Abstract
Mites of 3 genera—Myobia, Myocoptes, and Radfordia—continue to plague laboratory mouse facilities, even with use of stringent biosecurity measures. Mites often spread before diagnosis, predominantly because of detection difficulty. Current detection methods have suboptimal sensitivity, are time-consuming, and are costly. A sensitive serodiagnostic technique would facilitate detection and ease workload. We evaluated whether total IgE increases could serve as a serodiagnostic marker to identify mite infestations. Variables affecting total IgE levels including infestation duration, sex, age, mite species, soiled-bedding exposure, and ivermectin treatment were investigated in Swiss Webster mice. Strain- and pinworm-associated effects were examined by using C57BL/6 mice and Swiss Webster mice dually infested with Syphacia obvelata and Aspiculuris tetraptera, respectively. Mite infestations led to significant increases in IgE levels within 2 to 4 wk. Total IgE threshold levels and corresponding sensitivity and specificity values were determined along the continuum of a receiver-operating characteristic curve. A threshold of 81 ng/mL was chosen for Swiss Webster mice; values above this point should trigger screening by a secondary, more specific method. Sex-associated differences were not significant. Age, strain, and infecting parasite caused variability in IgE responses. Mice exposed to soiled bedding showed a delayed yet significant increase in total IgE. Treatment with ivermectin reduced total IgE levels within 2 wk. Our data suggest that increases in total IgE in Swiss Webster and C57BL/6 mice warrant investigation, especially because mite infestations can rapidly elevate total IgE levels. We propose that using total IgE levels routinely in serologic panels will enhance biosecurity.
Abbreviation: ROC curve, receiver-operating characteristic curve; AUC, area under the time–concentration curve
Both myobiid and myocoptid mites continue to plague laboratory mouse colonies, even in the face of stringent biosecurity measures.6 Unfortunately, because infestations may go undetected for varying periods of time, fur mites often have time to propagate and spread extensively. Diagnostic methods and intrinsic factors such as the mites’ minute size and cyclical nature of infestations limit the ability to detect these pests.10,39 In addition, host characteristics affect detection, because mite populations vary depending on differences in host strain, sex, age, health status, grooming tendencies, and housing density.10,20,25,38-40 The most prevalent detection methods include superficial skin scrapes, tape tests, and postmortem pelt exams.1,4,20,38 These methodologies, although highly specific, have a low sensitivity, frequently resulting in false-negative results.12 Furthermore, detection is time-consuming and tedious, requiring highly trained staff to examine numerous slides or pelts.
The 3 major species of mites found on laboratory mice— Myocoptes musculinus, Myobia musculi, and Radfordia affinis—are similar in appearance and behavior and occupy overlapping ecological niches, but they belong to different phylogenetic suborders.20 Myocoptid mites (M. musculinus) are found within the suborder Astigmata, superfamily Sarcoptoidea, along with Sarcoptid and Psoroptid mites.21 Myobiid mites, M. musculi and R. affinis, are in the suborder Prostigmata, family Myobiidae, indicating a wide taxonomic separation from Myocoptes.3
One of the many sequels of mite infestations is an increase in total serum IgE.19,27,33 Mite antigen exposure occurs predominantly through the skin, likely through microabrasions caused by scratching or other mild trauma, rather than through ingestion or inhalation.33 All 3 murine fur mite species are superficial skin dwellers and do not penetrate the epidermis, unlike mites such as Sarcoptes spp. Production of mite-responsive IgE results from sensitization of CD4+ cells, resulting in a Th2 cytokine-mediated response, primarily IL4, IL13, and CD40L.33 Several factors influence serum IgE concentrations in mice, including strain, sex, and age.2,5,13,23 Different strains of mice are known to have differing immunologic responses to allergenic stimulation, particularly regarding Th1–Th2-type cytokine balance. One of the most commonly used mouse strains, C57BL/6, is considered to be a Th1-type cytokine responder.17 Th1-cell-predominated immune responses lead to the downregulation of IgE production.31 A sex-associated bias in IgE response to murine mite infestation has not been reported; however, murine atopy models reveal sex-associated differences in total and allergen-specific IgE response, given that female mice have higher IgE levels.2,23,28 Both cellular and humoral immunologic responses, including IgE levels, decrease with age in various species, including mice.11,15,30
Although elevation of total IgE is nonspecific, there are no reports of naturally occurring IgE increases in laboratory mice. All reported increases appear to result from intentional experimental induction, such as that in atopy studies. In human clinical and animal research studies, stimuli reported to increase IgE include infection with nematode parasites, certain neoplasias, and exposure to specific chemicals and pollen.29 In mice, inoculation with many pathogenic nematodes, such as Trichinella spiralis, leads to increases in total IgE; however, infection with Syphacia obvelata in mice does not.14,26,32 The etiology of an increase in total IgE in a laboratory mouse should be discernable; however, the potential for confounding allergens such as contact bedding remains.
A more sensitive and less laborious method to detect mite infestations is clearly needed.25 A diagnostic test capable of detecting a nascent infestation, with low mite burden, as well as confirming eradication after treatment would be extremely valuable. Detection of mites by PCR has recently become available.7,35 The sensitivity of mite-specific PCR is considered greater than that of visual inspection of pelts, but these data have not yet been presented in peer-reviewed literature.35 The use of PCR in confirming the elimination of an infestation may be limited, given that egg casings and mite parts can remain on mice for more than 8 mo.36 PCR also incurs a greater cost per sample than other mite diagnostic techniques.
The majority of research conducted on murine fur mites has involved treatment schema. Currently, the only method for validating treatment success requires use of the previously mentioned diagnostic methodologies with the described limitations.37 In addition, the ability of soiled-bedding sentinel programs to detect murine fur mites is under debate; data conflict regarding whether a soiled-bedding sentinel program can reliably detect fur mites in a resident colony.22,36
We hypothesized that total IgE or that specific to mite antigens could be used as a surrogate marker (total IgE) or definitive method (mite-antigen–specific IgE) to detect murine mite infestations. We proposed that the use of whole-mite antigens would aid in developing a more specific ELISA. Confirmation and quantification of changes in total IgE concentration after exposure to myobiid, myocoptid, and mixed mite infestations were performed in Swiss Webster mice, an outbred stock commonly used as soiled bedding sentinels, as well as in C57BL/6 mice, the most commonly used inbred strain and genetic background in genetically engineered mice. These data allowed us to evaluate several variables that may affect IgE concentrations, including monospecific and multispecies infestations as well as the role of sex, age, and genetic background. We examined whether the sensitivity of infestation detection in soiled-bedding sentinels improved by measuring their total serum IgE. We also investigated the effect of mite treatment on posttherapy IgE concentrations to determine whether total serum IgE concentration can be used to confirm eradication. Finally, we assessed the effect of pinworm infection on total IgE.
Materials and Methods
Experimental animals.
Male and female Swiss Webster mice (Tac:SW; age, 6 wk) and C57BL/6NTac (age, 6 wk) were obtained from a commercial supplier (Taconic Farms, Germantown, NY). Female retired breeders (Tac:SW; age 12 mo) were obtained from the same source. Mice were free of mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, Mycoplasma pulmonis and ectoparasites including Myobia spp., Myocoptes spp., and Radfordia spp. and endoparasites including Syphacia spp. and Aspiculuris spp. All mice were housed under Animal Biosafety Level 2 conditions in solid-bottom, polysulfone, individually ventilated cages (Thoren Systems, Hazelton, PA) on autoclaved aspen chip bedding (PWI Industries Canada, Quebec, Canada); γ-irradiated feed (LabDiet 5058, PMI, St Louis, MO) and acidified water (pH, 2.5 to 2.8) were provided ad libitum. Mice were provided at least 3 d to acclimate. Cages were changed weekly in a class II type 2A biologic safety cabinet (NU S602-500, Series SP, Nuaire, Plymouth, MN). Experimental (mite) groups were changed on different days to prevent cross contamination. Animal-handling tongs were disinfected with chlorine dioxide (1:18:1 dilution; Clidox-S, Pharmacal Research Laboratories, Naugatuck, CT) between cages. The holding room was ventilated with 95% filtered outside air at 15 air changes hourly, temperature was maintained at 72 ± 2 °F (21.5 ± 1 °C), relative humidity was between 30% and 70%, and a 12:12-h light:dark cycle was used. Animal use was approved by the Memorial Sloan-Kettering Cancer Center's Animal Care and Use Committee. The animal care and use program is AAALAC-accredited, and all animals were housed according to the recommendations provided in the Guide for the Use and Care of Laboratory Animals.16
Mites.
A colony of female Swiss Webster mice dually infested by Myobia musculi and Myocoptes musculinus has been maintained in an isolated quarantine room after a naturally occurring mite outbreak in our vivaria. Mice infested by only M. musculinus and mice infested with both M. musculi and Radfordia affinis were gifts from outside institutions. The mite populations on these mice were used to infest naïve Swiss Webster and C57BL/6 mice used for this study.
Infestation procedure.
A known infested mouse was housed with naïve female Swiss Webster mice (4 per cage) for 3 mo to allow for contact mite transmission. At that time, Swiss Webster mice were confirmed to be mite-positive and mite burden estimated by skin scrape technique (3 regions). Mite and egg populations were counted directly from a standardized 2 × 2 cm piece of cellophane tape. Mice with similar mite burdens were introduced to the naïve male and female Swiss Webster or C57BL/6 mice used in each study arm. Naïve mice were housed in groups of 4, for a total of 12 female or 12 male mice for each exposure type. Prior to introduction of a mite-infested mouse to each group, naïve animals were bled by using the submandibular technique to quantify initial IgE levels, and a skin scrape (3 regions) was performed. Mite-infested mice remained with the naïve mice for 14 d, after which they were removed.
Mite-detection techniques.
Skin scrape (3 regions).
Skin scrapes were performed as previously described.36 Briefly, the mouse was gently restrained by the scruff, and a no. 10 scalpel was used to scrape at 3 locations on the mouse: the scalp rostral to and between the ears, dorsal midline immediately cranial to the lumbosacral junction, and midline along the ventrum at the level of the femoral triangle. An approximately 1-cm2 area was scraped by holding the blade perpendicular to the skin surface and applying gentle pressure. A 2 × 2 cm piece of cellophane tape was applied to the scraped area to adhere to loosened hair and debris. This process was repeated for each scraped area. The 3 pieces of tape were affixed to a glass slide. Slides were examined under a scanning microscope at 40× magnification with the condenser raised; A grid pattern was used to evaluate the entire piece of tape. A slide was counted as positive when an egg or mite of any developmental stage was seen. The numbers of mites and mite eggs were recorded; mites were not further distinguished by stage of development (adult or juvenile). All slides were read by a single investigator (GR).
Postmortem pelt exam.
After euthanasia of mice by using CO2 asphyxiation, we used a secondary method to confirm mite infestations. Pelts were removed by making a ventral midline incision from the pubis to the mandible. The skin was removed from each mouse by using digital pressure to undermine the subcutis from its body wall attachment. The pelt was placed into a culture dish (100 × 15 mm, Fisher Scientific, Waltham, MA) and allowed to cool for 1 to 2 h prior to stereoscopic microscopic examination at 20× magnification. Mouse fur was separated gently by using a dental brush (Pocket Pick Action Brush, CVS, Woonsocket, RI) to visualize the skin of the mouse. The initial exam was performed at 7 locations: on the midline dorsal surfaces of the head, thoracolumbar, and lumbosacral regions and on the midline ventral neck, abdomen, and bilateral inguinal regions. If no mites were noted in these 7 locations, the entire pelt was examined according to a continuous grid pattern progressing from cranial to caudal.
Submandibular blood collection.
Submandibular blood collection was performed by using a 5-mm lancet (GoldenRod Animal Lancet, Medipoint, Mineola, NY). Mice were gently restrained by the scruff, the temporomandibular joint identified, and the cheek and underlying vascular bundle punctured immediately caudal to the joint. Drops of blood were collected into a serum separation tube (BD Microtainer Tubes, BD Vacutainer Systems, Franklin Lake, NJ). Gentle pressure was applied by using a 3 × 3 in. gauze sponge until bleeding stopped. Approximately 100 μL blood was collected from each mouse at each designated time point. Blood was collected from alternating submandibular veins on consecutive collections. Blood was allowed to sit at room temperature for 45 to 60 min and centrifuged at 1207 × g for 10 min. Serum was transferred to a 0.5-mL screwcap microcentrifuge tube (VWR International, Radnor, PA) and stored at −80 °C until analyzed.
Total IgE analysis.
Total IgE concentrations were determined by using a commercially available total mouse IgE ELISA kit (Mouse Total IgE ELISA Kit, XpressBio Life Science Products, Frederick, MD) according to instructions from the assay manufacturer. In brief, serum samples were diluted 1:50 in sample diluent, dispensed into microtiter wells coated with antimouse IgE antibody, and incubated for 60 min. Diluent was aspirated and wells washed prior to addition of antimouse IgE conjugated to horseradish peroxidase enzyme. Conjugate was aspirated and wells washed before Substrate Solution (tetramethylbenzidine) added. Reaction was stopped by addition of Stop Solution (1 N H2SO4) and absorbance values read at 450 nm. Samples were run in duplicate, and results determined from a known standard curve were used to determine total IgE concentration.
Development of mite antigen ELISA.
After euthanasia by CO2 asphyxiation, pelts from mice infested with myocoptid and myobiid mites were removed from known infested mice and placed into culture dishes. Pelts were allowed to cool to room temperature to facilitate collection of mites. A fine paintbrush was used to gently remove mites from the pelt. Mites were placed into a 0.5-mL screwtop microcentrifuge tube and frozen at −80 °C. Approximately 0.1 g mites was collected per tube. Two different protocols were followed to extract proteins. For the first extraction method, PBS (5:1 v/w) was added to 0.1 g mites. Mites were homogenized in a Dounce homogenizer on ice. Mites and PBS were centrifuged at 14,000 × g for 15 min at 4 °C. The supernatant was transferred to a fresh tube and centrifuged again at 14,000 × g for 10 min at 4 °C. The supernatant was transferred to a fresh tube for protein determination by using a commercially available colorimetric assay (Bio-Rad DC Protein Assay, Bio-Rad Laboratories, Hercules, CA) according to instructions for microtiter plates. Samples were read on a microplate reader (VersaMax Tunable Microplate Reader, Molecular Devices, Sunnyvale, CA) at 595 nm. Assays were run in duplicate.
For the second protein extraction protocol, tissue cell lysis solution (1:20 w/v, T-PERTissue Protein Extraction Reagent, Thermoscientific, Thermo Fisher Scientific, Rockford, IL) was added after homogenization of mites. Mites and lysis solution were centrifuged at 10,000 × g for 5 min. Protein determination was performed as described for extraction method 1. In addition, whole-mite antigen was extracted from Dermatophagoides pteronyssinus and D. farinae (GREER, Greer Laboratories, Lenoir, NC) to see whether these antigens could serve as surrogate antigens for those generated from murine fur mites. Briefly, 600 mites were suspended in 120 µL 1% SDS in PBS, homogenized with mortar and pestle, sonicated in a water bath, centrifuged at 14,000 × g for 10 min, and the supernatant removed and dialyzed against PBS. Protein determination was performed as described for extraction method 1.
ELISA were performed at XpressBio (Frederick, MD). Plastic 96-well microtiterplates (Costar® High Binding, Corning, Corning, NY) were coated separately with protein extract from each mite species generated by both extraction methods in 0.5 M carbonate bicarbonate buffer, pH 9.6 (XpressBio) by overnight incubation at 4 °C. Plates were incubated with serum samples from known Myocoptes and Myocoptes+Myobia mite-positive mice (undiluted and 1:1 and 1:2 dilutions). After washing of the plates with a Tris–Tween wash buffer (Tris-buffered saline [pH, 7.8 to 8.0] containing 0.05% Tween 20; XpressBio), specific IgE antibodies were detected by incubation with antimouse IgE conjugated to horseradish peroxidase enzyme (Immunology Consultants Laboratory, Newberg, OR). After washing of the wells, tetramethylbenzidine (Highly Reactive TMB, Moss, Pasadena, MD) was added. Reactions were read at 450 nm on a microtiter plate reader (Biochrom Anthos, Biochrom, Holliston, MA).
Study design.
Determination of total IgE in Swiss Webster mice infested with fur mites.
The effect of mite infestation on serum IgE concentrations was analyzed by using female (n = 48) and male (n = 48) Swiss Webster mice evenly distributed among 3 experimental groups for contact exposure to M. musculinus, M. musculi + M. musculinus, or M. musculi + R. affinis. Control mice were exposed to clean bedding. Mice were evaluated throughout 1 y, because this is the longest period that sentinel mice remain in our facility. Skin scrapes and blood for total IgE analysis were collected at the following time points for all mice in this study: days 0 (prior to infestation), 14, 28, 42, 56, 90, 180, 270, and 360 (after infestation). In addition, blood and skin scrapes were collected from controls on days 120 and 150. Mice were euthanized by using CO2 asphyxiation on day 360 after infestation.
Total IgE determination in C57BL/6 mice infested with fur mites.
The effect of genetic background was analyzed by using male (n = 48) and female (n = 48) C57BL/6NTac mice. These mice were evenly distributed among 3 experimental groups for contact exposure to M. musculinus, M. musculi + M. musculinus, or M. musculi + R. affinis. Control mice were exposed to clean bedding. Experimental mice were introduced to mite-infested mice on day 0, as described earlier. Blood for IgE analysis and 3-region skin scrapes were collected on days 0, 14, 28, 42, 56, 90, and 180. On day 180, mice were euthanized by CO2 asphyxiation.
Total IgE determination in mice exposed to fur-mite–contaminated bedding.
Contaminated bedding was collected at cage change from all cages in the Swiss Webster IgE determination study described earlier. Bedding from cages housing mice infested with the same mite species was mixed in a plastic bag; 100 mL was collected from each of the 6 infested cages per mite group. Contaminated bedding was placed into a cage containing clean bedding (100 mL contaminated bedding added to 400 mL clean bedding per cage; 6 cages per experimental group) and used to infest male (n = 36) and female (n = 36) Swiss Webster mice evenly distributed among 3 experimental groups for contaminated bedding exposure to M. musculinus, M. musculi + M. musculinus, and M. musculi and R. affinis. Contaminated bedding was used until at least one mouse per cage per mite type was mite-positive on skin scrape or until 6 mo had elapsed. Blood for total IgE analysis and skin scrapes were collected at the following time points for all mice in this study: days 0 (prior to infestation), 14, 28, 42, 56, 90, 120, 150, 180, 270, and 360 (after infestation). The additional time points were added to more closely identify the day of initial infestation. Mice were euthanized by using CO2 asphyxiation on day 360 after infestation.
Total IgE determination in aged Swiss Webster mice infested with fur mites.
To examine the effect of age on mice's capacity to mount an IgE response, female Tac:SW mice (n = 48) were obtained at 6 wk of age and allowed to age in the facility to 6 mo of age. They then were evenly distributed among 3 experimental groups for contact exposure to M. musculinus, M. musculi + M. musculinus, or M. musculi + R. affinis. Control mice were exposed to clean bedding. Female Tac:SW female retired breeders (n = 48) were obtained from the vendor at 1 y of age (day 0) and placed into the same 4 experimental groups for mite infestation. Blood for IgE analysis and 3-region skin scrapes were collected from both study arms on days 0, 14, 28, 42, 56, 90, and 180. On day 180, mice were euthanized by CO2 asphyxiation.
Female Swiss Webster mice that had been retired from our sentinel program (n = 36) were transferred from our barrier facility into a dedicated quarantine room at approximately 12 mo of age. These mice were determined free of endo- and ectoparasites as well as serologic evidence of adventitious diseases. Mice were evenly distributed between 2 experimental groups for contact exposure to M. musculinus or M. musculi + R. affinis. Control mice were exposed to clean bedding. After acclimatization for 3 d, the retired sentinel mice were introduced to mite-infested mice (day 0) as described earlier. Blood for IgE analysis and skin scrapes were collected on days 0, 14, 28, 42, 56, 90, and 180. On day 180, mice were euthanized by CO2 asphyxiation.
Effect of fur-mite eradication on total IgE.
The effect of ivermectin treatment on IgE levels was evaluated in mice exposed to mites. Female (n = 24) and male (n = 24) Swiss Webster mice were contact-exposed to Myocoptes, Myocoptes+Myobia, or myobiid mites for approximately 90 d prior to ivermectin treatment. All mice were confirmed to be mite-infested prior to the start of ivermectin treatment. All mice received a γ-irradiated closed-formula rodent feed (LabDiet 5058, PMI) containing 12 ppm ivermectin (Test Diet, PMI) ad libitum for 8 wk as previously described.37 Treated feed then was removed and regular rodent diet (LabDiet 5058) provided for the remainder of the study. Blood was collected for IgE analysis and skin scrapes (3 regions) were performed on days 0 (prior to treatment initiation), 14, 28, 42, 56, and 90 after treatment start.
Determination of total IgE in pinworm-infected mice.
The effect of pinworm infection with Syphacia obvelata and Aspiculuris tetraptera on serum IgE concentrations over time was analyzed by using female (n = 12) and male (n = 12) Swiss Webster mice. Retired sentinels reportedly infected with A. tetraptera only were a gift from an outside institution. Testing by fecal float and anal tape analysis at our institution prior to the use of these mice for infection of naïve mice confirmed the presence of A. tetraptera but not S. obvelata. Initially, feces from these mice were introduced once to naïve mice by oral gavage. Subsequently, feces from known pinworm-positive mice were transferred to experimental cages at weekly cage change. Fecal flotations were performed on fresh-caught fecal samples pooled by cage (5 to 7 pellets). For each cage, feces transfer and fecal floatations were continued until pinworm eggs were detected. Use of this diagnostic technique was based on the assumption that infection was limited to A. tetraptera. Blood for IgE analysis, skin scrapes, and feces for fecal flotation (as necessary) were collected on days 0, 14, 28, 42, 56, 90, 120, 150, and 180. At euthanasia, fecal material (2 to 3 pellets) was collected from the colon of each mouse and examined by fecal flotation, and intestinal contents underwent microscopic examination (10×) to confirm the presence or absence of pinworms. Pinworms were speciated based on the morphology and size of ova, larvae, and adult worms.
Determination of total IgE in vivarium sentinel mice.
IgE levels were determined in Swiss Webster mice used as sentinels in a soiled-bedding colony health-monitoring program (n = 169). Sentinel mice from 13 holding rooms were bled on days 0 (day of introduction to facility), 14, 28, 42, 56, 90, 180, 270, and 360 and serum IgE levels determined. Each sentinel cage was exposed to 500 to 600 mL of undiluted, soiled bedding collected weekly from approximately 40 cages (15 mL per cage) housing mice free of all excluded pathogens including endo- and ectoparasites.
Statistical methods.
IgE levels over time were compared within and between infestation groups. Differences in IgE levels at day 0 compared with later time points within infestation groups were evaluated by using the Wilcoxon signed rank test for all groups. The following tests were performed for the Swiss Webster mice total IgE determination group. Differences in IgE levels between infestation groups and between male and female mice at various time points were evaluated by using the Wilcoxon rank sum test. Median time to detection of mites, eggs, or elevation of IgE above 81 ng/mL within infestation groups was calculated by using the Kaplan–Meier method. Correlations between IgE levels and egg or mite counts at various time points were examined by using the Spearman rank correlation coefficient. Receiver-operating characteristic (ROC) curves were plotted to examine the predictive ability of IgE levels regarding mite infestation. The skin scrape was used as the ‘gold standard.’ ROC curves were plotted at various time points by using the control mice and those from infestation groups that were confirmed as having mites based on the skin scrape at the time point of interest. Area under the time–concentration curve (AUC) was calculated at each time point, and estimates of sensitivity and specificity were calculated based on various threshold IgE levels. Associations and differences were considered significant when the P value was less than or equal to 0.05.
Results
Development of mite antigen ELISA.
Creation of a mite-antigen-specific ELISA using Myocoptes and Myobia antigen was unsuccessful. Although measurable protein was extracted by both extraction methods, incubation with serum from mite-positive animals produced no readable ELISA signal. D. pteronyssinus and D. farinae antigen used as surrogate antigens for detection of mouse mite antibodies also failed to detect measurable IgE levels.
Total IgE in Swiss Webster mice infested with fur mites.
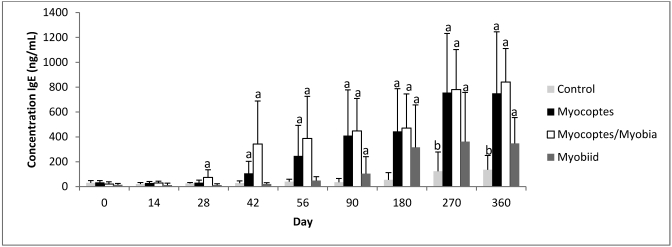
Mean total IgE levels rose significantly (P ≤ 0.05) over time in all groups exposed to Myocoptes, myobiid or Myocoptes+Myobia mites in the longitudinal (yearlong) study regardless of exposure technique (Figures 1 and 2). Confirmation of mite infestations was temporally variable within each mite infestation group. Across all groups, only one third of mice exposed by direct contact became infested by day 14. The majority of mice became infested by day 56. By day 90, mean total IgE levels for all contact-infested groups reached significance (P ≤ 0.05) when compared with control mice at the same time point (Figure 1). IgE levels in control mice also increased over time, first reaching significance at day 150 when compared with time 0 (Figure 2). These values rarely reached values equivalent to those of infested mice at the same time point. No difference in IgE levels was noted due to sex.
Figure 1.
Temporal changes in total IgE concentration (mean ± 1 SD) in Swiss Webster mice after exposure to mites by direct contact with infested mice. a, P ≤ 0.05 as compared with control at same time point; b, P ≤ 0.05 compared with day 0 control.
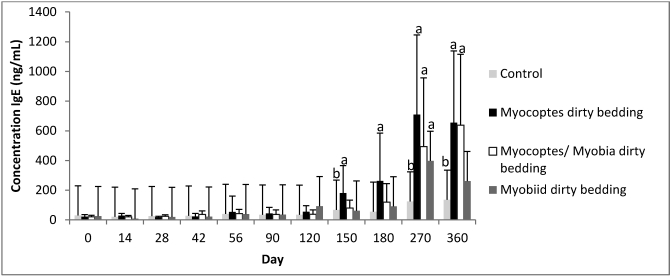
Figure 2.
Temporal changes in total IgE concentration (mean ± 1 SD) in Swiss Webster mice after exposure to mites by using bedding from cages housing infested mice. a, P ≤ 0.05 as compared with control at same time point; b, P ≤ 0.05 as compared with time 0 control.
Use of IgE as screening test.
ROC curves as well as AUC values were determined for each mite species individually and in aggregate across all 3 species at 5 time points (Table 1). Table 1 also indicates the sensitivity and specificity for a threshold value based on the aggregate data from all mite infestations. A threshold level of 81 ng/mL was selected based on this data to optimize sensitivity and specificity. Three false-negative results were detected at euthanasia. These mice had both positive total IgE titers and postmortem pelt exams but tested negative by skin scrape.
Table 1.
AUC calculated from the ROC curve for each mite infestation group as well as aggregate data for all groups
| Test accuracy |
||||||
| Day | Myocoptes | Myocoptes/ Myobia | Myobiid | Aggregate data | Sensitivity (%)a | Specificity (%)a |
| 14 | not done | 0.73 | not done | 0.69 | 0 | 100 |
| 28 | 0.68 | 0.84 | not done | 0.63 | 20 | 100 |
| 42 | 0.77 | 0.97 | not done | 0.77 | 20 | 100 |
| 56 | 0.88 | 0.98 | 0.65 | 0.86 | 57 | 96 |
| 90 | 0.96 | 0.98 | 0.82 | 0.94 | 81 | 88 |
| 180 | 0.96 | 0.95 | 0.91 | 0.95 | 92 | 61 |
| 270 | 0.93 | 0.98 | 0.81 | 0.91 | 89 | 64 |
| 360 | 0.92 | 0.998 | 0.83 | 0.93 | 95 | 55 |
Day represents days after initial exposure to infested mice.
Sensitivity and specificity were calculated for aggregate data and based on a cut off value of 81 ng/mL.
IgE as a function of mite or egg infestation.
Eggs were consistently seen on skin scrapes prior to mites of any maturation stage (Table 2). A median time to egg or mite appearance could not be determined for the myobiid dirty-bedding group because of the large number of mice (n = 12) that were never confirmed to be infested with mites. The median time to elevation of total IgE levels above threshold value varied from 0 to 28 d after visual detection of mite or egg infestation (Table 2). A positive correlation between the number of eggs or mites present and the magnitude of IgE levels was demonstrated at several time points for Myocoptes (day 56: Spearman correlation coefficient [ρ] = 0.57, P = 0.004; day 90: ρ = 0.73, P ≤ 0.0001; day 180: ρ = 0.54, P = 0.006; day 270 ρ = 0.55, P = 0.007) and Myocoptes+Myobia-infested mice (day 90: ρ = 0.44, P = 0.03; day 180: ρ = 0.46, P = 0.03). Myobiid infestations did not show a correlation between increases in egg or mite populations and IgE levels except at day 180 (ρ = 0.59, P = 0.004).
Table 2.
A comparison of the median time (d) to detection of eggs or mites by skin scrape or elevation of total IgE above the threshold (81 ng/mL) established as an indicator of mite infestation
| Median time (d) to |
|||
| Infestation species | Eggs | Mites | Elevation of total IgE |
| Myocoptes | 28 | 42 | 42 |
| Myocoptes/Myobia | 14 | 42 | 42 |
| Myobiid | 180 | 360 | 180 |
| Myocoptes dirty bedding | 150 | 270 | 150 |
| Myobia/Myocoptes dirty bedding | 270 | 270 | 270 |
| Myobiid dirty bedding | not done | not done | not done |
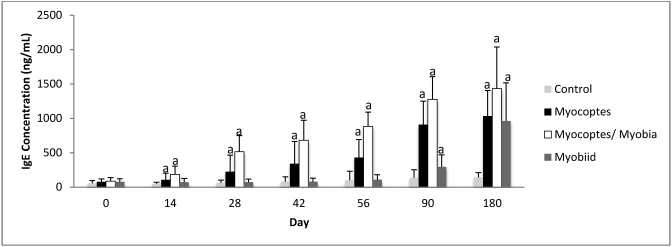
Total IgE in C57BL/6 mice infested with fur mites.
C57BL/6 mice infested with different species of mites showed a significant increase in IgE levels as compared with same-day controls (Figure 3). Although the trend in total IgE elevation over time is similar to that of mite infested Swiss Webster mice (Figures 1 and 3), the IgE values were significantly lower in C57BL/6 mice for the Myocoptes-infested mice for days 42 onward (P ≤ 0.05 at all points) and the Myocoptes+Myobia-infested mice for days 28 onwards (P ≤ 0.05 at all points). No differences were noted between the myobiid-infested groups for either strain or stock.
Figure 3.
Temporal changes in total IgE concentration (mean ± 1 SD) in C57BL/6 mice after exposure to mite infested mice. a, P ≤ 0.05 as compared with control value at same time point.
Total IgE in mice exposed to fur-mite-contaminated bedding.
Figure 2 demonstrates the observed changes in mean total IgE concentrations among mice exposed to mite-contaminated bedding. Mite infestation, and consequently increased IgE levels, occurred at earlier time points for mice exposed to mites through direct contact when compared with contaminated bedding exposure (Figures 1 and 2 and Table 2). Examining data from both contact-infested and dirty-bedding-infested mice shows that mean total IgE levels for all mite groups had the capacity to increase to values greater than 5 times the corresponding control value (Figures 1 and 2).
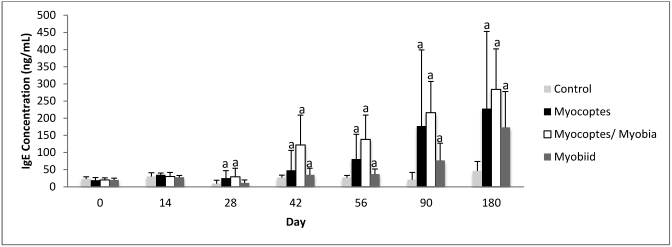
Total IgE in aged Swiss Webster mice infested with fur mites.
Mice introduced to mites at 6 mo of age mounted total IgE responses to Myocoptes and Myocoptes+Myobia infestations. By day 14, significant (P ≤ 0.05) differences were seen between infested and control mice. Compared with control mice, myobiid-infested mice did not mount a significantly (P ≤ 0.05) different total IgE response until day 90 (Figure 4).
Figure 4.
Temporal changes in total IgE concentration (mean ± 1 SD) in 6-mo-old Swiss Webster mice after exposure to mite-infested mice. a, P ≤ 0.05 as compared with control value at same time point.
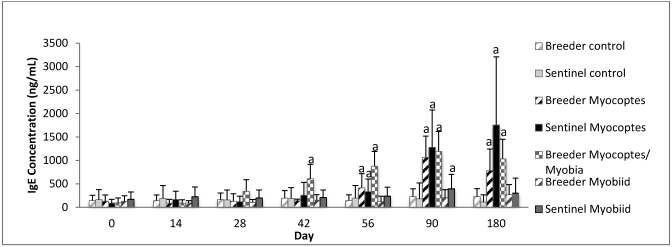
Retired Swiss Webster female breeders infested at 1 y of age and sentinel female mice transferred to this study after 1 y of residence developed significant IgE levels at later time points than did the other contact-infested mice evaluated (Figure 5). Significantly (P ≤ 0.05) elevated total IgE levels did not occur by day 180 for myobiid-infested retired breeders (Figure 5).
Figure 5.
Temporal changes in total IgE concentration (mean ± 1 SD) in 1-y-old Swiss Webster mice after exposure to mite-infested mice. a, P ≤ 0.05 as compared with control value at same time point. ‘Breeder’ represents retired female Swiss Webster breeders, and ‘sentinel’ represents retired female Swiss Webster sentinel mice. Sentinel–Myocopes/Myobia was not investigated.
Effect of fur mite eradication on total IgE.
IgE levels decreased after provision of ivermectin-impregnated feed for 8 wk to infested mice. By day 14 after treatment initiation, IgE values for each infestation type were significantly (P ≤ 0.05) reduced as compared with pretreatment levels (Table 3). To determine whether IgE values returned to baseline 30 d after 8 wk of treatment concluded, each ivermectin-treated group was compared with day 180 control mice (mean total IgE, 55 ng/mL; Table 3). This group was selected to serve as controls because they matched the length of time that the ivermectin-treated mice had been housed in the facility. All mite-infested groups except the myobiid-infested group had IgE values that were significantly (P ≤ 0.05) different from those of age-matched control mice at day 180. Live mites were last detected by skin scrapes (3 regions) on day 14 after treatment initiation. By day 28, only egg casings and mite exoskeleton sections were seen. By day 90, only egg casings were seen on 2 Myocoptes-infested mice. No other evidence of mite infestation was detected on skin scrapes.
Table 3.
Mean IgE levels (ng/mL) in mite-infested Swiss Webster mice immediately before treatment, 14 d after treatment initiation, and 30 d after completion of treatment with ivermectin
| Infestation type | Before treatment | day 14 | day 30 |
| Myocoptes | 940 | 531a | 219b |
| Myobia/Myocoptes | 1081 | 601a | 284b |
| Myobiid | 185 | 134a | 136 |
| Control | 41 | 92 | 38 |
P ≤ 0.05 compared with pretreatment value for each mite-infested group.
P ≤ 0.05 compared with overall mean for day 180 control mice (55 ng/mL).
Total IgE in pinworm-infected mice.
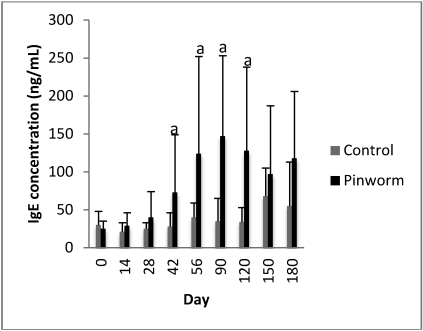
Five of 6 cages were A. tetraptera-positive on pooled (by cage) fecal flotation by day 42. Female mice in a single cage never tested positive for pinworms by any method. At necropsy, all female mice were found to be negative on both intestinal content exam and fecal float. Unexpectedly, all male mice had confirmed S. obvelata infections by one or both of these methods. Only 1 male animal showed a dual infection of A. tetraptera and S. obvelata at necropsy. Compared with control mice of the same age, mice infected with pinworms demonstrated significant (P ≤ 0.05) elevation in IgE levels by day 42 (Figure 6). By day 150 the difference was no longer significant. The highest mean total IgE level was noted at day 90 (147 ng/mL), which was significantly (P ≤ 0.05) lower than that of Myocoptes- (410 ng/mL) and Myocoptes+Myobia- (448 ng/mL) infested Swiss Webster mice at the same time point (day 90). All mite-infested groups had IgE levels significantly (P ≤ 0.05) greater than those of pinworm-infected mice at day 180. All mice were mite free.
Figure 6.
Temporal changes in total IgE concentration (mean ± 1 SD) after exposure to pinworms. a, P ≤ 0.05 as compared with control value at same time point.
Total IgE in vivarium sentinel mice.
Total IgE values for age-matched sentinels did not differ significantly from total IgE values measured in control mice (male and female) except at days 42 (total IgE: control, 28 ng/mL; sentinel, 4 ng/mL; P ≤ 0.05) and 56 (total IgE: control, 40 ng/mL; sentinel, 25 ng/mL; P ≤ 0.05). Because all sentinel mice were female, total IgE values of female control mice were compared with IgE values of sentinels. Similarly significant (P ≤ 0.05) differences were present in sera collected at days 42 (total IgE: control, 26 ng/mL; sentinel, 4 ng/mL; P ≤ 0.05) and 56 (total IgE: control, 43 ng/mL; sentinel, 25 ng/mL; P ≤ 0.05). Vivarium sentinel mice showing total IgE elevations had all been present in the facility for more than 90 d—most more than 180 d. All of these mice tested negative for endo- and ectoparasites by using a secondary screening method.
Discussion
Diagnostic methods used to identify mite-infested animals in a timely and efficient manner have consistently been associated with low sensitivity and are laborious.36 We show here that total IgE determination appears to be an accurate and robust screening modality for mite detection. We envision incorporating the total IgE assay into the routine serologic panel used to screen mice for adventitious infective agents. Use of the assay to screen for fur mite infestation will reduce technician time for both sample collection and analysis, as compared with more commonly used methods. Because greater scrutiny can be paid to animals with elevated total IgE levels and the samples collected from them, diagnostic accuracy likely will improve.
Among diagnostic tests, 100% accurate tests (high sensitivity and specificity) are rare; a viable alternative is to screen animals initially by using a high-sensitivity, low-specificity test, followed by a low-sensitivity, high-specificity test for those samples that are positive in the initial screen.18 With respect to the total IgE assay, several options are available, including the use of skin scrapes or determining total IgE levels for cage mates. In instances, when we detected false-positive total IgE levels among controls in our study, the IgE levels of cage mates generally remained beneath the selected threshold. PCR would be an excellent confirmatory test also.
The use of a ROC curve to establish thresholds for serodiagnostic tests is common.41 The threshold is the level above which the test is considered positive; below this point, the test is considered negative. The ROC curve graphically represents the sensitivity and specificity calculated at multiple data points. Each data point can be considered a threshold value.41 The optimal threshold point can be chosen by moving along the continuum of the curve, until the desired sensitivity and specificity are identified. An increase in sensitivity causes a decrease in specificity. For this IgE assay, sensitivity could be increased to 100% for all time points and mite infestation types, but the corresponding decrease in the specificity would make this screening test no more efficient than using a confirmatory test for every animal. Test accuracy is determined from an ROC curve by calculating the AUC value; the closer the AUC approaches 1.0, the more accurate is the test compared with a gold standard (skin scrape). A value of 0.5 indicates an inaccurate test. In the current study, the IgE values determined at each time point (Table 2) indicate a highly accurate test, especially for later time points. Accuracy improved for the total IgE assay as more experimental animals developed mite infestations over time.
Using the current aggregate data for accuracy, we chose a threshold point from the day-90 data (81 ng/mL) to provide appropriate sensitivity for time points greater than 90 d (Table 1). This threshold point also allows for a specificity greater than 50% for all time points. Because they more closely resemble typical control values, using lower threshold values also increases the ambiguity of result interpretation.
Sensitivity and specificity data for the total IgE assay were calculated by using skin scrape diagnosis as the gold standard. Mite diagnosis by using skin scrapes is imperfect. Postmortem pelt exams revealed 3 additional positive mice that were not detected by skin scrape. These 3 mice all had positive IgE titers. The data also reveal multiple instances where total IgE levels rose prior to detection of mites by skin scrape; however, premature euthanasia would have been required to confirm whether these were classified erroneously as negative by skin scrape. A greater number of confirmed positives would increase the calculated sensitivity of the total IgE assay.
Once a detectable infestation manifests on Swiss Webster mice, the majority of animals mount a strong antigenic response that results in IgE levels that surpass the threshold within 4 wk. This delay is an intrinsic test weakness. However, many facilities test colony or sentinel mice every 2 or 3 months, a practice that would allow sufficient time for antibody formation to mite antigen, as occurs with other adventitious pathogens.
Myobiid mite infestations took longer to establish and cause an increase in total IgE levels as compared with Myocoptes and Myocoptes+Myobia infestations. The Myocoptes+Myobia infestations reflected colonization with Myocoptes rather than Myobia (data not shown). Myobiid mites had a longer median time to the appearance of eggs and mites than did the other 2 infestation groups (Table 2); this result is attributable to the myobiid mites’ longer life cycle (23 d for Myobia compared with as short as 8 d for Myocoptes).20 The life cycle for R. affinis is presumed to be similar to that of M. musculi. Detailed studies of this mite have not been conducted.
There was a correlation between Myocoptes and Myocoptes+Myobia egg or mite counts and total IgE levels at multiple time points. Myobiid mites showed a correlation at day 180 only. We surmise that greater populations of mites result in greater increases in total IgE levels; however, IgE levels still increase with smaller mite populations. It appears that the minimal detectable threshold by microscopic exam parallels the minimal population needed to mount an IgE response. Myocoptid mite populations increase rapidly above this threshold, resulting in a detection lag when using total IgE as compared with skin scrapes (Table 2). Detection of myobiid mites by using either total IgE or skin scrape testing occurs simultaneously.
Some animal use facilities evaluate colony animals as well as sentinels. To examine whether genetic background influences IgE responses to mites, we measured the IgE response to mites in C57BL/6 mice because it is one of the most common strains used. Given that C57BL/6 mice are reported Th1-type cytokine responders, we hypothesized that they would mount lower total IgE levels after mite infestation than would Swiss Webster mice.17 This notion appeared to hold true as the total IgE titer in C57BL/6 mice was lower for Myocoptes and Myocoptes+Myobia as compared with the same Swiss Webster mouse groups (mite burdens were comparable between strains [data not shown]). The titers were not significantly different between C57BL6 and Swiss Webster mice for the myobiid-infested mice which may reflect the increased time it takes Myobiid mites to cause an elevated IgE titer. The differences could potentially have become significant had later time points been investigated. Total IgE titers in C57BL/6 mice exposed to mites were increased significantly compared with those of C57BL/6 controls, indicating test validity in this strain, although different threshold points may be needed.
Recent findings on the ability of soiled bedding sentinels to detect ectoparasites have been contradictory.22,36 Although the primary aim of the current studies was not to investigate the efficacy of soiled bedding transfer, several observations are noteworthy. Myocoptid infestations transferred most rapidly and effectively on soiled bedding, either singly or in a mixed infestation. Soiled bedding transfer of myobiid mites was relatively inefficient, given that only approximately half of exposed cages developed infestations; this finding likely reflects mite ecology. Our experimental conditions created an initial low parasite load in soiled bedding, which may contribute to the delay in establishing infestation. This situation reproduces a natural infestation, because not all cages contributing bedding to a sentinel cage will be infested. Mice exposed to soiled bedding that became positive for mite infestations developed increases in total IgE for both myocoptid and myobiid mites. However, due to the length of time that may transpire prior to infestation, age may confound interpretation and, in programs that replace their soiled bedding sentinels frequently (for example, quarterly), sentinels may not have sufficient time to develop a sufficient mite burden and a significant increase in total IgE. However, determination of total IgE concentration in soiled bedding sentinels may be valuable in colony health monitoring programs in which the sentinels are exposed to soiled bedding and maintained for at least 6 mo.
Increases in total IgE with age were observed in multiple experimental arms, including the soiled bedding sentinels. We posit that this age-related increase is due to chronic immune stimulation from weakly antigenic sources such as bedding. Immunosenescence may be responsible for the delay in significant increases in IgE in older (greater than 180 d) Swiss Webster mice (Figure 5).15 Age-related changes most likely account for the decrease in the AUC seen at days 270 and 360. The majority of vivarium sentinel animals with IgE levels above the cut-off value had been in the facility for more than 180 d. To alleviate the challenge in interpreting IgE levels in older mice and reduce the number of animals requiring secondary testing, one could consider implementing a secondary threshold value for older mice to account for the age related rise in total IgE.
One potential advantage of screening total IgE levels would be the ability to validate treatment efficacy. Treatment with ivermectin for 8 wk after a 3-mo mite infestation decreased IgE levels within 2 wk (Table 3). Our findings correlate with previous findings indicating that mite egg casings or mite exoskeleton parts can remain on mice for 3 mo or more and that this propensity may interfere with some testing modalities such as PCR.36 Although IgE levels after treatment did not return to comparable control levels, total IgE decreased rapidly due to efficacious treatment.
Pinworms are another commonly encountered parasitic infection of laboratory mice.34 We therefore investigated whether pinworm infections also elevate total IgE. The difficulty of confirming pinworm infections was exemplified in that the mice used as a pinworm infection source were reported to, and initially appeared to, harbor only A. tetraptera. However, S. obvelata was observed at study termination, and infection from another source was highly unlikely because no other pinworm-positive mice were housed in the holding room. The recognized decrease in S. obvelata ova excretion with increasing age and duration of infection time likely contributed to the initial false-negative results.8 A presumptive mixed A. tetraptera and S. obvelata infection led to increases in mean total IgE in mice between days 42 and 120, although these values were less than those of corresponding mite-infested mice. The experimental design did not allow for assessment of several variables affecting total IgE levels. The population size and species of pinworms within each mouse were unknown until the last time point. Unknown alterations in pinworm populations may affect IgE levels, given that pinworm burden can decrease with age and sex, and outbred strains of laboratory mice may resist pinworm infection.9,24 It is also possible that one pinworm species (potentially A. tetraptera) may be more antigenic than another, because S. obvelata is not reported to increase total IgE.26 This may account for low IgE levels by day 180 as most male mice were observed to be infected with only S. obvelata at necropsy. Further investigation is required to determine the accuracy and validity of total IgE as a screening test for pinworms and whether intensive testing for pinworms should be conducted in animals with elevated IgE levels.
Attempts to create a mite-specific ELISA using either the specific murine fur mites or dust mite (D. farinae and D. pteronyssinus) antigens were unsuccessful. Dust mites are reported to potentially cross react with fur mites.27 There are a number of possibilities for this failure, including insufficient protein sample, denaturation or loss of the antigenic protein during processing, secretion of the antigenic protein by the mite, and, in the case of Dermatophagoides, insufficient cross reactivity. Further work into the specific nature of the mite antigen is required to develop a reliable immunoassay.
In conclusion, we found that assaying total IgE levels may be a useful tool in fur mite diagnostics. The robust nature of the mouse's IgE response to mite infestation facilitates identification of either sentinel or colony animals that would undergo further confirmatory analysis. Evaluating total IgE screening on a larger scale is necessary, especially to determine statistical indices such as positive and negative predictive values, but we believe its use will reduce the time and costs and the improve accuracy associated with screening colonies for fur mites. In addition, this method likely is applicable across all immunocompetent strains; additional investigation should be conducted to assess IgE responses to mite infestation in other commonly used strains and stocks.
Acknowledgments
We thank Lisa Eldred and Antonio Bravo for their invaluable help in caring for the mite-infested mice. We also thank the staff of the Laboratory for Comparative Pathology (MSKCC/WCMC/RU) and Greg Baseler (XpressBio) for their diagnostic support. Our gratitude to Kelly Pate and Kevin Prestia for their donations of mite-positive mice and to Tanya Burkholder for her donation of pinworm-positive mice. We also acknowledge Theresa Cunningham for her gift of ivermectin-impregnated rodent chow and thank Drs Felix Wolf and Rodolfo Ricart-Arbona for their insights. Thank you to Dr Frances Weis-Garcia for her assistance with protein extraction techniques.
William Boteler is the Scientific Director and cofounder of XpressBio, which created and sells the mouse total IgE ELISA kit used in this work. He has a direct commercial interest in this work in that the published manuscript can be cited as part of the marketing strategy for this serodiagnostic test.
References
- 1.Baumans V, Havenaar R, van Herck H, Rooymans TP. 1988. The effectiveness of Ivomec and Neguvon in the control of murine mites. Lab Anim 22:243–245 [DOI] [PubMed] [Google Scholar]
- 2.Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. 2010. Airway inflammation and remodeling in 2 mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol 153:173–181 [DOI] [PubMed] [Google Scholar]
- 3.Bochkov A, Arbobi M, Malikov V. 2000. Notes on mites of the family Myobiidae (Acari: Prostigmata) parasitising rodents (Mammalia: Rodentia) in Iran. Folia Parasitol (Praha) 47:73–77 [DOI] [PubMed] [Google Scholar]
- 4.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of 5 treatment regimens and 5 diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 5.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. 2007. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy 37:1392–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 7.Charles River Laboratories International, Inc. [Internet]. Fur mite PCR now available from Charles River. [Cited 23 August 2011]. Available at: http://www.criver.com/en-US/NewsEvents/WhatsNew/Pages/Fur_Mite_PCR.aspx.
- 8.Clifford CB, Watson J. 2008. Old enemies, still with us after all these years. ILAR J 49:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derothe JM, Loubes C, Orth A, Renaud F, Moulia C. 1997. Comparison between patterns of pinworm infection (Aspiculuris tetraptera) in wild and laboratory strains of mice, Mus musculus. Int J Parasitol 27:645–651 [DOI] [PubMed] [Google Scholar]
- 10.Friedman S, Weisbroth SH. 1975. The parasitic ecology of the rodent mite Myobia musculi. II. Genetic factors. Lab Anim Sci 25:440–445 [PubMed] [Google Scholar]
- 11.Fujiwara M, Kishimoto S. 1979. IgE antibody formation and aging. I. Age-related changes in IgE antibody formation and avidity for the DNP-determinant in mice. J Immunol 123:263–268 [PubMed] [Google Scholar]
- 12.Gaertner DJ. 2004. Speculations on why some lab rodent pathogens continue to be prevalent. Contemp Top Lab Anim Sci 43:8. [PubMed] [Google Scholar]
- 13.Gelfand EW, Joetham A, Cui ZH, Balhorn A, Takeda K, Taube C, Dakhama A. 2004. Induction and maintenance of airway responsiveness to allergen challenge are determined at the age of initial sensitization. J Immunol 173:1298–1306 [DOI] [PubMed] [Google Scholar]
- 14.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. 2004. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol 172:1139–1145 [DOI] [PubMed] [Google Scholar]
- 15.Ichihara Y, Okano M, Nishioka K, Manabe N, Ichihara N, Jitsunari F, Fujiwara T, Nishizaki K. 2009. Aging exacerbates restraint stress-induced inhibition of antigen-specific antibody production in mice. Allergol Int 58:119–124 [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 17.Kanai N, Min WP, Ichim TE, Wang H, Zhong R. 2007. Th1/Th2 xenogenic antibody responses are associated with recipient dendritic cells. Microsurgery 27:234–239 [DOI] [PubMed] [Google Scholar]
- 18.Lalkhen A, McCluskey A. 2008. Clinical tests: sensitivity and specificity. Continuing Education in Anesthesia Critical Care and Pain 8:221–223 [Google Scholar]
- 19.Laltoo H, Van Zoost T, Kind LS. 1979. IgE antibody response to mite antigens in mite-infested mice. Immunol Commun 8:1–9 [DOI] [PubMed] [Google Scholar]
- 20.Letscher RM. 1970. Observations concerning the life cycle and biology of Myobia musculi (Schrank) and Myocoptes musculinus (Koch). [MS thesis]. College Station (TX): Texas A&M University. [Google Scholar]
- 21.Liana M, Witalinski W. 2005. Sperm structure and phylogeny of Astigmata. J Morphol 265:318–324 [DOI] [PubMed] [Google Scholar]
- 22.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled-bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60 [PMC free article] [PubMed] [Google Scholar]
- 23.Matheu V, Barrios Y, Arnau MR, Navikas V, Issazadeh-Navikas S. 2010. Similar response in male and female B10.RIII mice in a murine model of allergic airway inflammation. Inflamm Res 59:263–269 [DOI] [PubMed] [Google Scholar]
- 24.Mathies AW., Jr 1959. Certain aspects of the host-parasite relationship of Aspiculuris tetraptera, a mouse pinworm. I. Host specificity and age resistance. Exp Parasitol 8:31–38 [DOI] [PubMed] [Google Scholar]
- 25.Metcalf Pate KA, Rice KA, Wrighten R, Watson J. 2010. Effect of sampling strategy on the detection of fur mites within a naturally infested colony of mice (Mus musculus). J Am Assoc Lab Anim Sci 50:337–343 [PMC free article] [PubMed] [Google Scholar]
- 26.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43 [DOI] [PubMed] [Google Scholar]
- 28.Okuyama K, Wada K, Chihara J, Takayanagi M, Ohno I. 2008. Sex-related splenocyte function in a murine model of allergic asthma. Clin Exp Allergy 38:1212–1219 [DOI] [PubMed] [Google Scholar]
- 29.Pate MB, Smith JK, Chi DS, Krishnaswamy G. 2010. Regulation and dysregulation of immunoglobulin E: a molecular and clinical perspective. Clin Mol Allergy 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauwels R, Bazin H, Platteau B, van der Straeten M. 1979. The effect of age on IgE production in rats. Immunology 36:145–149 [PMC free article] [PubMed] [Google Scholar]
- 31.Peng HJ, Su SN, Chang ZN, Chao PL, Kuo SW, Tsai LC. 2002. Induction of specific Th1 responses and suppression of IgE antibody formation by vaccination with plasmid DNA encoding Der f 11. Vaccine 20:1761–1768 [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer P, Konig W, Bohn A. 1983. Genetic dependence of IgE antibody production in mice infected with the nematode Nippostronglyus brasiliensis. I. Modulation of the IgE antibody response in vivo by serum factors. Int Arch Allergy Appl Immunol 72:347–355 [DOI] [PubMed] [Google Scholar]
- 33.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur mite-infested mice. Eur J Immunol 36:2434–2445 [DOI] [PubMed] [Google Scholar]
- 34.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 35.RADIL. [Internet]. PCR Services. [Cited 23 August 2011]. Available at: http://www.radil.missouri.edu/pcr.html.
- 36.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587 [PMC free article] [PubMed] [Google Scholar]
- 37.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: III. Treatment of a large mouse colony with ivermectin-compounded feed. J Am Assoc Lab Anim Sci 49:633–637 [PMC free article] [PubMed] [Google Scholar]
- 38.Weisbroth SH, Friedman S, Powell M, Scher S. 1974. The parasitic ecology of the rodent mite Myobia musculi. I. Grooming factors. Lab Anim Sci 24:510–516 [PubMed] [Google Scholar]
- 39.Weisbroth SH, Friedman S, Scher S. 1976. The parasitic ecology of the rodent mite, Myobia musculi. III. Lesions in certain host strains. Lab Anim Sci 26:725–735 [PubMed] [Google Scholar]
- 40.Whiteley HJ, Horton DL. 1965. Further observations on the effect of Myobia musculi on the skin of the mouse. J Pathol Bacteriol 89:331–335 [PubMed] [Google Scholar]
- 41.Zweig MH, Campbell G. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 39:561–577 [PubMed] [Google Scholar]