Abstract
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors (AMPARs) are of fundamental importance in the brain. They are responsible for the majority of fast excitatory synaptic transmission, and their overactivation is potently excitotoxic. Recent findings have implicated AMPARs in synapse formation and stabilization, and regulation of functional AMPARs is the principal mechanism underlying synaptic plasticity. Changes in AMPAR activity have been described in the pathology of numerous diseases, such as Alzheimer’s disease, stroke, and epilepsy. Unsurprisingly, the developmental and activity-dependent changes in the functional synaptic expression of these receptors are under tight cellular regulation. The molecular and cellular mechanisms that control the postsynaptic insertion, arrangement, and lifetime of surface-expressed AMPARs are the subject of intense and widespread investigation. For example, there has been an explosion of information about proteins that interact with AMPAR subunits, and these interactors are beginning to provide real insight into the molecular and cellular mechanisms underlying the cell biology of AMPARs. As a result, there has been considerable progress in this field, and the aim of this review is to provide an account of the current state of knowledge.
I. Introduction
A. Classes of Glutamate Receptors
The amino acid glutamate is the major excitatory neurotransmitter in the mammalian central nervous system (CNS1), and it exerts its physiological effects by binding to a number of different types of glutamate receptors (GluRs). Glutamate receptors can be divided into two functionally distinct categories: those that mediate their effects via coupling to G-protein second messenger systems, the metabotropic glutamate receptors (mGluRs) and ionotropic ligand-gated ion channels (Simeone et al., 2004). Based largely upon the work of Watkins and coworkers (Watkins, 1981, 1991; Watkins et al., 1981), the ionotropic glutamate receptors have been separated into three distinct sub-groups based upon their pharmacology (for related reviews, see Dingledine et al., 1999; Barnes and Slevin, 2003; Mayer and Armstrong, 2004). These are the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, N-methyl-D-aspartate (NMDA) receptors, and kainate (KA) receptors. However, it is important to note that AMPARs are responsive to kainate.
The pharmacologically isolated families of receptors were subsequently found to be encoded by distinct gene families. AMPARs comprise four subunits named GluR1 to GluR4 (also called GluRA–GluRD). The GluR1 subunit was first cloned after screening an expression library (Hollmann et al., 1989), and subsequent cDNA homology screens revealed the remaining GluR2, GluR3, and GluR4 homologs (Boulter et al., 1990; Keinanen et al., 1990; Nakanishi et al., 1990; Sakimura et al., 1990).
B. AMPA Receptor Topology
The schematic topology of an AMPAR subunit is illustrated in Fig. 1. This structure has been based largely on biochemical investigations as well as homology between the AMPARs and prokaryotic amino acid receptors (reviewed in Paas, 1998). The molecular architecture of each AMPAR subunit (GluR1–4) is very similar; each comprises ~900 amino acids and has a molecular weight of ~105 kDa (Rogers et al., 1991). There is approximately 70% sequence homology between genes encoding each subunit, although genes may undergo alternative splicing in two distinct regions, resulting in subunits that have either long or short C termini, and flip or flop variants in an extracellular domain (for review, see Black and Grabowski, 2003).
FIG. 1.
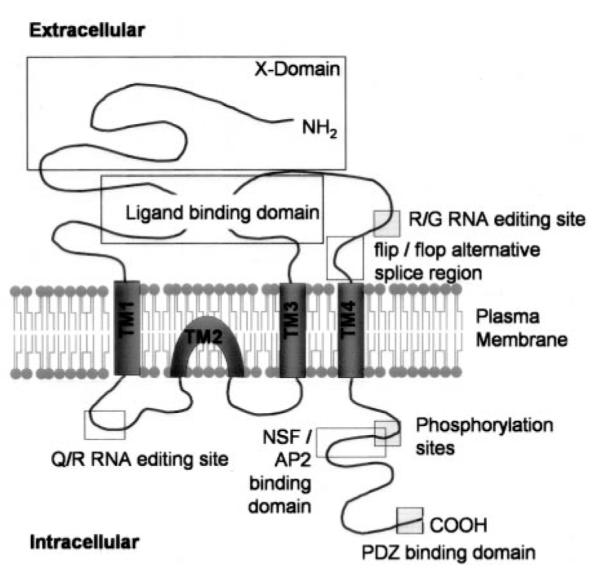
Schematic showing the topology of an AMPA receptor subunit. Each subunit consists of an extracellular N-terminal domain, four hydrophobic regions (TM1–4), and an intracellular C-terminal domain. The ligand-binding site is a conserved amino acid pocket formed from a conformational association between the N terminus and the loop linking TM3 and TM4. A flip/flop alternative splice region and R/G RNA editing site are also present within the TM3/TM4 loop. TM2 forms an intracellular re-entrant hairpin loop which contributes to the cation pore channel and is also the site for Q/R RNA editing in the GluR2 subunit. The intracellular C terminus contains phosphorylation sites and conserved sequences that have been shown to interact with a number of intracellular proteins, for example, PDZ domain-containing proteins and the ATPase NSF.
1. The N Terminus
Each subunit includes an extracellular N terminus, four hydrophobic domains (TM1–4), and an intracellular C terminus (Fig. 1). In eukaryotes, the N terminus contains the N-terminal domain of ~400 amino acids and a ~150 amino acid ligand-binding core. The N-terminal domain is also known as the X-domain because of its unknown function (Kuusinen et al., 1999). Suggestions for X-domain function include receptor assembly, allosteric modulation of the ion channel, and binding of a second ligand. The X-domains of GluR4 form dimers in solution (Kuusinen et al., 1999) and confer specificity for AMPARs, as opposed to KARs upon coassembly with other subunits (Leuschner and Hoch, 1999). However, deletion of the entire X-domain of GluR4 did not alter the function of homomers expressed in human embryonic kidney 293 cells, indicating that it is not involved in homomeric assembly of this subunit (Pasternack et al., 2002). The structure of this part of AMPARs is suggestive of a ligand-binding site, but no endogenous ligands have been found to bind here, although Zn2+ modulates at a similar site on NMDARs (Mayer and Armstrong, 2004). Intriguingly, the N terminus of GluR2 is involved in dendritic spine morphogenesis, perhaps through a receptor-ligand complex (Passafaro et al., 2003).
The ligand-binding core of AMPARs confers pharmacological specificity to the receptors; indeed, swapping the domains of AMPA and KARs swapped both their affinity for the ligand and desensitization properties and proved that this is the glutamate binding site (Stern-Bach et al., 1994, 1998). The structures of the ligand binding cores of GluR2 and GluR4 have been studied intensively (Jayaraman et al., 2000; Kubo and Ito, 2004; McFeeters and Oswald, 2004), and this is the only part of any AMPAR to be crystallized so far (Armstrong et al., 1998). For GluR2, the ligand-binding core has been crystallized with various pharmacological agents (Johansen et al., 2003). This approach gives an insight into the mode of action of some AMPAR agonists and antagonists and promises to be a valuable tool for the rational design of future drugs (reviewed in Stensbol et al., 2002; Mayer and Armstrong, 2004).
2. Hydrophobic Regions
The transmembrane orientation of the AMPAR subunits was initially elucidated by the use of specific antibodies, N-glycosylation pattern, and proteolytic sites (Molnar et al., 1994; Wo et al., 1995). Together, these studies demonstrated that the mature N terminus is expressed on the exterior surface of the neuron (Hollmann et al., 1994; Bennett and Dingledine, 1995; Seal et al., 1995), and subsequent work showed that the TM1, TM3, and TM4 regions are all transmembrane spanning domains, whereas TM2 forms a hairpin loop on the intracellular side of the cell membrane (see also Wo and Oswald, 1994) (Fig. 1). Similar to K+ channels, the re-entrant loop contributes to the cation pore channel (Kuner et al., 2003), although the specificity of AMPARs differ in that they gate Na+ and Ca2+ in preference to K+, perhaps because of a comparatively larger pore size (Tikhonov et al., 2002).
3. The Intracellular C Terminus
The intracellular C terminus of eukaryotic AMPARs has been shown to be the interaction site for a range of different proteins, many of which are involved in the receptor trafficking (reviewed in Henley, 2003) and synaptic plasticity (reviewed in Malenka, 2003; Sheng and Hyoung Lee, 2003). The functions of AMPAR interactors are under intense scrutiny and will be discussed in greater detail below.
C. Pharmacology
AMPARs gate Na+ and Ca2+ in response to ligand binding, with conductance and kinetic properties of the receptor depending on the subunit composition (Mat Jais et al., 1984; Hollmann et al., 1991; Jonas, 1993). This influx of ions causes a fast excitatory postsynaptic response, and the Ca2+ component can also activate second messenger pathways (Michaelis, 1998), including many protein kinases (Wang et al., 2004); indeed, AMPARs have been reported to have metabotropic as well as ionotropic properties (Wang and Durkin, 1995; Wang et al., 1997). For researchers investigating the function of AMPARs in in vitro preparations, it may also be interesting to note that AMPARs may be potentiated by serum factors (Nishizaki et al., 1997).
There are many drugs available that act on AMPARs, and listing them is beyond the scope of this review (but see Stensbol et al., 2002; Stone and Addae, 2002; Weiser, 2002; McFeeters and Oswald, 2004; O’Neill et al., 2004; Stromgaard and Mellor, 2004).
D. Post-Transcriptional Modification
1. Splice Variants
All four AMPAR subunits undergo alternative splicing in an extracellular region N-terminal to the fourth transmembrane domain to give “flip” and “flop” splice variants (Sommer et al., 1990) (see Fig. 1). This modifies the channel’s kinetic and pharmacological properties with flip splice variants desensitizing four times slower than flop (Mosbacher et al., 1994; Koike et al., 2000) and confers different sensitivity to allosteric modulators cyclothiazide (Partin et al., 1994; Kessler et al., 2000), 4-[2-(phenylsulfonylamin-o)ethylthio]-2,6-difluoro-phenoxyacetamide (Sekiguchi et al., 1997, 1998), zinc (Shen and Yang, 1999), and lithium (Karkanias and Papke, 1999), although affinity to AMPA is unchanged (Arvola and Keinanen, 1996). Expression levels of the different splice variants is region and cell-type specific (Sommer et al., 1990; Fleck et al., 1996; Lambolez et al., 1996) and developmentally regulated, for example, in the cerebellum (Mosbacher et al., 1994), as well as modified by physiological insults (Zhou et al., 2001b), lesions (Pires et al., 2000), and disease (Seifert et al., 2002, 2004; Tomiyama et al., 2002). This means that the number of permutations of AMPARs is very large giving a potential to fine-tune the kinetic properties of the channel.
Further to the flip and flop splice variants, GluR1, −2, and −4 can also undergo alternative splicing in the C terminus to give “long” isoforms (Gallo et al., 1992; Kohler et al., 1994); however the “short” isoform of GluR1 has not been reported. The short isoform of GluR2 is the most abundant, accounting for over 90% of total GluR2 (Kohler et al., 1994), and the long form of GluR4 is predominant (Gallo et al., 1992). GluR3 has a short C terminus due to a lack of splice sites in the C terminus. The alternative splice variants are able to bind different interacting proteins because the PDZ binding motif is only present in the short form (Dev et al., 1999). In consequence, much of the work in the field of AMPAR interactors has focused on the short form of GluR2.
2. RNA Editing
The genomic DNA of the GluR2 subunit of AMPARs contains a glutamine (Q) residue at amino acid 607. However, the vast majority of neuronal cDNA contains an arginine (R) at this position and occurs via a process of nuclear RNA editing (Sommer et al., 1991; Cha et al., 1994; Seeburg et al., 1998; Seeburg and Hartner, 2003). The edited residue is found in the channel-forming segment of the receptor, and the amount and presence of the edited subunit alters the kinetics and divalent ion permeability of the resulting receptors (Burnashev et al., 1992, 1995; Geiger et al., 1995), although it is not linked to receptor desensitization (Thalhammer et al., 1999). GluR2(R)-containing AMPARs have a low permeability to Ca2+ and low single-channel conductance because of the size and charge of the amino acid side chain in the edited form (Burnashev et al., 1992, 1996; Swanson et al., 1997). Nonetheless, GluR2(R)-containing AMPARs can still participate in intracellular Ca2+ signaling (Utz and Verdoorn, 1997) and can be trafficked in a Ca2+-dependent way (Liu and Cull-Candy, 2000). Furthermore, editing at this position has been shown to regulate endoplasmic reticulum retention (Greger et al., 2002) and tetramerization of AMPARs (Greger et al., 2003). Edited and unedited forms of the receptor have different sensitivity to the pharmacological agents Joro Spider toxin and adamantine derivatives (Meucci et al., 1996; Magazanik et al., 1997; McBain, 1998), and editing at this position is developmentally, region- and cell-specifically regulated (Lerma et al., 1994; Nutt and Kamboj, 1994). Indeed, changes in the levels of editing occur during differentiation of neurons and glia (Meucci et al., 1996; Lai et al., 1997).
Because of the link between calcium influx and excitotoxicity, changes in the amount of edited GluR2 have been implicated in a number of diseases including schizophrenia, Huntington’s disease, Alzheimer’s disease (Akbarian et al., 1995), epilepsy (Brusa et al., 1995), and malignant glioma (Maas et al., 2001) with most research being focused on amyotropic lateral sclerosis (Takuma et al., 1999; Kawahara et al., 2003b, 2004), although the process is not involved in ischemia (Kamphuis et al., 1995; Paschen et al., 1996; Rump et al., 1996; but see Tanaka et al., 2000). Editing at this position is not essential for brain development (Kask et al., 1998), but mice have neurological deficits when editing does not take place (Feldmeyer et al., 1999).
Further, to the Q/R site of GluR2, GluR2, −3, and −4 may be edited at another site, the R/G site, in a region that immediately precedes the flip/flop splice module in the N terminus of the molecule (Lomeli et al., 1994; Fig. 1). This modification changes the desensitization and resensitization of the resulting AMPAR (Lomeli et al., 1994; Krampfl et al., 2002) and may be involved in epilepsy (Vollmar et al., 2004) and, in contrast to the Q/R site, ischemia (Yamaguchi et al., 1999).
The RNA-dependent adenosine deaminase 2 carries out RNA editing of AMPARs (Higuchi et al., 2000; Ohman et al., 2000), and like the edited and unedited forms of AMPAR subunits, expression levels of the enzyme are developmentally and regionally regulated. Indeed, it has been shown that RNA-dependent adenosine deaminase 2 abundance regulates levels of edited GluR2 (Kawahara et al., 2003a).
E. Post-Translational Modification
1. Glycosylation
All AMPARs have sites for asparagine (N)-linked glycosylation (Hullebroeck and Hampson, 1992; Breese and Leonard, 1993; Keinanen et al., 1994) in the extracellular domains of the protein, with two conserved sites in the S1 domain that forms part of the ligand-binding domain (Arvola and Keinanen, 1996; reviewed in Standley and Baudry, 2000; Pasternack et al., 2003). The functional consequence of the addition of these oligosaccharides is not clear. Inhibition of glycosylation with tunicamycin was found to prevent functional expression of recombinant AMPARs (Musshoff et al., 1992; Kawamoto et al., 1994), but later the drug itself was reported to inhibit AMPARs regardless of glycosylation state (Maruo et al., 2003). Studies of recombinantly expressed S1-S2 domain fusion proteins show that this form of post-translational modification does not affect the ligand-binding site (Arvola and Keinanen, 1996; Pasternack et al., 2003), however, the desensitizing lectin concanavalin A potentiates AMPAR currents by binding to these carbohydrates, with GluR2 remaining unaffected (Everts et al., 1997). This suggests that glycosylation of different AMPAR subunits may have different functional effects. It appears that only surface and synaptically expressed AMPARs possess the mature glycosylated form (Hall et al., 1997; Standley et al., 1998), and the exact nature of the oligosaccharides involved has been identified (Clark et al., 1998). It is likely that this protein modification is involved in the maturation and transport of the receptor or could protect AMPARs from proteolytic degradation. Indeed, the aberrant glycosylation of GluR3 can lead to proteolysis and release of an autoimmunogen leading to Rasmussen’s encephalitis (Gahring et al., 2001).
2. Phosphorylation
Phosphorylation of ligand-gated ion channels can regulate the properties of the channel, its intermolecular interactions, and trafficking of the protein (reviewed in Swope et al., 1999). The regulation of AMPAR phosphorylation adds a further complex level of receptor modulation beyond subunit composition, splice variants, and other post-translational modifications (Carvalho et al., 2000; Gomes et al., 2003). Phosphorylation of receptor subunits can occur basally or in response to particular types of synaptic activity, and each AMPAR subunit has its own calcium and kinase profile (Wyneken et al., 1997). In particular, the role of GluR1 phosphorylation in synaptic plasticity has been studied in detail (reviewed in Roche et al., 1994; see Yakel et al., 1995). However, some phosphorylation sites are thought to be shared by all AMPARs: a residue between 620 and 638 of GluR1 and the equivalent sites in all other AMPARs is phosphorylated in vitro by CaMKII leading to enhanced responses in synaptic plasticity (Yakel et al., 1995), and although most research has centered on the role of CaMKII, it is possible that CaMKIV may play a similar role (Kasahara et al., 2000). There also appears to be a general role for developmental regulation of AMPAR properties by phosphorylation (Shaw and Lanius, 1992; Li et al., 2003). Furthermore, a GluR2 Ser 696 phosphospecific antibody helped demonstrate phosphorylation of this and analogous sites in all other AMPARs in response to agonist by PKC (Nakazawa et al., 1995a,b). This phosphorylation is thought to underlie long-term desensitization.
In addition to modulation of AMPAR phosphorylation in response to glutamatergic synaptic activity, PKA phosphorylation of AMPARs, primarily GluR1, is enhanced by D1 dopamine receptor activation (Price et al., 1999; Snyder et al., 2000; Chao et al., 2002) and may play a role in Parkinson’s disease (Chase et al., 2000; Oh et al., 2003). Indeed, dopamine receptors may influence synaptic plasticity of AMPARs (Wolf et al., 2003) by increasing PKA-mediated AMPAR insertion (Mangiavacchi and Wolf, 2004). Serotonin hydroxytryptamine 1A receptors can, on the other hand, inhibit CaMKII phosphorylation of AMPARs (Cai et al., 2002b).
a. GluR1
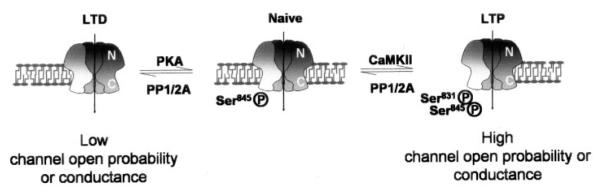
GluR1 is phosphorylated at multiple sites on the C terminus. PKC and CaMKII phosphorylate Ser 831 (Roche et al., 1996; Mammen et al., 1997), whereas PKA phosphorylates Ser 845 (Mammen et al., 1997). Differential phosphorylation of both sites occurs according to activity (Blackstone et al., 1994) leading to changes in synaptic efficacy (Fig. 2) and phosphorylation by PKA and PKC may play a role in nociception (Fang et al., 2003a,b; Nagy et al., 2004). Furthermore, phosphorylation at both PKA and CaMKII sites controls synaptic incorporation of these receptors (Esteban et al., 2003) and may be enhanced by the constitutive activity of phospholipase A2 (Menard et al., 2005).
FIG. 2.
Schematic illustrating that phosphorylation of the GluR1 AMPA receptor subunit may act as a bidirectional switch in synaptic plasticity.
AMPARs present in the postsynaptic density are phosphorylated by CamKII, and there is evidence that the PKA site is occluded in this location (Figurov et al., 1993; Vinade and Dosemeci, 2000). CaMKII phosphorylation plays a role in synaptic unsilencing (Liao et al., 2001) and enhancement (Figurov et al., 1993; Nishizaki and Matsumura, 2002), ischemia (Takagi et al., 2003; Fu et al., 2004), inflammation (Guan et al., 2004), and LTP (Hayashi et al., 1997; of depressed synapses: Strack et al., 1997; Lee et al., 2000) perhaps by increasing the AMPAR-mediated current (Derkach, 2003; Vinade and Dosemeci, 2000) and/or by modulating an interaction between GluR1 and a PDZ-containing protein (Hayashi et al., 2000). Interestingly, amyloid β protein prevents the activation of CaMKII and AMPAR phosphorylation during LTP (Zhao et al., 2004). Dephosphorylation of this site by protein phosphatase 1 leads to depotentiation (Lee et al., 2000; Vinade and Dosemeci, 2000; Huang et al., 2001).
PKA phosphorylation leads to potentiation of homomeric peak current (Roche et al., 1996; Vinade and Dosemeci, 2000) by increasing the peak open probability (Banke et al., 2000) and leads to LTP in naive synapses (Lee et al., 2000). Dephosphorylation occurs via a calcineurin-mediated pathway (Snyder et al., 2003) and is a feature of NMDA-dependent LTD expression at naive synapses (Kameyama et al., 1998; Ehlers, 2000; Lee et al., 2000; Launey et al., 2004). The interacting proteins SAP-97 and A-kinase-anchoring protein 79 are thought to direct basal PKA phosphorylation of GluR1 and calcium-dependent dephosphorylation at this site (Colledge et al., 2000; Lisman and Zhabotinsky, 2001; Tavalin et al., 2002).
It is clear that the effects of GluR1 (de)phosphorylation at the major CaMKII and PKA sites on synaptic plasticity depends on the history of the synapse (Lee et al., 2000), and it is becoming increasingly evident that these kinases and phosphatases play important roles in development, synaptic plasticity, learning, and memory (Vianna et al., 2000; Genoux et al., 2002; D’Alcantara et al., 2003; Lee et al., 2003a; Lu et al., 2003), although a role for GluR1 dephosphorylation in LTD maintenance rather than induction has been postulated (Brown et al., 2005). See Fig. 2 for a model of how the phosphorylation state of GluR1 relates to the plastic state of the synapse. GluR1 phosphorylation by the Src family tyrosine kinase Fyn also occurs in vitro and protects the receptors from calpain digestion which could modulate trafficking events or channel properties (Rong et al., 2001).
b. GluR2
GluR2 is phosphorylated by PKC at Ser 880 in the PDZ binding site. This differentially regulates binding of AMPAR binding protein (ABP)/glutamate receptor-interacting protein 1 (GRIP1) and protein interacting with C kinase (PICK)1, with a decrease in ABP/GRIP binding but not PICK1 in response to phosphorylation (Matsuda et al., 1999; Seidenman et al., 2003). Furthermore, ABP binding to GluR2 prevents this phosphorylation (Fu et al., 2003). Phosphorylated GluR2 has been shown to recruit PICK1 to synapses apparently causing the release of the GluR2-PICK1 complex from synapses facilitating internalization (Chung et al., 2000; Seidenman et al., 2003) and LTD (Matsuda et al., 2000; Xia et al., 2000; Kim et al., 2001; Chung et al., 2003). Interestingly, cerebellar LTD has also been shown to involve a mitogen-activated protein kinase step that converts transiently phosphorylated AMPARs to stably phosphorylated ones (Kuroda et al., 2001), and LTD-inducing protocols lead to tyrosine phosphorylation of GluR2 and, consequently, endocytosis of the receptors (Ahmadian et al., 2004). Indeed, phosphorylation of Tyr 876 near the C terminus of the receptor by a src family tyrosine kinase has a similar effect on GRIP1 and PICK1 binding to PKC phosphorylation of Ser 880, although this phosphorylation is involved in AMPA- and NMDA-induced internalization of these receptors (Hayashi and Huganir, 2004). Furthermore, potential PKC phosphorylation sites have also been identified in the C terminus of GluR2, including Ser 863 (Hirai et al., 2000; McDonald et al., 2001).
c. GluR3
There has been no systematic study into the regulation of GluR3 subunit phosphorylation.
d. GluR4
Recombinant homomeric GluR4 is the most rapidly desensitizating of the AMPARs, a property that may be regulated by (de)phosphorylation. This subunit is phosphorylated by PKC in response to activation of the kinase/receptor (Carvalho et al., 2002). PKC directly interacts with the membrane-proximal C terminus of the receptor, phosphorylating at Ser 482, and leading to increased surface expression of recombinant receptors (Correia et al., 2003; Esteban et al., 2003), and Thr 830 is also a potential PKC site (Carvalho et al., 1999). Ser 842 can be phosphorylated by PKA which modulates surface expression of the receptor (Gomes et al., 2004).
F. Expression Patterns
1. Regional Distribution in the Brain
In situ hybridization studies (Keinanen et al., 1990; Pellegrini-Giampietro et al., 1991), receptor autoradiography using [3H]AMPA and [3H]glutamate (Monaghan et al., 1984), and immunocytochemistry using antibodies directed against individual AMPAR subunits (Petralia and Wenthold, 1992; Martin et al., 1993) demonstrated the widespread and varied distribution of AMPARs in the brain (reviewed in Hollmann and Heinemann, 1994). The hippocampus, outer layers of the cortex, olfactory regions, lateral septum, basal ganglia. and amygdala of the CNS are enriched in GluR1, GluR2, and GluR3 (Keinanen et al., 1990; Beneyto and Meador-Woodruff, 2004). In contrast, GluR4 mRNA and immunolabeling is low to moderate throughout the rat CNS, except in the reticular thalamic nuclei and the cerebellum where levels are high (Petralia and Wenthold, 1992; Martin et al., 1993; Spreafico et al., 1994).
2. Neuronal and Glial Expression
Interestingly, AMPARs have also been found on glial cells (Gallo and Russell, 1995; Garcia-Barcina and Matute, 1998; Janssens and Lesage, 2001), where they appear to be involved in excitotoxicity (Yoshioka et al., 1996; Park et al., 2003) and ischemia pathology (Gottlieb and Matute, 1997; Meng et al., 1997). Activation of these receptors on some glia can lead to the release of ATP or nitric oxide, which may act as autocrine or paracrine messengers (Queiroz et al., 1999; Comoletti et al., 2001) and can affect glial morphology (Ishiuchi et al., 2001). It has recently been demonstrated that mouse hippocampal astrocytes may be categorized into AMPAR-expressing cells or glutamate transporter cells, which adds yet another layer of complexity (Wallraff et al., 2004). Glia-glia coupling (Muller et al., 1996) and neuron-glia signaling is an emerging area of interest in this field, with work centering on the Bergmann glia of the cerebellum (Mennerick et al., 1996; Clark and Barbour, 1997; Iino et al., 2001; Dziedzic et al., 2003; Millan et al., 2004). Indeed, it appears to be possible to induce a form of LTP in cerebellar glia by stimulating neighboring neurons (Linden, 1997), and a form of epilepsy appears to be coupled to a change in AMPAR splice variant expression in hippocampal glia (Seifert et al., 2002). It has been proposed that neuron-glia signaling in the hypothalamus may also control sexual development (Dziedzic et al., 2003).
3. Developmental Regulation
AMPAR mRNA can be detected at very early stages of development. In embryonic rat brain, GluR2 mRNA is near ubiquitous, with GluR1, GluR3, and GluR4 more differentially expressed (Monyer et al., 1991). GluR1 protein was found in rat brain as early as E15.5 and GluR4 at E11 in mouse brain (Durand and Zukin, 1993; Martin et al., 1998). Later in development, studies on postnatal tissue have suggested that expression levels of GluR1–4 increase gradually, concurrent with synapse development, and appear to peak in the third postnatal week (Insel et al., 1990; Pellegrini-Giampietro et al., 1991; Durand and Zukin, 1993; Standley et al., 1995; Arai et al., 1997; Martin et al., 1998). However, immunoreactivity to GluR4 in rat brain was not apparent until P14, after which its levels of immunoreactivity increased gradually until adulthood (Hall and Bahr, 1994). Developmental changes in the expression levels of AMPARs in hippocampal organotypic slice cultures (Fabian-Fine et al., 2000) and living cultured hippocampal neurons have also been reported (Pickard et al., 2000; Molnar et al., 2002), concluding that maturation of synapses may be retarded in vitro. AMPAR expression is also developmentally regulated in the spinal cord (Jakowec et al., 1995; Kalb and Fox, 1997), the visual system (Silveira dos Santos Bredariol and Hamassaki-Britto, 2001; Batista et al., 2002; Hack et al., 2002), and the auditory system (Sugden et al., 2002). During neonatal development, AMPAR incorporation into the plasma membrane occurs prior to synaptogenesis when GluR1-containing AMPARs cluster at potential postsynaptic sites (Martin et al., 1998). As well as developmental regulation of receptor subunit expression, splicing of AMPARs changes during development (Monyer et al., 1991; Tonnes et al., 1999), as can the kinetics of channel opening (Koike-Tani et al., 2005; Wall et al., 2002). The Ca2+-permeability of some glia also appears to be developmentally regulated (Backus and Berger, 1995).
4. Subcellular Expression Patterns
Subcellular fractionation experiments have indicated an enrichment of AMPARs in both synaptic membrane and postsynaptic density preparations (Rogers et al., 1991; Blackstone et al., 1992; Archibald and Henley, 1997). For a diagram of the morphology of an excitatory synapse at the electron-micrograph level, please see Fig. 3. Microscopy confirmed these findings in many brain areas (Petralia and Wenthold, 1992; Craig et al., 1993; Baude et al., 1994; Spreafico et al., 1994; Petralia et al., 1997). However, some AMPARs have also been detected extrasynaptically and within the cytoplasm of individual neurons (Baude et al., 1994, 1995). Consistent with this observation, a large number of intracellular AMPARs have been identified by biochemical studies (Barnes and Henley, 1993; Hall and Soderling, 1997; Hall et al., 1997; Lee et al., 2001). Overall, these studies suggest 60 to 70% of the total AMPAR population is intracellular. As discussed in more detail below, it has been speculated that this intracellular pool of AMPARs may play a role in synaptic plasticity and development via the “silent synapse” hypothesis.
FIG. 3.
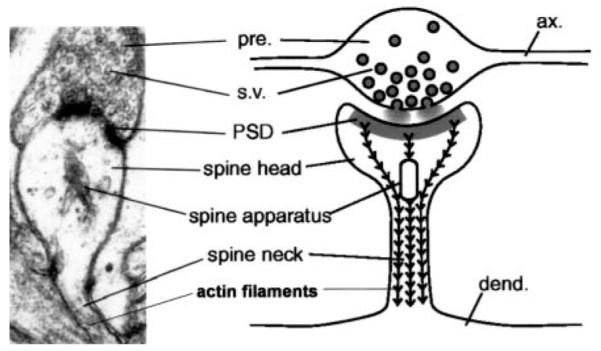
Electron micrograph and corresponding diagram depicting a single excitatory synapse. The electron dense PSD directly opposes the neurotransmitter release sites located on the presynaptic bouton and the spine neck and spine apparatus are clearly defined (reproduced with permission from Fischer et al., 2000).
G. Plasma Membrane Distribution of AMPA Receptors
1. Postsynaptic Membrane
It is well established that AMPARs reside in the postsynaptic compartment and that their appropriate targeting and clustering in this region is critical for the formation and maintenance of excitatory synapses. The mechanisms of receptor insertion and removal at the postsynaptic membrane and of incorporation and maintenance in functional clusters within the postsynaptic density have been the topics of intensive research (Nusser, 2000; Savtchenko et al., 2000; Sheng, 2001; Franks et al., 2003). The interactions between AMPARs and many accessory proteins are of critical importance in these trafficking steps, and insertion and removal of synaptic AMPARs plays an important role in synaptic plasticity (Lu et al., 2001a). These interactors will be discussed later in the review.
GluR2/3 and GluR4 subunits colocalize throughout the postsynaptic density in the rat organ of Corti, with higher concentrations of receptors located around the periphery of the PSD. These subunits were not detected at extrasynaptic membranes, but some GluR4 subunits appeared to be presynaptic (Matsubara et al., 1996). This suggests that AMPARs are inserted into the postsynaptic membrane in a very precise manner and that receptor density increases upon moving away from the center of the synapse. In the rat hippocampus, it was estimated that there were 3 to 140 individual AMPAR present at synapses on CA3 pyramidal spines (Nusser et al., 1998). However, more recent investigations into the relationship between spine morphology and AMPAR distribution suggest that different types of spines contain differing amounts of AMPARs (Matsuzaki et al., 2001). In addition to the differential expression of AMPAR subunits within and between brain regions (McBain and Dingledine, 1993; Hack et al., 2001), AMPARs of different composition may be targeted to different synapses within a single cell (Rubio and Wenthold, 1997) or the distribution of AMPARs inside a neuron may be heterogeneous (Andrasfalvy and Magee, 2001).
It has been shown that the abundance of postsynaptic AMPARs correlates with both the size of the synapse and the dimensions of the dendritic spine head (Matsuzaki et al., 2004). These findings suggest that silent Schaffer collateral-commissural (SCC) synapses (see below) are smaller than the majority of SCC synapses at which AMPA and NMDARs are colocalized and that synapse size may determine important properties of SCC synapses (Takumi et al., 1999). Furthermore, NMDAR activation has been reported to cause the formation of new spines as well as synaptic delivery of AMPARs and perhaps is involved in the mechanism of LTP (Shi et al., 1999). Recently, it has been reported that the N-terminal domain of GluR2 increases spine size and density in hippocampal neurons suggesting that, in addition to being involved in rapid neurotransmission, GluR2 is important for spine growth and/or stability (Passafaro et al., 2003).
2. Extrasynaptic AMPA Receptors
Electrophysiological experiments indicate that AMPARs are widely distributed throughout the cell surface plasma membrane. However, antibody surface labeling of neurons indicates that surface-expressed AMPARs do not have a homogenous distribution. Typically, distinct immunopositive areas are observed that are thought to correspond to synaptic puncta. This discrepancy may be explained if nonsynaptic AMPARs are present at an insufficient density to be detected efficiently by immunocytochemistry.
Recent studies have focused on the mechanisms of receptor recruitment to the plasma membrane, incorporation of receptors into the synapse, and their clustering, both during synaptogenesis and synaptic plasticity (for example, see Cottrell et al., 2000; Andrasfalvy and Magee, 2004). This will be discussed in more detail in the trafficking sections below.
3. Presynaptic Terminal
The investigation into presynaptic AMPARs has not received much attention to date. Nonetheless, some years ago it was reported that AMPA increased glutamate release from rat hippocampal synaptosomes. This effect was shown to be related specifically to AMPARs and suggests that AMPARs are present on presynaptic terminals and that they may play a role in the regulation of neurotransmitter release (Barnes et al., 1994). It has also been shown that the movement of axonal filopodia is strongly inhibited by glutamate and requires the presence of axonal AMPA/ kainate glutamate receptors (Chang and De Camilli, 2001). Furthermore, functional GluR1 and GluR2 are expressed in axonal growth cones of hippocampal neurons (Martin et al., 1998; Hoshino et al., 2003), and a pool of presynaptic AMPAR subunits have also been isolated biochemically (Pinheiro et al., 2003; Schenk et al., 2003).
II. Trafficking of AMPA Receptors
A. Assembly
Each mature AMPAR is assembled from four individual subunits (Wu et al., 1996; Mano and Teichberg, 1998; Rosenmund et al., 1998; Safferling et al., 2001), although early work had indicated that the receptors may be pentamers (Ferrer-Montiel and Montal, 1996). Assembly is thought to occur through dimer pairing (Armstrong and Gouaux, 2000; Ayalon and Stern-Bach, 2001; Mansour et al., 2001; Robert et al., 2001). The early stages of assembly may be mediated by the proximal extracellular N-terminal domain of the subunits (Leuschner and Hoch, 1999; Ayalon and Stern-Bach, 2001; Horning and Mayer, 2004), but involvement of other regions, such as the membrane region and S2 portion, could also be necessary for formation of the mature tetrameric receptor (for differing viewpoints, see Wells et al., 2001; Pasternack et al., 2002).
Functional assembly of AMPAR subunits expressed in mammalian cells or Xenopus oocytes is selective, with no association with kainate or NMDAR subunits (Brose et al., 1994; Leuschner and Hoch, 1999), although coassembly with the orphan GluRδ2 with GluR1 and GluR2 has been reported (Kohda et al., 2003). The stoichiometry of the receptor complexes in these systems seems to be largely controlled by the expression levels of individual subunits, and it remains to be determined what processes govern assembly in neurons, especially since the type of complex can vary dramatically between synapses on a single neuron. Evidence that GluR2 is the preferred binding partner of GluR1 during heteromeric receptor assembly was uncovered by Mansour et al. (2001) in an elegant study involving physiological tagging of recombinant subunits and modeling of the results. They suggest that mature receptors are composed of a dimer of heteromers (i.e., that a GluR1/2 dimerizes with another GluR1/2), not a pair of homomers, and that they are arranged with identical subunits on opposite sides of the pore and not side by side, the stoichiometry and spatial arrangements of subunits affecting the phenotype. However, assembly of homomeric receptors is a stochastic process. Furthermore, recent studies in GluR2 knockout mice reported AMPAR complexes comprising abnormal heteromers of GluR1 and GluR3 as well as increased numbers of GluR1 and GluR3 homomers (Sans et al., 2003) with less efficient synaptic expression. This confirms that GluR2 is the preferred subunit partner in the assembly process and that it is important for synaptic expression of AMPARs.
There is considerable evidence that the subunit composition of functional AMPARs at specific synapses can change rapidly in response to synaptic activation (Shi et al., 2001; Liu and Cull-Candy, 2002; Lee et al., 2004), possibly due to targeted delivery of specific AMPAR complexes or subunit rearrangement of existing receptors in the spine. For example, it was observed that AMPARs containing only long C-terminal tails (i.e., GluR1) require plasticity-inducing synaptic activity for delivery to synapses, and those containing only short tails (i.e., GluR2/3) are constitutively expressed there (Ehrlich and Malinow, 2004). An attractive but as yet untested hypothesis is that binding of interacting proteins could favor the assembly of certain subunit combinations and prevent or disfavor others.
B. Visualizing AMPA Receptor Translocation
The isolation of green fluorescent protein (GFP) and its variants has enabled the experimenter to label cell structures and proteins for use in microscopy. For example, the morphology of neurons expressing GFP can be visualized using confocal microscopy, down to the shape and size of individual spines (Fig. 4). GFP-tagged AMPAR subunits may be expressed in neurons using a variety of techniques, and this approach has provided new insight into trafficking (Sheridan et al., 2002; Ashby et al., 2004), e.g., by viral transfer (Okada et al., 2001). For example, tetanic stimulation of hippocampal slice cultures causes the rapid NMDAR-dependent delivery of GFP-GluR1 into dendritic spines (Shi et al., 1999). GFP-labeled subunits have also been visualized in combination with electrophysiological tagging, where the channel rectification properties of recombinant AMPARs comprising specific subunits are altered by point mutations, e.g., GluR2 (R586Q)-GFP. Furthermore, expression of GFP-tagged AMPAR subunits in knockout mice has been used to “rescue” the wild-type phenotype, giving an insight into the cell biology of GluR1-containing heteromers (Mack et al., 2001). Using these approaches, it has been proposed that there are differential targeting mechanisms for AMPARs comprising either GluR1/GluR2 or GluR2/GluR3 subunit combinations (for example, see Hayashi et al., 2000). More specifically, GluR1/GluR2 receptors are added to synapses during plasticity via interactions between GluR1 and group I PDZ domain proteins, and CaMKII and LTP drive the synaptic expression of GluR1-containing AMPARs. In contrast, GluR2/GluR3 receptors replace existing synaptic receptors in a constitutive manner dependent on interactions between GluR2 with N-ethylmaleimide-sensitive factor (NSF) and group II PDZ domain proteins (Shi et al., 2001; Malinow and Malenka, 2002).
FIG. 4.
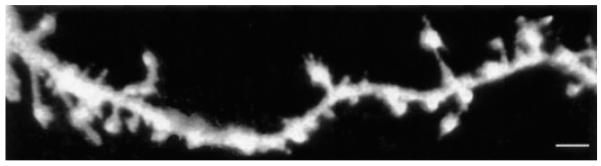
3D reconstruction of confocal image stack showing an area of spiny dendrite from a cultured pyramidal hippocampal neuron expressing a variant of GFP. The surface rendering of this reconstruction allows clear visualization of the varied structure of different spines (reproduced courtesy of Dr. M.C. Ashby, MRC Centre for Synaptic Plasticity, University of Bristol). Scale bar 4 μm.
Direct visualization of the intracellular transport of AMPARs using dispersed cultures of hippocampal neurons expressing GFP-GluR1 and GFP-GluR2 showed intracellular GFP-tagged AMPARs are widely distributed throughout the somatodendritic compartment (Perestenko and Henley, 2003). No evidence for intracellular clusters of receptors either in the soma or the dendrites was observed, suggesting the relatively free translocation of AMPARs in the soma and dendrites of neurons. AMPARs appear to be transported at rates comparable with fast axonal transport and move in a predominantly, but not exclusively, proximal to distal anterograde direction. Further data suggest that the intracellular transport of GFP-GluR1-containing AMPARs is not activity regulated but that various subunit combinations of the receptor complex are likely to be translocated throughout the soma and dendrites. Synapses may then “capture” these passing receptors as and when required (Perestenko and Henley, 2003). Furthermore, live imaging of GluR2 tagged with a pH-sensitive version of GFP that allows visualization of surface receptors only at the working (high) pH (pHlorin) demonstrated that extrasynaptic AMPARs containing the modified subunit react differently to the application of NMDA to synaptic receptors. The extrasynaptic receptors were internalized more quickly than those contained within the synapse (Ashby et al., 2004). This data confirms the model whereby extrasynaptic (or juxtasynaptic) receptors are more mobile than their synaptic counterparts, also demonstrated by particle tracking experiments (see below).
C. Lateral Diffusion of AMPA Receptors in the Membrane
Although the majority of interest in AMPAR dynamics has focused upon mechanisms of endo- and exocytosis, particularly with regard to plasticity, an increasing body of evidence suggests that diffusion within the postsynaptic membrane must account at least in part for some AMPAR movement (reviewed in Choquet and Triller, 2003). First, upon activation, AMPARs dissociate from their membrane anchors and diffuse away from the synapse prior to entering the constitutive endocytotic pathway, which is initiated either at the edge of the PSD or further afield in the extrasynaptic membrane (Zhou et al., 2001a). Second, studies involving stargazin suggest that the protein may recruit AMPARs from extrasynaptic to synaptic sites by binding synaptic PSD-95, then trapping freely diffusing AMPARs from outside the PSD (Chen et al., 2000; Schnell et al., 2002).
It is unsurprising that new research has centered on the kinetics of receptor movement during lateral diffusion in the neuronal plasma membrane. Interacting molecules outside, inside, and within the membrane can modulate receptor movement, and the subsynaptic cytoskeleton appears to be spatially organized into “corrals”, for example, rafts that limit diffusion of receptors within certain boundaries (Choquet and Triller, 2003).
Recently, innovative imaging methods involving single-particle tracking have permitted the study of the movement of a single AMPA receptor in the plasma membrane by the attachment of a specific fluorophore-conjugated antibody (Borgdorff and Choquet, 2002). GluR2-containing receptors appear confined to the regions surrounding the synapse, and their movement is controlled by basal neuronal activity. GluR2 diffusion ceased after influx of calcium, such as that observed after membrane depolarization, and the receptor appeared tethered. Increased Ca2+ levels may therefore change receptor binding to scaffolding proteins, stabilizing the receptor. This method was used to demonstrate that a proportion of AMPARs are able to exchange rapidly between synaptic and juxtasynaptic sites and that this diffusion is regulated (Tardin et al., 2003). This process may also be involved in synaptic plasticity mechanisms (Groc et al., 2004). While this study concentrated on the GluR2 subunit, tracking of other AMPAR species would be invaluable for establishing if differential movement took place in line with evidence gained from other studies (Passafaro et al., 2001; Shi et al., 2001).
D. AMPA Receptor Delivery to Synapses
The mechanisms by which AMPARs are brought to and inserted at the postsynaptic membrane represent a major research interest for many workers in the field. In particular, the activity-dependent components of this regulation which underlie changes in synaptic plasticity are an intensely studied area of neuroscience. In essence, there are two basic processes by which AMPARs could be delivered to the correct postsynaptic location: direct exocytosis of receptors to the site of action, or insertion into the membrane at a separate location with subsequent diffusion to the PSD. In fact, evidence to date suggests that both mechanisms can occur.
Single-particle tracking and video microscopy of the lateral membrane mobility of native AMPARs containing GluR2 in rat-cultured hippocampal neurons revealed that AMPARs alternate between rapid diffusive and stationary behavior. In older neurons, the stationary periods increased in frequency and length and were usually associated with synaptic sites. Increasing intracellular calcium causes rapid receptor immobilization and local accumulation on the surface, suggesting that calcium influx can inhibit AMPAR diffusion and that lateral receptor diffusion to and from synapses is important for the regulation of receptor numbers at synapses (Borgdorff and Choquet, 2002; Choquet and Triller, 2003). Using different methods, it has also been reported that surface insertion of GluR1 occurs slowly in basal conditions and is stimulated by NMDA receptor activation, whereas GluR2 exocytosis is constitutively rapid. In addition, GluR1 and GluR2 show different spatial patterns of surface accumulation, consistent with GluR1 being inserted initially at extrasynaptic sites and GluR2 inserted more directly at synapses (Passafaro et al., 2001; Gomes et al., 2003; Sheng and Hyoung Lee, 2003).
E. AMPA Receptor Turnover at Synapses
In the past, it was generally accepted that AMPARs within the postsynaptic membrane are relatively static, at least under basal conditions, with a constitutive turnover of surface-expressed receptors in the order of hours to days (Archibald et al., 1998; Huh and Wenthold, 1999). However, electrophysiological recordings predicted a more rapid emergence of AMPARs to the cell surface, such as that seen during the acquisition of AMPARs at silent synapses (Luscher et al., 1999; Kim and Lisman, 2001). Evidence that receptor internalization may also occur with equal rapidity was initially gathered during experiments involving blockade of the GluR2-NSF interaction (Nishimune et al., 1998; Song et al., 1998). However, as set out below, it was discovered that GluR2-containing AMPARs undergo rapid NSF-dependent cycles of internalization and reinsertion into the postsynaptic membrane with a half-life in the order of a few minutes. The principle of rapid NSF-dependent recycling has also been extended to G-protein-coupled receptors via β-arrestins (Miller and Lefkowitz, 2001) and GABAA receptors via GABA receptor-activating protein (Kittler et al., 2001), suggesting that this may be an important general regulatory synaptic mechanism.
The involvement of AMPAR interactors in receptor cycling has now been extensively studied (Ehlers, 2000; Osten et al., 2000; Ehrlich and Malinow, 2004; Lee et al., 2004; Nakagawa et al., 2004) and has had major implications for understanding the cellular processes underlying synaptic plasticity (for review, see Collingridge and Isaac, 2003; Collingridge et al., 2004; Lee et al., 2004). The application of glutamate to cultured hippocampal neurons causes a significant reduction in AMPARs, but not NMDARs from synaptic sites (Lissin et al., 1999), supporting the hypothesis that dynamic movement of AMPARs occurs in response to spontaneous synaptic activity. Subsequent work demonstrated that loss of AMPARs from synapses can also be promoted by NMDA, AMPA, insulin, or mGluR receptor activation (Carroll et al., 1999; Lin et al., 2000; Snyder et al., 2001; Ehlers, 2003; Lee et al., 2004).
Several studies have focused on identifying the molecular basis of AMPAR cycling at the postsynaptic membrane. Increasing synaptic activity has been shown to cause a decrease in the number of surface-expressed AMPARs and size of AMPAR clusters in cultured neurons (Lissin et al., 1999). The internalization of GluR2-containing AMPARs and subsequent targeting for lysosomal degradation can be triggered by NMDA receptor activation and is mediated through the formation of clathrin-coated pits (Carroll et al., 1999; Ehlers, 2000; Lee et al., 2004). Furthermore, GluR2-containing receptors are internalized and recycled in response to the application of AMPA (Lee et al., 2004), but probably only in a subset of synapses as miniature excitatory postsynaptic currents decrease in frequency but not size (Carroll et al., 1999; Lissin et al., 1999; Beattie et al., 2000). GluR2 appears to be the dominant subunit when deciding the fate of internalized receptors, with GluR3 homomers being constitutively targeted to lysosomes and GluR1 homomers being continually recycled, and the GluR2-NSF interaction is important for the correct targeting of these receptors (Lee et al., 2004). These findings are in contrast to those of Ehlers, perhaps because of the different use of tetrodotoxin in these investigations (Ehlers, 2000; Lin et al., 2000). Similar results have also been reported for the effects of AMPA and insulin stimuli on the fate of internalized receptors (Lin et al., 2000).
F. Trafficking, Learning, and Memory
Changes in surface-expressed receptor populations are mirrored in LTD. Consistent with the synaptic loss of strength observed electrophysiologically, LTD, in part, is caused by the NMDA receptor-induced internalization of synaptic AMPARs (Carroll et al., 1999; Beattie et al., 2000) and shares a common mechanism with clathrin-mediated endocytosis (Man et al., 2000; Wang and Linden, 2000). It has also been shown that the clathrin adaptor protein (AP)2 associates with GluR2 in a similar region to that of NSF and is required for NMDA-dependent LTD (Lee et al., 2002b).
Research has also focused upon the intracellular signaling cascades that trigger the removal of AMPARs from the surface. NMDA-dependent LTD appears to require the activation of a calcium-dependent protein phosphatase cascade involving calcineurin and protein phosphatase I (PPI) (Lisman, 1989; Mulkey et al., 1993, 1994). Specific inhibition of calcineurin appears to block NMDA-dependent internalization as well as that which is observed after application of AMPA or insulin (Beattie et al., 2000; Ehlers, 2000; Lin et al., 2000). The situation is less clear when PPI is inhibited. Endocytosis has been reported to be both blocked (Ehlers, 2000) and enhanced after pharmacological inhibition of PPI (Beattie et al., 2000; Lin et al., 2000), although different techniques were employed in each set of experiments. More recently, roles for the small GTPases Ras, Rap, and Rab5 have been reported for AMPAR trafficking and synaptic plasticity. Both Rap and Rab5 are thought to mediate the activity-dependent removal of AMPARs during LTD expression, with Rap removing short-tail subunit-containing AMPARs and Rab5 involved in the clathrin-dependent removal of GluR1- and GluR2-containing AMPARs from synapses (Zhu et al., 2002; Brown et al., 2005). Conversely, Ras has a role in activity-dependent long-tail AMPAR insertion and may play a role in LTP (Zhu et al., 2002). Future work will no doubt focus on linking the induction mechanisms of synaptic plasticity to the functional up- or down-regulation of the AMPAR number at synapses.
A cellular mechanism for learning and memory was initially hypothesized by Bliss and Lomo (1973) who demonstrated that, in the rabbit hippocampus, repeated high frequency electrical stimulation resulted in a strengthening of synaptic transmission in target cells. This “long-term potentiation” of transmission after such a short period of stimulation was expressed as an experimental phenomenon in which “the coincident activation of a pre and postsynaptic neuron resulted in a long-lasting increase in synaptic strength” (Bliss and Collingridge, 1993). It was hypothesized that this endurance of transmission could be the underlying mechanism of learning and memory. The transition from observing such occurrences to unraveling the cellular mechanisms beneath them has led to a huge field of investigation, much of which centers on the modulation of synaptic AMPARs.
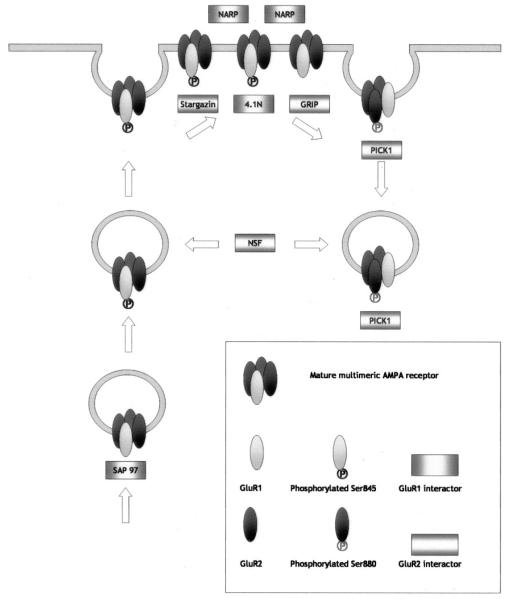
As already described, the synaptic expression of functional AMPARs is highly regulated both during development and by neuronal activity. Furthermore, an important characteristic of glutamatergic synapses is that they can exhibit NMDAR responses in the absence of functional AMPARs, and because of the magnesium block of NMDARs at resting membrane potentials, these postsynaptic membranes are functionally inactive or silent (Isaac et al., 1995; Liao et al., 1995). Silent synapses contain NMDARs but no AMPARs (Liao et al., 1999; Petralia et al., 1999; Pickard et al., 2000), and AMPARs can be recruited to these synapses within minutes by either spontaneous or stimulated NMDAR activation (Fitzjohn et al., 2001; Liao et al., 2001; Lu et al., 2001a; Pickard et al., 2001). The “unsilencing” of such synapses by the rapid insertion of functional AMPARs is likely to be a key determinant for NMDAR-dependent synaptic plasticity and neuronal development (Durand et al., 1996). The role of AMPAR trafficking in synaptic plasticity is a matter of intense investigation and has been reviewed extensively (Henley, 2003; Malenka, 2003; Sheng and Hyoung Lee, 2003). Aspects of current research are discussed elsewhere, since they relate to specific interacting proteins and phosphorylation events involved in exo- and endocytosis (Fig. 5).
FIG. 5.
Simplified schematic diagram illustrating the protein interactions at GluR1 and GluR2 containing AMPA receptors.
III. Interacting Proteins
The regulation of AMPAR surface expression is undoubtedly a complex process that requires multiple protein interactions (Fig. 5). Many different proteins interact selectively with individual AMPAR subunits and can be divided into PDZ domain and non-PDZ domain-containing proteins (Ziff, 1997; Henley, 2003). Furthermore, a comparative study in macaques into the expression patterns of AMPARs and their interaction partners has addressed whether some of these interactions are relevant in higher mammals (Srivastava et al., 1998; Srivastava and Ziff, 1999).
A. PDZ-Containing Proteins
PDZ domains are modular protein interaction motifs that bind in a sequence-specific fashion predominantly to short C-terminal peptides, but also to internal peptides that fold into a β-finger or to other PDZ domains (reviewed in Sheng and Sala, 2001; Hung and Sheng, 2002). The acronym PDZ is derived from the three proteins first identified as containing this motif, namely PSD-95/SAP90, the Drosophila discs large tumor suppressor gene Dlg-A, and an epithelial tight junction protein ZO-1 (Woods and Bryant, 1991; Cho et al., 1992; Itoh et al., 1993). PDZ domain-containing proteins are characteristically involved in the assembly of supramolecular structures that can perform specific signaling tasks at particular cellular locations, for example, the subcellular targeting of receptor subunit complexes to the cell surface (for reviews, see O’Brien et al., 1998; Garner et al., 2000). The motif has been found both as a single domain and in a repeated format in different proteins (Ponting and Phillips, 1995; Songyang et al., 1997). Evidence of variable sequences encoding for amino acids lining the peptide binding groove of the domain reflects the diverse nature of PDZ interactions and their functional roles (Bezprozvanny and Maximov, 2001).
1. PDZ Architecture
PDZ domains exhibit a similar architecture throughout the range of proteins within which they are found (Doyle et al., 1996). Each PDZ domain binds only one ligand. A carboxylate-binding loop with the conserved sequence R/K-XXX-GLGF (X = any residue) ensures that the terminal carboxylate group of the peptide ligand is orientated in the correct fashion along the binding groove. Specific selectivity of PDZ motifs for different binding peptides is thought to be dependent upon small changes in the size and geometry of the hydrophobic pocket.
PDZ domains may be classified according to their specificity for C-terminal peptides. Each class of PDZ domain has been identified as recognizing a specific consensus sequence of the last four amino acids of the C-terminal peptide. Class I PDZ domains recognize either the sequence X-S/T-X-L or X-S/T-X-V, for example, the C terminus of NMDAR2a by PSD-95 (exact sequence E-D-D-V; Doyle et al., 1996). Class II domains recognize the sequence X-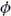 -X-
-X-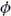 where X = unspecified amino acid and
where X = unspecified amino acid and 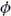 = hydrophobic amino acid. Within this class are the PDZ domain-containing proteins GRIP and PICK1 which have both been shown to interact with the S-V-K-I sequence at the extreme C terminus of GluR2 (Dong et al., 1997; Dev et al., 1999).
= hydrophobic amino acid. Within this class are the PDZ domain-containing proteins GRIP and PICK1 which have both been shown to interact with the S-V-K-I sequence at the extreme C terminus of GluR2 (Dong et al., 1997; Dev et al., 1999).
Brain-derived neurotrophic factor treatment leads to an increase in expression of PDZ-containing AMPAR interactors SAP97, GRIP1, and PICK1 (Jourdi et al., 2003), and the control of GRIP expression by neurotrophins may be involved in light adaptation (Cotrufo et al., 2003). Although AMPARs don’t bind PSD-95 directly, this PDZ-containing protein can drive GluR1-containing AMPARs into synapses and is involved in LTP (DeSouza et al., 2002). Truncation of the last 10 amino acids of GluR2 removes the PDZ ligand and reduces synaptic incorporation of the receptor (Dong et al., 1999; Osten et al., 2000).
2. AMPA Receptor Binding Protein/Glutamate Receptor-Interacting Protein
ABP is a relative of GRIP and both proteins bind to the C terminus of GluR2/3 via a PDZ domain (Dong et al., 1997). This binding site is also shared by PICK1, and the interaction is differentially modulated by PKC/tyrosine kinase phosphorylation of Ser 880/Ser 876 within the ligand (Seidenman et al., 2003). ABP and GRIP, like SAP-97, have multiple PDZ domains and are major anchoring proteins in the postsynaptic density. Furthermore, ABP and GRIP can form homo- and heteromultimers via their PDZ domains (Srivastava et al., 1998; Fu et al., 2003). Blocking the ABP/GRIP interaction with GluR2 in cultured neurons did not affect surface targeting or constitutive recycling of the receptor, but prevented surface accumulation of the receptor over time (Osten et al., 2000) and reduces the amount of receptor internalized after AMPA stimulation (Braithwaite et al., 2002).
ABP exists as two splice variants, one short (containing six PDZ domains and known as ABP-S) (Srivastava et al., 1998; Dong et al., 1999) and one long (containing seven PDZ domains and called ABP-L) (Wyszynski et al., 1999). The long splice variant is sometimes confusingly referred to as GRIP2 and can be palmitoylated (to give pABP-L), which facilitates membrane and GluR2 association in spines (DeSouza et al., 2002). Its expression pattern was found to parallel AMPARs, and immunoreactivity was detected in the postsynaptic density and at synaptic plasma membranes (Gabriel et al., 2002). Interestingly, the nonpalmitoylated version of ABP-L is intracellular (Fu et al., 2003), which points to different sites of AMPAR anchoring according to the expression levels of the splice variants. In addition to this, ABP present on intracellular membranes can bind and retain GluR2 that has been internalized and can also prevent phosphorylation of GluR2 Ser 880 to stabilize this interaction (Fu et al., 2003). Immunocytochemistry studies demonstrated that ABP is predominantly expressed by pyramidal neurons of the neocortex, where it colocalizes with two thirds of GluR2/3-containing puncta in spines (Burette et al., 2001). A protein called KIAA1719 could be the human homolog of ABP, and studies into its expression in macaque brain suggest that overlapping distribution with GluR2/3-containing AMPARs may be restricted to particular areas of the forebrain and cerebellum, e.g., CA3 and dentate gyrus of the hippocampus (Beneyto and Meador-Woodruff, 2004).
GRIP is predominantly expressed in GABAergic interneurons (Burette et al., 1999; Wyszynski et al., 1999), where ABP is expressed at lower levels and expression of these proteins is almost mutually exclusive in some synapses (Dong et al., 1999; Burette et al., 2001). Differential expression was also found in the retina (Gabriel et al., 2002). Furthermore, an interaction between GRIP and GABA receptor-activating protein implies a role for GRIP in GABAA receptor trafficking or stabilization (Kittler et al., 2004).
GRIP exists as a variety of splice variants to give long and short forms (Wyszynski et al., 1998). Full-length GRIP has seven PDZ domains, with the fourth and fifth domain being involved in GluR2/3 binding (Dong et al., 1997). Interestingly, two forms of GRIP, GRIP1-a and -b are thought to have different transcriptional start sites resulting in potential differential palmitoylation of the protein and hence membrane and protein interactions (Yamazaki et al., 2001). GRIP1b, the version that can be palmitoylated in heterologous cells, appears to be the dominant isoform in the forebrain and cerebellum and is soley associated with membranes. Confusingly, another GRIP splice variant, also called GRIP1-b and DLX-interacting protein, has been isolated as a truncated form of GRIP. This protein retains the PDZ domains necessary for AMPAR interaction, although one of these is required for its function as a transcriptional coactivator (Yu et al., 2001). Interestingly, this function can be blocked by activation of GluR2. Another isoform of GRIP, called GRIP1τ has also been isolated and acts as a testis-specific transcription factor (Nakata et al., 2004). GRIP1-c is a short isoform of GRIP containing only four PDZ domains, which can interact with GluR2/3 and is found concentrated in both GABAergic and glutamatergic synapses, although this protein can also be detected presynaptically (Charych et al., 2004).
GRIP protein is widely expressed in the brain throughout development and can be detected early in embryonic development, before the appearance of AMPARs (Dong et al., 1999). This is in contrast to the expression pattern of ABP, which parallels AMPARs (Dong et al., 1999). Indeed, Northern blotting demonstrated that mouse GRIP is down-regulated early in postnatal development (Wyszynski et al., 1998). GRIP is enriched in postsynaptic densities and is mostly associated with membranes throughout the neuron (Wyszynski et al., 1998), the intracellular version of the protein being associated with the rough endoplasmic reticulum, the Golgi apparatus and recycling endosomes (Burette et al., 1999; Lee et al., 2001).
GRIP is a substrate for the Ca2+-dependent protease calpain, and activation of this enzyme disrupts the GluR2-GRIP interaction. Given that both LTP and LTD have calcium dependencies, activation of calpain and the subsequent effects on this interaction could play a role in these phenomena (Lu et al., 2001b).
Consistent with its role as a scaffolding protein, GRIP itself can interact with many other proteins. GRIP binding to Shank 2 could link AMPARs to mGluRs in the cerebellum (Uemura et al., 2004), although little colocalization was detected in retinal synapses (Brandstatter et al., 2004). An interaction with liprinα links GluR2/3-containing AMPARs to receptor tyrosine phosphatases, GTPases, and motor proteins and may therefore modulate receptor trafficking and clustering, the actin cytoskeleton, and synaptic maturation (Wyszynski et al., 2002; Ko et al., 2003). GRIP binding to GRIP-associated proteins link AMPARs to activity-dependent Ras signaling and trafficking events (Ye et al., 2000), as well as to the caspase pathway (Ye et al., 2002). The interaction with the Ephrin B receptor, a receptor tyrosine kinase, may be involved in LTP and recruitment of cytoplasmic GRIPs to membrane lipid rafts (Torres et al., 1998; Bruckner et al., 1999), and an interaction with kinesin heavy chain and microtubule-associated protein-1B may be involved in AMPAR trafficking (Setou et al., 2002; Seog, 2004). Furthermore, GRIP binding to the proteoglycan NG2 in immature glia could play a role in glial-neuronal signaling in the immature brain (Stegmuller et al., 2003), as well as diseases such as multiple sclerosis and melanoma (Stegmuller et al., 2002), and an interaction with Fras1 protein implicates GRIP in Fraser syndrome (Takamiya et al., 2004).
3. LIN-10
LIN-10 is a Caenorhabditis elegans membrane-associated guanlylate kinase (MAGUK) family protein. The human homolog, mLIN-10 or X11L can bind directly to GluR1 via a PDZ domain interaction and could direct trafficking of receptors containing this subunit (Stricker and Huganir, 2003). Interestingly, X11L can bind and modulate the activity of the transcription factor NF-κB and plays a role in Alzheimer’s disease (Tomita et al., 2000).
4. Protein Interacting with C Kinase
PICK binds to the C terminus of GluR2 at the synapse via a PDZ domain that is also required for PICK1 binding to active PKCα (Dev et al., 1999; Xia et al., 1999). The role of PICK1 binding to AMPARs may be to target this active PKC to the receptors (Perez et al., 2001), indeed, it is possible to differentiate between PICK1 binding to PKC and GluR2 (Dev et al., 2004). Phosphorylation of the PDZ ligand Ser 880 by PKC causes PICK1 to traffic to synapses and rapid internalization of GluR2-containing receptors, perhaps because this phosphorylation releases GluR2 from GRIP1 binding (Chung et al., 2000), but not PICK1. Similarly, phosphorylation of Tyr 876 in the PDZ ligand differentially affects the ability of GluR2 to bind ABP/GRIP and PICK1 (Hayashi and Huganir, 2004). The PKC-dependent internalization is implicated in cerebellar and hippocampal LTD (Matsuda et al., 2000; Xia et al., 2000; Kim et al., 2001; Seidenman et al., 2003), and PICK1 has been detected in recycling endosomes (Lee et al., 2001). However, overexpression of PICK1 in the hippocampus in vitro reduces the amount of synaptic GluR2, but not GluR1, and increases synaptic strength at resting potentials (Terashima et al., 2004). The PICK1-GluR2 interaction can itself be disassembled by NSF and other proteins (Hanley et al., 2002), and PICK is capable of interacting with at least an additional 13 proteins besides GluR2 (Meyer et al., 2004).
5. Synapse-Associated Protein-97
The SAP family of proteins are members of the MAGUK family that contain many protein interaction domains. These have undergone extensive investigation and are thought to have roles beyond anchoring and trafficking of transmembrane receptors (Fujita and Kurachi, 2000). SAP-97 is the mammalian homolog of the Drosophila protein discs large tumor suppressor protein, the human homolog of which is often called hDlg (Lin et al., 1997). SAP-97 expression is not restricted to the central nervous system, for example, the role of this protein in the heart and epithelium is of interest to many researchers (Godreau et al., 2003). In particular, the role of SAP-97 in cell-cell contact within epithelial tissues is an extensive area of study (Muller et al., 1995; Wu et al., 1998; Firestein and Rongo, 2001). Within the brain, the majority of SAP-97 may be detected at the postsynaptic density, but some of the protein can also be found cytoplasmically and presynaptically (Aoki et al., 2001); indeed, it has been shown that the protein plays a fundamental role in the architecture of synapses (Thomas et al., 1997). The very C terminus of GluR1 binds to the second PDZ domain of SAP-97 via an interaction that confers specificity to this particular AMPAR (Leonard et al., 1998; Cai et al., 2002a). This interaction potentially allows GluR1-containing AMPARs to associate with many other proteins including PKA, PKC and calcineurin, calmodulin (Paarmann et al., 2002), NMDARs (Bassand et al., 1999; Gardoni et al., 2003) and KARs (Mehta et al., 2001), stargazin (Ives et al., 2004), guanylate kinase-activating protein (Kuhlendahl et al., 1998; Wu et al., 2000), a kinesin superfamily motor protein (Mok et al., 2002), and the actin-based motor myosin VI, which plays a role in endocytosis (Wu et al., 2002; Hasson, 2003) provided that the interaction sites do not overlap. The interaction with SAP-97 also promotes the stabilization and synaptic incorporation of GluR1-containing AMPARs (Valtschanoff et al., 2000; Nakagawa et al., 2004). The Drosophila homolog of SAP-97, Dlg, with the cooperation of another protein called Strabismus promotes incorporation of proteins into newly formed plasma membrane (Lee et al., 2003b). SAP-97 is capable of multimerization with itself and other MAGUKs, which is consistent with a role as an anchoring protein (Karnak et al., 2002; Lee et al., 2002a; Feng et al., 2004) and controls synaptic targeting of AMPARS (Nakagawa et al., 2004). Synaptic targeting of SAP-97 is modulated by CamKII (Mauceri et al., 2004), and SAP-97 itself is a target for the kinase, which can modulate some interactions (Yoshimura et al., 2002; Gardoni et al., 2003). SAP-97 occurs as different splice variants (Mori et al., 1998; Godreau et al., 2003). Overexpression of the synaptic form increases the number of functional AMPARs at synapses (Rumbaugh et al., 2003) and can occlude LTP (Nakagawa et al., 2004), although NMDA-dependent internalization of GluR1 doesn’t involve SAP-97 (Sans et al., 2001). The SAP-97-A-kinase-anchoring protein 79 interaction promotes basal PKA phosphorylation of GluR1 Ser 845 and may be involved in the calcium-dependent dephosphorylation of this residue and hence LTD (Tavalin et al., 2002). Interestingly, blocking NMDARs leads to an up-regulation of SAP-97 expression in some areas of the cortex (Linden et al., 2001). Interaction with SAP-97 can occur early in the secretory pathway (Sans et al., 2001). SAP-97 has been implicated in cleft palate (Caruana and Bernstein, 2001), Alzheimer’s disease (Wakabayashi et al., 1999), and schizophrenia (Toyooka et al., 2002). SAP-97 also plays a role in trafficking of potassium channels (Kim and Sheng, 1996; Tiffany et al., 2000; Leonoudakis et al., 2001; Folco et al., 2004), plasma membrane Ca2+-ATPases (DeMarco and Strehler, 2001; Schuh et al., 2003), the receptor tyrosine kinase ErbB4 (Huang et al., 2002), and interacts with tumor necrosis factor α-converting enzyme (Peiretti et al., 2003).
6. SemaF Cytoplasmic Domain-Associated Protein-3 and PDZ-Regulator of G-Protein Signaling-3
An interaction between the C terminus of GluR2 and SemaF cytoplasmic domain associated protein-3 (a protein with similar domains to E3 ubiquitin ligase LNX) and PDZ-RGS3 (PDZ-regulator of G-protein signaling-3, a GTPase-activating protein for trimeric G-proteins) has been reported in the yeast two-hybrid system, the functional implications of which are not clear (Meyer et al., 2004).
7. Syntenin
Syntenin is a protein that has been reported to interact with all AMPARs in vitro, but not in the yeast two-hybrid system. The affect of syntenin binding to AMPARs is not clear, but the interaction with KARs has been investigated (Hirbec et al., 2002, 2003). Syntenin is able to interact directly with the phosphatidylinositol biphosphate component of the plasma membrane (Zimmermann et al., 2002) and with syndecans, implicating them in cell-adhesion and synaptogenesis mechanisms (Zimmermann et al., 2001).
8. Transmembrane AMPA Receptor Regulatory Proteins
Stargazin was the first transmembrane interactor isolated for AMPARs (Chen et al., 2000) and is the only AMPAR interactor found associated with AMPARs in native gel preparations of cerebellar extracts (Vandenberghe et al., 2005). Null mutant mice exhibit the stargazer phenotype of absence seizures and cerebellar ataxia, which was attributed to the lack of functional AMPARs in cerebellar granule cells (Chen et al., 2000; Schnell et al., 2002). Stargazin is a member of a family of L-type calcium channel modulatory γ subunits and is also called γ-2. Stargazin, γ-3, γ-4, and γ-8 together form the transmembrane AMPAR regulatory protein family (Moss et al., 2003; Tomita et al., 2003). These proteins are differentially expressed throughout the nervous system, with γ-4 being predominant early in development (Tomita et al., 2003). Different TARPs may be expressed by the same neuron, but they do not form heteromeric complexes with each other. Furthermore, stargazin is the only TARP expressed by cerebellar granule cells (which explains the stargazer phenotype), and γ-8 has striking expression throughout the hippocampus, whereas stargazin is absent from CA1 (Tomita et al., 2003). This has implications for the differential control of activity-dependent trafficking events in different regions of the brain, for example, cerebellar and hippocampal LTD. TARPs comprise a conserved N-terminal AMPAR-binding domain and a more variable C-terminal PDZ binding domain that can bind to MAGUK proteins, for example, PSD-95 and PSD-93 (Dakoji et al., 2003). TARPs play a dual role in the trafficking of AMPARs, first enabling surface expression of AMPARs and second via interaction with PDZ proteins, facilitating synaptic incorporation of these receptors (Chen et al., 2000). The role of PSD-95 and stargazin in functional expression of AMPARs has been studied in the most detail, and it transpires that the TARP-MAGUK interaction can be regulated by phosphorylation of the PDZ binding site with phosphorylated stargazin being unable to bind PSD-95, resulting in a loss of synaptic AMPAR clusters (Chetkovich et al., 2002; Choi et al., 2002). Clustering of the tertiary complex is also regulated by the palmitoylation state of PSD-95, with activity-dependent depalmitoylation resulting in the loss of synaptic AMPARs (El-Husseini Ael et al., 2002). It has also been demonstrated that TARPs, unlike AMPARs, are stable at the plasma membrane and that agonist binding to AMPARs results in detachment from TARPs by an allosteric mechanism and internalization of the receptors (Tomita et al., 2004). The interaction between stargazin and PSD-95 also serves to link AMPARs to NMDARs and signaling cascades (Chetkovich et al., 2002; Lim et al., 2003; Mi et al., 2004). Other stargazin interactors include neuronal protein interacting specifically with TC10, which is enriched in the Golgi and is involved in trafficking of transmembrane proteins (Cuadra et al., 2004) and LC2, which is involved in microtubule trafficking events (Ives et al., 2004).
B. Other Interactors
1. Neuronal Activity-Regulated Pentraxin
Narp (neuronal activity-regulated pentraxin) is a neuronal intermediate-early gene product secreted upon synaptic activity (O’Brien et al., 1999). Narp is enriched in a subset of synapses within the hippocampus and spinal cord, but unlike other AMPAR interactors, Narp is extracellular and is believed to interact with the N terminus of AMPA subunits and can also form homomultimers. The protein is more predominant in presynaptic regions, with accumulation opposing excitatory synapses on dendritic shafts rather than on spines. Narp is postulated to promote aggregation of AMPARs via direct interaction, although in neuronal models this has only been demonstrated using exogenously applied Narp and dominant-negative mutant experiments (O’Brien et al., 1999, 2002). Interestingly, Narp secretion by hippocampal axons may selectively target the aggregation of AMPARs only in interneurons (Mi et al., 2002). Further research has shown that Narp may be involved in enduring forms of neuronal plasticity (Reti and Baraban, 2000).
2. N-Ethylmaleimide-Sensitive Factor
The interaction between the C terminus of GluR2 and weaker ones with GluR3 and GluR4c and NSF were first reported in 1998 (Nishimune et al., 1998; Osten et al., 1998; Song et al., 1998). The interaction domain on GluR2 comprises Lys 844-Gln 853 with Asn 851 playing a critical role, and inclusion of a peptide with this sequence (called pep2m/r or G10) or a monoclonal antibody against NSF blocks the interaction and causes a decrease in functional AMPAR responses (Noel et al., 1999). It is important to note, however, that later studies revealed that the clathrin adaptor protein AP2 associates with GluR2 in an overlapping region with NSF and that the pep2m/r peptide also blocks this interaction (Lee et al., 2002b). Interpretation of pep2m/r data must therefore proceed with caution. Because of the link between pep2m/r and a decrease in AMPAR responses, NSF has been suggested to play a role in the regulated delivery or removal of GluR2-containing AMPARs to the synapse in LTD (Lee et al., 2004). Indeed, infusion of pep2m and the expression of de novo homosynaptic LTD in the hippocampus are mutually occlusive (Luscher et al., 1999; Luthi et al., 1999), although this could be due to the role of AP2 in NMDA-dependent internalization of AMPARs and LTD (Lee et al., 2002b). Viral expression of pep2m in cultured hippocampal neurons led to a decrease in surface expression of AMPARs but not total levels of the receptor (Noel et al., 1999), which can be neuroprotective (Ralph et al., 2001), and more selective peptides have been used to show that NSF is required for the maintenance of synaptic AMPARs (Lee et al., 2002b). Blocking the NSF interaction also leads to aberrant targeting of GluR2-containing AMPARs after AMPA-induced and constitutive internalization to the lysosomal pathway for degradation, which is usually targeted only after NMDA stimulation (Lee et al., 2004). NSF and GluR2 are also part of a reversible complex with α and β soluble NSF attachment proteins (Osten et al., 1998; Osten and Ziff, 1999). The identification of PICK1 as part of the functional complex led to the proposal that, similar to soluble NSF attachment protein receptor complex disassembly presynaptically, the NSF ATPase acts to disrupt the PICK1-GluR2 interaction postsynaptically with the aid of soluble NSF attachment proteins (Hanley et al., 2002), furthering the idea that NSF acts as a stabilizer of synaptic GluR2. A role for NSF as a modulator of activity-dependent internalization of GluR2-containing AMPARs is emerging, with NSF blocking the AP2 interaction and hence internalization. However, a role for NSF in the constitutive cycling of GluR2/3 has also been postulated and remains controversial (Lee et al., 2002b). Interestingly, there appears to be a component of AMPAR transmission that is resistant to the actions of pep2m (Kim and Lisman, 2001), and NSF has many more interaction partners that suggest more far-reaching roles than the one discussed here (Haas, 1998).
3. 4.1
Protein 4.1 was originally isolated as a cytoskeletal protein in erythrocytes. A neuronal homolog has been identified (4.1N) that exists as several splice variants and can be detected in the postsynaptic density of some neurons (Walensky et al., 1999). Both GluR1 and GluR4 have 4.1 binding sites in the proximal region of their intracellular C termini (Shen et al., 2000; Coleman et al., 2003). This interaction between 4.1 and AMPARs is necessary for the surface expression of the receptors, links them to the actin cytoskeleton, and is involved in the formation of postsynaptic signaling complexes.
IV. Future Directions
The immense variety and scope of current research on AMPARs makes it almost impossible to predict where future advances are likely to occur. Nonetheless, much remains to be discovered before a reasonable working knowledge of the mechanisms underlying AMPAR structure, function, and trafficking can be assembled. One clear goal is the need for the complexity surrounding the temporal and spatial arrangement of AMPAR-interacting proteins to be clarified and specific functions assigned to each. Once more information becomes available, it seems reasonable that one or more of these interacting proteins may represent a potential target for the development of specific drugs designed to counteract a loss of synaptic AMPARs (of importance in Alzheimer’s disease) and/or AMPAR overexpression (of importance in epilepsy and excitotoxic damage). Moreover, it is conceivable that eventually such drugs would be capable of targeting AMPARs of a specific subunit composition and possibly even specific subunit splice isoforms. With these possibilities in mind, this area of neuroscience promises an exciting and challenging time ahead.
In summary, despite the highly complex series of interactors and modulators, it seems that in the hippocampus, at least, GluR2-containing AMPARS are constitutively delivered to synapses and cycle continuously (Malinow and Malenka, 2002), whereas GluR1-containing AMPAR are inserted into synapses in response to NMDAR activation and the subsequent activation of CaMKII. The GluR4 subunit plays the role of GluR1 earlier in development (Zhu et al., 2000). This activity-dependent insertion plays a role in the mechanism of LTP, with GluR1 being dominant over GluR2 (Hayashi et al., 2000; Passafaro et al., 2001; Shi et al., 2001).
Acknowledgments
We would like to acknowledge Laura-Jane King for advice on the manuscript. We are grateful to the Medical Research Council, the Wellcome Trust, Human Frontiers Science Foundation, and the European Union Grant QLRT-2000-02089 for financial support to our laboratory. L.C. was funded by a Medical Research Council–Novartis Collaborative Studentship.
Footnotes
Abbreviations: CNS, central nervous system; GluR, glutamate receptor; mGluR, metabotropic glutamate receptor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N-methyl-D-aspartate; KA, kainate; AMPAR, AMPA receptor; TM, transmembrane; KAR, KA receptor; NMDAR, NMDA receptor; PDZ, postsynaptic density 95-discs large-zona occludens 1 domain; Dlg, discs large; CaMK, calcium-calmodulin-dependent protein kinase; PKC, protein kinase C; PKA, protein kinase A; LTP, long-term potentiation; LTD, long-term depression; SAP, synapse-associated protein; ABP, AMPAR binding protein; GRIP, glutamate receptor-interacting protein; PICK1, protein interacting with C kinase 1; PSD, postsynaptic density; SCC, Schaffer collateral-commissural; GFP, green fluorescent protein; NSF, N-ethylmaleimide-sensitive factor; AP2, clathrin adaptor protein 2; PPI, protein phosphatase I; MAGUK, membrane-associated guanlylate kinase; TARP, transmembrane AMPAR regulatory protein; Narp, neuronal activity-regulated pentraxin.
References
- Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO (Eur Mol Biol Organ) J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer’s disease, Huntington’s disease and schizophrenia. Brain Res. 1995;699:297–304. doi: 10.1016/0006-8993(95)00922-d. [DOI] [PubMed] [Google Scholar]
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Magee JC. Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones. J Physiol. 2004;559:543–554. doi: 10.1113/jphysiol.2004.065219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Miko I, Oviedo H, Mikeladze-Dvali T, Alexandre L, Sweeney N, Bredt DS. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40:239–257. doi: 10.1002/syn.1047. [DOI] [PubMed] [Google Scholar]
- Arai Y, Mizuguchi M, Takashima S. Developmental changes of glutamate receptors in the rat cerebral cortex and hippocampus. Anat Embryol. 1997;195:65–70. doi: 10.1007/s004290050025. [DOI] [PubMed] [Google Scholar]
- Archibald K, Henley JM. Subcellular localization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor subunits in rat cortex. Biochem Soc Trans. 1997;25:541S. doi: 10.1042/bst025541s. [DOI] [PubMed] [Google Scholar]
- Archibald K, Perry MJ, Molnar E, Henley JM. Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacology. 1998;37:1345–1353. doi: 10.1016/s0028-3908(98)00135-x. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature (Lond) 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Arvola M, Keinanen K. Characterization of the ligand-binding domains of glutamate receptor (GluR)-B and GluR-D subunits expressed in Escherichia coli as periplasmic proteins. J Biol Chem. 1996;271:15527–15532. doi: 10.1074/jbc.271.26.15527. [DOI] [PubMed] [Google Scholar]
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon G, Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron. 2001;31:103–113. doi: 10.1016/s0896-6273(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Backus KH, Berger T. Developmental variation of the permeability to Ca2+ of AMPA receptors in presumed hilar glial precursor cells. Pflugers Arch. 1995;431:244–252. doi: 10.1007/BF00410197. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GN, Slevin JT. Ionotropic glutamate receptor biology: effect on synaptic connectivity and function in neurological disease. Curr Med Chem. 2003;10:2059–2072. doi: 10.2174/0929867033456800. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Dev KK, Henley JM. Cyclothiazide unmasks AMPA-evoked stimulation of [3H]-L-glutamate release from rat hippocampal synaptosomes. Br J Pharmacol. 1994;113:339–341. doi: 10.1111/j.1476-5381.1994.tb16902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Henley JM. Autoradiographic distribution of glutamatergic ligand binding sites in Xenopus brain: evidence for intracellular [3H]AMPA binding sites. Brain Res. 1993;626:259–264. doi: 10.1016/0006-8993(93)90585-b. [DOI] [PubMed] [Google Scholar]
- Bassand P, Bernard A, Rafiki A, Gayet D, Khrestchatisky M. Differential interaction of the tSXV motifs of the NR1 and NR2A NMDA receptor subunits with PSD-95 and SAP97. Eur J Neurosci. 1999;11:2031–2043. doi: 10.1046/j.1460-9568.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Batista SS, Pires RS, Britto LR. Differential expression of AMPA-type glutamate receptor subunits during development of the chick optic tectum. Braz J Med Biol Res. 2002;35:973–978. doi: 10.1590/s0100-879x2002000800015. [DOI] [PubMed] [Google Scholar]
- Baude A, Molnar E, Latawiec D, McIlhinney RA, Somogyi P. Synaptic and nonsynaptic localization of the GluR1 subunit of the AMPA-type excitatory amino acid receptor in the rat cerebellum. J Neurosci. 1994;14:2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnar E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Expression of transcripts encodingAMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol. 2004;468:530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Maximov A. PDZ domains: more than just a glue. Proc Natl Acad Sci USA. 2001;98:787–789. doi: 10.1073/pnas.98.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DL, Grabowski PJ. Alternative pre-mRNA splicing and neuronal function. Prog Mol Subcell Biol. 2003;31:187–216. doi: 10.1007/978-3-662-09728-1_7. [DOI] [PubMed] [Google Scholar]
- Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J Neurosci. 1994;14:7585–7593. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992;58:1118–1126. doi: 10.1111/j.1471-4159.1992.tb09370.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature (Lond) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature (Lond) 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Boulter J, Hollmann M, O’Shea-Greenfield A, Hartley M, Deneris E, Maron C, Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science (Wash DC) 1990;249:1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Xia H, Malenka RC. Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proc Natl Acad Sci USA. 2002;99:7096–7101. doi: 10.1073/pnas.102156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter JH, Dick O, Boeckers TM. The postsynaptic scaffold proteins ProSAP1/Shank2 and Homer1 are associated with glutamate receptor complexes at rat retinal synapses. J Comp Neurol. 2004;475:551–563. doi: 10.1002/cne.20194. [DOI] [PubMed] [Google Scholar]
- Breese CR, Leonard SS. Glutamate receptor subtype expression in human postmortem brain. J Mol Neurosci. 1993;4:263–275. doi: 10.1007/BF02821558. [DOI] [PubMed] [Google Scholar]
- Brose N, Huntley GW, Stern-Bach Y, Sharma G, Morrison JH, Heinemann SF. Differential assembly of coexpressed glutamate receptor subunits in neurons of rat cerebral cortex. J Biol Chem. 1994;269:16780–16784. [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science (Wash DC) 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- Burette A, Khatri L, Wyszynski M, Sheng M, Ziff EB, Weinberg RJ. Differential cellular and subcellular localization of ampa receptor-binding protein and glutamate receptor-interacting protein. J Neurosci. 2001;21:495–503. doi: 10.1523/JNEUROSCI.21-02-00495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burette A, Wyszynski M, Valtschanoff JG, Sheng M, Weinberg RJ. Characterization of glutamate receptor interacting protein-immunopositive neurons in cerebellum and cerebral cortex of the albino rat. J Comp Neurol. 1999;411:601–612. [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion perme-ability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol. 1996;496:165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Coleman SK, Niemi K, Keinanen K. Selective binding of synapse-associated protein 97 to GluR-A alpha-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. J Biol Chem. 2002a;277:31484–31490. doi: 10.1074/jbc.M204354200. [DOI] [PubMed] [Google Scholar]
- Cai X, Gu Z, Zhong P, Ren Y, Yan Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J Biol Chem. 2002b;277:36553–36562. doi: 10.1074/jbc.M203752200. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol. 2001;21:1475–1483. doi: 10.1128/MCB.21.5.1475-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AL, Correia S, Faro CJ, Duarte CB, Carvalho AP, Pires EM. Phosphorylation of GluR4 AMPA-type glutamate receptor subunit by protein kinase C in cultured retina amacrine neurons. Eur J Neurosci. 2002;15:465–474. doi: 10.1046/j.0953-816x.2001.01881.x. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Duarte CB, Carvalho AP. Regulation of AMPA receptors by phosphorylation. Neurochem Res. 2000;25:1245–1255. doi: 10.1023/a:1007644128886. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JH, Kinsman SL, Johnston MV. RNA editing of a human glutamate receptor subunit. Brain Res Mol Brain Res. 1994;22:323–328. doi: 10.1016/0169-328x(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Chang S, De Camilli P. Glutamate regulates actin-based motility in axonal filopodia. Nat Neurosci. 2001;4:787–793. doi: 10.1038/90489. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Chase TN, Oh JD, Konitsiotis S. Antiparkinsonian and antidyskinetic activity of drugs targeting central glutamatergic mechanisms. J Neurol. 2000;247(Suppl 2):II36–II42. doi: 10.1007/pl00007759. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature (Lond) 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Chen L, Stocker TJ, Nicoll RA, Bredt DS. Phosphorylation of the postsynaptic density-95 (PSD-95)/discs large/zona occludens-1 binding site of stargazin regulates binding to PSD-95 and synaptic targeting of AMPA receptors. J Neurosci. 2002;22:5791–5796. doi: 10.1523/JNEUROSCI.22-14-05791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Choi J, Ko J, Park E, Lee JR, Yoon J, Lim S, Kim E. Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J Biol Chem. 2002;277:12359–12363. doi: 10.1074/jbc.M200528200. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science (Wash DC) 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Barbour B. Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices. J Physiol. 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Gurd JW, Bissoon N, Tricaud N, Molnar E, Zamze SE, Dwek RA, McIlhinney RA, Wing DR. Identification of lectin-purified neural glycoproteins, GPs 180, 116 and 110, with NMDA and AMPA receptor subunits: conservation of glycosylation at the synapse. J Neurochem. 1998;70:2594–2605. doi: 10.1046/j.1471-4159.1998.70062594.x. [DOI] [PubMed] [Google Scholar]
- Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinanen K. Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci. 2003;23:798–806. doi: 10.1523/JNEUROSCI.23-03-00798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT. Functional roles of protein interactions with AMPA and kainate receptors. Neurosci Res. 2003;47:3–15. doi: 10.1016/s0168-0102(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Comoletti D, Muzio V, Capobianco A, Ravizza T, Mennini T. Nitric oxide produced by non-motoneuron cells enhances rat embryonic motoneuron sensitivity to excitotoxins: comparison in mixed neuron/glia or purified cultures. J Neurol Sci. 2001;192:61–69. doi: 10.1016/s0022-510x(01)00607-4. [DOI] [PubMed] [Google Scholar]
- Correia SS, Duarte CB, Faro CJ, Pires EV, Carvalho AL. Protein kinase C gamma associates directly with the GluR4 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. Effect on receptor phosphorylation. J Biol Chem. 2003;278:6307–6313. doi: 10.1074/jbc.M205587200. [DOI] [PubMed] [Google Scholar]
- Cotrufo T, Viegi A, Berardi N, Bozzi Y, Mascia L, Maffei L. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. J Neurosci. 2003;23:3566–3571. doi: 10.1523/JNEUROSCI.23-09-03566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell JR, Dube GR, Egles C, Liu G. Distribution, density and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. J Neurophysiol. 2000;84:1573–1587. doi: 10.1152/jn.2000.84.3.1573. [DOI] [PubMed] [Google Scholar]
- Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Cuadra AE, Kuo SH, Kawasaki Y, Bredt DS, Chetkovich DM. AMPA receptor synaptic targeting regulated by stargazin interactions with the Golgi-resident PDZ protein nPIST. J Neurosci. 2004;24:7491–7502. doi: 10.1523/JNEUROSCI.1255-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakoji S, Tomita S, Karimzadegan S, Nicoll RA, Bredt DS. Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases. Neuropharmacology. 2003;45:849–856. doi: 10.1016/s0028-3908(03)00267-3. [DOI] [PubMed] [Google Scholar]
- D’Alcantara P, Schiffmann SN, Swillens S. Bidirectional synaptic plasticity as a consequence of interdependent Ca2+-controlled phosphorylation and dephosphorylation pathways. Eur J Neurosci. 2003;17:2521–2528. doi: 10.1046/j.1460-9568.2003.02693.x. [DOI] [PubMed] [Google Scholar]
- DeMarco SJ, Strehler EE. Plasma membrane Ca2+-atpase isoforms 2b and 4b interact promiscuously and selectively with members of the membrane-associated guanylate kinase family of PDZ (PSD95/Dlg/ZO-1) domain-containing proteins. J Biol Chem. 2001;276:21594–21600. doi: 10.1074/jbc.M101448200. [DOI] [PubMed] [Google Scholar]
- Derkach VA. Silence analysis of AMPA receptor mutated at the CaM-kinase II phosphorylation site. Biophys J. 2003;84:1701–1708. doi: 10.1016/S0006-3495(03)74978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza S, Fu J, States BA, Ziff EB. Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. J Neurosci. 2002;22:3493–3503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakanishi S, Henley JM. The PDZ domain of PICK1 differentially accepts protein kinase C-alpha and GluR2 as interacting ligands. J Biol Chem. 2004;279:41393–41397. doi: 10.1074/jbc.M404499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature (Lond) 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci. 1999;19:6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature (Lond) 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Durand GM, Zukin RS. Developmental regulation of mRNAs encoding rat brain kainate/AMPA receptors: a northern analysis study. J Neurochem. 1993;61:2239–2246. doi: 10.1111/j.1471-4159.1993.tb07465.x. [DOI] [PubMed] [Google Scholar]
- Dziedzic B, Prevot V, Lomniczi A, Jung H, Cornea A, Ojeda SR. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci. 2003;23:915–926. doi: 10.1523/JNEUROSCI.23-03-00915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini Ael D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-Glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol. 1997;52:861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Volknandt W, Fine A, Stewart MG. Age-dependent pre- and postsynaptic distribution of AMPA receptors at synapses in CA3 stratum radiatum of hippocampal slice cultures compared with intact brain. Eur J Neurosci. 2000;12:3687–3700. doi: 10.1046/j.1460-9568.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res. 2003a;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003b;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Kask K, Brusa R, Kornau HC, Kolhekar R, Rozov A, Burnashev N, Jensen V, Hvalby O, Sprengel R, Seeburg PH. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci. 1999;2:57–64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- Feng W, Long JF, Fan JS, Suetake T, Zhang M. The tetrameric L27 domain complex as an organization platform for supramolecular assemblies. Nat Struct Mol Biol. 2004;11:475–480. doi: 10.1038/nsmb751. [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel AV, Montal M. Pentameric subunit stoichiometry of a neuronal glutamate receptor. Proc Natl Acad Sci USA. 1996;93:2741–2744. doi: 10.1073/pnas.93.7.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Boddeke H, Muller D. Enhancement of AMPA-mediated synaptic transmission by the protein phosphatase inhibitor calyculin A in rat hippocampal slices. Eur J Neurosci. 1993;5:1035–1041. doi: 10.1111/j.1460-9568.1993.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Firestein BL, Rongo C. DLG-1 is a MAGUK similar to SAP97 and is required for adherens junction formation. Mol Biol Cell. 2001;12:3465–3475. doi: 10.1091/mbc.12.11.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Pickard L, Duckworth JK, Molnar E, Henley JM, Collingridge GL, Noel J. An electrophysiological characterisation of long-term potentiation in cultured dissociated hippocampal neurones. Neuropharmacology. 2001;41:693–699. doi: 10.1016/s0028-3908(01)00128-9. [DOI] [PubMed] [Google Scholar]
- Fleck MW, Bahring R, Patneau DK, Mayer ML. AMPA receptor heterogeneity in rat hippocampal neurons revealed by differential sensitivity to cyclothiazide. J Neurophysiol. 1996;75:2322–2333. doi: 10.1152/jn.1996.75.6.2322. [DOI] [PubMed] [Google Scholar]
- Folco EJ, Liu GX, Koren G. Caveolin-3 and SAP97 form a scaffolding protein complex that regulates the voltage-gated potassium channel Kv1.5. Am J Physiol. 2004;287:H681–H690. doi: 10.1152/ajpheart.00152.2004. [DOI] [PubMed] [Google Scholar]
- Franks KM, Stevens CF, Sejnowski TJ. Independent sources of quantal variability at single glutamatergic synapses. J Neurosci. 2003;23:3186–3195. doi: 10.1523/JNEUROSCI.23-08-03186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, deSouza S, Ziff EB. Intracellular membrane targeting and suppression of Ser880 phosphorylation of glutamate receptor 2 by the linker I-set II domain of AMPA receptor-binding protein. J Neurosci. 2003;23:7592–7601. doi: 10.1523/JNEUROSCI.23-20-07592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XZ, Zhang QG, Meng FJ, Zhang GY. NMDA receptor-mediated immediate Ser831 phosphorylation of GluR1 through CaMKIIalpha in rat hippocampus during early global ischemia. Neurosci Res. 2004;48:85–91. doi: 10.1016/j.neures.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Fujita A, Kurachi Y. SAP family proteins. Biochem Biophys Res Commun. 2000;269:1–6. doi: 10.1006/bbrc.1999.1893. [DOI] [PubMed] [Google Scholar]
- Gabriel R, de Souza S, Ziff EB, Witkovsky P. Association of the AMPA receptor-related postsynaptic density proteins GRIP and ABP with subsets of glutamate-sensitive neurons in the rat retina. J Comp Neurol. 2002;449:129–140. doi: 10.1002/cne.10280. [DOI] [PubMed] [Google Scholar]
- Gahring L, Carlson NG, Meyer EL, Rogers SW. Granzyme B proteolysis of a neuronal glutamate receptor generates an autoantigen and is modulated by glycosylation. J Immunol. 2001;166:1433–1438. doi: 10.4049/jimmunol.166.3.1433. [DOI] [PubMed] [Google Scholar]
- Gallo V, Russell JT. Excitatory amino acid receptors in glia: different subtypes for distinct functions? J Neurosci Res. 1995;42:1–8. doi: 10.1002/jnr.490420102. [DOI] [PubMed] [Google Scholar]
- Gallo V, Upson LM, Hayes WP, Vyklicky L, Jr, Winters CA, Buonanno A. Molecular cloning and development analysis of a new glutamate receptor subunit isoform in cerebellum. J Neurosci. 1992;12:1010–1023. doi: 10.1523/JNEUROSCI.12-03-01010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barcina JM, Matute C. AMPA-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Brain Res Mol Brain Res. 1998;53:270–276. doi: 10.1016/s0169-328x(97)00318-5. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Mauceri D, Fiorentini C, Bellone C, Missale C, Cattabeni F, Di Luca M. CaMKII-dependent phosphorylation regulates SAP97/NR2A interaction. J Biol Chem. 2003;278:44745–44752. doi: 10.1074/jbc.M303576200. [DOI] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature (Lond) 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Godreau D, Vranckx R, Maguy A, Goyenvalle C, Hatem SN. Different isoforms of synapse-associated protein, SAP97, are expressed in the heart and have distinct effects on the voltage-gated K+ channel Kv1. J Biol Chem. 2003;278:47046–47052. doi: 10.1074/jbc.M308463200. [DOI] [PubMed] [Google Scholar]
- Gomes AR, Correia SS, Carvalho AL, Duarte CB. Regulation of AMPA receptor activity, synaptic targeting and recycling: role in synaptic plasticity. Neurochem Res. 2003;28:1459–1473. doi: 10.1023/a:1025610122776. [DOI] [PubMed] [Google Scholar]
- Gomes AR, Cunha P, Nuriya M, Faro CJ, Huganir RL, Pires EV, Carvalho AL, Duarte CB. Metabotropic glutamate and dopamine receptors co-regulate AMPA receptor activity through PKA in cultured chick retinal neurones: effect on GluR4 phosphorylation and surface expression. J Neurochem. 2004;90:673–682. doi: 10.1111/j.1471-4159.2004.02519.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366:201–205. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Haas A. NSF–fusion and beyond. Trends Cell Biol. 1998;8:471–473. doi: 10.1016/s0962-8924(98)01388-9. [DOI] [PubMed] [Google Scholar]
- Hack I, Frech M, Dick O, Peichl L, Brandstatter JH. Heterogeneous distribution of AMPA glutamate receptor subunits at the photoreceptor synapses of rodent retina. Eur J Neurosci. 2001;13:15–24. [PubMed] [Google Scholar]
- Hack I, Koulen P, Peichl L, Brandstatter JH. Development of glutamatergic synapses in the rat retina: the postnatal expression of ionotropic glutamate receptor subunits. Vis Neurosci. 2002;19:1–13. doi: 10.1017/s0952523801191017. [DOI] [PubMed] [Google Scholar]
- Hall RA, Bahr BA. AMPA receptor development in rat telencephalon: [3H]AMPA binding and western blot studies. J Neurochem. 1994;63:1658–1665. doi: 10.1046/j.1471-4159.1994.63051658.x. [DOI] [PubMed] [Google Scholar]
- Hall RA, Hansen A, Andersen PH, Soderling TR. Surface expression of the AMPA receptor subunits GluR1, GluR2, and GluR4 in stably transfected baby hamster kidney cells. J Neurochem. 1997;68:625–630. doi: 10.1046/j.1471-4159.1997.68020625.x. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience. 1997;78:361–371. doi: 10.1016/s0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Khatri L, Hanson PI, Ziff EB. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron. 2002;34:53–67. doi: 10.1016/s0896-6273(02)00638-4. [DOI] [PubMed] [Google Scholar]
- Hasson T. Myosin VI: two distinct roles in endocytosis. J Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Ishida A, Katagiri H, Mishina M, Fujisawa H, Manabe T, Takahashi T. Calcium- and calmodulin-dependent phosphorylation of AMPA type glutamate receptor subunits by endogenous protein kinases in the post-synaptic density. Brain Res Mol Brain Res. 1997;46:338–342. doi: 10.1016/s0169-328x(97)00073-9. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science (Wash DC) 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Henley JM. Proteins interactions implicated in AMPA receptor trafficking: a clear destination and an improving route map. Neurosci Res. 2003;45:243–254. doi: 10.1016/s0168-0102(02)00229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature (Lond) 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hirai H, Yoshioka K, Yamada K. A simple method using 31P-NMR spectroscopy for the study of protein phosphorylation. Brain Res Brain Res Protoc. 2000;5:182–189. doi: 10.1016/s1385-299x(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes. Analysis of PDZ binding motifs. J Biol Chem. 2002;277:15221–15224. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA–gated glutamate receptor channels depends on subunit composition. Science (Wash DC) 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Maron C, Heinemann S. N-Glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994;13:1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Hollmann M, O’Shea-Greenfield A, Rogers SW, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature (Lond) 1989;342:643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Tatsumi H, Nakashima T, Sokabe M. In vitro reconstitution of signal transmission from a hair cell to the growth cone of a chick vestibular ganglion cell. Neuroscience. 2003;120:993–1003. doi: 10.1016/s0306-4522(03)00224-0. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. Characterization of the mechanism underlying the reversal of long term potentiation by low frequency stimulation at hippocampal CA1 synapses. J Biol Chem. 2001;276:48108–48117. doi: 10.1074/jbc.M106388200. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Wang Q, Won S, Luo ZG, Xiong WC, Mei L. Compartmentalized NRG signaling and PDZ domain-containing proteins in synapse structure and function. Int J Dev Neurosci. 2002;20:173–185. doi: 10.1016/s0736-5748(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Huh KH, Wenthold RJ. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem. 1999;274:151–157. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- Hullebroeck MF, Hampson DR. Characterization of the oligosaccharide side chains on kainate binding proteins and AMPA receptors. Brain Res. 1992;590:187–192. doi: 10.1016/0006-8993(92)91094-u. [DOI] [PubMed] [Google Scholar]
- Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002;277:5699–5702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science (Wash DC) 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain–I. N-Methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Ishiuchi S, Tsuzuki K, Yamada N, Okado H, Miwa A, Kuromi H, Yokoo H, Nakazato Y, Sasaki T, Ozawa S. Extension of glial processes by activation of Ca2+-permeable AMPA receptor channels. Neuroreport. 2001;12:745–748. doi: 10.1097/00001756-200103260-00026. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives JH, Fung S, Tiwari P, Payne HL, Thompson CL. Microtubule-associated protein light chain 2 is a stargazin-AMPA receptor complex-interacting protein in vivo. J Biol Chem. 2004;279:31002–31009. doi: 10.1074/jbc.M402214200. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience. 1995;67:893–907. doi: 10.1016/0306-4522(95)00026-f. [DOI] [PubMed] [Google Scholar]
- Janssens N, Lesage AS. Glutamate receptor subunit expression in primary neuronal and secondary glial cultures. J Neurochem. 2001;77:1457–1474. doi: 10.1046/j.1471-4159.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- Jayaraman V, Keesey R, Madden DR. Ligand–protein interactions in the glutamate receptor. Biochemistry. 2000;39:8693–8697. doi: 10.1021/bi000892f. [DOI] [PubMed] [Google Scholar]
- Johansen TN, Greenwood JR, Frydenvang K, Madsen U, Krogsgaard-Larsen P. Stereostructure-activity studies on agonists at the AMPA and kainate subtypes of ionotropic glutamate receptors. Chirality. 2003;15:167–179. doi: 10.1002/chir.10177. [DOI] [PubMed] [Google Scholar]
- Jonas P. AMPA-type glutamate receptors–nonselective cation channels mediating fast excitatory transmission in the CNS. EXS (Basel) 1993;66:61–76. doi: 10.1007/978-3-0348-7327-7_4. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Iwakura Y, Narisawa-Saito M, Ibaraki K, Xiong H, Watanabe M, Hayashi Y, Takei N, Nawa H. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev Biol. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb RG, Fox AJ. Synchronized overproduction of AMPA, kainate and NMDA glutamate receptors during human spinal cord development. J Comp Neurol. 1997;384:200–210. doi: 10.1002/(sici)1096-9861(19970728)384:2<200::aid-cne3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, de Leeuw FE, Lopes da Silva FH. Ischaemia does not alter the editing status at the Q/R site of glutamate receptor-A, -B, -5 and -6 subunit mRNA. Neuroreport. 1995;6:1133–1136. doi: 10.1097/00001756-199505300-00015. [DOI] [PubMed] [Google Scholar]
- Karkanias NB, Papke RL. Lithium modulates desensitization of the glutamate receptor subtype gluR3 in Xenopus oocytes. Neurosci Lett. 1999;277:153–156. doi: 10.1016/s0304-3940(99)00878-2. [DOI] [PubMed] [Google Scholar]
- Karnak D, Lee S, Margolis B. Identification of multiple binding partners for the amino-terminal domain of synapse-associated protein 97. J Biol Chem. 2002;277:46730–46735. doi: 10.1074/jbc.M208781200. [DOI] [PubMed] [Google Scholar]
- Kasahara J, Fukunaga K, Miyamoto E. Activation of CA(2+)/calmodulin-dependent protein kinase IV in cultured rat hippocampal neurons. J Neurosci Res. 2000;59:594–600. doi: 10.1002/(SICI)1097-4547(20000301)59:5<594::AID-JNR2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kask K, Zamanillo D, Rozov A, Burnashev N, Sprengel R, Seeburg PH. The AMPA receptor subunit GluR-B in its Q/R site-unedited form is not essential for brain development and function. Proc Natl Acad Sci USA. 1998;95:13777–13782. doi: 10.1073/pnas.95.23.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature (Lond) 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Ito K, Sun H, Kanazawa I, Kwak S. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur J Neurosci. 2003a;18:23–33. doi: 10.1046/j.1460-9568.2003.02718.x. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kwak S, Sun H, Ito K, Hashida H, Aizawa H, Jeong SY, Kanazawa I. Human spinal motoneurons express low relative abundance of GluR2 mRNA: an implication for excitotoxicity in ALS. J Neurochem. 2003b;85:680–689. doi: 10.1046/j.1471-4159.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Hattori S, Oiji I, Hamajima K, Mishina M, Okuda K. Ligand-binding properties and N-glycosylation of alpha 1 subunit of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate(AMPA)-selective glutamate receptor channel expressed in a baculovirus system. Eur J Biochem. 1994;223:665–673. doi: 10.1111/j.1432-1033.1994.tb19039.x. [DOI] [PubMed] [Google Scholar]
- Keinanen K, Kohr G, Seeburg PH, Laukkanen ML, Oker-Blom C. High-level expression of functional glutamate receptor channels in insect cells. Biotechnology (NY) 1994;12:802–806. doi: 10.1038/nbt0894-802. [DOI] [PubMed] [Google Scholar]
- Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science (Wash DC) 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kessler M, Rogers G, Arai A. The norbornenyl moiety of cyclothiazide determines the preference for flip-flop variants of AMPA receptor subunits. Neurosci Lett. 2000;287:161–165. doi: 10.1016/s0304-3940(00)01180-0. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lisman JE. A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin and N-ethylmaleimide-sensitive factor. J Neurosci. 2001;21:4188–4194. doi: 10.1523/JNEUROSCI.21-12-04188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sheng M. Differential K+ channel clustering activity of PSD-95 and SAP97, two related membrane-associated putative guanylate kinases. Neuropharmacology. 1996;35:993–1000. doi: 10.1016/0028-3908(96)00093-7. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Arancibia-Carcamo IL, Moss SJ. Association of GRIP1 with a GABA(A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem Pharmacol. 2004;68:1649–1654. doi: 10.1016/j.bcp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neurosci. 2001;18:13–25. doi: 10.1006/mcne.2001.1005. [DOI] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K, Kamiya Y, Matsuda S, Kato K, Umemori H, Yuzaki M. Heteromer formation of delta2 glutamate receptors with AMPA or kainate receptors. Brain Res Mol Brain Res. 2003;110:27–37. doi: 10.1016/s0169-328x(02)00561-2. [DOI] [PubMed] [Google Scholar]
- Kohler M, Kornau HC, Seeburg PH. The organization of the gene for the functionally dominant alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor subunit GluR-B. J Biol Chem. 1994;269:17367–17370. [PubMed] [Google Scholar]
- Koike M, Tsukada S, Tsuzuki K, Kijima H, Ozawa S. Regulation of kinetic properties of GluR2 AMPA receptor channels by alternative splicing. J Neurosci. 2000;20:2166–2174. doi: 10.1523/JNEUROSCI.20-06-02166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Tani M, Saitoh N, Takahashi T. Mechanisms underlying developmental speeding in AMPA-EPSC decay time at the calyx of Held. J Neurosci. 2005;25:199–207. doi: 10.1523/JNEUROSCI.3861-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampfl K, Schlesinger F, Zorner A, Kappler M, Dengler R, Bufler J. Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur J Neurosci. 2002;15:51–62. doi: 10.1046/j.0953-816x.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- Kubo M, Ito E. Structural dynamics of an ionotropic glutamate receptor. Proteins. 2004;56:411–419. doi: 10.1002/prot.20154. [DOI] [PubMed] [Google Scholar]
- Kuhlendahl S, Spangenberg O, Konrad M, Kim E, Garner CC. Functional analysis of the guanylate kinase-like domain in the synapse-associated protein SAP97. Eur J Biochem. 1998;252:305–313. doi: 10.1046/j.1432-1327.1998.2520305.x. [DOI] [PubMed] [Google Scholar]
- Kuner T, Seeburg PH, Guy HR. A common architecture for K+ channels and ionotropic glutamate receptors? Trends Neurosci. 2003;26:27–32. doi: 10.1016/s0166-2236(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Schweighofer N, Kawato M. Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation. J Neurosci. 2001;21:5693–5702. doi: 10.1523/JNEUROSCI.21-15-05693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusinen A, Abele R, Madden DR, Keinanen K. Oligomerization and ligand-binding properties of the ectodomain of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunit GluRD. J Biol Chem. 1999;274:28937–28943. doi: 10.1074/jbc.274.41.28937. [DOI] [PubMed] [Google Scholar]
- Lai F, Chen CX, Lee VM, Nishikura K. Dramatic increase of the RNA editing for glutamate receptor subunits during terminal differentiation of clonal human neurons. J Neurochem. 1997;69:43–52. doi: 10.1046/j.1471-4159.1997.69010043.x. [DOI] [PubMed] [Google Scholar]
- Lambolez B, Ropert N, Perrais D, Rossier J, Hestrin S. Correlation between kinetics and RNA splicing of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in neocortical neurons. Proc Natl Acad Sci USA. 1996;93:1797–1802. doi: 10.1073/pnas.93.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launey T, Endo S, Sakai R, Harano J, Ito M. Protein phosphatase 2A inhibition induces cerebellar long-term depression and declustering of synaptic AMPA receptor. Proc Natl Acad Sci USA. 2004;101:676–681. doi: 10.1073/pnas.0302914101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature (Lond) 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003a;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lee OK, Frese KK, James JS, Chadda D, Chen ZH, Javier RT, Cho KO. Discs-Large and Strabismus are functionally linked to plasma membrane formation. Nat Cell Biol. 2003b;5:987–993. doi: 10.1038/ncb1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Fan S, Makarova O, Straight S, Margolis B. A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol. 2002a;22:1778–1791. doi: 10.1128/MCB.22.6.1778-1791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002b;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lee SH, Valtschanoff JG, Kharazia VN, Weinberg R, Sheng M. Biochemical and morphological characterization of an intracellular membrane compartment containing AMPA receptors. Neuropharmacology. 2001;41:680–692. doi: 10.1016/s0028-3908(01)00124-1. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Mailliard W, Wingerd K, Clegg D, Vandenberg C. Inward rectifier potassium channel Kir2.2 is associated with synapse-associated protein SAP97. J Cell Sci. 2001;114:987–998. doi: 10.1242/jcs.114.5.987. [DOI] [PubMed] [Google Scholar]
- Lerma J, Morales M, Ibarz JM, Somohano F. Rectification properties and Ca2+ permeability of glutamate receptor channels in hippocampal cells. Eur J Neurosci. 1994;6:1080–1088. doi: 10.1111/j.1460-9568.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Leuschner WD, Hoch W. Subtype-specific assembly of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is mediated by their N-terminal domains. J Biol Chem. 1999;274:16907–16916. doi: 10.1074/jbc.274.24.16907. [DOI] [PubMed] [Google Scholar]
- Li AJ, Suzuki M, Suzuki S, Ikemoto M, Imamura T. Differential phosphorylation at serine sites in glutamate receptor-1 within neonatal rat hippocampus. Neurosci Lett. 2003;341:41–44. doi: 10.1016/s0304-3940(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature (Lond) 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Zhang X, O’Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Lim IA, Merrill MA, Chen Y, Hell JW. Disruption of the NMDA receptor-PSD-95 interaction in hippocampal neurons with no obvious physiological short-term effect. Neuropharmacology. 2003;45:738–754. doi: 10.1016/s0028-3908(03)00276-4. [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lin L, Sahr KE, Chishti AH. Identification of the mouse homologue of human discs large and rat SAP97 genes. Biochim Biophys Acta. 1997;1362:1–5. doi: 10.1016/s0925-4439(97)00059-8. [DOI] [PubMed] [Google Scholar]
- Linden AM, Vasanen J, Storvik M, Lakso M, Korpi ER, Wong G, Castren E. Uncompetitive antagonists of the N-methyl-D-aspartate (NMDA) receptors alter the mRNA expression of proteins associated with the NMDA receptor complex. Pharmacol Toxicol. 2001;88:98–105. doi: 10.1034/j.1600-0773.2001.d01-89.x. [DOI] [PubMed] [Google Scholar]
- Linden DJ. Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron. 1997;18:983–994. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci. 2002;22:3881–3889. doi: 10.1523/JNEUROSCI.22-10-03881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature (Lond) 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science (Wash DC) 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical ‘barrel’ map development. Nat Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001a;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Lu X, Wyszynski M, Sheng M, Baudry M. Proteolysis of glutamate receptor-interacting protein by calpain in rat brain: implications for synaptic plasticity. J Neurochem. 2001b;77:1553–1560. doi: 10.1046/j.1471-4159.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Maas S, Patt S, Schrey M, Rich A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci USA. 2001;98:14687–14692. doi: 10.1073/pnas.251531398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science (Wash DC) 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- Magazanik LG, Buldakova SL, Samoilova MV, Gmiro VE, Mellor IR, Usherwood PN. Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J Physiol. 1997;505:655–663. doi: 10.1111/j.1469-7793.1997.655ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann NY Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Mano I, Teichberg VI. A tetrameric subunit stoichiometry for a glutamate receptor-channel complex. Neuroreport. 1998;9:327–331. doi: 10.1097/00001756-199801260-00027. [DOI] [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring RB, Clements JD, Rosenmund C. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–853. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience. 1993;53:327–358. doi: 10.1016/0306-4522(93)90199-p. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Furuta A, Blackstone CD. AMPA receptor protein in developing rat brain: glutamate receptor-1 expression and localization change at regional, cellular and subcellular levels with maturation. Neuroscience. 1998;83:917–928. doi: 10.1016/s0306-4522(97)00411-9. [DOI] [PubMed] [Google Scholar]
- Maruo K, Nagata T, Yamamoto S, Nagai K, Yajima Y, Maruo S, Nishizaki T. Tunicamycin inhibits NMDA and AMPA receptor responses independently of N-glycosylation. Brain Res. 2003;977:294–297. doi: 10.1016/s0006-8993(03)02838-5. [DOI] [PubMed] [Google Scholar]
- Mat Jais AM, Kerkut GA, Walker RJ. The ionic mechanisms associated with the excitatory response of kainate, L-glutamate, quisqualate, ibotenate, AMPA and methyltetrahydrofolate on leech Retzius cells. Comp Biochem Physiol C. 1984;77:115–126. doi: 10.1016/0742-8413(84)90139-7. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO (Eur Mol Biol Organ) J. 2000;19:2765–2774. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature (Lond) 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauceri D, Cattabeni F, Di Luca M, Gardoni F. Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines. J Biol Chem. 2004;279:23813–23821. doi: 10.1074/jbc.M402796200. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- McBain CJ. A short-term mechanism of plasticity for interneurones? J Physiol (Lond) 1998;511:331. doi: 10.1111/j.1469-7793.1998.331bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Dingledine R. Heterogeneity of synaptic glutamate receptors on CA3 stratum radiatum interneurones of rat hippocampus. J Physiol. 1993;462:373–392. doi: 10.1113/jphysiol.1993.sp019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- McFeeters RL, Oswald RE. Emerging structural explanations of ionotropic glutamate receptor function. FASEB J. 2004;18:428–438. doi: 10.1096/fj.03-0873rev. [DOI] [PubMed] [Google Scholar]
- Mehta S, Wu H, Garner CC, Marshall J. Molecular mechanisms regulating the differential association of kainate receptor subunits with SAP90/PSD-95 and SAP97. J Biol Chem. 2001;276:16092–16099. doi: 10.1074/jbc.M100643200. [DOI] [PubMed] [Google Scholar]
- Menard C, Valastro B, Martel MA, Chartier E, Marineau A, Baudry M, Massicotte G. AMPA receptor phosphorylation is selectively regulated by constitutive phospholipase A(2) and 5-lipoxygenase activities. Hippocampus. 2005;15:370–380. doi: 10.1002/hipo.20061. [DOI] [PubMed] [Google Scholar]
- Meng SZ, Ohyu J, Takashima S. Changes in AMPA glutamate and dopamine D2 receptors in hypoxic-ischemic basal ganglia necrosis. Pediatr Neurol. 1997;17:139–143. doi: 10.1016/s0887-8994(97)00087-8. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Benz A, Zorumski CF. Components of glial responses to exogenous and synaptic glutamate in rat hippocampal microcultures. J Neurosci. 1996;16:55–64. doi: 10.1523/JNEUROSCI.16-01-00055.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Holzwarth JA, Miller RJ. Developmental regulation of the toxin sensitivity of Ca(2+)-permeable AMPA receptors in cortical glia. J Neurosci. 1996;16:519–530. doi: 10.1523/JNEUROSCI.16-02-00519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N. The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–733. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Mi R, Sia GM, Rosen K, Tang X, Moghekar A, Black JL, McEnery M, Huganir RL, O’Brien RJ. AMPA receptor-dependent clustering of synaptic NMDA receptors is mediated by Stargazin and NR2A/B in spinal neurons and hippocampal interneurons. Neuron. 2004;44:335–349. doi: 10.1016/j.neuron.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Mi R, Tang X, Sutter R, Xu D, Worley P, O’Brien RJ. Differing mechanisms for glutamate receptor aggregation on dendritic spines and shafts in cultured hippocampal neurons. J Neurosci. 2002;22:7606–7616. doi: 10.1523/JNEUROSCI.22-17-07606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Millan A, Arias-Montano JA, Mendez JA, Hernandez-Kelly LC, Ortega A. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors signaling complexes in Bergmann glia. J Neurosci Res. 2004;78:56. doi: 10.1002/jnr.20237. [DOI] [PubMed] [Google Scholar]
- Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr Opin Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97 and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 2002;22:5253–5258. doi: 10.1523/JNEUROSCI.22-13-05253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar E, McIlhinney RA, Baude A, Nusser Z, Somogyi P. Membrane topology of the GluR1 glutamate receptor subunit: epitope mapping by site-directed antipeptide antibodies. J Neurochem. 1994;63:683–693. doi: 10.1046/j.1471-4159.1994.63020683.x. [DOI] [PubMed] [Google Scholar]
- Molnar E, Pickard L, Duckworth JK. Developmental changes in ionotropic glutamate receptors: lessons from hippocampal synapses. Neuroscientist. 2002;8:143–153. doi: 10.1177/107385840200800210. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Yao D, Cotman CW. Distribution of [3H]AMPA binding sites in rat brain as determined by quantitative autoradiography. Brain Res. 1984;324:160–164. doi: 10.1016/0006-8993(84)90636-x. [DOI] [PubMed] [Google Scholar]
- Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- Mori K, Iwao K, Miyoshi Y, Nakagawara A, Kofu K, Akiyama T, Arita N, Hayakawa T, Nakamura Y. Identification of brain-specific splicing variants of thehDLG1 gene and altered splicing in neuroblastoma cell lines. J Hum Genet. 1998;43:123–127. doi: 10.1007/s100380050052. [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science (Wash DC) 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Moss FJ, Dolphin AC, Clare JJ. Human neuronal stargazin-like proteins, gamma2, gamma3 and gamma4; an investigation of their specific localization in human brain and their influence on CaV2.1 voltage-dependent calcium channels expressed in Xenopus oocytes. BMC Neurosci. 2003;4:23. doi: 10.1186/1471-2202-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature (Lond) 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science (Wash DC) 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Muller BM, Kistner U, Veh RW, Cases-Langhoff C, Becker B, Gundelfinger ED, Garner CC. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Moller T, Neuhaus J, Kettenmann H. Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia. 1996;17:274–284. doi: 10.1002/(SICI)1098-1136(199608)17:4<274::AID-GLIA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Musshoff U, Madeja M, Bloms P, Musch-Nittel K, Speckmann EJ. Tunicamycin-induced inhibition of functional expression of glutamate receptors in Xenopus oocytes. Neurosci Lett. 1992;147:163–166. doi: 10.1016/0304-3940(92)90585-u. [DOI] [PubMed] [Google Scholar]
- Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Shneider NA, Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990;5:569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A, Ito T, Nagata M, Hori S, Sekimizu K. GRIP1tau, a novel PDZ domain-containing transcriptional activator, cooperates with the testis-specific transcription elongation factor SII-T1. Genes Cells. 2004;9:1125–1135. doi: 10.1111/j.1365-2443.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Mikawa S, Hashikawa T, Ito M. Transient and persistent phosphorylation of AMPA-type glutamate receptor subunits in cerebellar Purkinje cells. Neuron. 1995a;15:697–709. doi: 10.1016/0896-6273(95)90157-4. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Tadakuma T, Nokihara K, Ito M. Antibody specific for phosphorylated AMPA-type glutamate receptors at GluR2 Ser-696. Neurosci Res. 1995b;24:75–86. doi: 10.1016/0168-0102(95)00977-9. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Matsumura T. The aniracetam metabolite 2-pyrrolidinone induces a long-term enhancement in AMPA receptor responses via a CaMKII pathway. Brain Res Mol Brain Res. 2002;98:130–134. doi: 10.1016/s0169-328x(01)00331-x. [DOI] [PubMed] [Google Scholar]
- Nishizaki T, Matsuoka T, Nomura T, Sumikawa K. A serum factor potentiates ACh and AMPA receptor currents via differential signal transduction pathways. Biochem Biophys Res Commun. 1997;238:565–568. doi: 10.1006/bbrc.1997.7264. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Nusser Z. AMPA and NMDA receptors: similarities and differences in their synaptic distribution. Curr Opin Neurobiol. 2000;10:337–341. doi: 10.1016/s0959-4388(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Kamboj RK. Differential RNA editing efficiency of AMPA receptor subunit GluR-2 in human brain. Neuroreport. 1994;5:1679–1683. doi: 10.1097/00001756-199408150-00034. [DOI] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Lau LF, Huganir RL. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol. 1998;8:364–369. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Oh JD, Geller AI, Zhang G, Chase TN. Gene transfer of constitutively active protein kinase C into striatal neurons accelerates onset of levodopa-induced motor response alterations in parkinsonian rats. Brain Res. 2003;971:18–30. doi: 10.1016/S0006-8993(03)02348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman M, Kallman AM, Bass BL. In vitro analysis of the binding of ADAR2 to the pre-mRNA encoding the GluR-B R/G site. RNA (NY) 2000;6:687–697. doi: 10.1017/s1355838200000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Yamada N, Kakegawa W, Tsuzuki K, Kawamura M, Nawa H, Iino M, Ozawa S. Sindbis viral-mediated expression of Ca2+-permeable AMPA receptors at hippocampal CA1 synapses and induction of NMDA receptor-independent long-term potentiation. Eur J Neurosci. 2001;13:1635–1643. doi: 10.1046/j.0953-816x.2001.01523.x. [DOI] [PubMed] [Google Scholar]
- O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders (Review) Curr Drug Targets CNS Neurol Disord. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Osten P, Ziff EB. AMPA receptor forms a biochemically functional complex with NSF and alpha- and beta-SNAPs. Ann NY Acad Sci. 1999;868:558–560. doi: 10.1111/j.1749-6632.1999.tb11328.x. [DOI] [PubMed] [Google Scholar]
- Paarmann I, Spangenberg O, Lavie A, Konrad M. Formation of complexes between Ca2+.calmodulin and the synapse-associated protein SAP97 requires the SH3 domain-guanylate kinase domain-connecting HOOK region. J Biol Chem. 2002;277:40832–40838. doi: 10.1074/jbc.M205618200. [DOI] [PubMed] [Google Scholar]
- Paas Y. The macro- and microarchitectures of the ligand-binding domain of glutamate receptors. Trends Neurosci. 1998;21:117–125. doi: 10.1016/s0166-2236(97)01184-3. [DOI] [PubMed] [Google Scholar]
- Park E, Liu Y, Fehlings MG. Changes in glial cell white matter AMPA receptor expression after spinal cord injury and relationship to apoptotic cell death. Exp Neurol. 2003;182:35–48. doi: 10.1016/s0014-4886(03)00084-0. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Mayer ML. Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol. 1994;46:129–138. [PubMed] [Google Scholar]
- Paschen W, Schmitt J, Uto A. RNA editing of glutamate receptor subunits GluR2, GluR5 and GluR6 in transient cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1996;16:548–556. doi: 10.1097/00004647-199607000-00004. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature (Lond) 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Pasternack A, Coleman SK, Fethiere J, Madden DR, LeCaer JP, Rossier J, Pasternack M, Keinanen K. Characterization of the functional role of the N-glycans in the AMPA receptor ligand-binding domain. J Neurochem. 2003;84:1184–1192. doi: 10.1046/j.1471-4159.2003.01611.x. [DOI] [PubMed] [Google Scholar]
- Pasternack A, Coleman SK, Jouppila A, Mottershead DG, Lindfors M, Pasternack M, Keinanen K. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels lacking the N-terminal domain. J Biol Chem. 2002;277:49662–49667. doi: 10.1074/jbc.M208349200. [DOI] [PubMed] [Google Scholar]
- Peiretti F, Deprez-Beauclair P, Bonardo B, Aubert H, Juhan-Vague I, Nalbone G. Identification of SAP97 as an intracellular binding partner of TACE. J Cell Sci. 2003;116:1949–1957. doi: 10.1242/jcs.00415. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Bennett MV, Zukin RS. Differential expression of three glutamate receptor genes in developing rat brain: an in situ hybridization study. Proc Natl Acad Sci USA. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perestenko PV, Henley JM. Characterization of the intracellular transport of GluR1 and GluR2 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2003;278:43525–43532. doi: 10.1074/jbc.M306206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mayat E, Wenthold RJ. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J Comp Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Pickard L, Noel J, Duckworth JK, Fitzjohn SM, Henley JM, Collingridge GL, Molnar E. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology. 2001;41:700–713. doi: 10.1016/s0028-3908(01)00127-7. [DOI] [PubMed] [Google Scholar]
- Pickard L, Noel J, Henley JM, Collingridge GL, Molnar E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci. 2000;20:7922–7931. doi: 10.1523/JNEUROSCI.20-21-07922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Rodrigues RJ, Silva AP, Cunha RA, Oliveira CR, Malva JO. Solubilization and immunological identification of presynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in the rat hippocampus. Neurosci Lett. 2003;336:97–100. doi: 10.1016/s0304-3940(02)01217-x. [DOI] [PubMed] [Google Scholar]
- Pires RS, Reboucas NA, Duvoisin RM, Britto LR. Retinal lesions induce differential changes in the expression of flip and flop isoforms of the glutamate receptor subunit GluR1 in the chick optic tectum. Brain Res Mol Brain Res. 2000;76:341–346. doi: 10.1016/s0169-328x(00)00016-4. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Phillips C. DHR domains in syntrophins, neuronal NO synthases and other intracellular proteins. Trends Biochem Sci. 1995;20:102–103. doi: 10.1016/s0968-0004(00)88973-2. [DOI] [PubMed] [Google Scholar]
- Price CJ, Kim P, Raymond LA. D1 dopamine receptor-induced cyclic AMP-dependent protein kinase phosphorylation and potentiation of striatal glutamate receptors. J Neurochem. 1999;73:2441–2446. doi: 10.1046/j.1471-4159.1999.0732441.x. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kugelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Bienemann A, Ma J, Tan HK, Noel J, Henley JM, Uney JB. Disruption of the GluR2-NSF interaction protects primary hippocampal neurons from ischemic stress. Mol Cell Neurosci. 2001;17:662–670. doi: 10.1006/mcne.2000.0959. [DOI] [PubMed] [Google Scholar]
- Reti IM, Baraban JM. Sustained increase in Narp protein expression following repeated electroconvulsive seizure. Neuropsychopharmacology. 2000;23:439–443. doi: 10.1016/S0893-133X(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Robert A, Irizarry SN, Hughes TE, Howe JR. Subunit interactions and AMPA receptor desensitization. J Neurosci. 2001;21:5574–5586. doi: 10.1523/JNEUROSCI.21-15-05574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Roche KW, Tingley WG, Huganir RL. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994;4:383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Hughes TE, Hollmann M, Gasic GP, Deneris ES, Heinemann S. The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J Neurosci. 1991;11:2713–2724. doi: 10.1523/JNEUROSCI.11-09-02713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Lu X, Bernard A, Khrestchatisky M, Baudry M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J Neurochem. 2001;79:382–390. doi: 10.1046/j.1471-4159.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science (Wash DC) 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rump A, Sommer C, Gass P, Bele S, Meissner D, Kiessling M. Editing of GluR2 RNA in the gerbil hippocampus after global cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:1362–1365. doi: 10.1097/00004647-199611000-00034. [DOI] [PubMed] [Google Scholar]
- Safferling M, Tichelaar W, Kummerle G, Jouppila A, Kuusinen A, Keinanen K, Madden DR. First images of a glutamate receptor ion channel: oligomeric state and molecular dimensions of GluRB homomers. Biochemistry. 2001;40:13948–13953. doi: 10.1021/bi011143g. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Bujo H, Kushiya E, Araki K, Yamazaki M, Meguro H, Warashina A, Numa S, Mishina M. Functional expression from cloned cDNAs of glutamate receptor species responsive to kainate and quisqualate. FEBS Lett. 1990;272:73–80. doi: 10.1016/0014-5793(90)80452-o. [DOI] [PubMed] [Google Scholar]
- Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Vissel B, Petralia RS, Wang YX, Chang K, Royle GA, Wang CY, O’Gorman S, Heinemann SF, Wenthold RJ. Aberrant formation of glutamate receptor complexes in hippocampal neurons of mice lacking the GluR2 AMPA receptor subunit. J Neurosci. 2003;23:9367–9373. doi: 10.1523/JNEUROSCI.23-28-09367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtchenko LP, Korogod SM, Rusakov DA. Electrodiffusion of synaptic receptors: a mechanism to modify synaptic efficacy? Synapse. 2000;35:26–38. doi: 10.1002/(SICI)1098-2396(200001)35:1<26::AID-SYN4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Schenk U, Verderio C, Benfenati F, Matteoli M. Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO (Eur Mol Biol Organ) J. 2003;22:558–568. doi: 10.1093/emboj/cdg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh K, Uldrijan S, Gambaryan S, Roethlein N, Neyses L. Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK. J Biol Chem. 2003;278:9778–9783. doi: 10.1074/jbc.M212507200. [DOI] [PubMed] [Google Scholar]
- Seal AJ, Collingridge GL, Henley JM. An investigation of the membrane topology of the ionotropic glutamate receptor subunit GluR1 in a cell-free system. Biochem J. 1995;312:451–456. doi: 10.1042/bj3120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Hartner J. Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr Opin Neurobiol. 2003;13:279–283. doi: 10.1016/s0959-4388(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Schramm J, Steinhauser C. Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon’s horn sclerosis. J Neurosci. 2004;24:1996–2003. doi: 10.1523/JNEUROSCI.3904-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert G, Schroder W, Hinterkeuser S, Schumacher T, Schramm J, Steinhauser C. Changes in flip/flop splicing of astroglial AMPA receptors in human temporal lobe epilepsy. Epilepsia. 2002;43(Suppl 5):162–167. doi: 10.1046/j.1528-1157.43.s.5.10.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Fleck MW, Mayer ML, Takeo J, Chiba Y, Yamashita S, Wada K. A novel allosteric potentiator of AMPA receptors: 4–2-(phenylsulfonylamino)ethylthio–2,6-difluoro-phenoxyacetamide. J Neurosci. 1997;17:5760–5771. doi: 10.1523/JNEUROSCI.17-15-05760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M, Takeo J, Harada T, Morimoto T, Kudo Y, Yamashita S, Kohsaka S, Wada K. Pharmacological detection of AMPA receptor heterogeneity by use of two allosteric potentiators in rat hippocampal cultures. Br J Pharmacol. 1998;123:1294–1303. doi: 10.1038/sj.bjp.0701707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seog DH. Glutamate receptor-interacting protein 1 protein binds to the microtubule-associated protein. Biosci Biotechnol Biochem. 2004;68:1808–1810. doi: 10.1271/bbb.68.1808. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature (Lond) 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shaw CA, Lanius RA. Reversible kinase and phosphatase regulation of brain amino acid receptors in postnatal development. Brain Res Dev Brain Res. 1992;70:153–161. doi: 10.1016/0165-3806(92)90193-z. [DOI] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Yang XL. Zinc modulation of AMPA receptors may be relevant to splice variants in carp retina. Neurosci Lett. 1999;259:177–180. doi: 10.1016/s0304-3940(98)00938-0. [DOI] [PubMed] [Google Scholar]
- Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci USA. 2001;98:7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–134. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Sheridan DL, Berlot CH, Robert A, Inglis FM, Jakobsdottir KB, Howe JR, Hughes TE. A new way to rapidly create functional, fluorescent fusion proteins: random insertion of GFP with an in vitro transposition reaction. BMC Neurosci. 2002;3:7. doi: 10.1186/1471-2202-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science (Wash DC) 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Silveira dos Santos Bredariol A, Hamassaki-Britto DE. Ionotropic glutamate receptors during the development of the chick retina. J Comp Neurol. 2001;441:58–70. doi: 10.1002/cne.1397. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol. 2004;19:343–360. doi: 10.1177/088307380401900507. discussion 361. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Galdi S, Fienberg AA, Allen P, Nairn AC, Greengard P. Regulation of AMPA receptor dephosphorylation by glutamate receptor agonists. Neuropharmacology. 2003;45:703–713. doi: 10.1016/s0028-3908(03)00319-8. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science (Wash DC) 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science (Wash DC) 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Spreafico R, Frassoni C, Arcelli P, Battaglia G, Wenthold RJ, De Biasi S. Distribution of AMPA selective glutamate receptors in the thalamus of adult rats and during postnatal development. A light and ultrastructural immunocytochemical study. Brain Res Dev Brain Res. 1994;82:231–244. doi: 10.1016/0165-3806(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Ziff EB. ABP: a novel AMPA receptor binding protein. Ann NY Acad Sci. 1999;868:561–564. doi: 10.1111/j.1749-6632.1999.tb11329.x. [DOI] [PubMed] [Google Scholar]
- Standley S, Baudry M. The role of glycosylation in ionotropic glutamate receptor ligand binding, function and trafficking. Cell Mol Life Sci. 2000;57:1508–1516. doi: 10.1007/PL00000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley S, Tocco G, Tourigny MF, Massicotte G, Thompson RF, Baudry M. Developmental changes in alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor properties and expression in the rat hippocampal formation. Neuroscience. 1995;67:881–892. doi: 10.1016/0306-4522(95)00075-t. [DOI] [PubMed] [Google Scholar]
- Standley S, Tocco G, Wagle N, Baudry M. High- and low-affinity alpha-[3H]amino-3-hydroxy-5-methylisoxazole-4-propionic acid ([3H]AMPA) binding sites represent immature and mature forms of AMPA receptors and are composed of differentially glycosylated subunits. J Neurochem. 1998;70:2434–2445. doi: 10.1046/j.1471-4159.1998.70062434.x. [DOI] [PubMed] [Google Scholar]
- Stegmuller J, Schneider S, Hellwig A, Garwood J, Trotter J. AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration. J Neurocytol. 2002;31:497–505. doi: 10.1023/a:1025743731306. [DOI] [PubMed] [Google Scholar]
- Stegmuller J, Werner H, Nave KA, Trotter J. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells. Implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Stensbol TB, Madsen U, Krogsgaard-Larsen P. The AMPA receptor binding site: focus on agonists and competitive antagonists. Curr Pharm Des. 2002;8:857–872. doi: 10.2174/1381612024607090. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O’Hara PJ, Heinemann SF. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Stone TW, Addae JI. The pharmacological manipulation of glutamate receptors and neuroprotection. Eur J Pharmacol. 2002;447:285–296. doi: 10.1016/s0014-2999(02)01851-4. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem. 1997;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Stricker NL, Huganir RL. The PDZ domains of mLin-10 regulate its trans-Golgi network targeting and the surface expression of AMPA receptors. Neuropharmacology. 2003;45:837–848. doi: 10.1016/s0028-3908(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Stromgaard K, Mellor I. AMPA receptor ligands: synthetic and pharmacological studies of polyamines and polyamine toxins. Med Res Rev. 2004;24:589–620. doi: 10.1002/med.20004. [DOI] [PubMed] [Google Scholar]
- Sugden SG, Zirpel L, Dietrich CJ, Parks TN. Development of the specialized AMPA receptors of auditory neurons. J Neurobiol. 2002;52:189–202. doi: 10.1002/neu.10078. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Takagi N, Besshoh S, Miyake-Takagi K, Takeo S. Transient global ischemia enhances phosphorylation of the GluR1 subunit of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor in the hippocampal CA1 region in rats. Neurosci Lett. 2003;341:33–36. doi: 10.1016/s0304-3940(03)00153-8. [DOI] [PubMed] [Google Scholar]
- Takamiya K, Kostourou V, Adams S, Jadeja S, Chalepakis G, Scambler PJ, Huganir RL, Adams RH. A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1. Nat Genet. 2004;36:172–177. doi: 10.1038/ng1292. [DOI] [PubMed] [Google Scholar]
- Takuma H, Kwak S, Yoshizawa T, Kanazawa I. Reduction of GluR2 RNA editing, a molecular change that increases calcium influx through AMPA receptors, selective in the spinal ventral gray of patients with amyotrophic lateral sclerosis. Ann Neurol. 1999;46:806–815. doi: 10.1002/1531-8249(199912)46:6<806::aid-ana2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Grooms SY, Bennett MV, Zukin RS. The AMPAR subunit GluR2: still front and center-stage. Brain Res. 2000;886:190–207. doi: 10.1016/s0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO (Eur Mol Biol Organ) J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalhammer A, Morth T, Strutz N, Hollmann M. A desensitization-inhibiting mutation in the glutamate binding site of rat alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is dominant in heteromultimeric complexes. Neurosci Lett. 1999;277:161–164. doi: 10.1016/s0304-3940(99)00885-x. [DOI] [PubMed] [Google Scholar]
- Thomas U, Phannavong B, Muller B, Garner CC, Gundelfinger ED. Functional expression of rat synapse-associated proteins SAP97 and SAP102 in Drosophila dlg-1 mutants: effects on tumor suppression and synaptic bouton structure. Mech Dev. 1997;62:161–174. doi: 10.1016/s0925-4773(97)00658-8. [DOI] [PubMed] [Google Scholar]
- Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J Cell Biol. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov DB, Mellor JR, Usherwood PN, Magazanik LG. Modeling of the pore domain of the GLUR1 channel: homology with K+ channel and binding of channel blockers. Biophys J. 2002;82:1884–1893. doi: 10.1016/S0006-3495(02)75538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Fujita T, Kirino Y, Suzuki T. PDZ domain-dependent suppression of NF-kappaB/p65-induced Abeta42 production by a neuron-specific X11-like protein. J Biol Chem. 2000;275:13056–13060. doi: 10.1074/jbc.c000019200. [DOI] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science (Wash DC) 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Tomiyama M, Rodriguez-Puertas R, Cortes R, Pazos A, Palacios JM, Mengod G. Flip and flop splice variants of AMPA receptor subunits in the spinal cord of amyotrophic lateral sclerosis. Synapse. 2002;45:245–249. doi: 10.1002/syn.10098. [DOI] [PubMed] [Google Scholar]
- Tonnes J, Stierli B, Cerletti C, Behrmann JT, Molnar E, Streit P. Regional distribution and developmental changes of GluR1-flop protein revealed by monoclonal antibody in rat brain. J Neurochem. 1999;73:2195–2205. [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Iritani S, Makifuchi T, Shirakawa O, Kitamura N, Maeda K, Nakamura R, Niizato K, Watanabe M, Kakita A, et al. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M. Direct interaction of GluRdelta2 with Shank scaffold proteins in cerebellar Purkinje cells. Mol Cell Neurosci. 2004;26:330–341. doi: 10.1016/j.mcn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Utz AL, Verdoorn TA. Recombinant AMPA receptors with low Ca2+ permeability increase intracellular Ca2+ in HEK 293 cells. Neuroreport. 1997;8:1975–1980. doi: 10.1097/00001756-199705260-00036. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Burette A, Davare MA, Leonard AS, Hell JW, Weinberg RJ. SAP97 concentrates at the postsynaptic density in cerebral cortex. Eur J Neurosci. 2000;12:3605–3614. doi: 10.1046/j.1460-9568.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci USA. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Alonso M, Viola H, Quevedo J, de Paris F, Furman M, de Stein ML, Medina JH, Izquierdo I. Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat. Learn Mem (Cold Spring Harb) 2000;7:333–340. doi: 10.1101/lm.34600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinade L, Dosemeci A. Regulation of the phosphorylation state of the AMPA receptor GluR1 subunit in the postsynaptic density. Cell Mol Neurobiol. 2000;20:451–463. doi: 10.1023/A:1007019030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar W, Gloger J, Berger E, Kortenbruck G, Kohling R, Speckmann EJ, Musshoff U. RNA editing (R/G site) and flip-flop splicing of the AMPA receptor subunit GluR2 in nervous tissue of epilepsy patients. Neurobiol Dis. 2004;15:371–379. doi: 10.1016/j.nbd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Narisawa-Saito M, Iwakura Y, Arai T, Ikeda K, Takahashi H, Nawa H. Phenotypic down-regulation of glutamate receptor subunit GluR1 in Alzheimer’s disease. Neurobiol Aging. 1999;20:287–295. doi: 10.1016/s0197-4580(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci. 1999;19:6457–6467. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Robert A, Howe JR, Usowicz MM. The speeding of EPSC kinetics during maturation of a central synapse. Eur J Neurosci. 2002;15:785–797. doi: 10.1046/j.1460-9568.2002.01910.x. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Odermatt B, Willecke K, Steinhauser C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia. 2004;48:36–43. doi: 10.1002/glia.20040. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L. Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol Neurobiol. 2004;29:1–14. doi: 10.1385/MN:29:1:01. [DOI] [PubMed] [Google Scholar]
- Wang Y, Durkin JP. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, but not N-methyl-D-aspartate, activates mitogen-activated protein kinase through G-protein beta gamma subunits in rat cortical neurons. J Biol Chem. 1995;270:22783–22787. doi: 10.1074/jbc.270.39.22783. [DOI] [PubMed] [Google Scholar]
- Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature (Lond) 1997;389:502–504. doi: 10.1038/39062. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Watkins JC. Pharmacology of excitatory amino acid transmitters. Adv Biochem Psychopharmacol. 1981;29:205–212. [PubMed] [Google Scholar]
- Watkins JC. Some chemical highlights in the development of excitatory amino acid pharmacology. Can J Physiol Pharmacol. 1991;69:1064–1075. doi: 10.1139/y91-158. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Davies J, Evans RH, Francis AA, Jones AW. Pharmacology of receptors for excitatory amino acids. Adv Biochem Psychopharmacol. 1981;27:263–273. [PubMed] [Google Scholar]
- Weiser T. AMPA receptor antagonists with additional mechanisms of action: new opportunities for neuroprotective drugs? Curr Pharm Des. 2002;8:941–951. doi: 10.2174/1381612024607135. [DOI] [PubMed] [Google Scholar]
- Wells GB, Lin L, Jeanclos EM, Anand R. Assembly and ligand binding properties of the water-soluble extracellular domains of the glutamate receptor 1 subunit. J Biol Chem. 2001;276:3031–3036. doi: 10.1074/jbc.M006668200. [DOI] [PubMed] [Google Scholar]
- Wo ZG, Bian ZC, Oswald RE. Asn-265 of frog kainate binding protein is a functional glycosylation site: implications for the transmembrane topology of glutamate receptors. FEBS Lett. 1995;368:230–234. doi: 10.1016/0014-5793(95)00655-s. [DOI] [PubMed] [Google Scholar]
- Wo ZG, Oswald RE. Transmembrane topology of two kainate receptor subunits revealed by N-glycosylation. Proc Natl Acad Sci USA. 1994;91:7154–7158. doi: 10.1073/pnas.91.15.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann NY Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC. Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem. 2002;277:30928–30934. doi: 10.1074/jbc.M203735200. [DOI] [PubMed] [Google Scholar]
- Wu H, Reissner C, Kuhlendahl S, Coblentz B, Reuver S, Kindler S, Gundelfinger ED, Garner CC. Intramolecular interactions regulate SAP97 binding to GKAP. EMBO (Eur Mol Biol Organ) J. 2000;19:5740–5751. doi: 10.1093/emboj/19.21.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- Wu TY, Liu CI, Chang YC. A study of the oligomeric state of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring glutamate receptors in the synaptic junctions of porcine brain. Biochem J. 1996;319:731–739. doi: 10.1042/bj3190731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyneken U, Riquelme G, Villanueva S, Orrego F. Effect of glutamate receptor phosphorylation by endogenous protein kinases on electrical activity of isolated postsynaptic densities of rat cortex and hippocampus. Neurosci Lett. 1997;224:131–135. doi: 10.1016/s0304-3940(97)13442-5. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Yang FC, Sheng M. Biochemical and immunocytochemical characterization of GRIP, a putative AMPA receptor anchoring protein, in rat brain. Neuropharmacology. 1998;37:1335–1344. doi: 10.1016/s0028-3908(98)00120-8. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci. 1999;19:6528–6537. doi: 10.1523/JNEUROSCI.19-15-06528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Vissavajjhala P, Derkach VA, Brickey DA, Soderling TR. Identification of a Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in non-N-methyl-D-aspartate glutamate receptors. Proc Natl Acad Sci USA. 1995;92:1376–1380. doi: 10.1073/pnas.92.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Yamaguchi F, Miyamoto O, Hatase O, Tokuda M. The reversible change of GluR2 RNA editing in gerbil hippocampus in course of ischemic tolerance. J Cereb Blood Flow Metab. 1999;19:370–375. doi: 10.1097/00004647-199904000-00002. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fukaya M, Abe M, Ikeno K, Kakizaki T, Watanabe M, Sakimura K. Differential palmitoylation of two mouse glutamate receptor interacting protein 1 forms with different N-terminal sequences. Neurosci Lett. 2001;304:81–84. doi: 10.1016/s0304-3940(01)01766-9. [DOI] [PubMed] [Google Scholar]
- Ye B, Liao D, Zhang X, Zhang P, Dong H, Huganir RL. GRASP-1: a neuronal RasGEF associated with the AMPA receptor/GRIP complex. Neuron. 2000;26:603–617. doi: 10.1016/s0896-6273(00)81198-8. [DOI] [PubMed] [Google Scholar]
- Ye B, Sugo N, Hurn PD, Huganir RL. Physiological and pathological caspase cleavage of the neuronal RasGEF GRASP-1 as detected using a cleavage site-specific antibody. Neuroscience. 2002;114:217–227. doi: 10.1016/s0306-4522(02)00142-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Shinkawa T, Taoka M, Kobayashi K, Isobe T, Yamauchi T. Identification of protein substrates of Ca(2+)/calmodulin-dependent protein kinase II in the postsynaptic density by protein sequencing and mass spectrometry. Biochem Biophys Res Commun. 2002;290:948–954. doi: 10.1006/bbrc.2001.6320. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of oligodendroglial excitotoxicity. J Neurosci Res. 1996;46:427–437. doi: 10.1002/(SICI)1097-4547(19961115)46:4<427::AID-JNR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Yu G, Zerucha T, Ekker M, Rubenstein JL. Evidence that GRIP, a PDZ-domain protein which is expressed in the embryonic forebrain, co-activates transcription with DLX homeodomain proteins. Brain Res Dev Brain Res. 2001;130:217–230. doi: 10.1016/s0165-3806(01)00239-5. [DOI] [PubMed] [Google Scholar]
- Zhao D, Watson JB, Xie CW. Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol. 2004;92:2853–2858. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA. Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci USA. 2001a;98:1261–1266. doi: 10.1073/pnas.031573798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QQ, Imbe H, Zou S, Dubner R, Ren K. Selective upregulation of the flip-flop splice variants of AMPA receptor subunits in the rat spinal cord after hindpaw inflammation. Brain Res Mol Brain Res. 2001b;88:186–193. doi: 10.1016/s0169-328x(01)00041-9. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Aelst L Van, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Meerschaert K, Reekmans G, Leenaerts I, Small JV, Vandekerckhove J, David G, Gettemans J. PIP(2)-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol Cell. 2002;9:1215–1225. doi: 10.1016/s1097-2765(02)00549-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Tomatis D, Rosas M, Grootjans J, Leenaerts I, Degeest G, Reekmans G, Coomans C, David G. Characterization of syntenin, a syndecan-binding PDZ protein, as a component of cell adhesion sites and microfilaments. Mol Biol Cell. 2001;12:339–350. doi: 10.1091/mbc.12.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]