Abstract
Corynebacterium bovis is a common pathogen in athymic nude mouse colonies. Control and eradication of the organism are challenging because depopulation and restricted colony access are often not options within vivaria. We evaluated potential sources and dissemination routes of C. bovis in an enzootically infected colony. Immunocompetent mice and personnel were evaluated for their potential to carry C. bovis, and husbandry and sanitation methods were evaluated for their efficacy in preventing cross-contamination. C. bovis was detected in furred immunocompetent mice previously exposed to infected athymic nude mice and in the nasopharynx of humans. Microisolation cages were not effective in maintaining athymic nude mice C. bovis-free when they were housed in a room known to contain immunodeficient mice with C. bovis infections. A tunnel washer that provided a ≥180 °F final rinse provided effective elimination of C. bovis from cage components. Passive and active air sampling techniques showed airborne dispersal of C. bovis despite the use of individually ventilated caging systems and stringent operational standards. Bacterial growth was not observed in settle plates placed inside autoclaved individually ventilated microisolation cages on various ventilated racks for 24-h periods. C. bovis aerosolization was shown to be a means of spread of the bacterium during cage-change procedures inside a class II type A2 biosafety cabinet. Our findings indicate that C. bovis can be a pervasive environmental contaminant in infected rodent holding rooms and successful eradication strategies must include environmental decontamination and attention to air quality.
Abbreviation: BSC, biological safety cabinet; IVC, individually ventilated cage
Corynebacterium-associated hyperkeratosis, commonly known as scaly skin disease,3,6,10,19 has anecdotally been reported as early as 1976 in athymic nude mice6 with subsequent global outbreaks described in the 1980s and 1990s.6,11,17,19 In 1998, the causative agent was identified as Corynebacterium bovis via 16S rRNA sequence analysis.9,18 Nude mice exhibit primarily a transient hyperkeratotic dermatitis with diagnosis confirmed by microbiologic culture4 or PCR.9 Once established, variable success in management and eradication of C. bovis has been achieved through antibiotic regimens4,17 and colony depopulation and disinfection.19
The primary means of entrance of C. bovis into vivaria is often unknown, although several reservoirs of the bacterium have been suggested.6,19 Fomites and keratin flakes often are implicated in the transmission of the bacterium,6,10,11 but demonstration and extent of cross-contamination in an experimental setting have not been documented. We examined possible sources of entry and persistence of C. bovis in vivaria. Immunocompetent and athymic nude mice, as well as personnel, were monitored for their potential to harbor C. bovis. We also evaluated the effectiveness of various husbandry techniques to prevent the spread of this bacterium. Microisolation cage-change technique was evaluated for its efficacy to maintain athymic nude mice free of C. bovis when newly introduced into an immunodeficient rodent holding room housing a high prevalence of C. bovis-infected mice. Tunnel washer efficacy, environmental bacterial burden, and bacterial aerosolization, including the potential for cross-contamination during cage change within a biologic safety cabinet, were evaluated also. We hypothesized that the critical evaluation of these parameters would elucidate steps that could be used to prevent the introduction and spread of C. bovis.
Materials and Methods
Animals.
Male (n = 39) and female (n = 98) athymic NCr-nu/nu mice (age, 4 to 6 wk; National Cancer Institute, Frederick, MD, and Wilmington, MA), specified by the supplier to be free of murine viruses, pathogenic bacteria including C. bovis, and endo- and ectoparasites were used. These mice were used in studies evaluating cage-change technique (n = 104) and as sentinels in immunocompetent rodent holding rooms (n = 33). Outbred athymic nude and immunocompetent mice, including 12 Swiss Webster (Tac:SW) mice, used on other approved institutional research protocols were also used as described. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals14 in an AAALAC-accredited facility. All procedures outlined in the study were approved by Memorial Sloan-Kettering Cancer Center's IACUC.
Animals were housed in individually ventilated cages (model nos. 19-140-10-14-1-4-7TMAL and 9-140-10-14-1-4-7TMAL, Thoren Caging Systems, Hazleton, PA) maintained at slight positive pressure (supply, 0.3 in. of water column; exhaust, 0.25 in. of water column) with respect to the holding room. The ventilated caging system distributed room air through a HEPA filter to individual cages whose effluent was exhausted directly into the building's HVAC system. Mice were housed on autoclaved aspen chip bedding (PJ Murphy Forest Products, Montville, NJ) and provided γ-irradiated commercial diet (PicoLab Rodent Diet 20, 5053 LabDiet, PMI Nutrition International, St Louis, MO) and acidified water (pH, 2.5 to 2.8) ad libitum. Feed bags were flash-autoclaved as described to disinfect the bags’ surface.20 Mice were housed at a population density that ranged from 1 to 5 mice per cage in an environment providing a temperature of 21.1 to 22.2 °C (70 to 72 °F), 30% to 70% relative humidity, 10 to 15 fresh air exchanges hourly, and a 12:12-h light:dark cycle. Cage components were sanitized without use of chemicals in mechanical washers (Basil 6000 and 4602 Series, Steris, Mentor, OH) that provided a ≥180 °F water final rinse.
Mouse cages were changed weekly within a 5′ class II type A2 biosafety cabinet (BSC; NU602-500, Nuaire, Plymouth, MN) in immunodeficient rodent holding rooms or an animal transfer station (NU-S612-400, Nuaire) in immunocompetent rodent holding rooms as previously described4 and further detailed following.
Enzootically infected rodent room.
Studies evaluating cage-change technique, environmental contamination, and potential aerosolization and cross-contamination of C. bovis were conducted in a single immunodeficient rodent holding room. The rodent holding room contained approximately 450 rodent cages (maximal capacity, 770 cages) maintained in individually ventilated caging systems housing immunodeficient mice including athymic nude, beige-nude-xid (NIHS-LystbgFoxn1nuBtkxid), NOD-scid (NOD.CB17-Prkdcscid), NOD-scid IL2Rγnull (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ)], and SCID-beige (CB17. Cg-PrkdcscidLyst bg) mice. As previously described, C. bovis infection prevalence was 65% when a representative 10% of the room population was sampled.4 The holding room had been used to house immunodeficient mice for approximately 2 y.
Microbiologic techniques.
Collection methods used to detect C. bovis infection in mice included skin and buccal swab techniques as previously described.4 In brief, the skin swab technique consisted of firmly rubbing a dry, sterile cotton-tipped applicator (Puritan Medical Products, Guilford, ME) over the dorsum and flanks of mice, and the buccal swab technique used a sterile urethrogenital calcium-alginate–tipped applicator (Puritan Medical Products) to sample along the caudal buccal mucosa. Both clinically affected and apparently healthy mice were sampled. All mice within a cage were sampled with the same applicator to generate a single pooled sample per cage. Human volunteers, having provided informed consent, were evaluated through throat and nasopharyngeal cultures; both techniques used sterile cotton-tipped applicators to either sample at the back of the throat or in the nasopharynx by passage through the nostril. To evaluate disinfection efficacy, cage components were sampled with dry, sterile cotton-tipped applicators.
Microbiologic culture and isolate identification were performed as previously described.4 Briefly, samples were streaked onto Columbia colistin–nalidixic-acid agar with 5% sheep blood (BBL, BD Diagnostic Systems, Sparks, MD) and incubated at 37 °C with 5% CO2 for as long as 7 d. Colonies that displayed characteristic morphology (1 to 2 mm, smooth, punctiform, white, nonhemolytic) and gram-stain appearance (short, gram-positive rods arranged in V forms)13 were subcultured onto Columbia agar with 5% sheep blood (BBL, BD Diagnostic Systems) for speciation by colorimetric biochemical testing (API Coryne, bioMerieux, Marcy l'Etoile, France) after 48 h of incubation at 37 °C. Columbia colistin–nalidixic-acid agar with 5% sheep blood was also used for environmental air sampling, but plates were not streaked after collection to allow for enumeration of discrete colony formation after 48 h incubation at 37 °C with 5% CO2.
Representative human and air samples identified as C. bovis by colorimetric biochemical testing were submitted to a commercial laboratory (Research Animal Diagnostic Laboratory, University of Missouri, Columbia, MO) for confirmatory testing with 16S rRNA sequencing. Either a generic bacterial PCR assay or one specific to C. bovis that amplifies a segment of the 16S rRNA gene was performed; the resulting product was sequenced and compared with sequences in GenBank by using BLAST software.
Potential sources of C. bovis.
Immunocompetent mice of various strains and stocks, newly received athymic nude mice, and select staff were cultured to ascertain whether they harbored C. bovis. Approximately 5% of the population of 2 mouse holding rooms housing predominantly furred immunocompetent mice (total: 41 cages, 135 rodents) was cultured by collecting a pooled buccal and skin swab from all mice to yield one swab per cage. The holding rooms contained approximately 460 and 440 rodent cages maintained in individually ventilated caging systems. The mice were included on other approved research protocols within the institution and housed at a density of 1 to 5 mice per cage. Mouse strains were primarily of C57BL/6 and BALB/c background, although in one holding room, there was the potential for a transient athymic nude mouse population because some researchers used both immunocompetent mice as well as immunodeficient mice housed elsewhere in the facility.
Female athymic nude mice were placed as sentinels in 6 additional immunocompetent mouse holding rooms each housing approximately 690 rodent cages. In 2 of these holding rooms, immunodeficient mice were housed transiently, and in an additional holding room, immunocompetent mice were irradiated. In 5 holding rooms, 2 sentinel cages each housing 3 athymic nude mice were placed. Sentinels were maintained by using standard husbandry methods (further description to follow) for 2 mo; subsequently, these cages received pooled soiled bedding collected from different resident cages (40 resident cages per cage change; approximately 15 mL soiled bedding from each) during weekly cage changes for as long as 13 mo. In 1 of the 6 rooms, 3 athymic nude mice were cohoused on arrival with Swiss Webster (Tac:SW) dirty-bedding sentinels for approximately 8 mo. Three sentinel cages were each composed of one athymic nude and 4 Swiss Webster mice. All athymic nude sentinels were confirmed to be culture-negative for C. bovis on arrival and then cultured weekly by skin swab to monitor for the presence of C. bovis. All athymic nude mice within a cage were sampled with the same applicator to generate a single pooled culture per cage. Culture frequency was reduced to monthly after 4 mo for the nude mice housed alone and after 2 mo for the nude mice cohoused with Swiss Webster mice. Mice were cultured until a minimum of 2 consecutive C. bovis-positive cultures were obtained or for as long as 8 to 13 mo as indicated.
In addition, 12 shipments of athymic nude mice from 2 vendor colonies were evaluated for C. bovis on arrival using both the skin swab and buccal swab techniques. All mice (8 shipments) or a subset of mice (4 shipments) from each shipment were cultured. A minimum of 5 mice were sampled from the subset-tested 4 shipments; shipment size ranged from 15 to 30 mice.
The nasopharynx and throat of personnel (n = 13) with daily access to immunodeficient rodents and the diagnostic laboratory were sampled for C. bovis. Persons harboring C. bovis were recultured every 2 wk, with no treatment or instructed modification to work practices, until 2 consecutive C. bovis-negative cultures were obtained.
Evaluation of cage-change technique.
The possibility of cross-contamination during cage change was evaluated in an immunodeficient mouse holding room housing C. bovis-infected mice. Studies were initiated by using intensified husbandry techniques. Techniques subsequently were relaxed in an attempt to elucidate critical steps in the cage-change process associated with cross-contamination between mice housed in different cages. Four trials were conducted, each beginning with 5 cages containing 3 to 5 male or female 4- to 6-wk-old C. bovis-free athymic nude mice. Each trial was conducted for 26 cage changes, with weekly cage changes performed for trials 1 through 3 and daily cage changes during trial 4. In trial 4, after 26 cage changes, mice in culture-negative cages again were sampled 1 and 2 wk later to detect latent infections. Seven cages (total, 24 mice) maintained by using standard husbandry technique served as controls.
Standard husbandry technique consisted of weekly cage changes performed in a class II type A2 BSC. Personal protective equipment included dedicated scrubs, a disposable gown, face mask (Tecnol Procedure Mask, Kimberly-Clark, Roswell, GA), hair bonnet, shoe covers, and latex gloves. Prior to use, the BSC was operated for a minimum of 5 min to ensure appropriate airflow and magnahelic gauge readings, and the interior of the BSC was disinfected with chlorine dioxide solution (1:18:1; Clidox-S, Pharmacal Research Laboratories, Naugatuck, CT) and then lined with chlorine dioxide-soaked absorbent ‘blue pads’ (Dukal Corporation, Hauppauge, NY). Chlorine dioxide disinfectant was made fresh every 14 d.
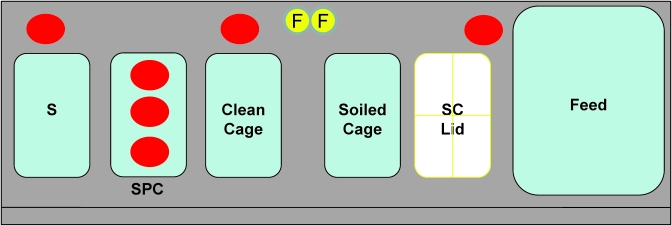
Sanitized cage components were transported into the animal holding room as 3 separate entities: individual cage units each containing a shoebox cage with autoclaved bedding, wire bar lid, and a filter top; crates of water bottles; and bags of flash-autoclaved irradiated feed. Each entity was covered with a plastic drape in clean cage wash before distribution to the animal holding room. Figure 1 shows placement of equipment within the BSC, with one soiled cage changed at a time. Irradiated feed bags were opened in the room and poured into a clean feed bin maintained within the BSC. Clean water bottles were removed individually during cage change from the bottle crates stacked immediately adjacent to the BSC and placed into the wire bar lid of each clean cage within the cabinet. Only closed cage units fitted with a filter top lid were handled outside the BSC.
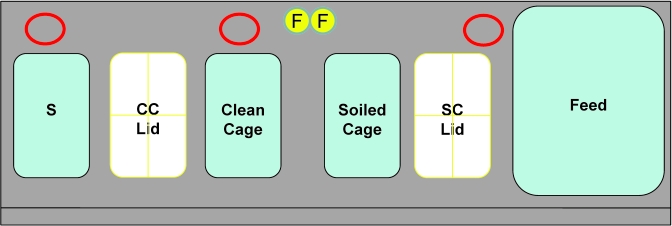
Figure 1.
Placement of materials within a class II type A2 BSC for cage changes by using standard technique. Red circular outlines at the back of the BSC represent positioning of settle plates (3) in evaluation of aerosolization of C. bovis during cage change. F, forceps in chlorine dioxide solution; S, sentinel cage; CC Lid, clean cage lid; SC Lid, soiled cage filter top.
Mice were transferred from the soiled to clean cage by using forceps that had been soaked in chlorine dioxide solution (1:18:1), alternating between 2 forceps with each cage change to allow more disinfectant contact time. Forceps remained in the chlorine dioxide solution for approximately 3 min between cage changes and were wet with disinfectant when used to transfer mice. Fresh chlorine dioxide solution was used at the start of each cage change to prevent the accumulation of organic debris. Gloves were disinfected with alcohol foam (Alcare Antiseptic Handrub, Steris) between cages and were changed after each side of the ventilated cage rack had been changed (maximum, 70 cages) or when obviously soiled. Soiled bedding samples (approximately 15 mL) were collected from 40 of every 280 soiled cages and provided as contact bedding to the respective sentinel cage.
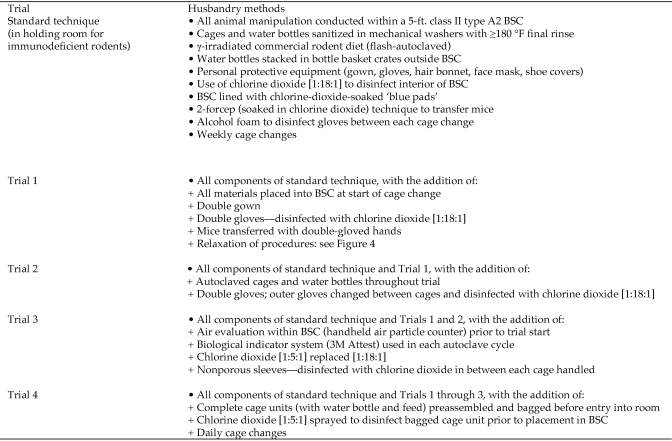
Experimental trials consisted of intensified husbandry techniques that deviated from our standard cage-change technique as outlined in Figure 2 and depicted in Figure 3. Personal protective equipment used is described in Figure 2. For all trials, mice were transferred from the soiled to clean cage by grasping their tail with double-gloved chlorine-dioxide–moistened hands and concurrently, each mouse's skin was cultured for C. bovis and evaluated for hyperkeratosis. For all trials, excluding the end of trial 1 as described following, the outer pair of gloves were changed and moistened with chlorine-dioxide solution between each cage change. Within a trial, cages were always changed in successive order from cage 1 to 5 until a specific cage became culture-positive, at which time the cage was removed from the study. Chlorine dioxide activity, equivalent to at least 19 ppm for 1:18:1 concentration and at least 63 ppm for 1:5:1 concentration (as provided by Pharmacal Research Laboratories) was confirmed for all trials by using chlorine-dioxide test strips (Insta-Test, LaMotte, Chestertown, MD). Chlorine dioxide concentration was increased from 1:18:1 for trials 1 and 2 to 1:5:1 for trials 3 and 4.
Figure 2.
Cumulative additional husbandry measures to standard cage-change technique added during trials 1 through 4. Evaluation of cage-change processes necessary to maintain athymic nude mice free of C. bovis in an enzootically infected immunodeficient mouse holding room.
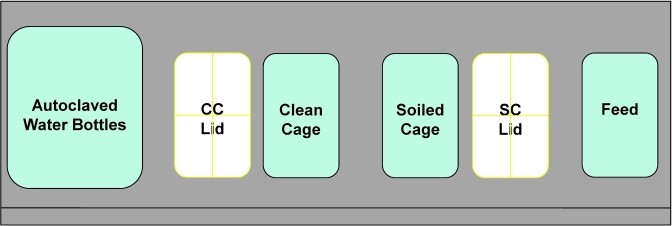
Figure 3.
Placement of materials within a class II type A2 BSC for cage changes during trials 1 through 3 to evaluate cage-change technique. In trial 1, when use of autoclaved cage components was discontinued, water bottles were placed in the BSC on absorbent ‘blue pads’ in the location shown. In trial 4, cages were similarly changed with the clean cage on the left and the soiled cage on the right, but cage units were preassembled.
Autoclaved materials including individual cage units consisting of bedding, wire bar lid, and filter top; water bottles containing acidified (pH, 2.5 to 2.8) reverse-osmosis–filtered water; and flash-autoclaved irradiated feed bags were used in trials 1 to 4, detailed following. Individual cage units were autoclaved for 30 min and water bottles for 15 min at 125 °C. In trials 3 and 4, a biologic monitoring indicator (3M Attest, St Paul, MN) was used with each autoclave cycle to verify effective sterilization.
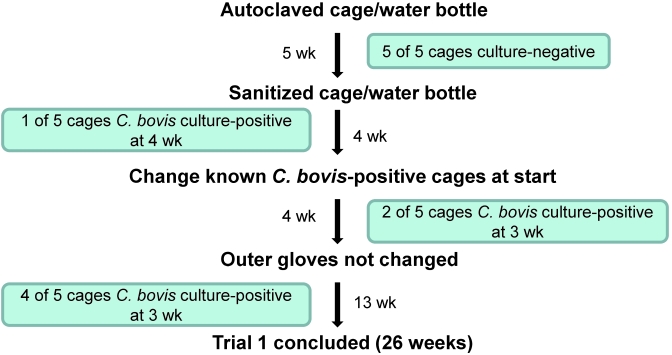
In trial 1 (Figure 4), autoclaved materials were used for 5 wk; subsequently the technique was relaxed to use cage components that had been sanitized in a tunnel washer, without use of chemicals, providing a ≥180 °F water final rinse. Nonsterile materials used in trial 1 were obtained from a clean materials supply bulk truck that was covered with a protective drape and maintained within the holding room. Materials were positioned within the BSC for cage change as shown in Figure 3. After 4 wk of using non-autoclaved supplies, the technique was altered further to include the changing of 5 cages housing known C. bovis-infected mice prior to the experimental cages at each cage change. After an additional 4 wk, the outer pair of latex gloves was no longer changed between cages. Gloves continued to be disinfected with chlorine dioxide and cages known to contain C. bovis-infected mice continued to be changed first for the duration of the 26-wk study. In trials 2 through 4, autoclaved materials were maintained throughout the 26-wk cage-change period, and husbandry methods were not relaxed.
Figure 4.
Illustration of methods used in trial 1 to evaluate cage-change technique. Husbandry measures were altered over time with the number of cages detected to house C. bovis-infected mice at each step indicated.
In trials 1 through 3, autoclaved cage components were transported into the animal holding room as separate entities: individual cage units consisting of bedding, wire bar lid, and filter top; water bottles inside a large microisolation cage consisting of a cage bottom and filter top (R20 Series Cage and Micro Filter Top, Ancare, Bellmore, NY); and flash-autoclaved irradiated feed bags, with each of these 3 entities covered with a plastic drape. Cage units containing water bottles and feed were assembled in the BSC. Water bottles and feed were exposed briefly to holding room air during initial placement into the BSC. Feed was scooped, by using an autoclaved scoop, from the feed bag just outside of the BSC into a sterile cage inside the BSC for subsequent distribution to cages during changing within the BSC. The filter top was removed from the microisolation cage containing the autoclaved water bottles immediately prior to placement of the cage with water bottles into the BSC.
In trial 4, autoclaved cage units consisting of a cage with bedding, wire bar lid, irradiated feed, water bottle, and filter top were assembled in an empty holding room in a pass-through BSC (NU-610-SPEC, Nuaire) and then packaged in plastic bags. Individually bagged cage units were transported to the immunodeficient mouse holding room at the time of cage change, surface-sprayed with chlorine dioxide (1:5:1), and opened for use within a class II type A2 BSC. In addition, the filter top was not removed from the clean cage during cage change. Instead, the wire bar lid and filter top were pushed back to provide sufficient space to allow for the transfer of mice into the cage.
Additional measures implemented in trials 3 and 4 included the use of nonporous sleeves (Tyvek, DuPont, Wilmington, DE) that were moistened thoroughly with chlorine-dioxide (1:5:1) solution between cages handled and further evaluation of the BSC. In addition to yearly safety cabinet certification, prior to commencing these trials, the air inside the BSC was evaluated at 6 sites (left, right, and middle of the cabinet, both at the back of the BSC near the exhaust grille and in front near the air intake grille) for 1-min readings each to ensure effective removal of particles 0.3 to 3.0 µm in size (Met One 227B Handheld Air Particle Counter, Hach Ultra, Grants Pass, OR).
Disinfection efficacy.
To evaluate the adequacy of sanitization of cage materials, complete cage units that had previously housed C. bovis-infected athymic nude mice (n = 8) were sanitized in a tunnel washer (Basil 6000 Series, Steris) without use of chemicals, providing a ≥180 °F water final rinse for an estimated contact time of 10 to 20 s. Four cage set-ups were cultured and sanitized each on 2 different days. Prior to disinfection, the interior walls of the shoebox cage and the wire-bar lid from each cage unit were sampled with a sterile cotton-tipped applicator to confirm the presence of C. bovis. After passage through the tunnel washer, separate cage components (including the filter top, wire bar lid, cage bottom, empty water bottle, and bottle cap–sipper tube from each cage unit) were cultured (cultures pooled; n = 4 cultures total per component) to monitor the efficacy of the tunnel washer in killing C. bovis.
Environmental contamination.
To evaluate environmental bacterial burden, passive and active air sampling was conducted in the same immunodeficient mouse holding room as that used to evaluate cage-change technique. In this holding room, all animal husbandry and research manipulation are performed in a class II type A2 BSC. Settle plates (n = 13) of Columbia colistin–nalidixic-acid agar with 5% sheep blood were positioned throughout the holding room on 4 d for 8-h periods. Placement location included counters and the tops of BSC. In addition, settle plates (n = 7) were placed inside empty autoclaved individually ventilated cages (IVC) located on the bottom 3 rows of 4 separate IVC racks for 24 h. A microbial air sampler (N6 Single-Stage Impactor [10-880 series], Andersen Instruments, Smyrna, GA) that impacted 28.3 L/min (1 cubic foot per minute) of air onto an agar plate was used on 2 d for four 20-min periods each day (total, 8 samples). The microbial air sampler was positioned on countertops and a 3-ft.-high tripod in multiple locations throughout the holding room. Air sampling was conducted over a 1-mo period.
Potential for C. bovis aerosolization during cage changes.
Air within a class II type A2 BSC was monitored during cage change for C. bovis. On each of 6 d, settle plates (total n = 18) of Columbia colistin–nalidixic-acid agar with 5% sheep blood were positioned in 3 locations (Figure 1) within the BSC during cage change performed by an animal care technician using standard husbandry technique. Settle plates were placed behind the dirty-bedding sentinel cage, behind the area where a clean cage was placed during the transfer of mice from a soiled cage, and next to the feed bin. Settle plates were positioned for 1-h periods, and the number of cages changed by the animal care technician during this time was recorded.
Potential for airborne cross-contamination within the BSC.
In addition to determining the potential for C. bovis to aerosolize during cage changes, we sought to determine whether C. bovis could linger within the BSC to infect subsequently manipulated cages. During each of six 1-h periods, 3 settle plates were positioned at the back of the BSC. In addition, 3 settle plates were positioned from front to back in a sanitized cage devoid of bedding periodically closed with a filter top lid (Figure 5). A soiled cage, housing athymic nude mice, was changed according to standard husbandry methods and then removed from the BSC. Next, a clean cage was placed in the BSC where the soiled cage had been located, and the filter top was removed from the cage containing the 3 settle plates to allow exposure to the BSC air. A mock cage change was performed by using the clean cage, with movement of arms and equipment undertaken to recreate the cage-change procedure. The clean cage then was closed and removed from the BSC, and the cage with settle plates was closed prior to placement of another soiled cage into the BSC. This study was conducted for 1-h periods, during which 30 to 40 soiled and clean cages were handled, on each of 6 d. New settle plates were provided for each of the 6 d. Some of the cages manipulated on each day were known to house C. bovis-infected mice; others were presumed to house infected mice, given the 65% infection prevalence in the holding room,4 the date of arrival of the mice into the vivarium, and clinical signs. The cage containing the settle plates was opened only during the mock-changing of the clean cages, whereas the settle plates positioned at the back of the BSC were exposed continuously throughout the 1-h period.
Figure 5.
Evaluation of C. bovis cross-contamination during cage changes within a class II type A2 BSC. The cage-change method mirrors that of our standard technique. Three settle plates (red circles) were positioned at the back of the BSC and 3 settle plates were placed in a standard cage without bedding (SPC). The settle plates within the SPC were exposed (filter top removed) only during a clean cage mock change (after a soiled cage was changed and removed from the BSC). During changing of soiled cages, the SPC lid was closed, and the clean cage lid rested on top of the SPC. F, forceps in chlorine dioxide solution; S, sentinel cage; SPC, settle plate cage; SC Lid, soiled cage filter top.
Statistical methods.
Trials 1 through 4 of the cage-change technique evaluation study, both individually and combined, were compared with the standard cage-change technique by using the permutation log-rank test. The application of the permutation procedure was due to the small number of mouse cages in these studies. We evaluated the median number of cage changes until infection (95% confidence interval) for each trial, along with the corresponding P value compared with controls. Associations and differences were considered significant if the P value was less than 0.05. All analyses were performed by using SAS 9.2 software (SAS Institute, Cary, NC).
Results
Potential sources of C. bovis.
Direct sampling of immunocompetent mice (5%) in 2 rodent holding rooms housing predominantly immunocompetent mice yielded 1 of 41 cages culture-positive for C. bovis. The culture-positive animals were C57BL/6 mice that previously had undergone a surgical procedure. In addition, after reexamination of the population in this holding room, 2 cages housing athymic nude mice that had a history of Corynebacterium-associated hyperkeratosis were identified.
Of the 6 immunocompetent rodent holding rooms evaluated, 3 yielded C. bovis-positive athymic nude sentinels. One sentinel cage, maintained by using standard husbandry (no dirty bedding), became culture-positive after 45 d, whereas 2 additional cages became culture-positive after approximately 2.5 mo in the facility, approximately 2 wk after beginning to receive dirty bedding. Approximately 4 mo later, an additional 3 cages became culture-positive. All sentinel cages within a holding room became culture-positive for C. bovis at the same collection time point, except those in the room that housed the only sentinel cage to become culture-positive while maintained without receiving dirty bedding. Each time C. bovis was detected, reexamination of the holding room identified a concurrent small population of either nude or immunodeficient stocks or strains or mice that had been irradiated as a component of their research utilization. The remaining 7 sentinel cages housed in the other 3 immunocompetent mouse holding rooms remained C. bovis culture-negative for the entire 13-mo period that they were evaluated; immunodeficient strains were not identified to be housed in these holding rooms during this time. All nude mice housed directly with Swiss Webster (Tac:SW) sentinels remained culture-negative for the approximately 8-mo monitoring period.
All 12 shipments of athymic nude mice sampled on arrival were negative for C. bovis on both skin and buccal culture. Interestingly, one partial shipment of male mice (those housed in 1 of 2 transport boxes) was culture-positive on arrival for a Corynebacterium sp., identified as Corynebacterium mastitidis with 16S rRNA sequencing, with no accompanying lesions or clinical signs. C. mastitidis recently was identified in preputial gland abscesses in mice.16
Nasopharyngeal cultures revealed that 1 of the 13 staff members was serially positive for C. bovis, confirmed with 16S rRNA sequencing. This person spent extensive time within an immunodeficient mouse holding room housing known C. bovis-infected mice while wearing appropriate personal protective equipment, including a face mask. After the initial positive culture, monitoring consisted of serial cultures at 2-wk intervals that began while the individual was working within the immunodeficient holding room and continued after discontinuation of the work. The carrier was known to be culture-positive for 72 d, including 55 d during which the individual had no exposure to rodents in the vivarium. All human throat cultures (n = 13) were negative for C. bovis.
Evaluation of cage-change technique.
Four trials were conducted to evaluate critical steps in the cage-change process necessary to prevent cross-contamination of C. bovis in nude mice within an enzootically infected immunodeficient mouse holding room. None of the trials were effective in preventing C. bovis infection in nude mice. C. bovis-infected mice were first detected in trials 1 through 4 after 9, 4, 6, and 15 cage changes, respectively (Table 1). Figure 4 illustrates the relaxation of the husbandry method during trial 1, with the number of cages that became culture-positive at each time point. In trial 2, cage change 2 was conducted with chlorine dioxide with insufficient activity (less than 10 ppm). Cage changes during trials 1 through 3 all were conducted with brief exposure of feed and water bottles to holding room air during placement inside the BSC. In each trial, cages of mice did not necessarily become C. bovis culture-positive in relation to the successive order in which they were changed. In addition, a single cage of mice remained culture-negative throughout each trial (Table 1).
Table 1.
Evaluation of cage-change methods to prevent cross-contamination of C. bovisin nude mice
| Order in which cages became infected (cage change at which infection was detected) |
|||||||
| Trial | No. of cages | No. of mice | First | Second | Third | Median no. of cage changes until infection (95% confidence interval) | Pa |
| 1 | 5 | 25 | cage 2 (9) | cage 1 (12) | cages 3 and 4 (16) | 16 (9–NA) | <0.001 |
| 2 | 5 | 25 | cages 4 and 5 (4) | cage 3 (7) | cage 1 (13) | 7 (4–NA) | 0.102 |
| 3 | 5 | 15 | cages 4 and 5 (6) | cage 1 (8) | cage 2 (14) | 8 (6–NA) | 0.02 |
| 4 | 5 | 15 | cage 2 (15) | cages 3 and 4 (16) | cage 1 (18) | 16 (15–NA) | 0.001 |
| Total for all trials | 20 | 80 | not applicable | 14.5 (7–16) | <0.001 | ||
| Control | 7 | 24 | not applicable | 5 (3–6) | |||
NA, not achieved.
In each trial, cages were changed in order from cage 1 to cage 5, with subsequent order (first, second, third) and cage change at which infection was detected by skin culture shown here. Cage changes were conducted weekly during trials 1 through 3 and daily in Trial 4. The standard cage-change technique served as the control.
Permutation log-rank test to evaluate median no. of cage changes until infection compared with control; a P value less than 0.05 is significant.
Particle count testing conducted prior to trials 3 and 4 detected zero 0.3- to 3.0-µm particles within the air at 1-min readings at various locations within the BSC.
Disinfection efficacy.
A tunnel washer providing a ≥180 °F water final rinse, without use of chemicals, demonstrated effective sterilization of C. bovis-contaminated cage components. All complete cage set-ups (n = 8) that had housed C. bovis-infected mice were culture-positive for C. bovis prior to sanitization. After passage through the tunnel washer, all cage components, including filter tops, wire bar lids, cage bottoms, water bottles, and bottle caps and sipper tubes, were C. bovis culture-negative (n = 4 cultures per component).
Environmental contamination.
To evaluate environmental contamination with C. bovis, passive and active air sampling was conducted in an immunodeficient mouse holding room. Approximately 54% (7 of 13) of the environmental settle plates and 50% (4 of 8) of the Andersen plates grew C. bovis. The number of pinpoint colonies consistent with Corynebacterium spp. ranged from 1 to 15 on plates from which C. bovis growth was speciated. C. bovis culture-positive locations included countertops, various room locations cultured 3 ft. above the floor, and the tops of both in use and idle BSC. No bacterial growth was observed in settle plates (n = 7) placed inside autoclaved IVC, maintained on various ventilated racks containing C. bovis-infected mice, for 24-h periods.
Potential for C. bovis aerosolization during cage changes.
Air within a class II type A2 BSC was found to be contaminated with C. bovis during cage changes. Among the 6 trials (3 settle plates each), C. bovis culture-positive results were obtained from all 3 plates during 3 trials, from 2 plates during 2 trials, and from 1 plate during the remaining trial. Settle plates positioned behind the sentinel cage that received dirty bedding were culture-positive 100% of the time, followed by those behind the clean cage (83%) and next to the feed bin (50%). On average, the animal care technician changed 40 cages within the 1-h testing period.
Potential for airborne cross-contamination within BSC.
Bacteria-laden air appears to remain in the BSC between cage changes, resulting in cross-contamination. Among the 6 trials (6 settle plates each), C. bovis culture-positive results were obtained from 4 plates in 1 trial, 3 plates in 2 trials, and 2 plates in the remaining 3 trials. Settle plates positioned behind the clean cage and next to the feed bin were culture-positive 100% of the time (all 6 trials). Settle plates positioned behind the sentinel cage were positive in 1 of the 6 trials. Settle plates contained within the settle plate cage were positive on 3 occasions. In 2 of 6 trials, the front settle plate within the settle plate cage was culture-positive, and in 1 of 6 trials, the middle settle plate was culture-positive.
Discussion
Once established, C. bovis is a difficult pathogen to manage and eliminate in colonies of nude and other immunodeficient mouse strains. As a result, the bacterium continues to present a major challenge to academic facilities because implementation of the necessary management measures can be difficult. Limited research addresses effective management and clearance of the bacterium.4,17,19 Reservoirs and fomites have been suggested as means of bacterial transmission,6,10,11,19 but various methods of transmission have not been demonstrated clearly. Our results reveal that contamination can be extensive in rooms housing immunodeficient mice. Transient carriers of C. bovis, including immunocompetent mice and personnel, further complicate management of the bacterium. Due to the potential for significant environmental contamination, cage changing and animal handling, even when performed using strict microisolation cage technique within a BSC, are not likely to prevent the spread of this bacterium if infected mice are present in the colony. We previously demonstrated limited success at eradication of C. bovis with antibiotic regimens.4 The greatest likelihood of eradication success would result from colony depopulation and extensive environmental decontamination, although published results have shown variable success.19
Speculated routes of entry of C. bovis into vivaria include immunodeficient and immunocompetent mice, biologics including tumor stocks, and personnel.6,10,12,19 Immunocompetent mice have been evaluated as potential carriers of C. bovis.6,12 The results of these studies are contradictory. We found that C. bovis culture-positive immunocompetent mice or athymic nude sentinels were detected only when other nude or immunodeficient mice with a history of Corynebacterium-associated hyperkeratosis were present in the rodent holding room. Furthermore, the single cage detected of C. bovis-infected furred, immunocompetent mice had undergone experimental surgical manipulation, which we believed to have compromised the immune status of the mice.
Athymic nude sentinels remained C. bovis culture-negative only in rooms that housed strictly immunocompetent mice, strengthening the conclusion that immunocompetent mice do not carry the bacterium without prior exposure to infected, immunodeficient mice. We did not evaluate the duration of C. bovis infection in immunocompetent mice after exposure, but believe that the infection is likely transient, given that athymic nude sentinels did not reveal transmission in rooms that held only immunocompetent mice.
Other sources of C. bovis examined included athymic nude mice and personnel. Mice are presumed to carry C. bovis in, among other locations, their upper alimentary tract;15 we therefore examined incoming nude mice from reputable vendors, and all were C. bovis-negative on skin and buccal swab cultures. C. bovis is a rare pathogen of humans, with most reported cases involving older adults with comorbidities.1,2,7,8 In our research, one staff member, who had no apparent immunosuppression but did have extensive prior exposure to an immunodeficient rodent holding room, transiently carried C. bovis in the nasopharynx. Although the evaluation conducted during our current study was limited, this finding further indicates potential complications to eradication and management efforts. Inapparent carriage of the bacterium in humans may be more prevalent than has been suspected and could account for transmission of the bacterium between vivaria. Dust or surgical masks, often included in the personal protective equipment worn in immunodeficient mouse rooms, may be ineffective at preventing bacterial colonization of personnel and spread of the bacterium. Further studies are necessary to determine whether exposure to C. bovis-infected mice is necessary for personnel colonization or, alternatively, whether personnel have become colonized from another source. Despite these findings, the initial source of infection in our vivarium remains undetermined, as is likely the case at many facilities.
The extreme difficulty in maintaining athymic nude mice free from C. bovis in colonies where the bacterium is present was illustrated by our current studies. In trial 1 of our study evaluating cage-change technique, the first infection occurred after discontinuing the use of autoclaved caging materials; therefore only autoclaved materials were used in all subsequent trials. However, we also evaluated the disinfection efficacy of a tunnel washer that provided a ≥180 °F water final rinse without the use of chemicals and found that it effectively killed C. bovis. Therefore although we could not definitively account for the infection break in this trial, we do not believe that autoclaving is necessary to prevent the spread of this bacterium on cages throughout a facility, as long as there is sufficient exposure of caging materials to a rinse of sufficient time and temperature in an automated washer.
In trial 2, cage-changing occurred once inadvertently with ineffective chlorine dioxide solution. Two cage changes (2 wk) later, 2 of 5 cages were C. bovis culture-positive. Although we have demonstrated other confounding factors that may account for the break in this study, we remain suspicious, given the rapidity of detection after its use, that ineffective disinfection was the cause in this case.
The additional measures in trial 3, including the use of nonporous sleeves and a higher concentration of chlorine dioxide solution, also failed to prevent C. bovis infection. Throughout trials 1 through 3, autoclaved water bottles and feed were exposed briefly to holding room air during their placement into the BSC at the start of the day's procedures. Therefore, we tested air quality in the immunodeficient mouse holding room to reveal that, despite stringent standards of operation and the use of IVC with HEPA-filtered supply and direct building exhaust, C. bovis was a persistent airborne contaminant, perhaps accounting for its continued transmission. The air contamination we detected was consistent and extensive regardless of room activity or observed clinical disease and despite the use of specialized equipment designed to provide product (animal) and personnel (environmental) protection, exceeding that previously postulated to result from aerosol formation and dispersal of C. bovis-laden keratin flakes from clinically diseased mice within a holding room.6
Although the IVC we used were operated at slightly different parameters, studies with this specific IVC system did not leak sulfur hexafluoride tracer gas; thus, we suspect our system would not leak particulates.21 Therefore, we believe that once housed in an IVC, infected mice do not contaminate rodent holding rooms. In addition, we have shown by using settle plates that intercage C. bovis contamination did not occur in the IVC system used.
In the immunodeficient mouse holding room sampled, stringent standards of operation required that all cage changing and animal manipulation be conducted in a class II type A2 BSC. Staff is trained and required to use the BSC in a manner that does not adversely affect BSC airflow dynamics and therefore its ability to provide both product and personnel protection. Our experience has shown that research staff do not consistently follow correct BSC use. We believe that these failures likely account for the initial as well as persistent room contamination observed, thus serving as the source of contamination for newly introduced immunocompromised mice and perpetuating the cycle of infection within the colony. We recently have shown that mice can remain infected for at least 7 mo,4 and the bacterium is anecdotally reported to survive more than 30 d on fomites.10
We conducted 2 experiments to evaluate air quality within a class II type A2 BSC during standard cage-change procedures. Both clearly detected the presence of aerosolized C. bovis within the cabinet, and the second illustrated the potential for cross-contamination between successive cage changes. C. bovis-positive air cultures within the BSC were routine when manipulating infected mice, regardless of whether the mice exhibited hyperkeratotic dermatitis. Settle plates positioned at the back of the BSC in a path of effluent airflow were routinely culture-positive. With mock cage changes, the front and middle settle plates within the settle plate cage were periodically culture-positive. Plates in these positions are most likely to become contaminated, given air flow dynamics within a BSC.
In trial 4 of the study evaluating cage-change technique, we attempted to prevent exposure of materials to contaminated holding room air by introducing only bagged, complete isolator cage units that were sprayed with chlorine dioxide disinfectant before placement into the BSC. In addition, cross-contamination within the BSC was thought to be inconsequential, because no infected mice were introduced into the BSC during this trial. Although a greater number of cage-change manipulations were performed before detecting C. bovis, the trial was ultimately unsuccessful in preventing infection. Given the very minimal manipulation of caging and mice and the measures taken to change gloves and thoroughly disinfect gloves and nonporous sleeves with chlorine dioxide solution prior to cage changes, we speculate disturbance of cabinet airflow dynamics to account for this contamination. The horizontal and vertical arm movements required to handle cages and mice within the BSC may exceed the ability of the cabinet's air curtain to prevent environmental contaminants from entering the cabinet. Guidelines recommend that work be delayed for approximately 1 min after placing hands inside the cabinet to allow for reestablishment of the front air curtain.5 With cage changes as conducted here and as routinely performed by animal care technicians, it would be difficult to implement this delay. It also is recommended that all operations be performed at least 4 in. back from the front grill.5 Due to space constraints and cage size, correct placement of equipment for manipulation is difficult to achieve.
In trial 4, the earliest detection of C. bovis was at the 15th cage change. Although it is difficult to pinpoint the exact time of exposure to the bacterium due to daily cage changes, we speculate that it occurred around the ninth cage change. This idea is based on earlier work demonstrating that athymic nude mice manifest hyperkeratotic dermatitis 12.5 d (on average) after exposure to either an infected mouse or dirty bedding from an infected cage.4 In our experience, nude mice exhibit hyperkeratotic dermatitis on average 6 d after detection of C. bovis infection. Therefore, we assume that mice in trial 4 had been exposed to the bacterium approximately 6 d earlier than when identified through skin culture.
Furthermore, our current cage-change technique evaluation study highlights the random nature at which cages became infected (Table 1). In each trial, cages were changed in successive order, but infection did not necessarily spread from one cage to the next. We feel this pattern further implicates air flow dynamics within the BSC during cage changing as a primary cause of contamination of cages and that measures conducted in between the handling of each cage were largely successful at preventing cross-contamination.
In summary, our findings indicate the C. bovis can be a pervasive environmental contaminant once introduced into rodent holding rooms. In addition to surface decontamination, air contamination must be considered when pursuing C. bovis eradication. Thorough environmental decontamination, restocking with C. bovis-free mice, and strict adherence to biosecurity measures are essential to successful maintenance of C. bovis-free mouse colonies.
Acknowledgments
We thank Junting Zheng (Department of Epidemiology and Biostatistics) for statistical analysis of data. We extend special thanks to Aziz Toma for assistance with microbiology and many thoughtful discussions. We also thank the animal care staff of the Memorial Sloan-Kettering Cancer Center for maintaining animal colonies used in this study and are grateful to many members of the Laboratory of Comparative Pathology for their support.
References
- 1.Achermann Y, Trampuz A, Moro F, Wust J, Vogt M. 2009. Corynebacterium bovis shoulder prosthetic joint infection: the first reported case. Diagn Microbiol Infect Dis 64:213–215 [DOI] [PubMed] [Google Scholar]
- 2.Bernard KA, Munro C, Wiebe D, Ongsansoy E. 2002. Characteristics of rare or recently described Corynebacterium species recovered from human clinical material in Canada. J Clin Microbiol 40:4375–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besch-Williford C, Franklin CL. 2007. Aerobic gram-positive organisms, p 389–406. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research, 2nd ed: diseases. New York (NY): Academic Press. [Google Scholar]
- 4.Burr HN, Lipman NS, White JR, Zheng J, Wolf FR. 2011. Strategies to prevent, treat, and provoke Corynebacterium-associated hyperkeratosis in athymic nude mice. J Am Assoc Lab Anim Sci 50:378–388 [PMC free article] [PubMed] [Google Scholar]
- 5.Chosewood LC, Wilson DE. 2007. Primary containment for biohazards: selection, installation, and use of biological safety cabinets, 3rd ed. Bethesda (MD): Department of Health and Human Services. [Google Scholar]
- 6.Clifford CB, Walton BJ, Reed TH, Coyle MB, White WJ, Amyx HL. 1995. Hyperkeratosis in athymic nude mice caused by a coryneform bacterium: microbiology, transmission, clinical signs, and pathology. Lab Anim Sci 45:131–139 [PubMed] [Google Scholar]
- 7.Coyle MB, Lipsky BA. 1990. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin Microbiol Rev 3:227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalal A, Urban C, Ahluwalia M, Rubin D. 2008. Corynebacterium bovis line-related septicemia: a case report and review of the literature. Scand J Infect Dis 40:575–577 [DOI] [PubMed] [Google Scholar]
- 9.Duga S, Gobbi A, Asselta R, Crippa L, Tenchini ML, Simonic T, Scanziani E. 1998. Analysis of the 16S rRNA gene sequence of the coryneform bacterium associated with hyperkeratotic dermatitis of athymic nude mice and development of a PCR-based detection assay. Mol Cell Probes 12:191–199 [DOI] [PubMed] [Google Scholar]
- 10.Field G. 2006. An update on scaly skin disease. ACLAM Newsl 37:5–8 [Google Scholar]
- 11.Field K, Greenstein G, Smith M, Herrman S, Gizzi J. 1995. Hyperkeratosis-associated coryneform in athymic nude mice. Lab Anim Sci 45:469 [Google Scholar]
- 12.Gobbi A, Crippa L, Scanziani E. 1999. Corynebacterium bovis infection in immunocompetent hirsute mice. Lab Anim Sci 49:209–211 [PubMed] [Google Scholar]
- 13.Hansen AK. 2000. Handbook of laboratory animal bacteriology. New York (NY): CRC Press. [Google Scholar]
- 14.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 15.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–120. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed. New York (NY): Academic Press. [Google Scholar]
- 16.Radaelli E, Manarolla G, Pisoni G, Balloi A, Aresu L, Sparaciari P, Maggi A, Caniatti M, Scanziani E. 2010. Suppurative adenitis of preputial glands associated with Corynebacterium mastitidis infection in mice. J Am Assoc Lab Anim Sci 49:69–74 [PMC free article] [PubMed] [Google Scholar]
- 17.Richter CB, Klingenberger KL, Hughes D, Friedman HS, Schenkman DI. 1990. D2 coryneforms as a cause of severe hyperkeratotic dermatitis in athymic nude mice. Lab Anim Sci 40:545 [Google Scholar]
- 18.Russell S, Riley L, Maddy A, Clifford C, Russell R, Franklin C, Hook R, Jr, Besch-Williford C. 1998. Identification of Corynebacterium bovis as the etiologic agent of hyperkeratosis in nude mice and development of a diagnostic polymerase chain reaction assay. Contemp Top Lab Anim Sci 37:93 [Google Scholar]
- 19.Scanziani E, Gobbi A, Crippa L, Giusti AM, Giavazzi R, Cavalletti E, Luini M. 1997. Outbreaks of hyperkeratotic dermatitis of athymic nude mice in northern Italy. Lab Anim 31:206–211 [DOI] [PubMed] [Google Scholar]
- 20.Thurlow RW, Arriola R, Soll CE, Lipman NS. 2007. Evaluation of a flash disinfection process for surface decontamination of γ-irradiated feed packaging. J Am Assoc Lab Anim Sci 46:46–49 [PubMed] [Google Scholar]
- 21.Tu H, Diberadinis LJ, Lipman NS. 1997. Determination of air distribution, exchange, velocity, and leakage in 3 individually ventilated rodent caging systems. Contemp Top Lab Anim Sci 36:69–73 [PubMed] [Google Scholar]