Abstract
The goal of this study was to evaluate the effectiveness of a new proprietary processed corncob bedding material (PCC) compared with standard corncob in ventilated and static mouse housing systems. Intracage ammonia levels, bacterial growth, and absorptive capacity of bedding were measured for cages of C57BL/6 mice under nonautoclaved and autoclaved conditions on static and ventilated racks in a barrier facility. Ammonia concentration was measured daily, and cages were removed from the study when measurements reached or exceeded 25 ppm. Bacterial growth in bedding was quantified and speciated before exposure to mice and at the time of cage removal. The absorptive capacity of all bedding material was determined under autoclaved and nonautoclaved conditions. Ventilated cages with PCC or autoclaved corncob took longer to reach ammonia concentrations of 25 ppm than did those with corncob or autoclaved PCC; PCC-filled cages remained below 25 ppm NH3 for at least 3 wk. The type of bedding material did not affect the number of days required to reach 25 ppm in static cages. Compared with other bedding types in the absence of mice, 1/4-in. PCC had a lower and 1/8-in. corncob a higher bacterial load. Autoclaving altered the absorptive capacity of 1/4-in. bedding materials, and for 1/8-in. bedding, corncob was more absorptive than PCC regardless of autoclaving. The results of this study indicate that PCC is comparable to autoclaved corncob in controlling intracage ammonia levels, and a cage-change interval of 3 wk is possible when ventilated cages are used with this bedding.
Abbreviation: PCC, processed corncob
The rodent microenvironment is a primary consideration for animal welfare. The Guide for the Care and Use of Laboratory Animals (The Guide) is the primary reference used for mice in AAALAC-accredited facilities and for those used in studies funded by the Public Health Service; this reference states that the cage environment should be dictated by performance standards, including temperature and humidity and concentration of gases and particulate matter.14 These parameters can alter metabolic and physiologic processes and disease susceptibility.14 Housing density, cage-change frequency, bedding material, and the use of ventilated caging contribute to the microenvironment.37,39 In addition to maximizing space and personnel resources, ventilated racks provide frequent HEPA-filtered air changes, remove excess humidity and waste gases from cages, and reduce disease transmission.8,37 Conversely, as cage density increases and cage-change frequency decreases, waste gases accumulate.39
The Guide states that rodents should be housed with bedding because it allows foraging, burrowing, digging, and nest building and absorbs urine and feces.14 Moreover, the type of bedding selected can influence animal wellbeing and experimental results.11,15,16,33 Several bedding materials are available for rodents, including corncob, wood chips, paper products, and grass fiber pellets. Bedding is evaluated for absorbency, biodegradability, toxicity, dust, palatability, comfort, cost, availability, damage to cage washers, and effect on research.43,50 Untreated softwood bedding can affect rodent metabolism,48,49 and the aromatic hydrocarbons of cedar shavings can induce hepatic microsomal enzymes and cytotoxicity.9,27 Aspen bedding is associated with sneezing and respiratory pathology in rats.7 In a study comparing aspen shavings, virgin pulp, recycled paper, corncob, reclaimed wood pulp, virgin cellulose, pine shavings, and hardwood chips, ammonia was detectable last in corncob.31 Rats have been shown to prefer paper bedding to corncob; however, corncob has been used to minimize dampness and ammonia concentrations.34 Mice prefer material suitable for nest building such as cloth, cotton, or paper.3,17,29,45 Although corncob bedding is not a sterile material,25,26,50 it is a common bedding choice due to its absorbency,26 biodegradability,26 and ability to control ammonia levels.43 In this study, corncob bedding was used as the control for comparison with a new processed corncob bedding material and because it is the most common bedding material used in our facilities.
The Guide also states that bedding should be changed frequently enough to keep animals clean and dry and to maintain acceptable concentrations of pollutants, including ammonia.14 The maximal acceptable concentration of ammonia in rodent housing has not been determined definitively; however, the 8-h exposure limit for humans (25 ppm)1 has been used as a guideline.37,39 Although subjective, human perception of cage cleanliness is still a factor in determining the need to change cages despite the lack of correlation between cage appearance and mouse health.39
Intracage ammonia concentration is influenced by temperature, humidity, ventilation, urine, and bacteria. Temperature and humidity are controlled at the room level. Humidity is influenced by bedding material, with highly absorbent materials decreasing the intracage humidity levels. Corncob bedding has been shown to be significantly more absorbent than other products.5 Likewise, in ventilated rodent cages, increased air flow within the cage decreased both humidity and ammonia concentrations.19 Intracage ammonia is produced by the combination of mouse urine and urease-producing bacteria, sources of which may include mouse feces and bedding material. Urine provides the urea substrate for ammonia production. Urease from bacteria catalyzes the conversion of urea to ammonia by cleaving urea to form 2 molecules of ammonia. The initial bacterial content in bedding can be controlled by husbandry practices.37 Autoclaving is a common method of minimizing the bacterial content of rodent bedding. The combination of steam at high pressure sterilizes the bedding material, killing bacteria, viruses, and fungi. Disadvantages of autoclaving bedding include personnel time, autoclave energy consumption and maintenance costs, and potential loss of absorptive capacity of the bedding material.
A novel, proprietary processed corncob bedding product (PCC; Green Pet Products, Conrad, IA) has been shown to contain fewer than 10 CFU per gram of Enterobacteriaceae, yeasts, molds, mesophilic spore, and thermophilic spores in the absence of autoclaving.13,24-26 Standard corncob bedding has a variable amount of bacteria, ranging from 23 to more than 1100 coliform CFU per gram of bedding.28,40 Bacteria present in bedding affect lung inflammation and other aspects of mouse health, contributing to stress of the animal.18,30,44 The choice of using PCC as promoted by the distributor can be assessed in terms of energy consumption, institutional cost, and animal stress.2 If bedding does not need to be autoclaved, the associated energy consumption and institutional cost will be eliminated.
If data published by the manufacturer indicating that PCC and autoclaved corncob share similar initial bacterial loads are accurate,24-26 then there should be less initial bacteria for production of ammonia and longer intervals until the ammonia threshold is reached. We hypothesized that intracage ammonia levels would be comparable between PCC and autoclaved corncob beddings and that both housing materials would result in an increased cage-change interval. Compared with a standard schedule, a prolonged cage-change interval would expose mice to fewer stressful events, husbandry staff would be exposed to fewer allergens, and facilities would benefit from cost savings.
This study was designed to compare the effectiveness of the corncob bedding currently used in our facilities with that of PCC. We determined the number of days required for cages on ventilated and static racks to reach aerosol concentrations of 25 ppm ammonia. Absorptive capacity and bacterial load and species were quantified to fully evaluate and compare these bedding materials. These studies primarily used 1/4-in. corncob bedding with mice because it is a common size used in research; however, 1/8-in. bedding was included in some aspects of the study design to determine whether and how the particle size of the same material affects absorption and bacterial content. These studies demonstrated that using autoclaved corncob or PCC bedding can extend the time required to reach maximal ammonia concentrations, as compared with nonsterile corncob or autoclaved PCC. In addition, corncob bedding is more absorptive than PCC and may therefore contribute to decreased humidity levels within cages.
Materials and Methods
Mice.
Female C57BL/6 mice (n = 100; age, 6 to 8 wk) were obtained from Harlan (Indianapolis, IN). The C57BL/6 strain was chosen because it is commonly used in research. Female mice were used to eliminate the variable of sex-associated differences and to decrease fighting among cagemates. Fighting could result in the removal of aggressive mice or the need for wound treatment, and the intracage ammonia concentration could be affected by removing mice or opening the cage repeatedly for treatment of wounded mice. Other similar studies have used only female mice.11,23,31,39
Mice were tested and found to be free from infections with cilia-associated respiratory bacillus, Citrobacter rodentia, Clostridium piliforme, Corynebacterium kutscheri, ectromelia virus, Encephalitozoon cuniculi, lymphocytic choriomeningitis virus, mouse minute virus, mouse adenovirus, mouse cyotomegalovirus, Theiler murine encephalomyelitis virus, mouse hepatitis virus, mouse parvovirus, mouse rotavirus, mouse thymic virus, Mycoplasma pulmonis, Myobia musculi, Pasteurella pneumotropica, pneumonia virus of mice, polyoma virus, reovirus 3, Salmonella spp., Streptococcus pneumoniae, Helicobacter spp., and endo- and ectoparasites. This study was approved by The Ohio State University IACUC and was conducted in AAALAC-accredited facilities.
Mice were housed in polysulfone microisolation cages (Super Mouse 750, Lab Products, Seaford, DE) measuring 29.8 cm × 15.6 cm × 14.1 cm, with an additional 0.7 cm beneath the filter-top lid. Cotton nesting pads (NES3600, Ancare, Bellmore, NY) measuring 5 cm × 5 cm were provided for environmental enrichment. Nesting pads were added to cages at the same time as bedding prior to autoclaving. Cages were maintained on an individually ventilated rack (Lab Products) or placed on a static free-standing wire shelving rack with 76 cm × 152.4 cm shelves spaced 38 cm apart vertically (3060NS, InterMetro Industries, Wilkes-Barre, PA). Rack ventilation was measured with a cage monitor unit (Enviro-Gard, Lab Products) and verified to be 41.2 ± 2.6 air changes hourly across all used rack locations. Air-change frequency in the static cage was not measured directly, but it is controlled at the room level, which was maintained at a ventilation rate of 10 to 15 air changes hourly. Room temperature was 71.8 ± 0.9 °F (22.1 ± 0.5 °C), and room humidity was 45% ± 13%. Mice were fed a standard irradiated diet (7292 Harlan Teklad, Harlan, Madison, WI) and had ad libitum access to chlorine-treated reverse-osmosis water. Cages were assigned into 4 groups (n = 5 each group) containing 300 mL bedding, with 5 mice per cage. The amount of bedding was chosen based on the ability to provide a uniform monolayer across the bottom of the cage. The 4 groups of mice received 1/4-in. corncob (Bed-o’ Cobs, The Andersons, Maumee, OH), autoclaved 1/4-in. corncob, 1/4-in. PCC (PureLite, 7073C, Harlan, Indianapolis, IN), or autoclaved 1/4-in. PCC bedding. Autoclave parameters were set at 121 °C for 20 min.
Bedding storage.
All bedding for these studies was accessed from the clean section of the vivarium cage preparation area. 1/4-in. Corncob bedding was accessed from the vivarium bedding dispenser (BD2000, TBJ, Chambersburg, PA); 1/4-in. and 1/8-in. PCC and 1/8-in. corncob bedding were stored within closed manufacturers’ bags inside 20-gal plastic containers (FG262000, Rubbermaid Commercial Products, Winchester, VA).
Ammonia measurement.
The American Conference of Governmental Industrial Hygienists has set 25 ppm ammonia as the 8-h limit for human exposure.1 This parameter was selected as the endpoint for the study on intracage ammonia levels. For ammonia sampling, a 1/4-in.-diameter hole was drilled on the midpoint of the front cage surface, 4 cm from the cage bottom. Autoclave tape was placed over each hole when measurements were not being made and was removed briefly for insertion of ammonia-detection tapes or tubes (Figure 1). Ammonia detection paper (pHydrion, Micro Essential Laboratory, Brooklyn, NY) was used for initial detection of ammonia levels in cages containing mice. In this test, a 2-cm piece of detection paper was moistened with sterile bacteriostatic water (Hospira, Lake Forest, IL) and inserted through the hole drilled in each cage for 15 s; the color change of the paper was matched to a standard list indicating approximate ammonia levels that was provided by the manufacturer. When ammonia levels detected with the detection paper reached 10 ppm, ammonia gas detector tubes (catalog no. 8014-105SC, Matheson Kitagawa, Montgomeryville, PA) were used with a toxic gas detector pump (model 8014-400A, Matheson Kitagawa) to provide more accurate gas measurement. When ammonia concentrations reached 25 ppm or greater as measured by the ammonia tubes, cages were changed; bedding was collected aseptically for bacterial culture. The experiment ended at 48 d, and all cages still measuring less than 25 ppm ammonia were changed.
Figure 1.
Cage modification. A 1/4-in.-diameter hole was drilled in the front of a microisolation mouse cage, 4 cm from the cage bottom. Dotted line indicates location of hole in cage wall.
Bacterial bedding quantification.
Cages were manipulated in a biosafety cabinet by using aseptic technique, with Spor-Klenz (Steris, St Louis, MO) as the surface disinfectant. Nonpopulated cages for each bedding group were prepared as controls for bacterial culture. For populated cages, mice were moved to a fresh cage, bedding was mixed in the used cage, and samples were collected aseptically from at least 9 sites in each cage. After aseptic removal of fecal pellets, feed, and nesting material, 5-g aliquots of bedding were obtained from each cage. All bedding samples were placed in 50-mL plastic tubes (Nunc 50-mL Centrifuge Tubes, Roskilde, Denmark) with 20 mL BHI medium (BBL Brain–Heart Infusion, Becton, Dickinson, Sparks, MD), vortexed for 10 s, and incubated at 35 °C for 24 h. Ten-fold serial dilutions of the bedding slurry were obtained by using BHI medium and plated onto trypticase soy agar (BD BBL Trypticase Soy Agar with 5% Sheep Blood [TSA II], Becton, Dickinson). Total cfu were determined after incubation at 37 °C for 24 h.
Bacterial speciation.
Inoculated media were streaked on trypticase soy agar by using sterile loops. Individual, unique colonies were Gram stained. Gram-positive cocci colonies were tested with hydrogen peroxide for catalase activity, and negative results were recorded as Streptococcus spp. Gram-positive and catalase-positive colonies were plated on mannitol, with positive reactions reported as Staphylococcus aureus and negative reactions as Staphylococcus spp. Gram-negative rods were speciated by using the API 20E enteric bacteria identification system (bioMérieux, Hazelwood, MO).
Absorptive capacity.
Clean bedding (5 g) was placed in a 50-mL plastic tube with 20 mL water, vortexed to wet all particle surfaces, and allowed to saturate for 2 min. Solid material was filtered out by using a 3-in. stainless steel strainer for 2 min, and bedding reweighed. The calculated weight difference was determined to represent absorptive capacity. The absorptive capacity was divided by the initial weight of bedding to create an absorption ratio.
Statistical analysis.
All experiments had 5 samples per group, with 5 mice per cage unless otherwise stated, and experiments were repeated once after randomization (www.randomizer.org). Data were analyzed by ANOVA multigroup comparison of means or by Kruskal–Wallis and Mann–Whitney nonparametric tests by using GraphPad InStat version 3.10 for Windows (GraphPad Software, La Jolla, CA). Bacterial data were analyzed after log conversion to normalize the data. Data are reported as mean ± SEM, with a P value less than 0.05 indicating statistical significance. Measurements exceeding 2 standard deviations and identified as outliers by Statistix 8 (Analytical Software, Tallahassee, FL) were excluded.
Results
Intracage ammonia concentration.
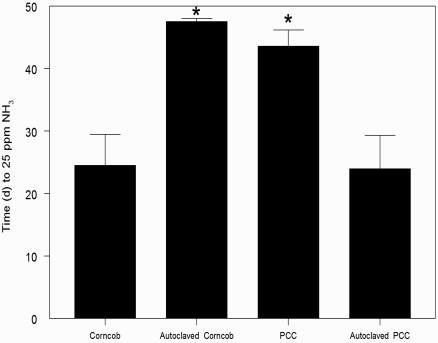
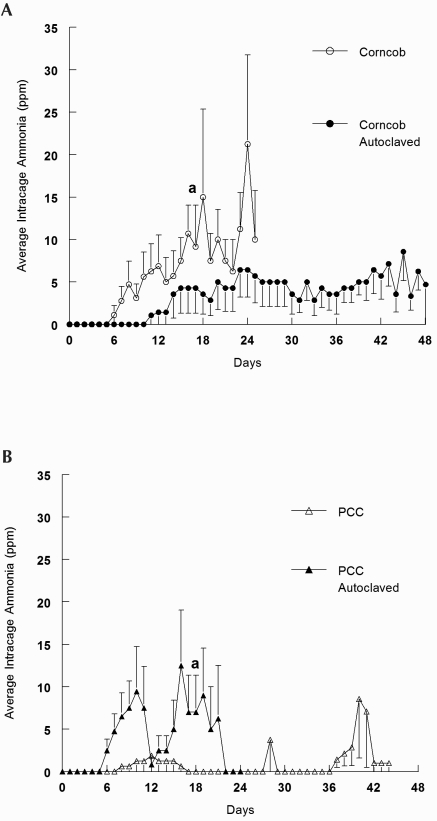
Ammonia levels in ventilated cages with PCC (43.6 ± 2.5 d) or autoclaved corncob (47.6 ± 0.4 d) bedding took twice as long to reach 25 ppm compared with those containing autoclaved PCC (24.0 ± 5.3 d) or corncob (24.6 ± 4.9 d) bedding (Figure 2). Ammonia levels in static cages reached 25 ppm ammonia in 5 to 7 d, regardless of bedding type (standard corncob, 5.7 ± 0.6 d; PCC, 5.7 ± 0.5 d; autoclaved corncob, 5.7 ± 0.7 d; autoclaved PCC, 5.0 ± 0.3 d; data not shown). The average daily ammonia concentration is illustrated in Figure 3.
Figure 2.
Time (d) for ventilated cages to reach 25 ppm ammonia: corncob bedding (n = 9), autoclaved corncob bedding (n = 9), PCC bedding (n = 8), autoclaved PCC bedding (n = 10) bedding. *, P < 0.05 (Kruskal–Wallis and Mann–Whitney tests) compared with values for corncob and autoclaved PCC.
Figure 3.
Average daily ammonia concentration (ppm). The number of cages measured changed over time because cages were removed when the intracage NH3 concentration reached or exceeded 25 ppm (data for the date of removal are included). Data are presented for each group only until the average day of cage removal for each group. (a) Day after which fewer than 50% of cages remain. (A) Corncob (n = 9; a, day 17) and autoclaved corncob (n = 7) bedding; note that 71% (5) of cages containing autoclaved corncob bedding remained on day 48. (B) PCC (n = 8) and autoclaved PCC (n = 10; a, day 18) bedding; note that 63% (5) of cages containing PCC remained on day 44.
Bacterial counts.
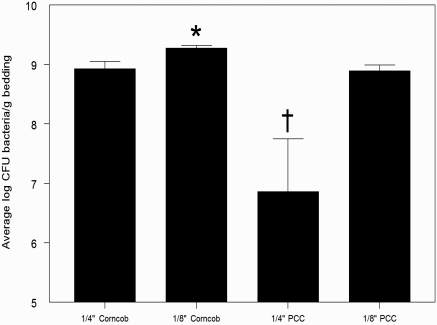
When cultured prior to use with mice, 1/4-in. PCC material contained less (P < 0.05) bacteria than did all other nonautoclaved beddings. Conversely, 1/8-in. corncob contained more (P < 0.05) bacteria than did all other types of bedding (Figure 4). No bacterial growth occurred in any of the autoclaved bedding groups prior to use with mice. Autoclaved PCC (9.50 ± 0.00 log CFU/g) cultured from populated ventilated cages contained 1614% more bacteria than did nonautoclaved PCC (8.27 ± 0.07 log CFU/g, P < 0.001) and 512% more than did autoclaved corncob (8.57 ± 0.23 log CFU/g, P = 0.0534). Although no differences in bacterial counts were noted between bedding groups for static cages, autoclaved PCC material (9.36 ± 0.14 log CFU/g) contained 159% more bacteria than did autoclaved corncob bedding (8.97 ± 0.16 log CFU/g, P = 0.0871, data not shown).
Figure 4.
Bacterial count (mean ± 1 SD; n = 10 for each group) of nonautoclaved, nonpopulated bedding. Bacterial count is expressed as log CFU bacteria per gram of bedding. †, P < 0.001 (ANOVA) compared with values for 1/4-in. corncob and 1/8-in. PCC; *, P < 0.05 (ANOVA) compared with values for 1/4-in. corncob and 1/8-in. PCC.
Bacterial species.
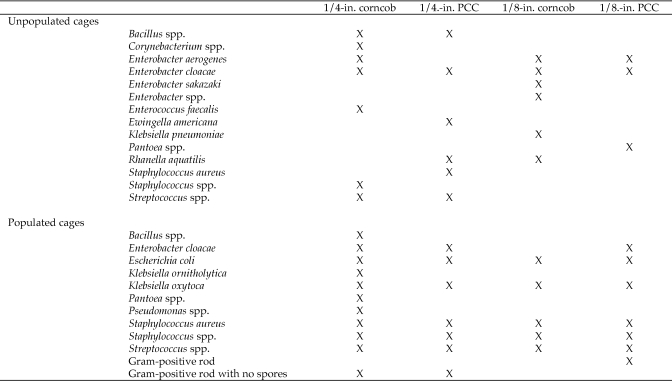
Bacteria isolated from unused, nonautoclaved bedding were similar in species to those isolated after exposure to mice (Figure 5). All bacteria isolated were considered to be nonpathogenic commensals. Enterobacter spp., Staphylococcus spp., Streptococcus spp. Klebsiella spp., and K. oxytoca were isolated from all bedding types only after exposure of bedding to mice.
Figure 5.
Bacteria isolated from bedding in unpopulated and populated cages. X, organism present.
Absorptive capacity.
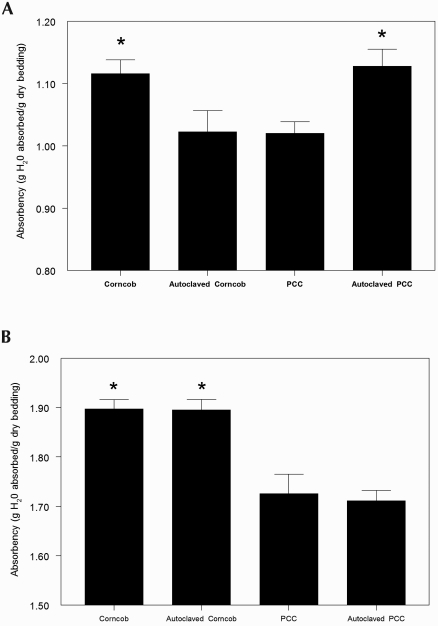
The absorption ratio was calculated for each bedding type and condition. For 1/4-in. beddings, corncob was significantly (P = 0.0045) more absorptive than PCC. Autoclaving decreased (P < 0.05) the absorptive capacity of corncob and increased (P < 0.05) the absorptive capacity of PCC (Figure 6 A). Between the two 1/8-in. beddings, corncob was significantly (P = 0.0014) more absorptive than was PCC, and autoclaving had no effect on this quality (Figure 6 B). In all bedding groups, the 1/8-in. bedding size was significantly (P < 0.05) more absorptive than was 1/4-in. bedding.
Figure 6.
Absorbency (g H2O absorbed/g dry bedding; n = 12 for each group) of bedding materials. (A) 1/4-in. Bedding materials; *, P < 0.05 (Kruskal–Wallis and Mann–Whitney tests) compared with values for autoclaved corncob and nonautoclaved PCC. (B) 1/8-in. Bedding materials; *, P < 0.05 (Kruskal–Wallis and Mann–Whitney tests) compared with nonautoclaved and autoclaved PCC.
Discussion
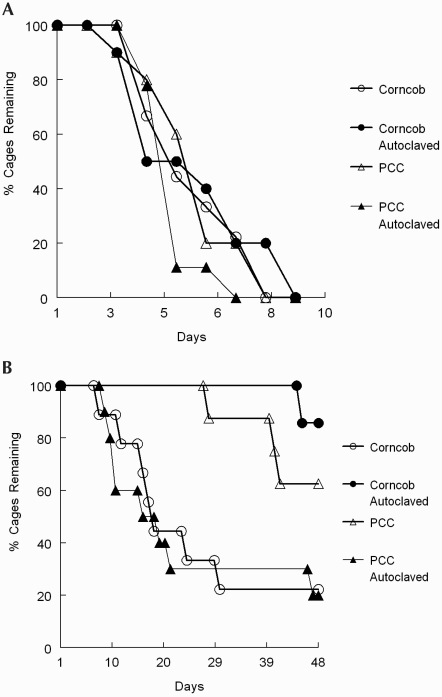
PCC bedding is touted as an alternative to autoclaving corncob material. In the current study, the first cage containing nonautoclaved PCC bedding to develop an ammonia concentration of 25 ppm in ventilated housing did so on day 28, and the first cage with autoclaved corncob material was removed at 14 d (Figure 7 A). These results regarding intracage ammonia levels indicate that the cage-change interval in ventilated cages likely could be increased to once every 3 wk (or more) with the use of PCC. For static cages, all types of bedding reached 25 ppm ammonia in at least one cage by day 4 (Figure 7 B), demonstrating that static cages should be changed at least weekly to control ammonia levels.
Figure 7.
Percentage of cages remaining on each day: (A) Ventilated housing: corncob bedding, n = 9 at start of study; autoclaved corncob bedding, n = 9; PCC bedding, n = 8; and autoclaved PCC bedding, n = 10. (B) Static housing: corncob bedding, n = 9; autoclaved corncob bedding, n = 10; PCC bedding, n = 10; and autoclaved PCC bedding, n = 9.
Several studies have evaluated different cage-change intervals for diverse bedding materials.19,31,32,36,38 Static cages had ammonia concentrations as high as 410 ppm when changed every 7 d, whereas cages with aspen shavings contained higher ammonia concentrations than did those with corncob bedding.31 Likewise, static cages with pine shavings exceeded 50 ppm ammonia concentration by day 5.32 Lower ammonia levels have been reported with increased air-change frequency in ventilated cages.19 In ventilated cages, ammonia concentrations for breeding mice were 25 ppm at 100 air-changes hourly or 50 ppm at 30 and 60 air-changes hourly at 14 d. Alternatively, ammonia levels due to groups of male mice were less than 25 ppm at 21 d for all air-change frequencies.36 Furthermore, mice in cages changed every 7 d exhibited more stereotypic behavior than did mice in cages changed every 14 d despite mild to moderate respiratory damage from exposure to elevated ammonia concentrations at this lower cage-change frequency.38
The recommendation to increase the length of time between cage changes also may markedly improve animal welfare by reducing stress associated with routine husbandry procedures. Previous research has shown that activities such as cage manipulation and bedding changes alter physiologic parameters in several species.2 Studies2,6,7,20,22,35,37,46,47 addressing mouse stress related to cage changes have demonstrated effects on behavior, pup mortality, and health. In response to routine husbandry procedures, mice have been reported to develop increased body temperature and corticosterone, prolactin, growth hormone, and glucose concentrations, with variable effects on immunoglobulin and lymphocytes.2 Cage changes increase serum corticosterone in C57BL/6 mice and induce anxiety-like behaviors during open-field testing.35 Increased pup mortality was reported in C57BL/6 mice in cages changed with greater frequency, with lower plasma corticosterone when cages were changed less frequently.37 Stress has been demonstrated to increase the prevalence of age-related amyloidosis in mice of several genotypes.20 Furthermore, cage changing has been associated with physiologic stress responses in rodents2,7,37,46 and increased fighting.46,47 The effects of cage changes and handling also have been studied in rats, with reports indicating increases in heart rate,22 mean arterial pressure,22 and pup cannibalization6 after routine husbandry procedures. In addition, the presence of familiar scents from intracage objects or cage lids has been reported to reduce the increases in heart rate and mean arterial pressure of rats during cage changing, suggesting that prolonging the interval between cage changes may produce a similar effect and reduce stress.22
In the current study, autoclaving of PCC material was actually detrimental to its performance, although the mechanism underlying this result could not be determined. One possible explanation for the apparent adverse effects of autoclaving PCC is physical alteration of the bedding as a result of high pressure and heat. After autoclaving, PCC and corncob bedding were both noted to darken in color (Figure 8). Alternatively, autoclaving may cause a reaction with possible residue remaining from the proprietary treatment. The specific steps of the proprietary process have not been disclosed, but the bedding is approved as ‘generally regarded as safe;’ further study is needed to determine whether autoclaving of PCC changes this status, as well as any possible effect on mouse health or experimental results due to this process. In comparison, autoclaving of standard corncob enhanced its performance, and autoclaved corncob bedding was as effective as PCC in delaying ammonia accumulation in the microenvironment. Because the autoclaving process represents a personnel and resource cost, the potential elimination of this step by implementing the use of PCC may represent a cost savings yet maintain an acceptable cage microenvironment. Another consideration regarding the choice of bedding relates to the risk of introducing bacterial contaminants into the microenvironment. In the current study, the bacterial counts and species in cages with PCC were similar to those with autoclaved corncob bedding, suggesting the feasibility of using PCC as a substitute for autoclaved corncob bedding. Although the bacterial species isolated before and after the addition of mice remained the same, the amounts of bacteria present seemed to make a difference in regard to the generation of ammonia by corncob bedding (autoclaved corncob remained below 25 ppm for a significantly longer time; Figure 2).
Figure 8.
Bedding samples before and after autoclaving. (A) Corncob, (B) autoclaved corncob, (C) PCC, and (D) autoclaved PCC bedding.
Bedding material serves to maintain a clean, dry surface via the absorption of excreta. Aspen bedding volumes of 250, 400, and 550 mL have been examined with the finding that lower volumes led to elevated intracage ammonia and humidity and a final recommendation of 400 mL bedding as the optimal amount.39 Dry bedding theoretically serves to limit the hydrolytic conversion of urea to ammonia; however, previous reports indicate that absorbency does not correlate with ammonia production.5 In the present study, absorbency was affected by particle size and autoclaving. For 1/4-in. bedding, corncob became more—and PCC became less—absorbent after autoclaving. Alternatively, 1/8-in. bedding was more absorbent overall than was 1/4-in. bedding, and although autoclaving had no additional effect on absorbency, corncob bedding was more absorbent than was PCC material. Interestingly, the least absorbent beddings (PCC and autoclaved corncob) provided the best ammonia control in ventilated caging, suggesting that bacterial numbers—and not absorbency—may be rate-limiting for ammonia production.
Animal welfare concerns involving the cage microenvironment include relative humidity, cleanliness, visibility (animal monitoring), health effects of bedding, and ammonia concentration. In the current studies, relative humidity remained within the Guide recommendation of 30% to 70%, and rack ventilation was within ranges used in previous studies.36,37,39 As has been reported previously, cages became increasingly soiled over time on study; however, the definition of when a cage is ‘too dirty’ is subject to individual perception and may not accurately reflect animal welfare.39 One concern is that decreased cage-change frequency could decrease the number of times that animals are observed and therefore diminish health monitoring. However, ensuring that cages are observed regularly without removing the cage from the rack or disrupting the cage interior should address this potential issue.
The effect of ammonia on rodent health and behavior has been evaluated, with mixed results. Increased ammonia exposure can irritate nasal passages and alter biologic responses.10,21 The ammonia concentration at which the respiratory rate in mice or rats is decreased by 50% has been reported as 303 ppm, with significant nasal epithelial pathology when mice were exposed for 6 h daily for 5 d.4 In a comparison of static with mechanically ventilated mouse cages, elevated ammonia levels for mice housed in static cages correlated with decreased body weight gain.23 Taken together, these cited studies suggest that increased ammonia levels are detrimental and that ammonia level is an appropriate parameter for determination of cage-change frequency. However, C57BL/6 mice exposed to ammonia concentrations of 400 ppm and 140 ppm had normal histology of eyes and nasal passages,42 and no behavioral abnormalities were noted when mice housed in individually ventilated cages were exposed to ammonia concentrations exceeding 50 ppm.41 Mice exhibited no location preference between areas with ammonia concentrations of 4 to 110 ppm.12 Overall, opinions differ regarding the type and severity of pathology related to ammonia concentrations in rodent housing. As a result, assumptions regarding exposure of rodents to ammonia have been based on data for human conditions,1 and 25 ppm was chosen for the current studies in light of the human correlate.
The choice of bedding used in a facility is frequently a result of several factors, including the ability to control ammonia levels, absorbency, microbial content, and cost. Costs associated with bedding materials include the material itself, personnel time associated with handling, and the use of processing equipment such as autoclaves. Autoclave costs also encompass utility, maintenance, repair, and purchase price. The manufacturer of PCC has touted its use in rodent housing based on decreased bacterial loads in the bedding. Our study confirms that PCC is equivalent in bacterial load to that of autoclaved standard corncob bedding. We further observed that the use of PCC extended the cage change interval to 3 wk, according to ammonia levels in the microenvironment.
Several options exist for the use of PCC within a facility. One option is the complete substitution of PCC for all existing corncob materials. Benefits of this approach include simplification and consistency of husbandry procedures, decreased energy and cost related to autoclaving, and the possible extended cage-change interval discussed earlier. Institutions must consider these factors against the necessity of complete microenvironment sterility for immunocompromised animals, increased cost of bedding, and the possible physiologic effects related to the proprietary manufacturing process, if any. Alternatively, PCC and corncob beddings can each be used according to need. Nonautoclaved PCC with standard enrichment material may be sufficient for immunocompetent animals, balanced against autoclaving the less-expensive corncob in cages for immunocompromised rodents. This approach avoids the diminished ammonia control of autoclaved PCC yet reduces the initial cost of materials. Although more flexible, this method complicates institutional operating procedure and may lead to miscommunication or confusion when preparing rodent cages. Any inconsistency of bedding introduces a variable to research and may confound experimental results.
The cost of implementing a change to PCC on the basis of the benefits identified in this study was estimated at our institution. Our institution uses 6 bulk sterilizers and 2 medium sterilizers as part of its animal care and use program. The busiest facility sterilizes approximately 27 loads each week, and each load requires an average personnel time of 45 min (loading the bulk truck for the cages, inserting the truck into the sterilizer, and removing when finished); these data translate into a salary-and-benefits cost of approximately $21 per load ($28,350 annually). The cost of PCC is currently $11 per bag, as compared with corncob bedding at $6 per bag. Replacement of all corncob within this single facility with PCC would result in a savings of approximately $4500 (7.7%) annually, when taking into account only personnel time and bedding cost. These calculations do not consider capital equipment and maintenance costs nor the increased time between cage changes, both of which might translate into additional savings for the animal care program overall. Likewise if PCC became generally accepted by more facilities, bulk production likely would decrease price and improve economic feasibility.
Overall, based on the results of the current study, the use of PCC instead of standard corncob likely improves the cage microenvironment and leads to cost savings. Further analysis of the use of PCC bedding in sterile housing rooms is under investigation at our institution, because autoclaving of this bedding is not recommended.
Acknowledgments
We thank Sally Wolfe for her technical work, Jason Chipps and Mackenzie Nicolaus for their laboratory support, Steve Green for his expertise in cage preparation, and Harlan Laboratories for providing the PureLite bedding used in this study.
References
- 1.American Conference of Governmental Industrial Hygienists. 2007. Threshold limit values (TLVs) and biological exposure indices (BEI). Cincinnati (OH): ACGIH. [Google Scholar]
- 2.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51 [PubMed] [Google Scholar]
- 3.Blom HJ, Van Tintelen G, Van Vorstenbosch CJ, Baumans V, Beynen AC. 1996. Preferences of mice and rats for types of bedding material. Lab Anim 30:234–244 [DOI] [PubMed] [Google Scholar]
- 4.Buckley LA, Jiang XZ, James RA, Morgan KT, Barrow CS. 1984. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol 74:417–429 [DOI] [PubMed] [Google Scholar]
- 5.Burn CC, Mason GJ. 2005. Absorbencies of 6 different rodent beddings: commercially advertised absorbencies are potentially misleading. Lab Anim 39:68–74 [DOI] [PubMed] [Google Scholar]
- 6.Burn CC, Mason GJ. 2008. Effects of cage-cleaning frequency on laboratory rat reproduction, cannibalism, and welfare. Appl Anim Behav Sci 114:235–247 [Google Scholar]
- 7.Burn CC, Peters A, Day MJ, Mason GJ. 2006. Long-term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare, and handleability: a cross-laboratory study. Lab Anim 40:353–370 [DOI] [PubMed] [Google Scholar]
- 8.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 9.Cunliffe-Beamer TL, Freeman LC, Myers DD. 1981. Barbiturate sleeptime in mice exposed to autoclaved or unautoclaved wood beddings. Lab Anim Sci 31:672–675 [PubMed] [Google Scholar]
- 10.Gordon AH, Hart PD, Young MR. 1980. Ammonia inhibits phagosome–lysosome fusion in macrophages. Nature 286:79–80 [DOI] [PubMed] [Google Scholar]
- 11.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68 [PubMed] [Google Scholar]
- 12.Green AR, Wathes CM, Demmers TG, Clark JM, Xin H. 2008. Development and application of a novel environmental preference chamber for assessing responses of laboratory mice to atmospheric ammonia. J Am Assoc Lab Anim Sci 47:49–56 [PMC free article] [PubMed] [Google Scholar]
- 13.Green Products Company. [Internet] 2011. PureLite—more accurate research results with PureLite-processed laboratory bedding. [Cited 01 November 2011]. Available at: http://www.purelitecob.com.
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 15.Iturrian WB, Fink GB. 1968. Comparison of bedding material: habitat preference of pregnant mice and reproductive performance. Lab Anim Care 18:160–164 [PubMed] [Google Scholar]
- 16.Kaliste E, Linnainmaa M, Meklin T, Torvinen E, Nevalainen A. 2004. The bedding of laboratory animals as a source of airborne contaminants. Lab Anim 38:25–37 [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Shimosaki S, Tongu M, Kobayashi Y, Nabika T, Nomura M, Yamada T. 2007. Evaluation of bedding and nesting materials for laboratory mice by preference tests. Exp Anim 56:363–368 [DOI] [PubMed] [Google Scholar]
- 18.Krop EJ, Doekes G, Stone MJ, Aalberse RC, van der Zee JS. 2007. Spreading of occupational allergens: laboratory animal allergens on hair-covering caps and in mattress dust of laboratory animal workers. Occup Environ Med 64:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipman NS, Corning BF, Coiro MAS. 1992. The effects of intracage ventilation on microenvironmental conditions in filter-top cages. Lab Anim 26:206–210 [DOI] [PubMed] [Google Scholar]
- 20.Lipman RD, Gaillard ET, Harrison DE, Bronson RT. 1993. Husbandry factors and the prevalence of age-related amyloidosis in mice. Lab Anim Sci 43:439–444 [PubMed] [Google Scholar]
- 21.Manninen A, Anttila S, Savolainen H. 1988. Rat metabolic adaptation to ammonia inhalation. Proc Soc Exp Biol Med 187:278–281 [DOI] [PubMed] [Google Scholar]
- 22.Meller A, Kasanen I, Ruksenas O, Apanaviciene N, Baturaite Z, Voipio H, Nevalainen T. 2011. Refining cage change routines: comparison of cardiovascular responses to 3 different ways of cage change in rats. Lab Anim 45:167–173 [DOI] [PubMed] [Google Scholar]
- 23.Memarzadeh F, Harrison PC, Riskowski GL, Henze T. 2004. Comparison of environment and mice in static and mechanically ventilated isolator cages with different air velocities and ventilation designs. Contemp Top Lab Anim Sci 43:14–20 [PubMed] [Google Scholar]
- 24.Mendonca AF.2009. [Internet] Destruction of microorganisms in regular- and PureLite-processed corncob particles using electron-beam irradiation. Final Project Report:1-9. [Cited 01 November 2011]. Available at: http://www.greenproducts.com.
- 25.Mendonca AF.2009. [Internet] Microbial destruction in regular- and PureLite-processed corncob particles: a study of autoclaving parameters (temperature and time combinations). Final Report:1-8. [Cited 01 November 2011]. Available at: http://www.greenproducts.com.
- 26.Mendonca AF, Sikinyi T, Byelashov O.2005. [Internet] Microbial quality of corncob particles and alternative commercial bedding materials for small animals. Initial Report:1-10. [Cited 01 November 2011]. Available at: http://www.greenproducts.com.
- 27.Nevalainen T, Vartiainen T. 1996. Volatile organic compounds in commonly used beddings before and after autoclaving. Scand J Lab Anim Sci 23:101–104 [Google Scholar]
- 28.NP Analytical Laboratories. 2010. [Internet] Bed-o'cobs quarterly report 08/09/2010. Quarterly Report 08/09/2010:1-3. [Cited 01 November 2011]. Available at: http://www.npal.com.
- 29.Olsson IA, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment’. Lab Anim 36:243–270 [DOI] [PubMed] [Google Scholar]
- 30.Pacheco KA, McCammon C, Thorne PS, O'Neill ME, Liu AH, Martyny JW, Vandyke M, Newman LS, Rose CS. 2006. Characterization of endotoxin and mouse allergen exposures in mouse facilities and research laboratories. Ann Occup Hyg 50:563–572 [DOI] [PubMed] [Google Scholar]
- 31.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98 [PubMed] [Google Scholar]
- 32.Perkins SE, Lipman NS. 1996. Evaluation of microenvironmental conditions and noise generation in 3 individually ventilated rodent caging systems and static isolator cages. Contemp Top Lab Anim Sci 35:61–65 [PubMed] [Google Scholar]
- 33.Potgieter FJ, Wilke PI. 1997. Effect of different bedding materials on the reproductive performance of mice. J S Afr Vet Assoc 68:8–15 [DOI] [PubMed] [Google Scholar]
- 34.Ras T, van de Ven M, Patterson-Kane EG, Nelson K. 2002. Rats’ preferences for corn- versus wood-based bedding and nesting materials. Lab Anim 36:420–425 [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen S, Miller MM, Filipski SB, Tolwani RJ. 2011. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J Am Assoc Lab Anim Sci 50:479–483 [PMC free article] [PubMed] [Google Scholar]
- 36.Reeb C, Jones R, Bearg D, Bedigan H, Myers D, Paigen B. 1998. Microenvironment in ventilated animal cages with differing ventilation rates, mice populations, and frequency of bedding changes. Contemp Top Lab Anim Sci 37:43–49 [PubMed] [Google Scholar]
- 37.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73 [DOI] [PubMed] [Google Scholar]
- 38.Reed B, Hawkins P, Latham N, Westwood K, van Driel K, Battram C, Golledge H, Farmer AM, Osborne N, Jennings M, Hubrecht R. 2008. Report of the 2006 RSPCA/UFAW rodent welfare group meeting. Lab Anim (NY) 37:216–222 [DOI] [PubMed] [Google Scholar]
- 39.Rosenbaum MD, VandeWoude S, Johnson TE. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. J Am Assoc Lab Anim Sci 48:763–773 [PMC free article] [PubMed] [Google Scholar]
- 40.Silliker. [Internet] 2009. Bed-o'cobs annual Silliker report 2009. Annual Report 2009:1–6. [Cited 01 November 2011]. Available at: http://www.silliker.com [Google Scholar]
- 41.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62 [PMC free article] [PubMed] [Google Scholar]
- 42.Smith AL, Mabus SL, Stockwell JD, Muir C. 2004. Effects of housing density and cage floor space on C57BL/6J mice. Comp Med 54:656–663 [PubMed] [Google Scholar]
- 43.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AL. 2004. Evaluation of cage microenvironment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43:12–17 [PubMed] [Google Scholar]
- 44.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. 2005. Endotoxin exposure is a risk factor for asthma: the National Survey of Endotoxin in United States Housing. Am J Respir Crit Care Med 172:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. 1997. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim 31:133–143 [DOI] [PubMed] [Google Scholar]
- 46.Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. 2004. Long-term effects of husbandry procedures on stress-related parameters in male mice of 2 strains. Lab Anim 38:169–177 [DOI] [PubMed] [Google Scholar]
- 47.Van Loo PL, Van Zutphen LF, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313 [DOI] [PubMed] [Google Scholar]
- 48.Vesell ES. 1967. Induction of drug-metabolizing enzymes in liver microsomes of mice and rats by softwood bedding. Science 157:1057–1058 [DOI] [PubMed] [Google Scholar]
- 49.Vesell ES, Lang CM, White WJ, Passananti GT, Tripp SL. 1973. Hepatic drug metabolism in rats: Impairment in a dirty environment. Science 179:896–897 [DOI] [PubMed] [Google Scholar]
- 50.Wirth H. 1983. Criteria for the evaluation of laboratory animal bedding. Lab Anim 17:81–84 [DOI] [PubMed] [Google Scholar]