Abstract
We performed the initial assessment of an alternative pressurized intraventilated (PIV) caging system for laboratory mice that uses direct-current microfans to achieve cage pressurization and ventilation. Twenty-nine pairs of female SPF BALB/c mice were used, with 19 experimental pairs kept in PIV cages and 10 control pairs kept in regular filter-top (FT) cages. Both groups were housed in a standard housing room with a conventional atmospheric control system. For both systems, intracage temperatures were in equilibrium with ambient room temperature. PIV cages showed a significant difference in pressure between days 1 and 8. Air speed (and consequently airflow rate) and the number of air changes hourly in the PIV cages showed decreasing trends. In both systems, ammonia concentrations increased with time, with significant differences between groups starting on day 1. Overall, the data revealed that intracage pressurization and ventilation by using microfans is a simple, reliable system, with low cost, maintenance requirements, and incidence of failures. Further experiments are needed to determine the potential influence of this system on the reproductive performance and pulmonary integrity in mice.
Abbreviation: FT, filter-top; PIV, pressurized intraventilated
Comfort and quality requirements for laboratory animal environments have led to improvements in intracage,5,11,14,15 or microenvironmental, atmospheric control systems.2-4,9,10,18 Recent studies have demonstrated improved sanitary status in rodents experimentally bred and maintained under such ventilation systems,16 particularly when intraventilated and pressurized cages of recent design are used,14,15 providing low concentrations of ammonia, moisture13 and carbon dioxide.7 Here we present an alternative to classic pressurized intraventilated (PIV) cages. This alternative is based on the use of direct-current microfans for ventilating and pressurizing these compartments. Previous experiments demonstrated that microfans similar to those used as cooling fans for conventional desktop computers were suitably adaptable to voltage fluctuations, allowing for precise control of airflow.8 In addition to their low implementation costs, microfans are readily commercially available, require little to no maintenance, and have an expected mean lifespan of 20,000 h under constant operation.8 The purpose of the current study was to design and test a technical alternative to PIV-type microenvironmental systems that used direct-current microfans, which are compatible with existing thermoelectric technology.9
Materials and Methods
Animals and group formation.
We used 29 male–female pairs of SPF BALB/c mice (weight, approximately 15 g; age, approximately 21 d); 19 experimental pairs were kept in PIV cages, and 10 control pairs were kept in standard polypropylene FT cages (28 cm × 17 cm × 12 cm). Both groups were housed in a standard holding room for laboratory animals that was equipped with a conventional atmospheric control system under general diluting ventilation. Excluded agents were cilia-associated respiratory bacillus, Clostridium piliforme, ectromelia virus, lymphocytic choriomeningitis virus, minute virus of mice, mouse hepatitis virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus, Sendai virus, mouse parvovirus, Citrobacter freundii, Pasteurella pneumotropica, Pasteurella multocida, Salmonella spp., Streptobacillus moniliformis, β-hemolytic Streptococcus spp., Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Myobia musculis, Myocoptes musculinus, Polyplax spp., Giardia spp., Tritrichomonas muris, Spironucleus muris, Rodentolepis nana, Syphacia spp., Aspicurulis tetraptera, Eimeria spp., Entamoeba muris, and Chilomastix bettencourt.
Husbandry.
The mice were housed and assayed under conditions of controlled temperature (22 ± 2 °C), humidity (45% to 65%), and artificial light (12:12-h light:dark cycle, lights on at 0700) with free access to rodent chow (Nuvilab CR1 Autoclavavel, Nuvital, Colombo, Brazil) and water (autoclaved and acidified, approximately pH 4.0). Bedding (Pinus spp. 20-mm shavings, autoclaved) and cages were autoclaved and changed weekly for both groups. Control (FT) cages had the same amount of food, water, and bedding as did experimental (PIV) cages. On day 1, the room was ventilated at approximately 22 air changes hourly and PIV cages at 174 air changes hourly.
The study occurred over 21 consecutive days. Temperature (wet and dry bulb), intracage pressure, intracage ammonia levels, and air speed were evaluated daily throughout the study in 3 equal 8-d periods (each one starting on Monday at 1600 and finishing on next Monday at 1300), for which the first day—first measurement—corresponds to experimental day 1, just after cage changes, and day 8 measurement was performed just before cage changes.
Mice were housed and used in accordance to the guidelines of the Committee on Care and Use of Laboratory Animal Resources of the School of Veterinary Medicine, University of São Paulo; these guidelines are similar to those of the Guide for the Care and Use of Laboratory Animals.6
Cage and microenvironmental ventilation system description.
Prototype PIV-type microenvironmental ventilation cages (n = 19; polycarbonate; 28 cm × 17 cm × 12 cm) were equipped with microfans (direct current, 12 V, 2.6 W, 3000 revolutions per minute, airflow rate of 18.06 L/s, pressure of 4.83 mm WC [water column at 4° C], 34 dB; model no. M712DCS1, Ventisilva, São Paulo, Brazil). A single stabilized direct-current (13.8 V, 10 Amp) power source was used to supply all microfans. In each cage, a microfan was installed horizontally on top of a filter holder (8 cm × 8 cm), which diffused the intake air through a polyester filter. Each fan was serially connected to a rotation measurement circuitry with an LED readout. On one of the vertical sides of the cage top, away from the insufflation aperture, 2 exhaust ports (diameter, 2.5 cm; both with air exhaust filters) were installed and were cleaned each week. The number of air changes hourly was determined multiplying the average exhaust air speed by the total area of the exhaust ports (0.0098125 m2) by 3600 s and dividing it by the total volume of the PIV cage (cage base plus cage top, 5712 cm3). The cage top had an internal neoprene sealing ring around the rim; this seal had to be included to ensure positive pressure within the cage and to guarantee that all air passed through the exhaust ports without leaking into the room air. Cage tops were fastened to the bottoms by using 4 drawbolts (Figure 1).
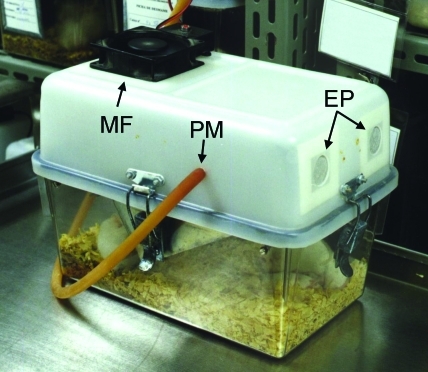
Figure 1.
PIV cage equipped with microfan (MF), exhaust ports (EP), and latex tube for intracage pressure measurement (PM).
Room air was captured by the microfans, insufflated into the cages, and then released through the exhaust ports toward the back of the rack, where the secondary exhaust ducts of the room were installed. The cages were placed on a standard cage rack made of steel bars, to which the electric cabling was affixed (Figure 2).
Figure 2.
Rack holding FT cages (top and bottom) and PIV cages (center).
Evaluated parameters.
For both types of caging, we measured were wet- and dry-bulb temperatures, relative humidity, intracage pressure, and intracage ammonia concentration, and air speed at the exhaust ports (a variable that allowed calculation of the number of air changes) in the PIV cages only.
Wet- and dry-bulb temperatures were measured by using a psychrometer built inhouse from 2 new, factory-calibrated thermometers (Salvi-Casagrande, São Paulo, Brazil). The instrument was positioned at the exhaust port of the PIV cages and at the volumetric center of the FT cages until wet- and dry-bulb temperatures stabilized. Relative humidity at the local altitude and barometric pressure was obtained from a psychrometric chart. The pressure inside the PIV cages was measured by using an inclined-column pressure gauge (in mm WC; Salvi-Casagrande) connected to the cage top through a latex tube.
Air speed at the exhaust ports was measured by using a thermal anemometer (Air Flow, Buckinghamshire, UK) that was programmed to obtain 20 sequential measurements at 5-s intervals. Intracage ammonia levels (in ppm) were determined by using a Matheson–Kitagawa toxic-gas detector system (Matheson Tri-Gas, Montgomeryville, PA). In the holding room, the background level of ammonia—a typical feature in general diluting ventilation systems—fluctuated primarily according to the time elapsed since the latest bedding change.3 To control for this variable, and because it has a far more pronounced influence on PIV than on FT systems,17 the ammonia level background was subtracted from those actually measured within the PIV cages, and the net values were compared with those measured in the FT system, which are less sensitive to external fluctuations.17
Statistical analysis.
Statistical analysis was performed by using the software package SyStat (version 7.0, SPSS, Chicago, IL). The data were analyzed according to unpaired Student t and Mann–Whitney tests. For all comparisons, a P value of less than 0.05 was defined as statistically significant. Data are presented as mean ± 1 SD.
Results
Throughout the study period, the temperature and relative humidity averages in both the PIV and FT systems were in equilibrium with those in the holding room, except for the dry-bulb temperature on day 5 in the FT cages (Table 1). Pressure measurements (Table 2) showed a trend toward progressive increase in intracage PIV values, with a significant difference between those for days 1 and 8. Air speed values (Table 2) were used to calculate the number of air changes hourly in the cages. These data showed a trend toward decreased air speed (and consequently airflow rate) and number of air changes hourly in the PIV compared with FT cages.
Table 1.
Wet- (WB) and dry-bulb (DB) temperatures (°C; mean ± 1 SD) and relative humidity (RH; %, mean ± 1 SD) within pressurized intraventilated (PIV, n= 19) and filter-top (FT, n= 10) cages each containing 2 mice
| PIV |
FT |
Holding room |
|||||||
| Day | DB | WB | RH | DB | WB | RH | DB | WB | RH |
| 1 | 23.1 ± 0.4 | 16.5 ± 0.4 | 45 | 21.5 ± 0.2 | 17.0 ± 0.3 | 67 | 21.5 | 15.0 | 52 |
| 2 | 23.3 ± 0.4 | 18.1 ± 0.7 | 61 | 23.0 ± 0.0 | 18.2 ± 0.4 | 61 | 22.0 | 16.0 | 53 |
| 3 | 23.7 ± 0.4 | 19.8 ± 0.4 | 69 | 23.3 ± 0.4 | 19.8 ± 0.3 | 76 | 22.0 | 18.0 | 68 |
| 4 | 24.2 ± 0.4 | 19.9 ± 0.5 | 69 | 24.2 ± 0.5 | 20.0 ± 0.3 | 69 | 23.0 | 18.0 | 61 |
| 5 | 23.0 ± 0.3 | 18.5 ± 0.4 | 70 | 23.3 ± 0.4a | 18.2 ± 0.5 | 70 | 22.0 | 17.0 | 60 |
| 6 | 22.9 ± 0.3 | 19.5 ± 0.4 | 78 | 23.0 ± 0.3 | 19.0 ± 0.6 | 74 | 22.0 | 18.0 | 68 |
| 7 | 23.1 ± 0.3 | 19.4 ± 0.4 | 71 | 23.1 ± 0.2 | 19.3 ± 0.4 | 71 | 22.0 | 18.0 | 68 |
| 8 | 23.1 ± 0.2 | 19.3 ± 0.3 | 71 | 23.0 ± 0.0 | 19.3 ± 0.4 | 71 | 22.5 | 17.5 | 69 |
Significant (P < 0.05, Student t test) difference relative to room temperature.
Table 2.
Pressure (mm WC; mean ± 1 SD), air speed (m/s; mean ± 1 SD; 20 measurements per cage), and number of air changes hourly within PIV cages (n= 19) each containing 2 mice
| Day | Pressure | Air speed | Air changes hourly |
| 1 | 4.08 ± 0.29 | 0.49 ± 0.08 | 303 |
| 2 | 4.25 ± 0.20 | 0.37 ± 0.06 | 228 |
| 3 | 4.30 ± 0.16 | 0.36 ± 0.06 | 222 |
| 4 | 4.38 ± 0.18 | 0.34 ± 0.05 | 210 |
| 5 | 4.45 ± 0.16 | 0.33 ± 0.05 | 204 |
| 6 | 5.53 ± 0.18 | 0.30 ± 0.05 | 185 |
| 7 | 4.58 ± 0.17 | 0.29 ± 0.05 | 179 |
| 8 | 5.53 ± 0.22a | 0.28 ± 0.05a | 173 |
Significant (P < 0.05, Student t test) difference compared with measurements on day 1.
In both the PIV and FT systems, ammonia concentrations followed an ascending curve with experimental day; differences between groups were present since the first day of measurement (Table 3).
Table 3.
Ammonia concentration (ppm; mean ± 1 SD) in PIV (n= 19) and FT (n= 10) cages each containing 2 mice
| Day | PIV | FT | Holding room |
| 1 | 0.90 ± 0.39 | 2.00 ± 1.10 | 0 |
| 2 | 0.40 ± 0.66 | 2.33 ± 1.63 | 1.00 |
| 3 | 1.40 ± 1.43 | 4.00 ± 1.79 | 3.00 |
| 4 | 4.20 ± 4.05 | 24.67 ± 24.06 | 10.00 |
| 5 | 17.80 ± 12.66 | 50.00 ± 35.78 | 12.00 |
| 6 | 31.40 ± 12.94 | 78.33 ± 44.46a | 4.00 |
| 7 | 34.40 ± 15.85 | 103.33 ± 57.50a | 3.00 |
PIV data are net values (concentrations measured in PIV cages minus background ammonia concentration in the holding room).
Significant (P < 0.05, Mann–Whitney test) between PIV and FT groups.
Discussion
Microfans providing air flow from the top of mouse cages1 maintained low intracage concentrations of various air contaminants,7,13 similar to those in microenvironmental systems comprising plenum chambers and a single insufflation fan per cage. Low levels of ammonia that were present in the atmosphere of the holding room13,17 from which the air for the PIV cages was collected were subtracted from the intracage measurements.
Wet- and dry-bulb temperatures revealed that both FT and PIV systems were in thermal equilibrium with the holding room throughout the experimental period. Relative humidity was similarly consistent, with no differences between PIV and FT groups. These results illustrate that the observed differences in ammonia levels were influenced by the number of air changes hourly and not by the relative humidity.
The air pressure in the PIV cages was positive relative to the room; that is, the microfans effectively kept the cages pressurized, ensuring an optimally insulated microenvironment in terms of air circulation. We attribute the increase in pressure observed during the study period to cumulative saturation of the exhaust filters by debris, such as hair, bedding dust, and chow residue. These filters were cleaned during cage changing, to avoid disturbances in other evaluated parameters, such as ammonia levels and relative humidity average. Because of increased intracage pressure and progressive saturation of exhaust filters, air speed (and consequently airflow rate) decreased with the duration of the experiment, although air speed values remained high when viewed in terms of the number of air changes hourly. Pressure and airflow values were much higher than the recommended parameters (20 air changes hourly),12 suggesting that the exhaust filter areas should be increased. Enlarging the exhaust area likely will decrease filter saturation without compromising internal pressure, allowing this parameter to remain within the range of recommended values (minimum, 1 to 2 mm WC).
During more than 8800 h of operation, equipment failure was negligible, being limited to one burned-out fuse (a predictable event for any safety device) and halting of microfans (which required only simple maintenance procedures to resolve). Advantages to the use of microfans in PIV caging include: 1) accurate adjustment of air speed, and consequently of airflow and pressure values, 2) reduced costs of implementation and maintenance of the ventilation system, and 3) the possibility of adaptation and reuse of low-cost commercially available components, such as PVC tubing and the microfans themselves.8
Overall, the data acquired in the current study reveal that pressurization and microenvironmental ventilation of individual mouse cages by using microfans is a simple, reliable system, with low cost, reduced maintenance requirements, and rare occurrence of failure. This system imposes few changes to the current cage-caging routine in laboratory animal facilities. However, further experiments are needed to determine the potential influence of this type of ventilation system on the reproductive performance and pulmonary integrity of laboratory mice. In addition, using insufflation and exhaust ducts to collect air from outside of the holding room likely will avoid the presence of background ammonia concentration in the air source. The alternative PIV design we present here is technically feasible, even considering the nominal pressure provided by microfans, because flow losses along the ducts can be minimized by appropriate adjustment of duct diameter and design of the overall rack network.
Acknowledgments
The study reported in this paper was developed as part of a Master's thesis submitted by Alexandre Martinewski to the Graduate Program in Experimental and Compared Pathology (Department of Pathology, School of Veterinary Medicine and Animal Science, Universidade de São Paulo, Brazil). Financial support was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant no. 98/15314-9).
Patent filed under no. PI 0402095-2–INPI, Brazil; patent holders: Alexandre Martinewski, José Luiz Bernardino Merusse, and University of São Paulo.
References
- 1.Baumans V, Schlingmann F, Vonck M, Van Fith H. 2002. Individually ventilated cages: beneficial for mice and men? Contemp Top Lab Anim Sci 41:13–19 [PubMed] [Google Scholar]
- 2.Carissimi AS, Chaguri LCAG, Teixeira MA, Mori CMC, Macchioni M, Sant'Anna ETG, Saldiva PHN, Souza NL, Merusse JLB. 1999. Effects on intracage ammonia on ciliated epithelium of respiratory tract of rats maintained in 2 ventilated system for animal facilities with different frequency bedding changes. Braz J Vet Res Anim Sci 1:99–118 [Google Scholar]
- 3.Carissimi AS, Chaguri LCAG, Teixeira MA, Mori CMC, Macchioni M, Sant'Anna ETG, Saldiva PHN, Souza NL, Merusse JLB. 2000. Effects of 2 ventilation systems and bedding change frequency on cage environmental factors in rats (Rattus norvegicus). Anim Tech 51:161–171 [Google Scholar]
- 4.Chaguri LCAG. 1998 Ventilação microambiental para biotérios: estudo experimental em ratos (Rattus norvegicus). [Dissertation]. São Paulo (Brazil): University of São Paulo. [Google Scholar]
- 5.Gamble MR, Clough G. 1976. Ammonia build-up in animal boxes and its effect on rat tracheal epithelium. Lab Anim 10:93–104 [DOI] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 7.Krohn TC, Hansen AK. 2000. The effects and tolerances for carbon dioxide in relation to recent developments in laboratory animal housing. Scand J Lab Anim Sci 27:173–181 [Google Scholar]
- 8.Martinewski A. 1998. Projeto de ventilação para gabinete microambiental usando múltiplos motores de corrente contínua. [Dissertation]. Sorocaba (Brazil): Centro Estadual de Educação Tecnológica Paula Souza / Faculdade de Tecnologia de Sorocaba. [Google Scholar]
- 9.Martinewski A, Souza NL, Aranha EFC, Merusse JLB. 2010. Controle termo-higrométrico microambiental para animais de laboratório por meio de tecnologia termoelétrica: estudo experimental para roedores de laboratório. Braz J Vet Res Anim Sci 47:315–322 [Google Scholar]
- 10.Merusse JLB. 1995. Equipamento para criação e manutenção de animais utilizados em experimentação biomédica e respectivo processo de distribuição unidirecional do ar—PI9302341-3. Revista de Propriedade Industrial 1262:28–56 [Google Scholar]
- 11.Perkins SE, Lipman NS. 1996. Evaluation of microenvironmental conditions and noise generation in 3 individually ventilated rodent caging systems and static isolator cages. Contemp Top Lab Anim Sci 35:61–65 [PubMed] [Google Scholar]
- 12.Reeb CK, Jones RB, Bearg DW, Bedigian H, Paigen B. 1997. Impact of room ventilation rates on mouse cage ventilation and microenvironment. Contemp Top Lab Anim Sci 36:74–79 [PubMed] [Google Scholar]
- 13.Riskowski GL, Harrison PC, Memarzadeh F. 2006. Mass generation rates of ammonia, moisture, and heat production in mouse cages with 2 bedding types, 2 mouse strains, and 2 room relative humidities. ASHRAE Trans 112:134–144 [Google Scholar]
- 14.Serrano LJ. 1971. Carbon dioxide and ammonia in mouse cages: effect of cage covers, population, and activity. Lab Anim Sci 21:75–85 [PubMed] [Google Scholar]
- 15.Shaw BH. 1976 Air movements within animal houses, p 185–298. In: McSheehy T. Control of the animal house environment. [Google Scholar]
- 16.Silverman J, Bays DW, Copper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62 [PMC free article] [PubMed] [Google Scholar]
- 17.Small JD, Dietrich R. 2007. Environmental and equipment monitoring, p 410–436. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research: normative biology, husbandry and models. San Diego (CA): Academic Press. [Google Scholar]
- 18.Teixeira MA, Sinhorini IL, Souza NL, Merusse JLB. 1999. Microenvironmental ventilation system for laboratory animal facilities with air distribution by means of plennum chambers. Anim Tech 50:187–195 [Google Scholar]