Abstract
Schizophrenia is a grave psychiatric disorder with psychotic symptoms and an enigmatic etiology. Family studies have strongly indicated that genetic risk factors have a role in this disease. Recent findings, together with previously established evidence, highlight the PDZ-domain-containing protein interacting with C-kinase 1 (PICK1) as a promising candidate for a schizophrenia susceptibility gene. Here, we outline possible molecular mechanisms, discuss clinical case-studies that indicate an unexpected role of PICK1 in schizophrenia and discuss potential avenues for pharmacological manipulation of PICK1.
Introduction
Schizophrenia is a severe brain disease that triggers positive and negative symptoms, including psychotic characteristics such as hallucinations, delusions and paranoia (see Glossary), cognitive impairment, loss of motivation and impaired ability to manage emotions and relationships [1,2]. The illness presents in several forms, which are diagnosed based on specific symptoms, the most common being paranoia. Schizophrenia occurs in almost 1% of the US population and emerges earlier in men (teens to 25 years of age) than in women (ages 25–35), with an estimated 2.2 million Americans affected each year. Genetic association, viral infection, brain injury and drug abuse during embryonic brain development have all been associated with the etiology of this disease [1-4].
Schizophrenia is not linked to a solitary genetic mutation and probably involves a heterogeneous mechanism with polymorphisms in several genes and in a range of environmental susceptibility factors [3-8]. Individually, these abnormalities probably have sub-threshold effects that can be difficult to confirm in genetic studies but, in combination, might manifest in symptoms [5-8]. These genetic and environmental factors result in a complex pathophysiology that includes: (i) problems in brain development and the aberrant formation of neuronal circuits; (ii) abnormal regulation of neurotransmitter concentrations and receptor activity; (iii) defects in metabolic pathways and enzyme function; and (iv) reduced brain volumes that are probably coupled with neurotoxicity and neurodegeneration [4-8]. Some of the genetic, pharmacological and environmental evidence that has inferred risk factors for schizophrenia is described in Box 1. The diversity in origin connected to this illness provides potential for several therapies that could correct receptor and enzyme function, repair neuronal circuits and prevent the progress of neurodegeneration. Better understanding of the molecular mechanisms that underlie schizophrenia will improve the ability to stratify patients, make tailored drugs and improve therapeutic outcomes. Therefore, the continued discovery of novel genes that are involved in this disorder remains crucial for developing better drugs.
Box 1. Drug targets for schizophrenia.
Pharmacology and genetics of schizophrenia
Pharmacological evidence using modulators of glutamate-mediated, dopamine-mediated and 5-hydroxytryptamine (5-HT)-mediated neurotransmission has helped to develop models of schizophrenia for these receptor systems (Table I). Antipsychotics such as haloperidol, chlorpromazine (Largactil®), clozapine (Clozaril®) and risperidone (Risperdal®) have pharmacology profiles that include dopamine and 5-HT receptor antagonism but might also include antagonist activity at adrenoceptors, acetylcholine receptors and histamine receptors. These antipsychotics reduce delusions and hallucinations but might induce extrapyramidal-motoric uncontrolled body movements (tardive dyskinesia, dystonias, akathisia and pseudo-parkinsonism) due to dopamine action in nigrostriatal pathways [1,2]. Genetic studies have helped to identify putative risk factors for schizophrenia (Table I). Chromosomal hotspots for schizophrenia have been suggested and the chromosomal locations of some genes implicated in schizophrenia include regulator of G-protein signaling (RGS) (1q21-q22), dystrobrevin binding-protein 1 (DTNBP1) (6p24-p22), neuregulin (NRG) (8p22-p21), d-amino acid oxidase activator (DAOA) (13q32-q34) and catechol-O-methyl-transferase (COMT) (22q11-q22) [3-8].
PICK1-interacting proteins with strong links to schizophrenia
The three PICK1-interacting proteins for which there is strong evidence for roles in schizophrenia are: (i) glutamate (Glu) receptor subunits and subtypes (e.g. Glu2 receptor AMPA subunit, Glu5 receptor kainate subunit and mGlu7 receptor metabotropic receptor); (ii) DAT; and (iii) serine racemase (SR) (Table I).
PICK1-interacting proteins with possible roles in schizophrenia
Proteins that interact with PICK1 and have putative roles in schizophrenia include: (i) neuregulin (NRG) and ErbB tyrosine kinase receptors (e.g. ErbB2/HER2), which regulate neurodevelopment, neuronal migration and differentiation, and synaptic plasticity; (ii) presynaptic-vesicle-docking proteins such as syndecan-interacting protein (β-SNAP) and possibly N-ethylmaleimide-sensitive factor (NSF); (iii) Coxsackie and adenovirus receptor (CAR); and (iv) brain-derived neurotrophic factor (BDNF), which regulates PICK1 expression (Table I).
Proteins that interact with PICK1 but have no or weak evidence for roles in schizophrenia
These proteins include: (i) Eph tyrosine kinase receptor (e.g. EphB2) and ephrin ligands; (ii) brain Na+ channel (e.g. BNaC1) and acid-sensitive ion channel (ASIC); (iii) prolactin-releasing peptide receptor (PrRP); (iv) tetradecanoyl phorbol ester-induced primary response sequence (TIS21); (v) ADP ribosylation factor (e.g. ARF1); (vi) kalirin7, a guanine-nucleotide-exchange factor; (vii) syntenin; (viii) homolog of Caenorhabditis elegans UNC5 (UNC5H); (ix) aquaporin-9; (x) anion exchanger-1; and (xi) neuroligin [14].
Protein interacting with C-kinase 1 (PICK1) is a recently proposed candidate protein for involvement in schizophrenia [9,10]. The pick1 gene is located on chromosome 22q13.1, which is a genetic locus that frequently links to schizophrenia [8,11-13]. At the protein level, PICK1 has been studied extensively because of its role in regulating the cell biology and functional properties of several important neuronal proteins [14-16]. PICK1 is a scaffolding protein that is located at neuronal synapses and associates with a wide range of proteins via its functional domains, which include a PSD95–Disc-large–ZO-1 (PDZ) domain and a Bin–amphiphysin–Rvs (BAR) domain [14]. PICK1 interacts with protein kinase C (PKC) and regulates the phosphorylation of many PICK1-interacting partners, thereby altering their synaptic clustering, trafficking to the neuronal surface and membrane recycling. A search for convergent loci has located not only PICK1 but also many of its interacting proteins, including neuregulin receptors, glutamate receptors and ephrins, within linkageregions of schizophrenia [8]. The proteins with an accepted association with schizophrenia and their interaction with PICK1 are described in Box 1.
Interest in PICK1 has been heightened by three recent genetic studies that investigated a role for this protein in schizophrenia. The first of these case studies involved the examination of 225 schizophrenic and 260 control Han Chinese patients. It revealed an association between a single nucleotide polymorphism (SNP), rs3952 in intron 3 of pick1, and the diagnosis of schizophrenia [11]. A second case-study of 200 unrelated Japanese schizophrenic patients and 200 age-matched Japanese normal controls found an association between an SNP, rs2076369 in intron 4 of pick1, and schizophrenia but only when using ‘disorganized’ schizophrenics [12]. Associations of lower magnitude might be expected if disease susceptibility results from the combined effect of several variants in a gene. Significantly, both of the SNPs reported occurred in the PDZ domain of PICK1, with no evidence of SNPs in the BAR domain (Figure 1). A third study comparing elderly schizophrenia patients (36 patients) with matched normal controls (26 patients) showed no difference in levels of PICK1 mRNA in the occipital cortex of elderly schizophrenia patients compared with matched normal controls [13]. This observation leaves open the possibility that PICK1 SNPs are more crucial for regulating the function of the PDZ domain than are total mRNA or protein levels. Although these genetic findings are potentially important, they require evidence from a larger population to confirm that mutations in PICK1 are associated risk factors for schizophrenia.
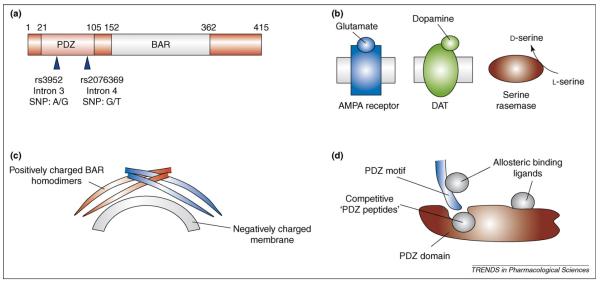
Figure 1.
PICK1-interacting proteins and regulation of PICK1 domains. (a) The structure of PICK1 and mutations linked to schizophrenia. Shown are the SNPs in the PDZ domain of PICK1 that are associated with schizophrenia. (b) Three proteins that interact with the PDZ domain of PICK1 and have a role in schizophrenia. The PDZ domain of PICK1 is unusual because it can bind to many types of PDZ motif (for review, see Ref. [14]). For example, it binds to PKCα via a type I PDZ motif [9,10], whereas it binds to Glu2 receptor (an AMPA receptor subunit) via a type II PDZ motif [15,31,32]. Although PICK1 interacts with ~20 different types of protein family [14], only the three proteins that have been associated with schizophrenia are shown: namely, AMPA receptors, DAT and serine racemase. PICK1 interacts with these proteins to regulate their function by PKC-mediated phosphorylation, synaptic clustering and/or membrane expression. (c) PICK1 harbors a BAR domain, in addition to its PDZ domain, that is involved in dimerization of PICK1 and interaction with glutamate-receptor-interacting protein (GRIP) [14]. GRIP is a PDZ-domain-containing protein that anchors AMPA receptors to the membrane [13,40,41]. The positively charged BAR homodimer of α-helical bundles is sufficient to support electrostatic forces with the negatively charged membrane and enable lipid–protein interactions. For example, a membrane containing 20% phosphatidylserine has a negative surface potential of approximately −30 mV, attracting K+ ions and clusters of basic protein residues (e.g. lysine). (d) Pharmacological approaches for regulating the PDZ domain interactions of PICK1. Competitive PDZ peptides match the sequences of PDZ motifs and fit into PDZ domains, thereby inhibiting the entry of endogenous PDZ motifs. PDZ peptides can be engineered to block specific interactions; for example, the PDZ peptide NVYGIESVKI, which is based on the PDZ-binding motif of the Glu2 receptor, blocks Glu2 receptor interaction with GRIP and PICK153. By contrast, the PDZ peptide NVYGIEEVKI, which is modeled on a phosphorylated Glu2 receptor mimic, blocks Glu2 receptor interaction with PICK1 but not GRIP53. Although no examples have been reported, the allosteric binding of drugs to PDZ motifs or PDZ domains could, in theory, alter the conformation of these interacting structures. This novel approach might be amenable to augment or attenuate PDZ interactions.
Further evidence of a role for PICK1 in schizophrenia comes from the proteins with which it interacts that have been associated with the illness (Figure 1) and there are different possible approaches to regulating these interactions (see later). Overall, collating evidence from genetic and protein interaction studies, we believe that one underlying biological cause of increased susceptibility for schizophrenia is the altered interactions between PICK1 and its associated synaptic proteins.
Functional domains of PICK1
PICK1 is 416 amino acids in length and has a single PDZ domain [14] (Figure 1). This domain contains ~90 residues and interacts with proteins that harbor PDZ-binding motifs at their C-termini. The PDZ domain of PICK1 accepts a range of PDZ-binding motifs and, thus, mediates protein–protein interactions with many different proteins [14]. PICK1 also has a sequence homology to the BAR domain of arfaptin2 [17-19]. BAR domains, which are present in many GTPases and other proteins, are involved in endocytosis. They are coiled-coil structures that dimerize to form banana-shaped structures that bind to phospholipids, sense and/or promote membrane curvature and promote endocytosis [17-19] (Figure 1). The two coiled-coil regions of a single PICK1 protein can dimerize and, following binding to its PDZ receptor or transporter, PICK1 dimers can target the receptor–transporter complexes to membrane areas in which endocytosis can occur [20,21]. Thus, via its BAR domain, PICK1 can direct PDZ-interacting proteins that are destined for internalization to membrane invaginations such as clathrin-coated pits. PICK1 is also a Ca2+-sensing protein – consistent with an active role in endocytosis and exocytosis, which are Ca2+ dependent [22]. When the PDZ domain is unoccupied, autodimerization of the PICK1 BAR domain can be inhibited by an intramolecular interaction with its own N-terminal PDZ domain [23]. Thus, the PICK1-mediated recruitment of PKCα can lead to phosphorylation of its interacting partners – which, together with its BAR-domain-mediated targeting of cargo to curved membranes, indicates a central role for PICK1 in receptor and transporter endocytosis and recycling.
Roles of PICK1 in glutamate receptor function
Disruption of the glutamate system has been implicated in the pathophysiology of bipolar I disorder and symptoms of schizophrenia [3,24-29]. Antagonists of NMDA glutamate receptors, such as phencyclidine and ketamine, induce psychotic signs and symptoms of schizophrenia, probably by increased glutamate release and activation of AMPA glutamate receptors [24,30]. Moreover, genetic studies indicate that NMDA, AMPA and kainate glutamate receptors are loci for schizophrenia [8,13]. The PDZ domain of PICK1 has been reported to interact with the C-terminal PDZ motifs of AMPA [31,32], kainate [33] and metabotropic [34-36] glutamate receptor subunits and subtypes. PICK1 regulates glutamate receptor phosphorylation by PKC and can control receptor clustering and synaptic expression [14-16,36-42]. Thus, PICK1 can regulate plasticity in the hippocampus and cerebellum, thus having a role in learning and memory [14-16,36-42]. The mutated PDZ domain of PICK1 in schizophrenia patients [11,12] might lead to the altered surface expression and/or trafficking of glutamate receptors and could increase susceptibility for schizophrenia.
PICK1 regulation of dopamine transporters
Monoamine transporters are located at perisynaptic sites and recycle monoamines via uptake and vesicle storage. In monoamine-transporter-knockout mice, the level of monoamines in intracellular stores and transmitter release are depleted [43]. Numerous pharmacological agents stimulate (e.g. amphetamine) or inhibit (e.g. cocaine) these transporters and induce hallucinogenic symptoms. They are also targeted by antidepressants and psychostimulants and are involved in psychiatric diseases [43]. The dopamine neurotransmitter system as a model for schizophrenia was developed based on pharmacological evidence that dopamine receptor agonists and antagonists trigger and reduce psychosis, respectively, and the suggestion that dopamine receptor polymorphisms are associated with the disease [44].
The PDZ domain of PICK1 also interacts with monoamine transporters of dopamine (DAT) and norepinephrine (NET), both of which have been associated with schizophrenia [43,45]. In dopamine-containing neurons, PICK1 co-localizes with DAT, and the expression of recombinant PICK1 results in DAT–PICK1 clusters that enhance dopamine uptake because of an increase in the number of plasma membrane DATs, probably through a PKC-dependent mechanism [45]. Therefore, it seems plausible that the regulation of DAT surface expression via PICK1 provides another potential mechanism for the involvement of PICK1 dysfunction in schizophrenia.
Interaction of PICK1 with serine racemase
Glutamate released from neurons activates AMPA receptors on astrocytes, which express serine racemase. This enzyme converts l-serine to d-serine, which is an endogenous ligand for the glycine site of NMDA receptors [46]. The treatment of schizophrenics with d-serine ameliorates some symptoms [47]. In addition, genes encoding the d-serine-degrading enzyme d-amino acid oxidase (DAAO) and its activator–regulator G72 contain SNPs that are associated with schizophrenia [48-51]. Recent studies show that the PDZ domain of PICK1 interacts with serine racemase [12], which provides additional support for a role of PICK1 in schizophrenia.
Pharmacological modulation of PICK1
Together, the genetic reports, chromosomal location and protein interaction studies implicate a role for PICK1 in schizophrenia. Therefore, an important challenge is to determine whether PICK1 can be therapeutically targeted to provide treatment for this disorder. Little is known about how to regulate the BAR domain of PICK1. By contrast, numerous examples indicate that PDZ domain interactions can be competitively antagonized by synthetic blocking peptides (for review, see Ref. [52]) (Figure 1). These ‘PDZ peptides’ can be designed to block specific PDZ interactions and are effective both in vitro [33,38,53-55] and in vivo [56,57]. However, although they are useful research tools, PDZ peptides cannot readily penetrate cellular membranes, are rapidly degraded and have poor pharmacokinetic properties. These limitations of PDZ peptides have fuelled interest in generating low molecular weight drugs that block PDZ domain interactions. Indeed, crystal structure analysis and modeling approaches [37,58], in addition to chemical library screening, have provided drugs that block PDZ domain interactions [59-62]. These early compounds provide proof that low molecular weight compounds can block PDZ-protein–protein interactions [60-62] and give hope that the PDZ domain of PICK1 can be modulated by drugs.
Concluding remarks
Outstanding questions that must be resolved to define unequivocally a role for PICK1 in schizophrenia include: (i) will studies using a larger number of patients confirm that PICK1 mutations are associated with an increased risk of schizophrenia? (ii) Do the currently reported SNPs in the PDZ domain of PICK1 alter its function and its interaction with all or just some of its associated proteins? (iii) Are SNPs in other regions of PICK1, for example its BAR domain, also associated with schizophrenia? (iv) Are other PICK1-interacting proteins also associated with schizophrenia? Overall, there are several possible avenues for future research aimed at modulating PICK1 PDZ interactions and/or modulating the levels of specific interacting partners with drugs. Obviously, a great deal of work must be carried out on each of these approaches to refine the methodology, define better the precise target sequences for enhanced selectivity and design improved delivery mechanisms. Further work is also needed to determine which combinations of PICK1 interactions should be regulated and which of these interactions should be enhanced or inhibited for beneficial effects in schizophrenia.
We believe that PICK1 is an exciting target for potential pharmaceutical intervention and that the capability to manipulate PICK1 interactions will lead to a greater understanding of its putative role in neuronal function and dysfunction; this, in due course, could lead to a novel therapy for schizophrenia.
Table I. Proteins that interact with PICK1 but for which there is no or only weak evidencea.
| Putative targets and models of schizophrenia | PICK1 interaction? | Refs |
|---|---|---|
| Receptors and transporters | ||
| Glu receptors: reduced NMDA receptor activity and glutamate release is associated with schizophrenia; NMDA receptor activators and glycine-site modulators limit symptoms |
Yes – PICK1 binds to many Glu receptors |
b |
| Dopamine receptors and transporters: increased D2 receptor expression and dopamine release in the striatum is linked to schizophrenia; D2 receptor antagonism reduces psychosis |
Yes – PICK1 interacts with DAT |
b |
| ErbB receptors and NRG: NRG expression is altered in schizophrenia; NRG is found in glutamate- containing vesicles; NRG binds to ErbB receptors and alters NMDA receptor expression |
Yes – PICK1 binds to ErbB receptors |
[5,6] |
| 5-HT receptors: the hypersensitivity of 5-HT receptors is linked to schizophrenia; 5-HT2A receptor antagonism reduces symptoms of schizophrenia |
Unknown – interaction not investigated |
[63,64] |
| GABA receptors: a lack of inhibitory GABA tone probably occurs in schizophrenia because of altered glutamate activity |
Unknown – interaction not investigated |
[65,66] |
| Nicotinic acetylcholine receptors: promoter variants and SNPs in the nicotinic α7 receptor are associated with schizophrenia |
Unknown – interaction not investigated |
[67,68] |
| Other synaptic proteins | ||
| SNAP-25: schizophrenia might be associated with abnormal levels of protein in presynaptic terminals and the SNARE complex, which is involved in vesicle docking |
Yes – PICK1 interacts with β-SNAP and NSF |
[69-72] |
| Dysbindin: altered expression and SNPs are linked to schizophrenia; dysbindin is a presynaptic protein that binds to dystrobrevin, and regulates synaptic structures and glutamate release |
Linked via glutamate receptors? |
[5,6] |
| RGS4: decreased expression and SNPs are associated with schizophrenia; RGS4 is a negative regulator of mGlu receptors |
Linked via glutamate receptors? |
[5,6] |
| Enzyme dysfunction and transmitter metabolism | ||
|
d-serine metabolism: d-serine activates the NMDA receptor glycine site; it is synthesized by SR and degraded by DAAO–G72; d-serine treatment ameliorates schizophrenia |
Yes – PICK1 binds to SR | b |
| COMT: COMT controls dopamine levels; SNPs in this enzyme are associated with schizophrenia | Linked via DAT? | [5,6] |
| Brain development abnormalities and viral infections | ||
| Coxsackie and adenovirus receptor: viral infections of Coxsackie B5 during the newborn period might increase the risk of schizophrenia |
Yes – PICK1 interacts with CAR |
[73,74] |
| Neurotrophic factors: low expression levels of NGF and SNPs of BDNF might alter neuronal development; atypical antipsychotics and AMPA receptor modulators release BDNF |
Yes – BDNF regulates PICK1 expression |
[75-78] |
| Inflammatory cytokines: increased release of cytokines during problems with birth, and fetal viral infections might be associated with increased risk of schizophrenia |
Unknown – can PICK1 alter cytokine release? |
[79,80] |
Abbreviations: NGF, nerve growth factor; SNAP, soluble NSF attachment protein; SNARE, SNAP receptor.
See main text.
Glossary
- AMPA receptor
subtype of ionotropic transmembrane receptor for glutamate that mediates most of the fast synaptic transmission in the CNS.
- BAR domain
crescent-shaped structure within proteins that is involved in membrane bending and tubulation.
- Disorganized schizophrenics
characterized by agitated and purposeless behavior.
- Dopamine receptor
G-protein-coupled metabotropic receptors that can be excitatory or inhibitory to the postsynaptic neuron.
- Ephrins
a family of protein ligands for ephrin receptor protein-tyrosine kinases that regulate axon guidance.
- G72
a protein that activates d-serine oxidation by DAAO.
- GTPases
a large family of enzymes that bind to and hydrolyze GTP. They have important functions in many aspects of cell function, including signaling and protein trafficking.
- Neuregulin
a family of four structurally related growth factors that are highly expressed in the developing and adult brain.
- NMDA receptor
subtype of ionotropic transmembrane glutamate receptor. Calcium flux through NMDA receptors has a crucial role in synaptic plasticity, which is a cellular mechanism for learning and memory.
- Occipital cortex
visual-processing region at the back of the mammalian brain.
- Paranoia
a psychotic disorder characterized by delusions of persecution.
- PDZ domain
protein-binding module within proteins that consists of 80-90 amino acids that fold into six β-strands and two α-helices.
- PICK1
a single-PDZ-domain protein that mediates interaction with the C termini of several receptors, transporters, ion channels and kinases.
- Polymorphisms
differences in DNA sequence among individuals. Genetic variations occurring in >1% of a population would be considered useful polymorphisms for genetic linkage analysis.
- SNP
DNA sequence variation at a single nucleotide. SNPs make up 90% of all human genetic variations.
References
- 1.Sadock BJ, Sadock VA. Schizophrenia. In: Kaplan HI, Sadock BJ, editors. Synopsis of Psychiatry. 9th edn Lippincott Williams and Wilkins; 2003. pp. 471–504. [Google Scholar]
- 2.Buchanan RW, Carpenter WT., Jr . Schizophrenia. In: Sadock BJ, Sadock VA, editors. Textbook of General Psychiatry. 8th edn Vol. 1. Lippincott Williams and Wilkins; 2005. pp. 1329–1558. [Google Scholar]
- 3.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 4.Goff DC. Pharmacologic implications of neurobiological models of schizophrenia. Harv. Rev. Psychiatry. 2005;13:352–359. doi: 10.1080/10673220500433262. [DOI] [PubMed] [Google Scholar]
- 5.Harrison PJ, Owen MJ. Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet. 2003;361:417–419. doi: 10.1016/S0140-6736(03)12379-3. [DOI] [PubMed] [Google Scholar]
- 6.Owen MJ, et al. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Collier DA, Li T. The genetics of schizophrenia: glutamate not dopamine? Eur. J. Pharmacol. 2003;480:177–184. doi: 10.1016/j.ejphar.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 8.Moises HW, et al. The glial growth factors deficiency and synaptic destabilization hypothesis of schizophrenia. BMC Psychiatry. 2002;2:1–14. doi: 10.1186/1471-244X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staudinger J, et al. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J. Cell Biol. 1995;128:263–271. doi: 10.1083/jcb.128.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staudinger J, et al. Specific interaction of the PDZ domain protein PICK1 with the COOH terminus of protein kinase C-α. J. Biol. Chem. 1997;272:32019–32024. doi: 10.1074/jbc.272.51.32019. [DOI] [PubMed] [Google Scholar]
- 11.Hong CJ, et al. Association study of PICK1 rs3952 polymorphism and schizophrenia. Neuroreport. 2004;15:1965–1967. doi: 10.1097/00001756-200408260-00026. [DOI] [PubMed] [Google Scholar]
- 12.Fujii K, et al. Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol. Psychiatry. 2006;11:150–157. doi: 10.1038/sj.mp.4001776. [DOI] [PubMed] [Google Scholar]
- 13.Dracheva S, et al. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J. Neurosci. Res. 2005;79:868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- 14.Dev KK. Making protein interactions druggable: targeting PDZ domains. Nat. Rev. Drug Discov. 2004;3:1047–1056. doi: 10.1038/nrd1578. [DOI] [PubMed] [Google Scholar]
- 15.Hanley JG, Henley JM. Picking out the details of cerebellar LTD. Neuron. 2006;49:778–780. doi: 10.1016/j.neuron.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collingridge GL, et al. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 17.Peter BJ, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 18.Habermann B. The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 2004;5:250–255. doi: 10.1038/sj.embor.7400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmerberg J, McLaughlin S. Membrane curvature: how BAR domains bend bilayers. Curr. Biol. 2004;14:250–252. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg JP, et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Jin W, et al. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J. Neurosci. 2006;26:2380–2390. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 25.Owen MJ, et al. Schizophrenia: a genetic disorder of the synapse? BMJ. 2005;330:158–159. doi: 10.1136/bmj.330.7484.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallin MD, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am. J. Hum. Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai GC, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 28.Scarr E, et al. Cortical glutamatergic markers in schizophrenia. Neuropsychopharmacology. 2005;30:1521–1531. doi: 10.1038/sj.npp.1300758. [DOI] [PubMed] [Google Scholar]
- 29.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 30.Heresco-Levy U. N-methyl-d-aspartate (NMDA) receptor based treatment approaches in schizophrenia: the first decade. Int. J. Neuropsychopharmacol. 2000;3:243–258. doi: 10.1017/S1461145700001978. [DOI] [PubMed] [Google Scholar]
- 31.Dev KK, et al. The protein kinase Cα binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 32.Xia J, et al. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 33.Hirbec H, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boudin H, et al. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- 35.Dev KK, et al. PICK1 interacts with and regulates PKC phosphorylation of mGluR7. J. Neurosci. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dev KK, et al. Regulation of mglu7 receptors by proteins that interact with the intracellular C-terminus. Trends Pharmacol. Sci. 2001;22:355–361. doi: 10.1016/s0165-6147(00)01684-9. [DOI] [PubMed] [Google Scholar]
- 37.Perez JL, et al. PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J. Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terashima A, et al. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J. Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim E, Sheng M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 40.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 41.Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr. Opin. Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 42.Xia J, et al. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 43.Torres GE, et al. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 44.Scharfetter J. Dopamine receptor polymorphisms and drug response in schizophrenia. Pharmacogenomics. 2001;2:251–261. doi: 10.1517/14622416.2.3.251. [DOI] [PubMed] [Google Scholar]
- 45.Torres GE, et al. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron. 2001;30:121–134. doi: 10.1016/s0896-6273(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 46.Mothet J-P, et al. d-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai G, et al. d-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. 1998;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 48.Schumacher J, et al. Examination of G72 and d-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol. Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- 49.Chumakov I, et al. Genetic and physiological data implicating the new human gene G72 and the gene for d-amino acid oxidase in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattori E, et al. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am. J. Hum. Genet. 2003;72:1131–1140. doi: 10.1086/374822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen YS, et al. Findings in an independent sample support an association between bipolar affective disorder and the G72/G30 locus on chromosome 13q33. Mol. Psychiatry. 2004;9:87–92. doi: 10.1038/sj.mp.4001453. [DOI] [PubMed] [Google Scholar]
- 52.Kim E, Sheng M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 53.Daw MI, et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 54.Park Y, et al. Long-term depression of kainate receptor-mediated synaptic transmission. Neuron. 2006;49:95–106. doi: 10.1016/j.neuron.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 55.Sans N, et al. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 56.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 57.Garry EM, et al. Specific involvement in neuropathic pain of AMPA receptors and adapter proteins for the GluR2 subunit. Mol. Cell. Neurosci. 2003;24:10–22. doi: 10.1016/s1044-7431(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 58.Madsen KL, et al. Molecular determinants for the complex binding specificity of the PDZ domain in PICK1. J. Biol. Chem. 2005;280:20539–20548. doi: 10.1074/jbc.M500577200. [DOI] [PubMed] [Google Scholar]
- 59.Ferrer M, et al. A PDZ domain-based detection system for enzymatic assays. Anal. Biochem. 2002;301:207–216. doi: 10.1006/abio.2001.5497. [DOI] [PubMed] [Google Scholar]
- 60.Fujii N, et al. A selective irreversible inhibitor targeting a PDZ protein interaction domain. J. Am. Chem. Soc. 2003;125:12074–12075. doi: 10.1021/ja035540l. [DOI] [PubMed] [Google Scholar]
- 61.Shan J, et al. Identification of a specific inhibitor of the Dishevelled PDZ domain. Biochemistry. 2005;44:15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- 62.Joshi M, et al. Discovery of low-molecular-weight ligands for the AF6 PDZ domain. Angew. Chem. Int. Ed. Engl. 2006;45:3790–3795. doi: 10.1002/anie.200503965. [DOI] [PubMed] [Google Scholar]
- 63.Svensson TH, et al. Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: role of 5-HT2 receptor and α1-adrenoreceptor antagonism. J. Clin. Psychopharmacol. 1995;15:11–18. doi: 10.1097/00004714-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 64.Bleich A, et al. The role of serotonin in schizophrenia. Schizophr. Bull. 1988;14:297–315. doi: 10.1093/schbul/14.2.297. [DOI] [PubMed] [Google Scholar]
- 65.Lewis DA, et al. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology. 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 66.Petryshen TL, et al. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol. Psychiatry. 2005;10:1074–1088. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- 67.Martin LF, et al. α-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 68.Leonard S, et al. Association of promoter variants in the 7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch. Gen. Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 69.Scarr E, et al. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–143. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 70.Tachikawa H, et al. Polymorphism of the 5′-upstream region of the human SNAP-25 gene: an association analysis with schizophrenia. Neuropsychobiology. 2001;43:131–133. doi: 10.1159/000054880. [DOI] [PubMed] [Google Scholar]
- 71.Young CE, et al. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb. Cortex. 1998;8:261–268. doi: 10.1093/cercor/8.3.261. [DOI] [PubMed] [Google Scholar]
- 72.Thompson PM, et al. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol. Psychiatry. 1998;43:239–243. doi: 10.1016/S0006-3223(97)00204-7. [DOI] [PubMed] [Google Scholar]
- 73.Rantakallio P, et al. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int. J. Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- 74.Excoffon KJ, et al. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J. Cell Sci. 2004;117:4401–4409. doi: 10.1242/jcs.01300. [DOI] [PubMed] [Google Scholar]
- 75.Gilmore JH, et al. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J. Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 76.Bai O, et al. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J. Neurosci. Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- 77.Hong CJ, et al. An association study of a brain-derived neurotrophic factor Val66Met polymorphism and clozapine response of schizophrenic patients. Neurosci. Lett. 2003;349:206–208. doi: 10.1016/s0304-3940(03)00828-0. [DOI] [PubMed] [Google Scholar]
- 78.Jourdi H, et al. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor associated PDZ proteins in developing cortical neurons. Dev. Biol. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown AS, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 80.Gilmore JH, et al. Prenatal infection and risk for schizophrenia: IL-1 β, IL-6, and TNFα inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]