Abstract
Disposable individually ventilated cages have lids that restrict air exchange when the cage is not mechanically ventilated. This design feature may cause intracage CO2 to increase and O2 to decrease (hypercapnic and hypoxic conditions, respectively) when the electrical supply to the ventilated rack fails, the ventilated rack malfunctions, cages are docked in the rack incorrectly, or cages are removed from the ventilated rack for extended periods of time. We investigated how quickly hypercapnic and hypoxic conditions developed within disposable individually ventilated cages after removal from mechanical ventilation and compared the data with nondisposable static cages, disposable static cages, and unventilated nondisposable individually ventilated cages. When disposable individually ventilated cages with 5 adult mice per cage were removed from mechanical ventilation, CO2 concentrations increased from less than 1% at 0 h to approximately 5% at 3 h and O2 levels dropped from more than 20% at 0 h to 11.7% at 6 h. The breathing pattern of the mice showed a prominent abdominal component (hyperventilation). Changes were similar for 4 adult mice per cage, reaching at least 5% CO2 at 4 h and 13.0% O2 at 6 h. For 3 or 2 mice per cage, values were 4.6% CO2 and 14.7% O2 and 3.04% CO2 and 17.1% O2, respectively, at 6 h. These results document that within disposable individually ventilated cages, a hypercapnic and hypoxic microenvironment develops within hours in the absence of mechanical ventilation.
Abbreviation: IVC, individually ventilated cage(s); dIVC, disposable IVC; ndIVC, nondisposable IVC; dSC, disposable static cage(s); ndSC, nondisposable SC
Our institution recently committed to exclusive use of disposable individually ventilated cages (dIVC) in a 26,000-cage capacity mouse barrier facility.32 dIVC are an appealing option for housing rodents for several reasons, including substantial reduction in labor costs; savings in capital expenditure otherwise allocated for tunnel cage washers, rack washers, and bulk autoclaves; reduction in electrical and water consumption; enhanced biosecurity due to the availability of irradiated, prebedded cages, irradiated food, and UV-irradiated and acidified water; and a healthier and ergonomically better working environment for the caretakers due to decreased exposure to allergens, lighter cages, and elimination of the need to install stoppers on water bottles.27,32 In addition, the disposable cages and water bottles are recyclable, thereby lessening the environmental impact of the vivarium.
dIVC have snap lids that form a tight seal to the cage bottom. When the dIVC is not ventilated mechanically, air exchange is restricted to a small surface area guarded by a filter. In the absence of mechanical ventilation, this restricted air exchange could result in an increased intracage CO2 and decreased intracage O2 (hypercapnic and hypoxic conditions, respectively). Two incidents at our institution suggested that hypercapnic and hypoxic conditions may develop quickly, depending on the ages and number of mice in the cage. One incident involved a ventilated rack that accidentally became unplugged for approximately 4 h; a researcher reported that mice that were about to be weaned were excessively lethargic. The second incident involved transporting mice in dIVC between facilities. Veterinary staff noted an increased abdominal component to the breathing pattern of the mice (hyperventilation) after being off mechanical ventilation for not more than 3 h. As part of our facilities’ Emergency Preparedness plan, we investigated how quickly hypercapnic and hypoxic conditions developed within dIVC after removal from mechanical ventilation. The results were compared with intracage conditions from the same cages with mechanical ventilation, and intracage conditions from disposable static cages (dSC), nondisposable static cages (ndSC), and nondisposable IVC (ndIVC) after removal from mechanical ventilation.
Materials and Methods
Mice.
Crl:CD1(ICR) female mice (n = 70) were obtained from Charles River Laboratories International (Hollister, CA). Health surveillance performed by the vendor showed that the mice were free from Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler murine encephalomyelitis virus, respiratory enteric virus III, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus, mouse norovirus, Mycoplasma spp., Pasteurella spp., Salmonella spp., Clostridium piliforme, Citrobacter rodentium, Corynebacterium kutscheri, Helicobacter spp., Streptococcus pneumoniae, Encephalitozoon cuniculi, and common mouse endo- and ectoparasites. The mice had an average weight of 39 g (range, 22 to 57 g). Great care was taken to ensure that the mouse weights did not exceed the available floor area of the various cages, as recommended by the Guide.13 Because the floor areas of nondisposable cages (ndSC, 67 in.2; ndIVC, 75 in.2) were smaller relative to that of disposable cages (dIVC and dSC, both 81 in.2), the total weight of the mice in nondisposable cages was less than that in disposable cages.
Husbandry.
Mice were housed in dIVC on ventilated racks (Innovive, San Diego, CA) under positive-pressure ventilation in an AAALAC-accredited facility. Room conditions included a temperature of 23 ± 2 °C, relative humidity of 30% to 40%, and a 12:12-h light:dark cycle (lights on, 0700 h). Mice were fed a commercial laboratory rodent diet (PicoLab Rodent Diet 20 5053, LabDiet, Brentwood, MO) and were provided with water ad libitum from disposable Aquavive water bottles prefilled with 300 mL acidified (pH, 2.5 to 3.0), reverse-osmosis–purified water (Innovive). Irradiated, 1/8-in. corncob bedding (Bed-o-Cobs, The Andersons, Maumee, OH) was dispensed under a cage changing hood to a depth of 0.75 cm in each cage and replaced after completion of testing.
Cages.
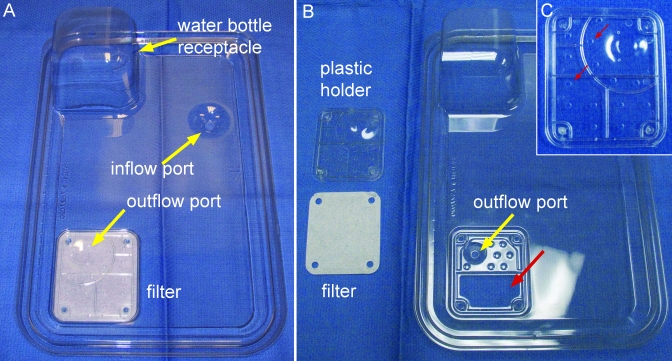
Disposable polyethylene terephthalate cage bottoms (Innocage, Innovive) with 523 cm2 (81 in.2) floor space and a calculated interior volume of approximately 7.7 L were used with 2 different types of lids. The first, designated the IVC lid (catalog no. MV1), is designed for use when the disposable cage is on the ventilated rack. This IVC lid has 2 ports (diameter, 4 to 6 mm) that provide for inflow and outflow of air when the cages are docked correctly on the ventilated rack (Figure 1 A). The inflow port does not have a filter. The outflow port is surrounded by a 6.2 × 7.3 cm, rectangular, Reemay spun-fiber filter. Air is mechanically exhausted through the outflow port and passively through a 3.0 × 5.6 cm trapezoid-shaped opening in the lid (Figure 1 B). The air must pass through 14 (diameter, approximately 3 mm) round holes in a rectangular plastic holder (total area of the openings, 98.9 mm2; Figure 1 C) that secures the Reemay filter to the inner surface of the lid. For ventilation, the cages were docked on a positive-air-pressure, double-sided rack (Innorack, Innovive) with dual HEPA filters. HEPA-filtered air was supplied at 52 air changes hourly, with the air inflow rate set at 25% higher than the exhaust outflow by using a blower mounted above the rack.
Figure 1.
A) Bottom view of the IVC cage lid for the dIVC, showing air inflow and outflow ports, receptacle for the water bottle, and the filter surrounding the outflow port. B) The plastic piece that holds the 6.2 × 7.3 cm, rectangular, Reemay spun-fiber filter surrounding the outflow port has been removed, showing the filter and the 3.0 × 5.6 cm trapezoid-shaped opening in the lid (large red arrow). C) Two of 14 approximately 3-mm-diameter holes in the plastic holder (small red arrows) that allow air exchange through the filter.
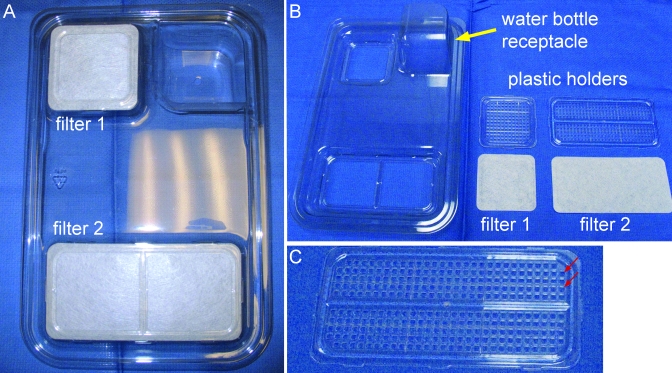
The second lid, designated the static lid (catalog no., MS1), is designed for using the disposable cage as a static cage. The static cage lid has 2, raised filter-carrying areas (6.3 × 6.3 cm; 6.5 × 14.2 cm) for air exchange (Figure 2 A) that prevent the cage from fitting into the ventilated rack. The filters are held in place by plastic holders with a grid of approximately 9-mm2 square holes that allow passive air exchange (Figure 2 B and C). The small and large holders have 140 and 286 holes, respectively (total area of the openings, 3834 mm2).
Figure 2.
A) Top view of cage lid for the dSC, showing the 2 raised filters. B) The plastic pieces that hold the Reemay spun filters have been removed. C) Larger plastic filter holder, showing the grid with 286 9-mm2 square holes through which air exchange occurs. Note that the holes in the plastic filter holder (small red arrows) of the dSC are more numerous per square area than are those in the plastic filter holder of the outflow port of dIVC lids (Figure 1 C).
Data also were obtained by using polysulfone ndIVC (model RC71MUD, Max 75, Altdesign, Siloam Springs, AK) and ndSC (Allentown, Model PC7115HT, Allentown, NJ). The ndIVC had a calculated volume of approximately 8 L and a floor space of 484 cm2 (75.0 in.2) and was designed to be used on a ventilated rack. The lid contains 2 spring-loaded, flap ports on the backside of the solid-top lid, with no provision for passive ventilation (Figure 3). When placed on the ventilated rack, HEPA-filtered air enters and exits the cage through the flap ports. The ndSC were used with a reduced-height, filter-carrying cage top (approximate area of openings, 180.0 cm2; model no. N10MBTRHPLF, Ancar, Bellmore, NY; approximate area of openings, 199.9 cm2; model no. FT71L-UD, Altdesign) and had a calculated volume of approximately 7.2 L and a floor space of 451 cm2 (67 in.2).
Figure 3.
Top view of cage lid for the ndIVC, showing the absence of any filter and the 2 spring-loaded, flap ports through which incoming and outgoing air exchange occurs once the IVC is mounted on the ventilation rack.
Experimental design.
Experiments were conducted between 0900 and 1600 h. Average room gas levels were: CO2, less than or equal to 0.02% vol; O2, approximately 20.9% vol; and NH3, 0 ppm. Intracage CO2, O2, and NH3 levels were measured at the start of the experiment (0 h) and then hourly for 6 consecutive hours by using a battery-operated, multigas analyzer (Eagle, RKI Instruments, Union City, CA). The gas sensor ranges were as follows: CO2, 0.00% to 5.00% vol; O2, 0.0% to 40.0% vol; NH3, 0 to 75 ppm. The analyzer was precalibrated by the manufacturer, and a 60-s selfcalibration test was conducted prior to each testing interval. On the day of testing, mice were transferred from the ventilated dIVC in which they were housed routinely into the cage types being tested. The cages had water bottles (Aquavive water bottles for disposable cages; 16-oz. reduced-height plastic water bottles for nondisposable cages) and wire lids (nondisposable cages only) or disposable feeders (disposable cages only) with commercial laboratory rodent diet. All cages contained irradiated, 1/8-in. corncob bedding dispensed to a depth of 0.75 cm. To sample intracage gas levels in the dIVC and dSC, the water bottle was removed from its receptacle (Figures 1 A and 2 B) and the receptacle manually dried. The probe then was placed through the water bottle hole and extended approximately 8 cm into each cage to the approximate level of the mice. For ndIVC and ndSC, sampling was through a 0.75-cm diameter hole drilled 5 cm above the cage floor in the front of the cage. When not sampling, the hole was covered with clear adhesive tape. CO2 levels were verified by using a manual analyzer (Gastec, Kanagawa, Japan) with a CO2 range of 0% to 10%. Each experiment was repeated 2 to 6 times on different days. During hourly testing, mice that showed clinical signs of lethargy, respiratory changes, or moribundity were noted. This study was approved by the Stanford University IACUC.
Statistical analysis.
Data were analyzed statistically by using InStat 3.0 for Macintosh (GraphPad Software, San Diego, CA). Groups were compared at each time point by using one-way ANOVA followed by a Dunnett Multiple Comparisons posttest by using ndSC, the cage type with which we have the most experience, as control and by defining a P value of less than 0.05 as being statistically significant. Graphs were drawn with Prism 5.0 for Macintosh (GraphPad Software).
Results
Intracage air quality with 5 mice per cage.
NH3 levels were undetectable within any of the experimental cages during the 6-h time frame. No water condensation was observed on any of the cage inner surfaces.
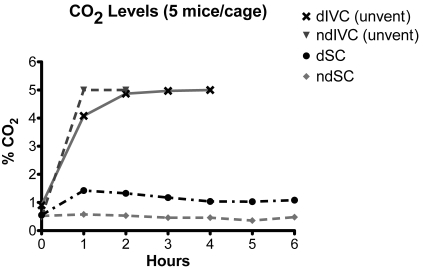
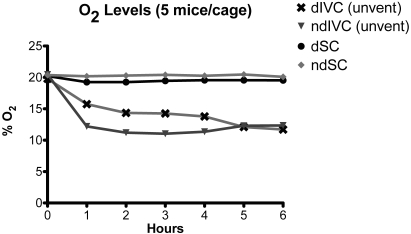
When dIVC were removed from mechanical ventilation, CO2 concentrations increased from less than 1% at 0 h to approximately 5% at 3 h (Table 1, Figure 4). The breathing pattern of the mice showed a prominent abdominal component (hyperventilation) that was most obvious when the mice were inactive. In 4 experiments, the CO2 concentrations obtained with the Eagle monitor were compared with readings obtained by using Gastec CO2 detector tubes. Results were comparable at all time points (0 h: Eagle, 0.9%; Gastec, 1.2%; 1 h: Eagle, 4.6%; Gastec, 4.5%; 2 h: Eagle, 4.8%; Gastec, 4.8%; 3 h: Eagle, 5.0% or greater; Gastec, 5.2%; 4 h: Eagle 5% or greater; Gastec, 5.2%). The actual concentration of CO2 achieved by the end of the 6-h experiment is unknown because 5% was the upper limit of the Eagle CO2 sensor. Concomitant with the increase in intracage CO2 concentrations, O2 levels decreased from greater than 20% at 0 h to 11.7% at 6 h (Figure 5, Table 2). In contrast, intracage mean CO2 remained below 0.6% and O2 above 20.1% for the entire 6-h time period in ndSC (Tables 1 and 2; Figures 4 and 5).
Table 1.
Carbon dioxide levels (%; mean ± 1 SD [n]) in various cage types at 0 to 6 h after removal from ventilation
| Time (h) |
|||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| dIVC, unventilated | 0.9 ± 0.3 (4) | 4.1 ± 1.0a (5) | 4.9 ± 0.3a (6) | 5.0 ± 0.1a (6) | ≥ 5%a (6) | ≥ 5%a (2) | ≥ 5%a (2) |
| ndSC | 0.5 ± 0.3 (6) | 0.6 ± 0.2 (6) | 0.5 ± 0.2 (6) | 0.5 ± 0.1 (6) | 0.5 ± 0.1 (3) | 0.4 ± 0.02 (3) | 0.5 ± 0.1(2) |
| dSC | 0.6 ± 0.3 (5) | 1.4 ± 0.3 (4) | 1.3 ± 0.3a (5) | 1.2 ± 0.2a (5) | 1.0 ± 0.1a (5) | 1.0 ± 0.2a (5) | 1.1 ± 0.2a (5) |
| ndIVC, unventilated | 0.5 ± 0.5 (3) | ≥ 5%a (3) | ≥ 5%a (3) | ≥ 5%a (3) | ≥ 5%a (2) | ≥ 5%a (2) | ≥ 5%a (2) |
Each cage contained 5 adult mice.
Data are given as ≥ 5% whenever the maximal limit of the monitor (5%) was reached.
Significantly (P < 0.05, ANOVA with Dunnett posttest) different from value for ndSC at the same time point.
Figure 4.
CO2 levels in dIVC after removal from the ventilation rack (unvent), dSC, ndIVC after removal from the ventilation rack (unvent), and ndSC. Each cage contained 5 adult mice.
Figure 5.
O2 levels in dIVC after removal from the ventilation rack (unvent), dSC, ndIVC after removal from the ventilation rack (unvent), and ndSC. Each cage contained 5 adult mice.
Table 2.
Oxygen levels (%; mean ± 1 SD [n]) in various cage types at 0 to 6 h after removal from ventilation
| Time (h) |
|||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| dIVC, unventilated | 19.8 ± 0.5 (4) | 15.7 ± 1.3a (5) | 14.4 ± 0.5a (6) | 14.3 ± 0.2a (6) | 13.8 ± 0.6a (6) | 12.1 ± 0.6a (2) | 11.7 ± 0.3a (2) |
| ndSC | 20.4 ± 0.6 (6) | 20.2 ± 0.4 (6) | 20.3 ± 0.4 (6) | 20.4 ± 0.4 (6) | 20.3 ± 0.2 (3) | 20.5 ± 0.3 (3) | 20.1 ± 0.3 (2) |
| dSC | 20.2 ± 0.8 (5) | 19.3 ± 0.3 (4) | 19.3 ± 0.4 (5) | 19.5 ± 0.4 (5) | 19.6 ± 0.3 (5) | 19.6 ± 0.3 (5) | 19.5 ± 0.4 (5) |
| ndIVC, unventilated | 20.5 ± 0.8 (3) | 12.2 ± 1.3a (3) | 11.2 ± 1.6a (3) | 11.0 ± 1.6a (3) | 11.4 ± 1.3a (2) | 12.3 ± 2.1a (2) | 12.4 ± 1.9a (2) |
Each cage contained 5 adult mice.
Significantly (P < 0.05, ANOVA with Dunnett posttest) different from value for ndSC at the same time point.
For dSC, intracage CO2 levels were slightly higher and intracage O2 levels were slightly lower than those of ndSC (Tables 1 and 2; Figures 4and 5). These differences were statistically significant only for CO2 after the 2-h time point. Intracage CO2 and O2 levels in dSC did not show the progressive increase and decrease, respectively, over time that occurred in unventilated dIVC (Tables 1 and 2).
ndIVC removed from mechanical ventilation showed similar changes in intracage CO2 and O2 concentrations as those in unventilated dIVC (Table 1, Figure 4). Intracage CO2 levels in unventilated ndIVC reached the maximal limit of the Eagle CO2 sensor (5% CO2) at 1 h, faster than what was observed with dIVC (Table 1, Figure 4), despite having a larger calculated cage volume (8 L compared with 7.7 L). Concomitantly, intracage O2 levels decreased to 12.2% at 1 h, dropped further to 11.0% at 3 h, and then rose to 12.4% at 6 h (Figure 5, Table 2).
Intracage air quality with fewer than 5 mice per cage.
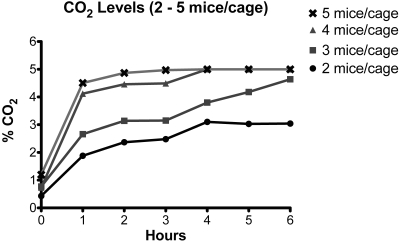
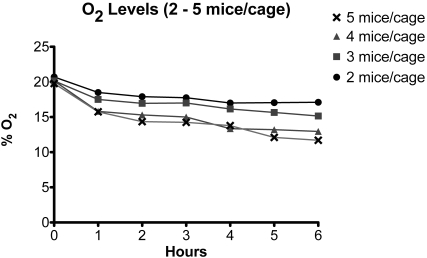
Housing 4 adult mice in each cage resulted in a slower but similar deterioration of intracage air quality in unventilated dIVC, as seen for 5 mice per cage, attaining more than 5% CO2 at 4 h and 13.0% O2 at 6 h (Figures 6 and 7; Tables 3 and 4). For 3 or 2 mice per cage, intracage CO2 and O2 levels deteriorated at an even slower rate. After removal of cages from mechanical ventilation for 6 h, intracage values were 4.6% CO2 and 14.7% O2 for 3 mice per cage and 3.0% CO2 and 17.1% O2 for 2 mice per cage (Figures 6 and 7; Tables 3 and 4).
Figure 6.
CO2 levels in dIVC after removal from the ventilation rack. Each cage contained 2 to 5 adult mice.
Figure 7.
O2 levels in dIVC after removal from the ventilation rack. Each cage contained 2 to 5 adult mice.
Table 3.
Carbon dioxide levels (%; mean ± 1 SD [n]) in unventilated dIVC each containing 2 to 5 adult mice at 0 to 6 h after removal from ventilation
| Time (h) | |||||||
| No. of mice | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 2 | 0.4 ± 0.2 (2) | 1.9 (1) | 2.4 ± 0.1 (2) | 2.5 ± 0.2 (2) | 3.1 ± 0.3 (2) | 3.0 ± 0.2 (2) | 3.0 ± 0.0 (2) |
| 3 | 0.8 ± 0.5 (2) | 2.7 (1) | 3.1 ± 0.6 (2) | 3.2 ± 0.0 (2) | 3.8 ± 0.0 (2) | 4.2 ± 0.8 (2) | 4.6 ± 0.4 (2) |
| 4 | 0.7 ± 0.4 (2) | 4.1 (1) | 4.5 ± 0.6 (2) | 4.5 ± 0.7 (2) | ≥ 5% (2) | ≥ 5% (2) | ≥ 5% (2) |
| 5 | 0.9 ± 0.3 (4) | 4.1 ± 1.0 (5) | 4.9 ± 0.3 (6) | 5.0 ± 0.1 (6) | ≥ 5% (6) | ≥ 5% (2) | ≥ 5% (2) |
Data are given as ≥ 5% whenever the maximal limit of the monitor (5%) was reached.
Table 4.
Oxygen levels (%; mean ± 1 SD [n]) in unventilated dIVC each containing 2 to 5 adult mice at 0 to 6 h after removal from ventilation
| Time (h) |
|||||||
| No. of mice | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| 2 | 20.7 ± 0.3 (2) | 18.5 (1) | 17.9 ± 0.0 (2) | 17.8 ± 0.1 (2) | 17.0 ± 0.0 (2) | 17.1 ± 0.2 (2) | 17.1 ± 0.0 (2) |
| 3 | 20.2 ± 1.1 (2) | 17.5 (1) | 17.0 ± 0.6 (2) | 17.0 ± 0.0 (2) | 16.2 ± 0.1 (2) | 15.7 ± 0.9 (2) | 14.7 ± 1.0 (2) |
| 4 | 20.2 ± 0.6 (2) | 15.8 (1) | 15.3 ± 0.7 (2) | 15.0 ± 1.3 (2) | 13.4 ± 1.1 (2) | 13.2 ± 1.3 (2) | 13.0 ± 0.5 (2) |
| 5 | 19.8 ± 0.5 (4) | 15.7 ± 1.3 (5) | 14.4 ± 0.5 (6) | 14.3 ± 0.2 (6) | 13.8 ± 0.6 (6) | 12.1 ± 0.6 (2) | 11.7 ± 0.3 (2) |
Discussion
Our primary concern with the removal of dIVC from the ventilation source was the death of mice. Deaths due to hypoxia have been reported within 1 h for weaning age and adult rats in unventilated ndIVC.12 After dIVC had been unhooked from the ventilation source for 6 h, we did not observe any deaths, excessive lethargy, or obvious signs of distress except hyperventilation. However, we used adult CD1 mice and intracage hypercapnic and hypoxic conditions may develop faster or the clinical effects more pronounced in mice of different ages, strains, or stocks. Indeed, CD1 and C57BL/6J mice differ in their metabolic response to acute hypoxia,33 and genetic differences among mouse strains and stocks relative to their physiologic response to hypoxia and hypercapnia have been published.2,3,6,7,29,30 Importantly, the C57BL/6J strain, a genetic background on which many mutations have been introduced, show spontaneous central apnea28 and are predisposed to dysrhythmic breathing.8,9 Whether mutant strains on this genetic background are more susceptible to hypercapnic and hypoxic conditions is unknown.
The manufacturer of the dIVC recommends that in the event of power failure mice should not be housed in unventilated cages for more than 12 h (http://www.disposablecages.com/disposable_rodent-cages-FAQ.php, 2011 no. 16). The data from the current study show that hypercapnic and hypoxic conditions worthy of concern can develop in far less time. Our data show that with 5 adult mice per cage, CO2 concentrations within unventilated dIVC quickly increased from less than 1% at 0 h to approximately 5% at 3 h, that O2 levels slowly decreased from approximately 20% at 0 h to 11.7% at 6 h, and that mice showed clinical signs of hyperventilation. It should be emphasized that the risk of developing hypercapnic and hypoxic conditions is not unique to dIVC. Depending on their design, certain models of ndIVC pose a similar risk of developing intracage hypercapnic and hypoxic conditions if removed from mechanical ventilation,16 and our current data confirmed these previous results for one brand of ndIVC. Hypercapnic and hypoxic conditions developed at least as quickly, if not more so, in ndIVC as in dIVC. This situation may be due in part to the absence of any filter on the cage lid of this particular ndIVC, thereby restricting all air exchange to where the cage lid rests on the brim of the cage bottom and around the 2 flap ports in the cage lid (Figure 3).
Progressively hypercapnic and hypoxic conditions in a cage will likely affect mice of various ages differently, and the effects will also depend on how long the conditions persist. Data in the literature on the effects of hypercapnic and hypoxic conditions vary with species, age, and length of exposure and often focus only on hypercapnia or hypoxia alone instead of simultaneously, as would occur in unventilated cages.
The effects of hypercapnia on adult laboratory rodents, primarily guinea pigs and rats, have been summarized previously.15 Acute (less than 24 h) exposure to greater than 3% CO2 under normoxic conditions results in respiratory acidosis, hyperventilation characterized by an increase in respiratory minute volume and tidal volume, and a stress response characterized by an elevation of blood corticosteroid levels and a decrease in white blood cells. At concentrations of 30% and more, CO2 shows anesthetic effects.15,25 If CO2 exposure is chronic, these parameters normalize after 3 to 4 d as the body compensates for the respiratory acidosis. Adult and neonatal CD1 mice exposed to 8% CO2 under normoxic conditions for 16 d (P2 to P17) did not show any significant difference in body weight, respiratory rate, oxygen saturation, pulse rate, blood pH, or mortality when compared with controls.24 Adult rats showed a decrease in heart rate if housed in 3% or 5% CO2 for 48 h; at 5% CO2, they also showed a decrease in systolic blood pressure.16 In adult animals, all effects were reversible after returning to normal atmospheric conditions for a few days.15 It has been recommended that rodents that are exposed to greater than 3% CO2 for 45 min or more should be given a few days of recovery to allow physiologic and hormonal parameters to return to normal.15,16
In contrast to hypercapnia, hypoxic conditions have more severe effects on rodents. Newborn rats raised in 10% O2 during the first 2 wk of life have a significant reduction in body weight.19,20,31 Exposure of preweanling rats to 4% O2 or less can trigger electroencephalographic seizure activity in the brain that may persist for days after return to normoxic conditions.14,21 Neonatal rats exposed to 10% O2 showed an immediate transient increase in ventilation that declined to a normal respiratory rate within a few minutes. However, continued exposure to 10% O2 again resulted in hyperventilation, which became statistically significantly at 8 h.23
These aforementioned hypercapnia and hypoxia studies were performed with either normal O2 or normal CO2 levels, respectively. Hypercapnia together with hypoxia, which develops in unventilated dIVC, may lead to more severe effects. Adult Wistar rats exposed to a combination of hypercapnic and hypoxic conditions for 32 d had significantly lower body weight and higher adrenal:body weight ratios relative to those of rats exposed to hypercapnic or hypoxic conditions alone.22 Treating neonatal mice with intermittent 4-min exposures to a combination of hypoxia (10% O2) and hypercapnia (8% CO2) alternating with 4 min of normoxia for 14 d, a model for obstructive sleep apnea, resulted in reduced body weight, increased hematocrit, and neuronal death in the cerebral cortex.4 Chronic intermittent hypercapnic hypoxia treatment of adult rats results in an increase in systemic and pulmonary arterial pressure in addition to a reduction of body weight and an increase in hematocrit.18
Mice can detect levels of CO2 as low as 0.067% and, in a T-maze assay, mice avoided CO2 concentrations above 0.2%.11 Our data and those of others26 show that CO2 levels in dSC with 5 mice per cage are approximately 1% (Table 1), a level that could be aversive according to previous data.11 Unlike mice, rats do not avoid cages with 1% CO2 but do avoid cages with greater than 3%.16 Field and laboratory data suggest that some fossorial mammalian species may tolerate some hypercapnic and hypoxic conditions. European rabbit (Oryctolagus cuniculus) kits are raised in earth-sealed, underground nesting chambers that achieve 7% to 8% CO2 and 13% to 14% O2; adults tolerate similar CO2 and O2 concentrations in their burrows.10 In addition, CO2 and O2 concentrations of 5.7% and 15.1%, respectively, have been recorded in burrows excavated in the laboratory by Syrian hamsters (Mesocricetus auratus).17 Some populations of wild Mus musculus are known to excavate complex burrows with several branches and exits.1 Further investigation is needed to determine the CO2 and O2 concentrations in burrows excavated in the laboratory or field by wild Mus musculus to elucidate the degree of hypercapnia and hypoxia that they might typically tolerate.
Our studies do not address whether CO2 and O2 concentrations within unventilated dIVC will continue to change with time after 6 h or whether there is sufficient air exchange through the filter in the lid to allow equilibration, thus avoiding potential lethal conditions. The answer to this question is crucial to our emergency preparedness plan. Our CO2 meter only registered to 5% CO2, and O2 levels were measured for 6 h only. Moreover, we used an outbred stock of adult mice (CD1), and various inbred strains or mutant stocks may be more susceptible to hypercapnic and hypoxic conditions, as has been demonstrated for rat strains exposed to hypoxia as models for retinopathy of prematurity.5 In addition, different breeding scenarios (for example, trio breeding) or particular ages (for example, weaning age) may be more susceptible to combined hypercapnic and hypoxic conditions. It is noteworthy that weaning age rats were reported to be more likely to die in unventilated IVC12 and that weaning-age mice were reported to be lethargic in the incident at our facility. Additional studies are required to determine whether weaning-age mice are more susceptible than are adults to combined hypercapnic and hypoxic conditions.
To avoid the variability of cages being soiled to different degrees and therefore increasing NH3 and decreasing O2 concentrations within the cages due to bacterial metabolism, we placed mice into cages with clean bedding at the start of each experiment. This practice had the effect of increasing the activity of the mice during the first 1 to 2 h and may explain the slightly higher CO2 and slightly lower O2 initial intracage readings relative to room atmospheric values (CO2, less than 0.02%; O2, 20.9%; Tables 1 and 2). It may have been better to allow the mice to acclimate to the new cages for 2 h before starting the experiment.
Previous studies on dSC concluded that there was no significant difference in intracage CO2 concentrations when compared with ndSC.26 Our data show that dSC have slightly higher CO2 and lower O2 concentrations when compared with ndSC although the differences are statistically significant only for the CO2 after the 2 h time point (Tables 1 and 2). Indeed, a closer examination of previous data26 showed that dSC tended to have overall higher, albeit not statistically significant, CO2 levels. This result is not unexpected, given that the filter area of the dSC took up only a portion of the area of the lid whereas that of the ndSC used in both the previous26 and current studies took up the entire lid surface. In addition, the tight fit of the static lid to the disposable cage allows for minimal, if any, air exchange at the lid–cage interface. In contrast, air exchange is possible at the lid–cage interface of ndSC.
The data and clinical signs in the current study suggest that dIVC with 5 adult mice per cage quickly result in hypercapnic and hypoxic conditions when removed from mechanical ventilation. Our results are consistent with previous data for unventilated ndIVC housing adult mice16 and rats.12 Although none of the mice died during the 6-h experiments, longer time periods and the long-term effects of the hypercapnic and hypoxic conditions on different ages of mice, pregnancy, and postnatal development have not been studied. These data are necessary for creating an emergency response plan for times when electricity is lost and back-up power fails, when part or all of a rack fails to provide adequate ventilation, and when a cage is docked in the ventilated rack incorrectly. Moreover, researchers should know how quickly suboptimal conditions develop within the cage if mice are maintained in dIVC cages without the appropriate lid. Even if animal deaths do not occur, the stress from prolonged periods of hypercapnic and hypoxic events may have adverse long-term effects on physiologic parameters, reproduction, or behavioral studies. For example, we have observed investigators leaving experimental mice in dIVC for extended periods of time before and after surgery. Having the cage lid tightly closed may have adverse effects on the survival of or results from these animals. To mitigate the potential for adverse events involving incorrect cage handling, we recommend that a supply of static lids be available and used to replace the IVC lid when mice in dIVC cages are to be taken to procedure rooms or labs. Recently the manufacturer of the disposable cages offered a new IVC lid (catalog no. MV6) with a larger ventilation grate than the version we used here. This modification may alleviate some of the concerns we reported. Appropriate education and training of all personnel involved in the use of dIVC are important steps in optimizing animal welfare.
Acknowledgment
This research was funded in part by the Department of Comparative Medicine (Stanford University School of Medicine).
References
- 1.Berry RJ. 1968. The ecology of an island population of the house mouse. J Anim Ecol 37:445–470 [Google Scholar]
- 2.Campen MJ, Tagaito Y, Jenkins TP, Balbir A, O'Donnell CP. 2005. Heart rate variability responses to hypoxic and hypercapnic exposures in different mouse strains. J Appl Physiol 99:807–813 [DOI] [PubMed] [Google Scholar]
- 3.Campen MJ, Tagaito Y, Li J, Balbir A, Tankersley CG, Smith P, Schwartz A, O'Donnell CP. 2004. Phenotypic variation in cardiovascular responses to acute hypoxic and hypercapnic exposure in mice. Physiol Genomics 20:15–20 [DOI] [PubMed] [Google Scholar]
- 4.Douglas RM, Ryu J, Kanaan A, Del Carmen Rivero M, Dugan LL, Haddad GG, Ali SS. 2010. Neuronal death during combined intermittent hypoxia–hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol 298:C1594–C1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floyd BN, Leske DA, Wren SM, Mookadam M, Fautsch MP, Holmes JM. 2005. Differences between rat strains in models of retinopathy of prematurity. Mol Vis 11:524–530 [PubMed] [Google Scholar]
- 6.Gaultier C, Gallego J. 2008. Neural control of breathing: insights from genetic mouse models. J Appl Physiol 104:1522–1530 [DOI] [PubMed] [Google Scholar]
- 7.Gaultier C, Matrot B, Gallego J. 2006. Transgenic models to study disorders of respiratory control in newborn mice. ILAR J 47:15–21 [DOI] [PubMed] [Google Scholar]
- 8.Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. 2001. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol 91:1962–1970 [DOI] [PubMed] [Google Scholar]
- 9.Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. 2002. Periodic breathing in the mouse. J Appl Physiol 92:1133–1140 [DOI] [PubMed] [Google Scholar]
- 10.Hayward JS. 1966. Abnormal concentrations of respiratory gases in rabbit burrows. J Mammal 47:723–724 [Google Scholar]
- 11.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. 2007. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317:953–957 [DOI] [PubMed] [Google Scholar]
- 12.Huerkamp MJ, Thompson WD, Lehner ND. 2003. Failed air supply to individually ventilated caging system causes acute hypoxia and mortality of rats. Contemp Top Lab Anim Sci 42:44–45 [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. Washington (DC): The National Academies Press. [Google Scholar]
- 14.Jensen FE, Applegate CD, Holtzman D, Belin TR, Burchfiel JL. 1991. Epileptogenic effect of hypoxia in the immature rodent brain. Ann Neurol 29:629–637 [DOI] [PubMed] [Google Scholar]
- 15.Krohn TC, Hansen AK. 2000. The effects of and tolerances for carbon dioxide in relation to recent developments in laboratory animal housing. Scand J Lab Anim Sci 27:173–181 [Google Scholar]
- 16.Krohn TC, Hansen AK. 2002. Carbon dioxide concentrations in unventilated IVC cages. Lab Anim 36:209–212 [DOI] [PubMed] [Google Scholar]
- 17.Kuhnen G. 1986. O2 and CO2 concentrations in burrows of euthermic and hibernating golden hamsters. Comp Biochem Physiol A 84:517–522 [DOI] [PubMed] [Google Scholar]
- 18.McGuire M, Bradford A. 2001. Chronic intermittent hypercapnic hypoxia increases pulmonary arterial pressure and haematocrit in rats. Eur Respir J 18:279–285 [DOI] [PubMed] [Google Scholar]
- 19.Mortola JP, Morgan CA, Virgona V. 1986. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol 61:1329–1336 [DOI] [PubMed] [Google Scholar]
- 20.Okubo S, Mortola JP. 1989. Respiratory mechanics in adult rats hypoxic in the neonatal period. J Appl Physiol 66:1772–1778 [DOI] [PubMed] [Google Scholar]
- 21.Owens J, Jr, Robbins CA, Wenzel HJ, Schwartzkroin PA. 1997. Acute and chronic effects of hypoxia on the developing hippocampus. Ann Neurol 41:187–199 [DOI] [PubMed] [Google Scholar]
- 22.Pepelko WE. 1970. Effects of hypoxia and hypercapnia, singly and combined, on growing rats. J Appl Physiol 28:646–651 [DOI] [PubMed] [Google Scholar]
- 23.Piazza T, Lauzon AM, Mortola JP. 1988. Time course of adaptation to hypoxia in newborn rats. Can J Physiol Pharmacol 66:152–158 [DOI] [PubMed] [Google Scholar]
- 24.Ryu J, Heldt GP, Nguyen M, Gavrialov O, Haddad GG. 2010. Chronic hypercapnia alters lung matrix composition in mouse pups. J Appl Physiol 109:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer KE, King CT, Mego JL, Williams EE. 1955. Effect of a narcotic level of CO2 on adrenal cortical activity and carbohydrate metabolism. Am J Physiol 183:53–63 [DOI] [PubMed] [Google Scholar]
- 26.Silverman J, Bays DW, Baker SP. 2009. Ammonia and carbon dioxide concentrations in disposable and reusable static mouse cages. Lab Anim (NY) 38:16–23 [DOI] [PubMed] [Google Scholar]
- 27.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62 [PMC free article] [PubMed] [Google Scholar]
- 28.Stettner GM, Zanella S, Huppke P, Gartner J, Hilaire G, Dutschmann M. 2008. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol 160:21–27 [DOI] [PubMed] [Google Scholar]
- 29.Tankersley CG. 2003. Genetic aspects of breathing: on interactions between hypercapnia and hypoxia. Respir Physiol Neurobiol 135:167–178 [DOI] [PubMed] [Google Scholar]
- 30.Tankersley CG, Elston RC, Schnell AH. 2000. Genetic determinants of acute hypoxic ventilation: patterns of inheritance in mice. J Appl Physiol 88:2310–2318 [DOI] [PubMed] [Google Scholar]
- 31.Vicencio AG, Eickelberg O, Stankewich MC, Kashgarian M, Haddad GG. 2002. Regulation of TGFβ ligand and receptor expression in neonatal rat lungs exposed to chronic hypoxia. J Appl Physiol 93:1123–1130 [DOI] [PubMed] [Google Scholar]
- 32.Zasio R. 2011. Case study: the Lorry I Lokey Stem Cell Research Building at Stanford University. ALN Mag 10:15–18 [Google Scholar]
- 33.Zwemer CF, Song MY, Carello KA, D'Alecy LG. 2007. Strain differences in response to acute hypoxia: CD1 versus C57BL/6J mice. J Appl Physiol 102:286–293 [DOI] [PubMed] [Google Scholar]