secondary Fah−/− recipients transplanted with ES-dervied hepatocytes derived by EB induction (n=4), secondary Fah−/− recipients transplanted with ES cell-derived hepatocytes derived by DE induction (n=4) and control Fah−/− mice (n=4, sera collected upon losing 20% of body weight). *: p < 0.05.
These results indicated that ES cell-derived hepatocytes had normal metabolic capacity in correcting the metabolic disorder of Fah−/− recipient mice. With corrected metabolic function by therapeutic liver repopulation, Fah−/− recipients were rescued from death. Remarkably, there was no significant difference between two populations of ES cell-derived hepatocytes (Fig 6A–L), which were obtained using distinct methods, in restoring the metabolic function of Fah−/− recipient mice and rescuing them from death.
3.6. Characterization of the metabolic function of ES cell-derived hepatocytes
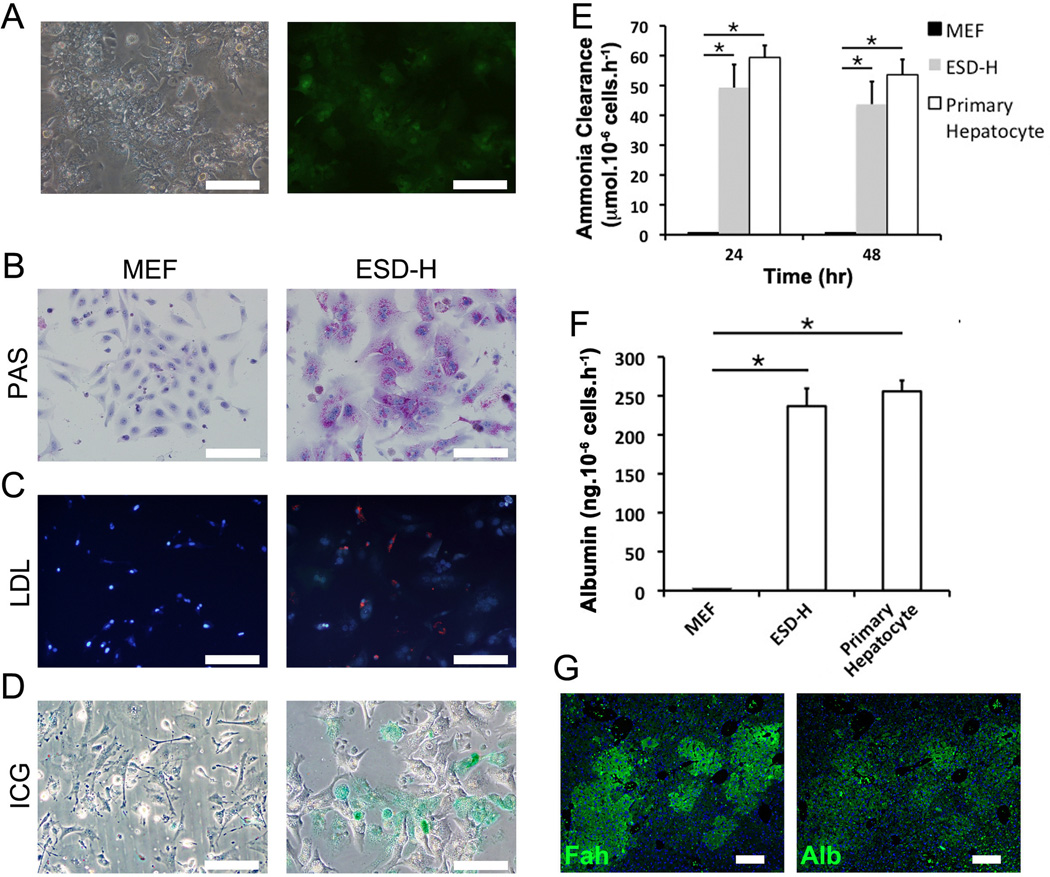
In order to further characterize the metabolic function of ES cell-derived hepatocytes in surviving secondary Fah−/− recipients, PAS stain, DiI-ac-LDL uptake assay, ICG uptake assay, Albumin ELISA and ammonia clearance assay were performed. ES cell-derived hepatocytes were sorted by FACS for GFP expression (Fig. 7A). Numerous PAS- positive granules were seen in the cytoplasm, indicating normal glycogen storage in these cells (Fig. 7B). The DiIac-LDL uptake assay evaluates fat metabolism, and ES cell-derived hepatocytes acquired the capability to transport ac-LDL from the medium into intracellular plasma (Fig. 7C). Furthermore, the cellular uptake of organic anion Indocyanine Green, which is eliminated exclusively by hepatocytes and clinically used as a test substance to evaluate liver function, was found in most of the ES cell-derived hepatocytes (Fig. 7D). Cellular ammonia clearance, a parameter related to the urea cycle, was observed in culture (Fig. 7E). ELISA assay showed that ES cell-derived hepatocytes also secreted high amount of albumin into the medium, reflecting the synthetic function of these cells (Fig. 7F). Albumin expression was further confirmed by immunohistochemistry (Fig. 7G).
Figure 7. In vitro metabolic function of ES cell-derived hepatocytes.
(A) GFP expression as the purification marker to enrich ES cells-derived hepatocytes (ESD-H) after sorting. (B) Glycogen storage in ESD-H was stained as purple by PAS staining. (C) ac-LDL transportation into ESD-H was analyzed by DiI-ac-LDL uptake assay (red staining). (D) Cellular uptake of ICG in ESD-H (green staining). (E) Cellular ammonia clearance was observed in ESD-H culture supernatant. (F) The levels of albumin protein levels secreted in ESD-H culture mediums were measured by ELISA assay. (G) Immunofluorescence staining of albumin and FAH in ESD-H. *: p < 0.05. Scale bars: 200 µm.
These results demonstrated ES cell-derived hepatocytes acquired the metabolic function of normal mature hepatocytes.
4. Discussion
Characterization of the in vivo physiological function of any ES cell derivative is a necessary step to prove completion of tissue-specific differentiation and potential for clinical application. Transplantation assays in animal models are generally regarded as an important for this purpose (Ishii et al., 2007; Murry and Keller, 2008; Yamamoto et al., 2003). For ES cell-derived hepatic cells, the capacity for liver engraftment and liver repopulation has already been demonstrated (Basma et al., 2009; Cai et al., 2007; Chinzei et al., 2002; Choi et al., 2002; Duan et al., 2007; Gouon-Evans et al., 2006; Haridass et al., 2009; Heo et al., 2006; Ishii et al., 2007; Kumashiro et al., 2005; Li et al., 2010; Shafritz and Oertel, 2011; Sharma et al., 2008; Teratani et al., 2005; Yamada et al., 2002; Yamamoto et al., 2003; Yin et al., 2002). However, the in vivo metabolic function and therapeutic action of ES cell-derived hepatocytes was not characterized in any previous study. We successfully performed an analysis of the metabolic function of ES cell-derived hepatocytes after serial transplantations in Fah−/− mice recipients. We found that the engrafted ES cell-derived hepatocytes could correct the metabolic disorders of Fah−/− recipients and rescue them from death after serial liver repopulation. Therefore, our findings significantly improve the understanding of the in vivo function of ES cell-derived hepatocytes, and support the application of ES cell-derived hepatocytes in treating liver diseases.
The levels of liver repopulation from ES cell-derived hepatocytes were variable among previous reports. For example, liver repopulation level varied from 0% to 20% in randomly selected liver sections, with an average of 1.94 ± 5.81% at 82 days in recipients of uPA transgenic mice (Heo et al., 2006; Shafritz and Oertel, 2011). In another report, transplanted alpha-fetoprotein (AFP)-producing cells repopulated more than 30% (32.8% as reported) of the liver tissue at 35 days after transplantation (Ishii et al., 2007). The level of liver repopulation in Fah−/− recipients transplanted with ES cell-derived c-Kit−;EpCAM+ cells reached 24 ± 15 % at ten weeks after transplantation in our previous study (Li et al., 2010). In fact, two frequently used methods, EB or DE methods, were used to derive ES cell-derived hepatic cells, and the transplantation assays were performed in different types of recipients for transplantation assay models. We directly compared the frequencies of liver engraftment and liver repopulation from the two populations of ES cell-derived hepatocytes induced by these two methods. In our study, the DE method generated higher quantities of ES-derived hepatic cells by stepwise induction, which was similar to previous studies (Cai et al., 2007; Duan et al., 2010; Gouon-Evans et al., 2006; Iwamuro et al., 2010; Yasunaga et al., 2005). There are probably two reasons for this improvement. First, the DE method with stepwise hepatic differentiation was more easily controllable and efficient, which is also shown in our recent publication (Li et al., 2010); Second, it is possible that the Alb+ hepatic cells did not contain other Alb+ cells of extra-embryonic tissues in DE method (Gouon-Evans et al., 2006; Keller, 2005), while ES cell-derived Alb+ hepatic cells could mix with ES cell-derived Alb+ visceral endoderm by the EB method (Arnold and Robertson, 2009; Barbacci et al., 1999; Haumaitre et al., 2003). After a parallel comparison of liver repopulation levels in Fah−/− mice recipients under the same conditions, however, we did not find a significant difference between the two populations of ES cell-derived Alb+ cells. On the other hand, both had liver repopulation capacities that were lower than normal wild-type hepatocytes. Therefore, our results suggested that both methods have room for improvement in future studies.
It is possible that only some of the ES cell-derived hepatocytes of the total of Alb+ cells achieved enough hepatic differentiation and maturation, and that only these cells were capable of liver engraftment and repopulation. This hypothesis was supported by our observation that FAH+ single cells predominate, and FAH+ nodules of less than 50 cells varied in size in the liver of primary recipients. Some ES cell-derived hepatic cells might be able to engraft, but may be hindered in ability to expand to repopulate the liver of recipients. With variable levels of 0.001% to 12.5% liver repopulation, and with requirements for NTBC to maintain their weight, all primary recipients were not completely cured of their metabolic defect. The outcomes with primary recipients suggested that both methods of hepatic differentiation have room for improvement in future translational medicine. Improvements in the yield of mature hepatic cells could be made by modifying the culture conditions, or by sorting for cells using more stringent markers of differentiation.
The cells harvested from primary recipients were enriched for mature ES cell-derived hepatocytes, which led to expanded levels of homogenous liver repopulation in secondary recipients. Liver repopulation requires complicated, simultaneous processes including cell homing into extra-cellular matrix, active cell migration and cell-cell interaction (Haridass et al., 2009; Sancho-Bru et al., 2009). It is possible that the in vivo environment in recipient livers may promote the late steps of hepatic maturation for ES cell-derived hepatic cells. It has been shown that ES cell-derived hepatic progenitor cells could become mature hepatocytes after cell transplantation into uPA transgenic mice or Fah−/− mice (Heo et al., 2006; Li et al., 2010), which suggested a continuum of hepatic differentiation and maturation. Therefore, the putative conditions for continuing differentiation and maturation inside the livers of Fah−/− mice recipients could be studied and mimicked in the in vitro micro-environment for generating mature hepatocytes from pluripotent stem cells such as ES cells. We verified this hypothesis by examining the pattern of gene expression of repopulating hepatic cells from primary recipients and secondary recipients, which suggested a gradual maturation of ES cell-derived Alb+ cells during serial transplantation. This is also consistent with our recent results from human liver progenitor cells repopulating the livers of Fah−/−Rag2−/− mice (He et al., 2010). The mechanisms that promote engraftment, differentiation and cell expansion of ES cell-derived Alb+ cells after transplantation into an in vivo situation is the focus of future investigations.
In summary, our transplantation assays reproduce previous findings that ES cell-derived hepatocytes have the capacity for liver engraftment and liver repopulation. For the first time, we studied the liver repopulation of ES cell-derived hepatocytes during serial transplantation, and proved the in vivo metabolic function of ES cell-derived hepatocytes and the capacity to rescue the recipients from liver disease and death. Our study demonstrates a useful method to characterize the functionality of ES cell-derived hepatocytes.
Supplementary Material
Acknowledgements
We thank Mr. Hu Wenbao and Yang Ke in our laboratory for immunohistochemical staining and hepatocytes isolation, and Dr. Huang Pengyu for in vitro metabolic function assay.
Funding:
This work was funded by National Natural Science Foundation of China (30801115, 30623003, 30901449, 31071298), National Key Basic Research and Development Program of China (2007CB947102, 2009CB941100, 2010CB945600, 2011CB966200), Chinese National 863 Plan Project (2006AA02Z474), The Chinese Academy of Sciences (KSCX2-YW-R-49), and National Institutes of Health Grant (DK074561 and AI065565, to X.W.).
Abbreviations
- ES cells
embryonic stem cells
- EB
embryoid body
- DE
definitive endoderm
- FAH
fumaryl acetoacetate hydrolase
- NTBC
2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
- GFP
green fluorescent protein
- Ab
antibody
- Alb
albumin
- Aat
α1-anti-trypsin
- Hnf4α
hepatic nuclear factor 4, alpha
- Ttr
tranthyretin
- Tat
tyrosine aminotransferase
- G-6-p
glucose 6-phosphate
- Cyp7a1
cholesterol 7-hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10(2):91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126(21):4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136(3):990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36(1):22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- Choi D, Oh HJ, Chang UJ, Koo SK, Jiang JX, Hwang SY, et al. In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant. 2002;11(4):359–368. [PubMed] [Google Scholar]
- Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25(12):3058–3068. doi: 10.1634/stemcells.2007-0291. [DOI] [PubMed] [Google Scholar]
- Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, et al. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28(4):674–686. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Hickey RD, Paulk NK, Culberson AJ, Olson SB, Finegold MJ, et al. Ploidy reductions in murine fusion-derived hepatocytes. PloS Genet. 2009;5(2):e1000385. doi: 10.1371/journal.pgen.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24(11):1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Haridass D, Yuan Q, Becker PD, Cantz T, Iken M, Rothe M, et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am J Pathol. 2009;175(4):1483–1492. doi: 10.2353/ajpath.2009.090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haumaitre C, Reber M, Cereghini S. Functions of HNF1 family members in differentiation of the visceral endoderm cell lineage. J Biol Chem. 2003;278(42):40933–40942. doi: 10.1074/jbc.M304372200. [DOI] [PubMed] [Google Scholar]
- He Z, Zhang H, Zhang X, Xie D, Chen Y, Wangensteen KJ, et al. Liver xeno-repopulation with human hepatocytes in Fah−/−Rag2−/− mice after pharmacological immunosuppression. Am J Pathol. 2010;177(3):1311–1319. doi: 10.2353/ajpath.2010.091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Factor VM, Uren T, Takahama Y, Lee JS, Major M, et al. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology. 2006;44(6):1478–1486. doi: 10.1002/hep.21441. [DOI] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- Ishii T, Yasuchika K, Machimoto T, Kamo N, Komori J, Konishi S, et al. Transplantation of embryonic stem cell-derived endodermal cells into mice with induced lethal liver damage. Stem Cells. 2007;25(12):3252–3260. doi: 10.1634/stemcells.2007-0199. [DOI] [PubMed] [Google Scholar]
- Iwamuro M, Komaki T, Kubota Y, Seita M, Kawamoto H, Yuasa T, et al. Comparative analysis of endoderm formation efficiency between mouse ES cells and iPS cells. Cell Transplant. 2010;19(6):831–839. doi: 10.3727/096368910X508951. [DOI] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Kumashiro Y, Asahina K, Ozeki R, Shimizu-Saito K, Tanaka Y, Kida Y, et al. Enrichment of hepatocytes differentiated from mouse embryonic stem cells as a transplantable source. Transplantation. 2005;79(5):550–557. doi: 10.1097/01.tp.0000153637.44069.c6. [DOI] [PubMed] [Google Scholar]
- Li F, He Z, Li Y, Liu P, Chen F, Wang M, et al. Combined Activin A/LiCl/Noggin treatment improves production of mouse embryonic stem cell-derived definitive endoderm cells. J Cell Biochem. 2011;112(4):1022–1034. doi: 10.1002/jcb.22962. [DOI] [PubMed] [Google Scholar]
- Li F, Liu P, Liu C, Xiang D, Deng L, Li W, et al. Hepatoblast-like progenitor cells derived from embryonic stem cells can repopulate livers of mice. Gastroenterology. 2010;139(6):2158–2169. doi: 10.1053/j.gastro.2010.08.042. [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151(5):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12(3):266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- Sancho-Bru P, Najimi M, Caruso M, Pauwelyn K, Cantz T, Forbes S, et al. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut. 2009;58(4):594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Sanderson ND, Thorgeirsson SS. Ploidy and karyotypic alterations associated with early events in the development of hepatocarcinogenesis in transgenic mice harboring c-myc and transforming growth factor alpha transgenes. Cancer Res. 1996;56(9):2137–2142. [PubMed] [Google Scholar]
- Shafritz DA, Oertel M. Model systems and experimental conditions that lead to effective repopulation of the liver by transplanted cells. Int J Biochem Cell Biol. 2011;43(2):198–213. doi: 10.1016/j.biocel.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AD, Cantz T, Vogel A, Schambach A, Haridass D, Iken M, et al. Murine embryonic stem cell-derived hepatic progenitor cells engraft in recipient livers with limited capacity of liver tissue formation. Cell Transplant. 2008;17(3):313–323. doi: 10.3727/096368908784153896. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gutiérrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, et al. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 2006;24(11):1412–1419. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- Teratani T, Yamamoto H, Aoyagi K, Sasaki H, Asari A, Quinn G, et al. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology. 2005;41(4):836–846. doi: 10.1002/hep.20629. [DOI] [PubMed] [Google Scholar]
- Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51(5):1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- Wang X, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Liver repopulation and correction of metabolic liver disease by transplanted adult mouse pancreatic cells. Am J Pathol. 2001;158(2):571–579. doi: 10.1016/S0002-9440(10)63999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. Am J Pathol. 2002;161(2):565–574. doi: 10.1016/S0002-9440(10)64212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422(6934):897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Wangensteen KJ, Wilber A, Keng VW, He Z, Matise I, Wangensteen L, et al. A facile method for somatic, lifelong manipulation of multiple genes in the mouse liver. Hepatology. 2008;47(5):1714–1724. doi: 10.1002/hep.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A, Wangensteen KJ, Chen Y, Zhuo L, Frandsen JL, Bell JB, et al. Messenger RNA as a source of transposase for sleeping beauty transposon-mediated correction of hereditary tyrosinemia type I. Mol Ther. 2007;15(7):1280–1287. doi: 10.1038/sj.mt.6300160. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20(2):146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37(5):983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23(12):1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- Yin Y, Lim YK, Salto-Tellez M, Ng SC, Lin CS, Lim SK. AFP(+), ESC-derived cells engraft and differentiate into hepatocytes in vivo. Stem Cells. 2002;20(4):338–346. doi: 10.1634/stemcells.20-4-338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.