Abstract
The neuron-specific G protein-coupled receptor interacting scaffold protein (GISP) is a multidomain, brain-specific protein derived from the A-kinase anchoring protein-9 gene. We originally isolated GISP as an interacting partner for the GABAB receptor subunit GABAB1. Here, we show that the protein tumour susceptibility gene 101 (TSG101), an integral component of the endosomal sorting machinery that targets membrane proteins for lysosomal degradation, also interacts with GISP. TSG101 co-immunoprecipitates with GISP from adult rat brain, and using GST pull-downs, we identified that the eighth coiled-coiled region of GISP is critical for TSG101 association. Intriguingly, although there is no direct interaction between GISP and the GABAB2 subunit, their co-expression in HEK293 cells increases levels of GABAB2. GISP also inhibits TSG101-dependent GABAB2 down-regulation in human embryonic kidney 293 cells whereas over-expression of a mutant GISP lacking the TSG101 binding domain has no effect on GABAB2 degradation. These data suggest that GISP can function as a negative regulator of TSG101-dependent lysosomal degradation of transmembrane proteins in neurons to promote receptor stability.
Keywords: A-kinase anchoring protein, degradation, endosomal sorting complex required for transport, G protein-coupled receptor, G protein-coupled receptor interacting scaffold protein, GABAB, receptor trafficking, tumour susceptibility gene 101
A-kinase anchoring proteins are a family of more than 50 separate multidomain scaffolding proteins that bind plasma membrane receptors, cytoskeletal proteins and signal transduction/termination enzymes to mediate a diverse array of cellular functions (Wong and Scott 2004). G protein-coupled receptor interacting scaffolding protein (GISP) is a brain-specific protein that has 90% homology with human A-kinase anchoring protein 450 (Kantamneni et al. 2007). GISP binds to the GABAB1 subunit and its co-expression in human embryonic kidney (HEK293) cells with GABAB1 and GABAB2 subunits significantly up-regulates the surface expression of recombinant GABAB receptor complexes (Kantamneni et al. 2007).
Effective trafficking of GABAB2 to the plasma membrane in the absence of GABAB1 is well established (Couve et al. 1998; Marshall et al. 1999; Pagano et al. 2001). However, to form functional receptors GABAB1 and GABAB2 subunits must heterodimerize, in part via coiled-coil domains in their cytoplasmic C termini (Marshall et al. 1999). Heterodimerization is also necessary to overcome GABAB1 retention in the endoplasmic reticulum (Margeta-Mitrovic et al., 2000; Pagano et al. 2001).
As GISP does not directly interact with the GABAB2 subunit or any other G protein-coupled receptor or ligand-gated ion channel receptor proteins tested, we reasoned that GISP increased the surface expression of GABAB receptors via a direct interaction with GABAB1 (Kantamneni et al. 2007). Subsequent experiments, however, have revealed that GISP increases expression levels of GABAB2 when co-expressed in HEK293 cells in the absence of GABAB1. We interpreted this finding to suggest that GISP could facilitate receptor synthesis or block receptor degradation. To identify GISP interactors that may mediate this effect we performed yeast 2-hybrid screens using GISP as a bait and isolated tumour susceptibility gene 101 (TSG101), a protein known to be involved in receptor down-regulation via endocytosis-lysosome pathway (Bache et al. 2004).
Tumour susceptibility gene 101 is the mammalian homologue of yeast Vps23 (Babst et al. 2000), a Class E Vps protein shown to function in the vacuolar sorting of membrane proteins (Li et al. 1999). In both yeast and mammals, Class E Vps proteins assemble into three large heteromeric complexes, termed the endosomal sorting complex required for transport (ESCRT) complexes, which orchestrate the sorting of endocytosed mono-ubiquitinated membrane proteins to the lysosome (Babst 2005). Activated cell surface receptors undergo endocytosis and mono-ubiquitination prior to transport to the peripheral sorting endosome where they are targeted to membrane subdomains containing components of the ESCRT machinery. Subsequent to the selection and concentration of ubiquitinated cargo proteins by the ESCRT machinery, the limiting membrane of the sorting endosome buds inwards, creating intralumenal vesicles. On fusion of this multivesicular body with lysosomes, the cargo residing on the intralumenal vesicles is delivered to the lysosomal lumen for degradation (for reviews see Katzmann et al. 2002; Gruenberg and Stenmark 2004; Slagsvold et al. 2006).
Numerous disorders result from aberrant protein degradation in neurons so proper regulation of the ESCRT machinery is critical. For example, a mutation in the charged multivesicular body protein 2b (CHMP2b) component of ESCRT-III has been reported in patients with autosomal dominant frontotemporal dementia (Skibinski et al. 2005) and amyotrophic lateral sclerosis (Parkinson et al. 2006). In addition, recent evidence suggests ESCRT dysfunction in neurons can result in neurodegenerative disorders resulting from aberrant autophagic protein degradation (Lee et al. 2007; Rusten et al. 2007; Lee and Gao 2008). TSG101 in particular appears critical for neuronal survival. For example mutations in Mahogunin, an E3 ubiquitin ligase that ubiquitinates TSG101 leading to its degradation, causes spongiform neurodegeneration in mice (Kim et al. 2007). In addition, mutations in the TSG101-binding protein small interval membrane protein of lysosome/endosome (SIMPLE) lead to Charcot–Marie–Tooth disease type 1C, a demyelinating disease affecting peripheral neurons (Shirk et al. 2005).
Here, we report that GISP and TSG101 are present in the same complex in both HEK293 cells and the brain. GISP increases levels of both GABAB receptor subunits when co-expressed in HEK293 cells despite the fact it does not bind directly to the GABAB2 subunit. Crucially, co-expression of a mutant GISP that cannot bind TSG101 has no effect on GABAB2 levels. These results suggest that GISP may function as a brain-specific down-regulator of lysosomal degradation via its interaction with TSG101.
Materials and methods
Plasmid constructs
Full-length GISP in pcDNA3.1 was produced as described previously (Kantamneni et al. 2007). A GISP mutant lacking the Δ8 region (GISPΔ8-Mut) was produced by overlapping PCR. cDNA encoding amino acids 1–779 was amplified using the primers CTCTGTAAGCTTGATATGGAGAGGACGGGCAAGG TC and TATGGCTTCGATGTCAGCGTCACCTGGCTGGCCAGT. cDNA encoding amino acids 851–1115 was amplified using the primers ACTGGCCAGCCAGGTGACGCTGACATCGAAGCC ATA and CTCAGAGGACTCGAGTTATCTCCTCATGCCAAAATG.
The resulting overlapping PCR products were then fused by selfpriming PCR before addition of the end primers and further cycling. The final product was then cloned into HindIII and XhoI linearized pcDNA3.1(+) (Invitrogen, Carlsbad, CA, USA), and the fidelity of this construct confirmed by sequencing.
Yeast 2-hybrid screening and analysis of TSG101-GISP interaction
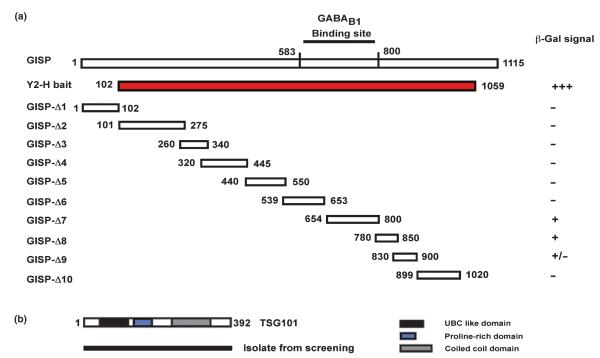
GISP (residues G102–Y1059; Fig. 1a) was subcloned into the pBTM-116ADE vector and used to screen an adult rat brain cDNA library (Clontech, Palo Alto, CA, USA) in the Saccharomyces cerevisiae L-40 reporter strain yeast as described previously (Nishimune et al. 1998).
Fig. 1.
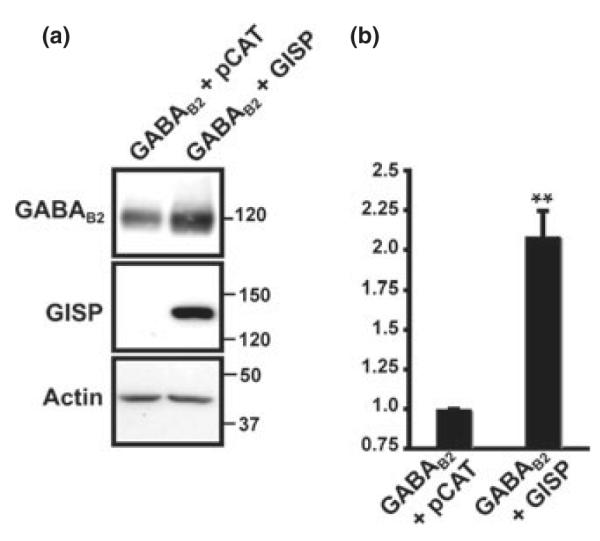
GISP increases levels of GABAB2. (a) GISP increases levels of GABAB2 in HEK293 cells. HEK293 cells were transfected with the receptor plasmid together with either a control vector (pCAT) or vector encoding GISP; 48 h post-transfection, the total receptor protein was determined by western blotting with anti-GABAB2 antibody. (b) The bar charts show normalized densitometric analysis of seven experiments. The increase in receptor expression when co-transfected with GISP is significant; **p < 0.0002.
HEK293 cell culture, transfection and lysis
Human embryonic kidney cells were cultured as previously described (Bouschet and Henley 2005). Cells were transfected using Lipofectamine 2000 (Invitrogen) and incubated for 24–48 h prior to harvesting. Transfected cells were washed twice with phosphate-buffered saline (Gibco, Rockville, MD, USA) and scraped into lysis buffer [0.125% (v/v) Nonidet P-40 (Sigma, St. Louis, MO, USA), 50 mM Tris, 125 mM NaCl, pH 7.4, containing EDTA-free protease inhibitors cocktail (Roche, Basel, Switzerland)] before being sonicated and mixed on a head-over-head shaker for 1 h at 4 °C. Lysates were then centrifuged at 16 000 g for 20 min and the pellets were discarded.
siRNA sequences and transfection
RNA sequences for TSG101 coding nt 413–433 were published previously (Garrus et al. 2001). Briefly, 21 nt RNA duplexes with symmetric 2 nt 3′ (2′-deoxy) thymidine overhangs were synthesized and purified by HPLC (Pro-Oligo; Sigma, St Louis, MO, USA). Oligonucleotides were annealed as described previously (Elbashir et al. 2001). HEK293 cells were transfected with 10 nM of siRNA nucleotides for 1 mL transfection mix and were further transfected again after 24 h. Cells were harvested after 24 h of second transfection.
Rat brain subcellular fractionation and developmental profiles
Fractions were obtained as described previously described (Kantamneni et al. 2007).
Co-immunoprecipitation
Human embryonic kidney 293 cells
Anti-myc conjugated agarose beads (5 μg of antibody; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were mixed with 20 μL of standard protein G beads (Sigma) and washed three times with lysis buffer. Lysates from HEK293 cells transiently expressing TSG101-myc and GISP were then diluted 1 : 1 (v/v) with 50 mM Tris, pH 7.4, and added to the beads. The beads were then mixed on a head-over-head shaker for 2 h at 4 °C before being washed five times with lysis buffer. Residual buffer was removed, 2x sodium dodecyl sulfate–polyacrylamide gel electrophoresis loading buffer added and the beads heated to 95 °C for 6 min prior to loading. For the control condition, 2.5 μg of mouse IgG (Neomarkers, Fremont, CA, USA) were immobilized on 20 μL of standard protein G beads.
Adult rat brain
The enriched microsomal membrane fraction from a whole adult rat brain was re-suspended in lysis buffer and sonicated. The membranes were then lysed on a head-over-head shaker for 2 h at 4 °C before being cleared by centrifugation at 16 000 g; 1 mL of lysate was then diluted in 9 mL of 50 mM Tris (containing protease inhibitors) and 15 μg of rabbit anti-TSG101 (Sigma) antibody or control rabbit IgG (Neomarkers) were added. Samples were mixed on a head-over-head shaker for 2 h at 4 °C. Next, 20 μL of pre-washed protein-G beads (Sigma) were added and the samples were mixed on a head-overhead shaker for 2 h at 4 °C. The beads were washed three times with lysis buffer (diluted 1 : 10 with 50 mM Tris, pH 7.4, containing protease inhibitors), and then treated as described above.
GST pull-downs
Truncated mutants of GISP (shown in Fig. 2a) were cloned into pGEX-4T-1 (Amersham, Piscataway, NJ, USA) and glutathione S-transferase (GST) pull-down experiments were performed as previously described (Kantamneni et al. 2007). In brief, each GST fusion protein was expressed in bacteria, lysed and then affinity purified using glutathione–agarose beads (Amersham); 10 μg of purified fusion protein was then immobilized on glutathione–agarose beads and mixed with either lysate from HEK293 cells expressing TSG101-myc or enriched microsomal lysate from rat brain for 1 h on a head-over-head shaker at 4 °C. The beads were then treated as described above.
Fig. 2.
TSG101 interacts with GISP in yeast 2-hybrid assays. (a) Identification of TSG101 binding domain on GISP. Diagram of the GISP truncation mutants tested. Only Δ7 and Δ8 (654–850) and the original cDNA fragment isolated from the GABAB1 yeast 2-hybrid screen (Kantamneni et al. 2007) robustly activated the β-galactosidase reporter signifying a positive interaction. (b) Schematic showing the domain structure of the TSG101 clone isolated.
Hippocampal culture, immunocytochemistry and confocal microscopy
Primary hippocampal culture and the immunocytochemistry experiments were performed as previously described (Corrêa et al. 2004). double staining, the concentration of the antibodies used were as follows: rabbit anti-GISP (15 μg/mL) and anti-mouse TSG101 μg/mL; Genetex, San Antonio, TX, USA). Secondary antibodies were used at 10 μg/mL. Images were obtained on a LSM510 Meta confocal microscope (Oberkochen, Germany). Fluorophores were excited with 488 or 568 nm wavelengths and emission from a single confocal plane was detected through 505–530 band pass and 560 long pass-filter. Images were processed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA, USA) and Corel DRAW 12.0 (Corel, Ottawa, ON, Canada). Quantification of the co-localization GISP and TSG101 was analysed along the 50 lm of three or more dendrites per neuron. Immunoreactive puncta were defined as discrete points along the dendrites with fluorescence intensity been twice the background staining of the neuron. Three cells were analysed per culture and five cultures were analysed. Data are expressed as puncta per 100 lm of dendrite.
Immunoblotting
Proteins were resolved by sodium dodecyl sulfate–polyacrylamide electrophoresis and immunoblotting was performed using rabbit polyclonal antibodies to GISP (1 μg/mL), NSF (N-ethylmaleinide sensitive factor) (1 μg/mL; Stressgen, Collegeville, PA, USA), β-adaptin antibody (1 μg/mL; Santa Cruz), GABAB2 (1 : 1000 dilution; Chemicon, Temecula, CA, USA), β-actin (1 μg/mL; Sigma), TSG101 (2 μg/mL; Abcam, Cambridge, UK) and TSG101 (2 μg/mL; Genetex). Quantitative densitometric analysis was performed using NIH Image J (NIH, Bethesda, MD, USA).
Results
GISP increases expression of GABAB2 in HEK293 cells
GISP enhances the expression of the GABAB2 subunit in the absence of GABAB1, despite the fact GISP and GABAB2 do directly interact (Kantamneni et al. 2007). We therefore tested the effect of GISP on the steady state levels of GABAB2 (Fig. 1). Co-expression of GISP increased the total levels of the GABAB2 by 100% (Fig. 1b). Significantly, GISP did not raise levels of β-actin, a cytosolic protein we used as controls (Fig. 1). These results suggest that the GISP-mediated increase in receptor level does not depend on any direct interaction with the receptors themselves and does not effect all proteins. We reasoned that this could be due to GISP acting to either promote forward trafficking and/or reduce receptor degradation pathways.
GISP interacts with TSG101
To identify GISP interactors that may mediate this effect we used the GISP clone originally obtained from the GABAB1 yeast 2-hybrid screen (GISP residues G102–Y1059; Kantamneni et al. 2007) as a bait to screen an adult rat brain cDNA library. TSG101 was isolated as a strong interacting partner that interacts in the region of 654–850 on GISP (Fig. 2). When transiently co-expressed in HEK293 cells GISP co-immunoprecipitated with myc-TSG101 (Fig. 3a). We next performed co-immunoprecipitation from rat brain extract using rabbit anti-TSG101 antibody. GISP was recovered in the co-immunoprecipitate, indicating that the interaction between TSG101 and GISP occurs in vivo (Fig. 3b). As TSG101 has a prominent role in receptor degradation (Babst 2005; Williams and Urbe 2007) our hypothesis was that the increase in receptor protein invoked by GISP might be due to an inhibitory action of GISP on TSG101-mediated lysosomal sorting of membrane proteins.
Fig. 3.
TSG101 and GISP exist in a complex. (a) HEK293 cells transiently expressing TSG101-myc and GISP were lysed and mixed with anti-myc antibody coupled to agarose beads. TSG101 complexes isolated by immunoprecipitation with 5 μg anti-myc antibody and rabbit IgG was used as a control. The data are representative of three separate experiments. (b) Microsomes enriched from rat brain were lysed and TSG101 complexes isolated by immunoprecipitation with 15 μg anti-TSG101 antibody. GISP was present in anti-TSG101 IPs but not control IPs, indicating that the proteins complex in brain. Rabbit IgG was used as a control for IPs and also anti-β-actin and anti-NSF antibodies were used as negative controls. β-Adaptin is used as a positive control that has been published previously (Camus et al. 2007) and as expected β-adaptin co-immunoprecipitates with TSG101 antibody. The data are representative of three separate experiments. (c) Top panel: Verification of the interaction between GISP and TSG101 by GST pull-down: GST was used as negative control. Myc-TSG101 binds only to GST-GISPΔ8. The input lane is from cell extracts transfected with myc-TSG101 and 10 μg of GST fusion protein was used for the pull-down. The blot is a representative of five different experiments. Middle panel: Coomassie brilliant blue staining showing the amount of GST fusion proteins. Lower panel: Schematic of GST truncations used for pull-downs. D, Verification of the interaction between GISP and TSG101 by GST pull-down using rat brain lysate: TSG101 binds only to GST-GISPΔ8. The input lane is from enriched microsomal extract and 10 μg of GST fusion protein was used for the pull-down. The blot is a representative of three different experiments.
The Δ8 region of GISP is critical for its interaction with TSG101
To identify the region of GISP critical for binding TSG101, we generated 10 GST-tagged GISP truncation constructs (Fig. 3c) and tested for binding with lysate from both rat brain (Fig. 3d) and HEK293 cells expressing myc-TSG101. GISP-Δ8 (residues 780–850) was the only region capable of interacting with TSG101. These data are consistent with the yeast 2-hybrid screening data described in Fig. 2.
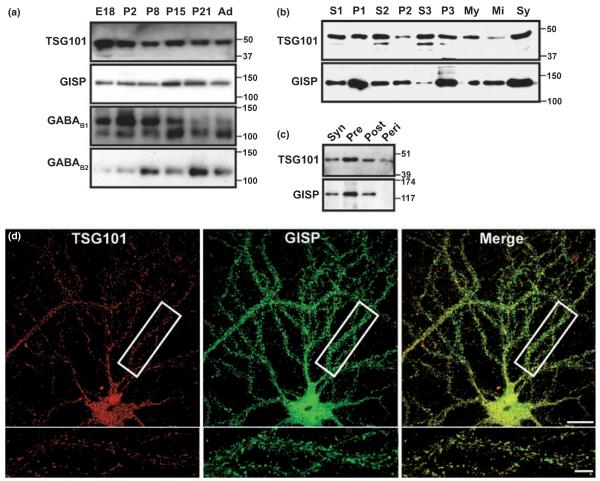
Comparison of GISP and TSG101 developmental profiles and subcellular localization
For GISP and TSG101 to form a functional complex in vivo, they must have overlapping temporal and spatial distributions. We therefore compared the developmental and subcellular fractionation profile of the two proteins in brain. GISP and TSG101 were both present at all ages with TSG101 expression highest at embryonic day 18 (Fig. 4a). TSG101 and GISP have distinct but overlapping subcellular fractionation profiles (Fig. 4b). Both proteins are most abundant in the microsomal membrane fraction (P3), which is consistent with the involvement of TSG101 in late endosomal sorting (Doyotte et al. 2005). Expression of GISP and TSG101 was also high in the synaptic fraction suggesting that both proteins could be involved in the regulation of synaptic transmission (Fig. 4c). Visualized by immunocytochemistry and confocal imaging, TSG101 has a widespread punctate distribution in hippocampal neurons, consistent with both a cytosolic and endosomal distribution. Furthermore, 41% of the TSG101 puncta co-localize with GISP puncta supporting the idea of a functional complex in vivo (Fig. 4d).
Fig. 4.
TSG101 is expressed in neurons. A, Developmental profile of TSG101 expression. Post-nuclear extracts were prepared from rat whole brain taken at the ages indicated in days (E = embryonic, P = postnatal, Ad = adult) and probed for TSG101 and GISP immunoreactivity. Both proteins were expressed in the every stage tested. The data are representative of three separate experiments. GABAB1 and GABAB2 were used as control and consistent with a previous report (Fritschy et al. 1999), expression of GABAB1a (upper band) and GABAB1b (lower band) subunit isoforms showed an inverse relationship, where GABAB1a is expressed most at early stages and GABAB1b at later stages of development. B, Distribution of TSG101 and GISP in subcellular fractions from rat brain; 50 μg protein was loaded per lane. The fractions are; S1, cell lysate minus nuclei and debris; P1, nuclei and debris; S2, crude microsomes and cytosol; P2, crude synaptosomes; S3, cytosol; P3, enriched microsomes; My, myelin; Mi, mitochondria; Sy, enriched synaptosomes. The data are representative of three separate experiments. C, Distribution of TSG101 and GISP in synaptic fractions. Purified synaptosomes (Syn) were further fractionated into Pre-, Post- and Peri-synaptic fractions as described previously (Feligioni et al. 2006); 3.5 μg of protein was loaded per lane and the blots were probed either with GISP or TSG101 antibodies. The blots are representative of three different experiments. (d) Localization of TSG101 and GISP in neurons. Representative images of an embryonic hippocampal neuron permeabilized with 0.1% Triton X-100 and stained with anti-GISP antibody (green) and anti-TSG101 antibody (red). The merged panel demonstrates the co-localization between TSG101 and GISP. Higher magnification of the region highlighted in the box is shown in the panels below. The images are representative of five different experiments. Scale bars, 10 μm.
Degradation of GABAB2 involves TSG101 in HEK293 cells
Having established that GISP and TSG101 interact in vivo, we hypothesized that the effect seen in Fig. 1 might be because of GISP-mediated inhibition of TSG101, resulting decreased receptor degradation via the lysosome. If is the case, the stability of GABAB2 should be susceptible to knockdown of TSG101. We therefore used siRNA to deplete endogenous HEK293 cell TSG101 and determined the effect on the levels of exogenously expressed GABAB2. As expected, siRNA-mediated knockdown of TSG101 led to a significant increase in GABAB2 expression (Fig. 5a and b), indicating that its degradation is mediated by TSG101. Importantly, the GISP mutant (GISPΔ8-Mut) lacking the critical binding region for TSG101 did not increase the expression of GABAB2 compared with wild type GISP (Fig. 5c and d). These results suggest that binding of GISP to TSG101 is crucial for down-regulating TSG101 protein function.
Fig. 5.
Effect of TSG101 siRNA and GISPΔ8-Mut protein on GABAB2 expression assessed by immunoblotting. (a) HEK293 cells were transfected with GABAB2, in combination with a control siRNA or TSG101 siRNA. After 24 h, the cells were again transfected with siRNA and left for further 24 h; 10 nM of siRNA was used for 1 mL of transfection mix. The data are representative of five separate experiments. Immunoblots were probed with anti-GABAB2 antibody; TSG101 antibody and anti-β-actin antibody lanes ensure equal loading. (b) Normalized densitometric analysis of the effect of TSG101 siRNA on the GABAB2 protein analysed from five independent experiments; **p < 0.0008. (c) GISP increases levels of GABAB2 in HEK293 cells compared with GISP mutant. HEK293 cells were transfected with the receptor plasmid together with either a control vector (pCAT) or vector encoding GISP or GISPΔ8-Mut; 48 h post-transfection, the total receptor protein was determined by immunoblotting and blots were probed with anti-GABAB2 antibodies. (d) The bar charts show normalized densitometric analysis of three blots. The increase in receptor expression when co-transfected with GISP is significant compared with GISPΔ8-Mut; *p < 0.01.
GABAB receptors are targeted to lysosomes in HEK293 cells
The observation that GABAB2 degradation is dependent on TSG101 and that the interaction of GISP with TSG101 is required to retard GABAB2 degradation imply that GABAB2 is subject to lysosomal degradation. To confirm this we used the lysosomal inhibitor NH4Cl. GABAB2 subunits are indeed protected from degradation by 50 mM NH4Cl confirming that they are targeted to lysosomes (Fig. 6a and b). This protection was not observed in cells expressing GABAB1 alone (Fig. 6a and b). We attribute this to the fact that GABAB1 expressed alone in HEK293 cells is retained in endoplasmic reticulum and is only trafficked to the membrane when co-expressed with GABAB2 (Couve et al. 1998; Marshall et al. 1999; Pagano et al. 2001). Consistent with a previous report (Grampp et al. 2007), when GABAB1 and GABAB2 are co-expressed in HEK293 cells NH4Cl protects both subunits from degradation.
Fig. 6.
Effect of lysosomal and proteosomal inhibitors on GABAB expression assessed by immunoblotting. (a) Individual expression of GABAB1a or GABAB2. HEK293 cells were transfected either with GABAB1a or GABAB2, plasmid; 42 h post-transfection, either 50 mM NH4Cl or 10 μM MG132 was added for 6 h and total protein was determined by western blotting with anti-GABAB subunit antibody. The data are representative of four separate experiments. Immunoblots were probed with anti-β-actin antibody to ensure equal loading. (b) Normalized densitometric analysis of the effect of inhibitors on the GABAB2 protein analysed from four independent experiments; **p < 0.007 and *p < 0.05. (c) Co-expression of GABAB1a and GABAB2. HEK293 cells were co-transfected with both GABAB1a and GABAB2, plasmids; 42 h post-transfection, either 50 mM NH4Cl or 10 μM MG132 was added for 6 h and the total receptor protein was determined by western blotting with anti-GABAB1 antibody and anti-GABAB2 antibody. The data are representative of three separate experiments. Immunoblots were probed with anti-β-actin antibody to ensure equal loading. (d) Normalized densitometric analysis of the effect of inhibitors on the GABAB receptor proteins analysed from three independent experiments; *p < 0.02 and §p < 0.04.
Unexpectedly, the proteosome inhibitor MG132 also afforded some protection. We reason that this is due to ubiquitin quenching as MG132 also resulted in a marked retardation in the degradation of activated epidermal growth factor receptor (EGFR), a process well established to be dependent on the lysosome (Longva et al. 2002). Indeed, several previous studies have reported that proteasome inhibitors can retard degradation through the lysosome. For example, degradation of the EGFR, which is not a proteasome substrate, is decreased or blocked by proteasome inhibitors (Bishop et al. 2002; Longva et al. 2002).
Discussion
Here, we show that GISP and TSG101 form a functional complex in vivo and that GISP can function as a neuron-specific regulator of TSG101-dependent membrane protein degradation. Exogenous expression of GISP in HEK293 cells leads to the increased expression of co-transfected GABAB2, despite the lack of any direct interaction. This is consistent with GISP acting either to globally enhance transcription/translation of this protein or to retard its degradation. On further investigation, we identified TSG101 as a GISP-interacting protein. TSG101 is an integral component of the ESCRT machinery that functions in the sorting of membrane proteins for degradation in the lysosome. We therefore reasoned that GISP binding to TSG101 might provide a mechanism to inhibit TSG101.
To test this, we first determined that degradation of GABAB2 is dependent on TSG101. Knockdown of TSG101 significantly increased the steady state levels of GABAB2, in a similar manner to GISP over-expression. Crucially, over-expression of GISP in cells lacking TSG101 had no effect on GABAB2 levels (Fig. S1), indicating that GISP is acting via TSG101 to retard GABAB2 degradation. In addition, the effect of GISP on GABAB2 levels requires the region of GISP essential for TSG101 binding, as deletion of this region prevents the effect of GISP on steady state GABAB2 levels. Thus, the effect of GISP on GABAB2 levels requires GISP binding to TSG101.
The observation that TSG101 was required for degradation of GABAB2 strongly suggests that GABAB2 is degraded by lysosomes. To confirm this we tested the lysosomal inhibitor NH4Cl. As expected, NH4Cl protects GABAB receptors from degradation indicating that they are targeted to lysosomes (see also Grampp et al. 2007). We also tested the proteosomal inhibitor MG132, which to our surprise also provided some protection against GABAB receptor degradation. However, MG132 also resulted in a marked retardation in the degradation of activated EGFR, a process known to be dependent on the lysosome (Longva et al. 2002). Further, several studies have reported that proteasome inhibitors can retard or block lysosomal degradation of, for example the EGFR (Bishop et al. 2002; Longva et al. 2002). Interestingly, other processes that are well established to be dependent on ESCRT function, such as viral budding, have been reported to be potently inhibited by proteasomal inhibitors (Patnaik et al. 2000; Schubert et al. 2000; Strack et al. 2000). Taken together, these data indicate that inhibition of the proteasome leads to the accumulation of multi-ubiquitinated cellular proteins, causing the depletion of free ubiquitin (Patnaik et al. 2000; Schubert et al. 2000; Strack et al. 2000; Bishop et al. 2002). This depletion of free ubiquitin, in turn, leads to a decrease in ESCRT- and ubiquitin-dependent lysosomal degradation.
Our results indicate that GISP performs a dual function in the regulation of GABAB receptors. Firstly, it promotes surface expression of GABAB receptor complexes via its interaction with GABAB1 (Kantamneni et al. 2007). Secondly, GISP enhances the steady state levels of GABAB receptors by retarding degradation via its interaction with TSG101. The retardation of lysosomal degradation may be an important general function of GISP, as GISP enhanced levels of GABAB2 despite there being not direct interaction.
Protein degradation is of fundamental importance for neuronal function. For example, perturbations of degradative systems and the resulting protein aggregations are implicated in a wide range of neuropathological conditions including Huntington’s, Parkinson’s and Alzheimer’s diseases (Ross and Poirier 2004). A prominent feature of these neurodegenerative disorders is the accumulation of misfolded proteins that can overwhelm degradative pathways (Kakizuka 1998; Aronin et al. 1999). Defects in degradative pathways themselves can also lead to toxicity and neuronal death. For example, defects in ESCRT-mediated degradation can cause neurodegeneration in both animal models and human patients (Skibinski et al. 2005; Parkinson et al. 2006; Lee et al. 2007; Rusten et al. 2007; Lee and Gao 2008). It is therefore likely that these pathways are highly regulated in neurons and we show that GISP can play an important role in the tight control of membrane proteins via down-regulation of ESCRT activity by inhibiting TSG101.
It is not yet clear how GISP acts to retard TSG101-dependent lysosomal degradation. TSG101 is recruited to the endosomal surface via its interaction with Hrs to perform its role in receptor down-regulation (Bache et al. 2003a). GISP is found in both the cytosolic and microsomal fractions, indicating it too can exist in both cytosolic and membrane bound forms (Kantamneni et al. 2007). Interestingly, GISP and TSG101 co-immunoprecipitate from membrane-enriched brain fractions, indicating that their interaction takes place at membranes. Therefore our data support a model in which GISP binds to and inhibits TSG101 in its membrane-bound, functional form, rather than by sequestering TSG101 in the cytosol.
Further work is required to determine precisely how GISP influences ESCRT-mediated degradation. It is possible that GISP could directly inhibit TSG101 function by blocking the binding of TSG101 to other members of the ESCRT complexes or by preventing TSG101 from binding ubiquitinated cargo. Alternatively, GISP may be acting as a scaffold, recruiting some other inhibitory factor to TSG101. A number of proteins have been reported to modulate TSG101 function by acting as E3 ubiquitin ligases, leading to the ubiquitination and degradation of TSG101 (Amit et al. 2004; Kim et al. 2007). However, GISP bears no similarity to known ubiquitin ligases, and GISP over-expression appears to have no effect on the steady state levels of TSG101.
Supplementary Material
Acknowledgements
We are grateful to the Wellcome Trust, the MRC and the EU (GRIPPANT, PL 005320) for financial support. We also thank Philip Rubin, Patrick Tidball and Yasuko Nakamura for technical assistance.
Abbreviations used
- EGFR
epidermal growth factor receptor
- ESCRT
endosomal sorting complex required for transport
- GISP
G protein-coupled receptor interacting scaffold protein
- GST
glutathione S-transferase
- HEK293
human embryonic kidney cells
Footnotes
Supporting information Additional supporting information may be found in the online version of this article.
Fig. S1 Effect of TSG101 siRNA and GISP protein on GABAB2 expression assessed by immunoblotting.
Please note: Blackwell Publishing is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Amit I, Yakir L, Katz M, et al. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 2004;18:1737–1752. doi: 10.1101/gad.294904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronin N, Kim M, Laforet G, DiFiglia M. Are there multiple pathways in the pathogenesis of Huntington’s disease? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:995–1003. doi: 10.1098/rstb.1999.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–258. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003a;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Slagsvold T, Stenmark H. Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 2004;23:2707–2712. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouschet T, Henley JM. Calcium as an extracellular signalling molecule: perspectives on the calcium sensing receptor in the brain. C. R. Biol. 2005;328:691–700. doi: 10.1016/j.crvi.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus G, Segura-Morales C, Molle D, Lopez-Vergès S, Begon-Pescia C, Cazevieille C, Schu P, Bertrand E, Berlioz-Torrent C, Basyuk E. The clathrin adaptor complex AP-1 binds HIV-1 and MLV Gag and facilitates their budding. Mol. Biol. Cell. 2007;18:3193–3203. doi: 10.1091/mbc.E06-12-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa SA, Munton R, Nishimune A, Fitzjohn S, Henley JM. Development of GABAB subunits and functional GABAB receptors in rat cultured hippocampal neurons. Neuropharmacology. 2004;47:475–484. doi: 10.1016/j.neuropharm.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J. Biol. Chem. 1998;273:26361–26367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- Doyotte A, Russell MR, Hopkins CR, Woodman PG. Depletion of TSG101 forms a mammalian ‘Class E’ compartment: a multicisternal early endosome with multiple sorting defects. J. Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Feligioni M, Holman D, Haglerod C, Davanger S, Henley JM. Ultrastructural localisation and differential agonist-induced regulation of AMPA and kainate receptors present at the presynaptic active zone and postsynaptic density. J. Neurochem. 2006;99:549–560. doi: 10.1111/j.1471-4159.2006.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur. J. Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Grampp T, Sauter K, Markovic B, Benke D. Gamma-aminobutyric acid type B receptors are constitutively internalized via the clathrin-dependent pathway and targeted to lysosomes for degradation. J. Biol. Chem. 2007;282:24157–24165. doi: 10.1074/jbc.M702626200. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- Kakizuka A. Protein precipitation: a common etiology in neurodegenerative disorders? Trends Genet. 1998;14:396–402. doi: 10.1016/s0168-9525(98)01559-5. [DOI] [PubMed] [Google Scholar]
- Kantamneni S, Correa SA, Hodgkinson GK, Meyer G, Vinh NN, Henley JM, Nishimune A. GISP: a novel brain-specific protein that promotes surface expression and function of GABA(B) receptors. J. Neurochem. 2007;100:1003–1017. doi: 10.1111/j.1471-4159.2006.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kim BY, Olzmann JA, Barsh GS, Chin LS, Li L. Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol. Biol. Cell. 2007;18:1129–1142. doi: 10.1091/mbc.E06-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Gao FB. Roles of ESCRT in autophagy-associated neurodegeneration. Autophagy. 2008;4:230–232. doi: 10.4161/auto.5384. [DOI] [PubMed] [Google Scholar]
- Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. 2007;17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Li Y, Kane T, Tipper C, Spatrick P, Jenness DD. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol. Cell. Biol. 1999;19:3588–3599. doi: 10.1128/mcb.19.5.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors – the first 7TM heterodimers. Trends Pharmacol. Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Pagano A, Rovelli G, Mosbacher J, et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson N, Ince PG, Smith MO, et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–1077. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- Patnaik A, Chau V, Wills JW. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl Acad. Sci. USA. 2000;97:13069–13074. doi: 10.1073/pnas.97.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10(Suppl.):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Filimonenko M, Rodahl LM, Stenmark H, Simonsen A. ESCRTing autophagic clearance of aggregating proteins. Autophagy. 2007;4 00. [Google Scholar]
- Schubert U, Ott DE, Chertova EN, Welker R, Tessmer U, Princiotta MF, Bennink JR, Krausslich HG, Yewdell JW. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl Acad. Sci. USA. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk AJ, Anderson SK, Hashemi SH, Chance PF, Bennett CL. SIMPLE interacts with NEDD4 and TSG101: evidence for a role in lysosomal sorting and implications for Charcot-Marie-Tooth disease. J. Neurosci. Res. 2005;82:43–50. doi: 10.1002/jnr.20628. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Strack B, Calistri A, Accola MA, Palu G, Gottlinger HG. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl Acad. Sci. USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.