Abstract
Laboratory mice typically are anesthetized by either inhalation of volatile anesthetics or injection of drugs. Here we compared the acute and postanesthetic effects of combining both methods with standard inhalant monoanesthesia using sevoflurane in mice. After injection of fentanyl–midazolam or S-ketamine as premedication, a standard 50-min anesthesia was conducted by using sevoflurane. Addition of fentanyl–midazolam (0.04 mg/kg–4 mg/kg) induced sedation, attenuation of aversive behaviors at induction, shortening of the induction phase, and reduced the sevoflurane concentration required by one third (3.3% compared with 5%), compared with S-ketamine (30 mg/kg) premedication or sevoflurane alone. During anesthesia, heart rate and core body temperature were depressed significantly by both premedications but in general remained within normal ranges. In contrast, with or without premedication, substantial respiratory depression was evident, with a marked decline in respiratory rate accompanied by hypoxia, hypercapnia, and acidosis. Arrhythmia, apnea, and occasionally death occurred under S-ketamine–sevoflurane. Postanesthetic telemetric measurements showed unchanged locomotor activity but elevated heart rate and core body temperature at 12 h; these changes were most prominent during sevoflurane monoanesthesia and least pronounced or absent during fentanyl–midazolam–sevoflurane. In conclusion, combining injectable and inhalant anesthetics in mice can be advantageous compared with inhalation monoanesthesia at induction and postanesthetically. However, adverse physiologic side effects during anesthesia can be exacerbated by premedications, requiring careful selection of drugs and dosages.
Abbreviation: FMS, fentanyl–midazolam–sevoflurane; KS, S-ketamine–sevoflurane; S, sevoflurane
Laboratory mice frequently are anesthetized by subcutaneous or intraperitoneal injection of hypnotic, analgesic, and muscle-relaxing agents.35 Although easy, practical and cost-effective, this method has its drawbacks. After injection of relatively long-acting drugs through the subcutaneous or intraperitoneal route, the course and depth of anesthesia is nearly uncontrollable once the initial dose has been administered. In addition, due to the considerable variability in dose requirements for mice of different age, strain, sex, and other specifics (for example, circadian rhythm, sociophysiologic conditions), the margin between reaching a state of anesthesia sufficiently deep to provide surgical tolerance and a lethal outcome is usually narrow.1 Moreover, most injection anesthesia protocols induce a prolonged recovery period accompanied by hypothermia and compromised physiologic function.
Such problems rarely are encountered with inhalation anesthesia, because this method has a short recovery phase and accommodates control of the duration and depth of anesthesia, including expeditious adjustment of the dosage of inhalation anesthetics tailored to the requirements of the individual animal. Therefore, in terms of survival rate, inhalation anesthesia generally is suggested to be safe in mice. However, negative effects on the cardiovascular system combined with depression of respiration are well-known side effects of halogenated volatile anesthetics.18,19,46 This situation, coupled with the fact that the analgesia provided by monoanesthesia with volatile anesthetics is still controversial,9,15 justifies a continued search for improvement.
By taking advantage of the well-known synergistic and additive interactions between injectable drugs (analgesics or sedatives) and volatile anesthetics, the dosages of each component can be decreased (relative to its use as a sole agent) while inducing general anesthesia of sufficient depth with fewer side effects.16,20,32 This approach, sometimes referred to as ‘balanced’ or ‘modular’ anesthesia,49 is used widely in human and veterinary medicine—but only recently has it has begun to be used in mice. Therefore, in the present study, 2 protocols of combined injection and inhalant anesthesia in laboratory mice were established and compared with a standard protocol of inhalant monoanesthesia with a commonly used volatile anesthetic.
Isoflurane and sevoflurane are the 2 of the volatile anesthetics most widely used in human and veterinary anesthesia. We decided to use sevoflurane to provide rapid induction and recovery. Because we considered that volatile anesthetics offer suboptimal analgesia, we focused on injectable agents that could provide sufficient analgesia to complement inhalant anesthesia. Ketamine is known for its ability to cause profound analgesia, which can occur even at subhypnotic dosages—particularly if the S(+)-enantiomer of ketamine is adminstered.36 Therefore, we chose S-ketamine for injection in one protocol. We calculated the dosage based on literature reports,17,34,36,37 with the aim of minimizing side effects such as catalepsy, slight respiratory depression, and stimulation of locomotor activity (restlessness) while inducing analgesia and taking advantage of the hypnotic and cardiovascular stimulatory effects of ketamine.17,30 Our second approach to combining inhalation anesthesia with injectable agents in mice was based on drugs that are used widely in human medicine, namely fentanyl and midazolam. Midazolam, which often is applied as premedication to anesthesia, belongs to the benzodiazepines, which typically induce sedation, anxiolysis, and muscle relaxation.28 Antinociceptive effects of midazolam have been reported in mice8 and rats.5 In humans, benzodiazepines frequently are administered with opioids to improve pain relief. Therefore, we combined midazolam with fentanyl—a potent synthetic opioid analgesic. Among the typical side effects of opioids,26 sedation, hypothermia, respiratory depression, and hypercapnia could be of relevance for the use of fentanyl during anesthesia in mice. Although opioids can cause bradycardia, vasodilation, and hypotension, they have mostly only mild effects on cardiovascular function. In addition, their effects on the genitourinary system and gastrointestinal tract26 (for example, constipation) are suggested to be tolerable side effects, which may be of only minor relevance for establishing an anesthesia protocol in mice. Fentanyl often is administered as an intravenous constant-rate infusion in the context of anesthesia in humans,48 but this technique is complex and difficult to manage in mice. Therefore, we attempted to achieve preemptive analgesia with subcutaneous injection of fentanyl with midazolam as premedication, with dosages selected on the basis of anecdotal evidence, clinical experience, and hints from the literature.47
To compare the 3 anesthesia protocols, 50 min of sevoflurane inhalant anesthesia was conducted either alone (S) or with subcutaneous injection of S-ketamine (KS) or a mixture of fentanyl and midazolam (FMS). Injections were administered as premedication, and their effects on behavior during induction of anesthesia and on the sevoflurane concentration required were noted. During anesthesia, heart rate, core body temperature, respiratory rate, arterial blood gases, and arterial pH were monitored. The long-term effect of the 3 protocols on recovery from anesthesia was investigated through telemetric measurements of heart rate, core body temperature, and locomotor activity for 3 d after anesthesia.
Materials and Methods
Animals and housing conditions.
Female C57BL/6J mice (n = 98; age, 6 wk) were obtained from our inhouse breeding colony. The 72 mice used for determination of minimal alveolar concentrations were later euthanized to obtain arterial blood for measuring acid–base balance and blood gas concentrations. The remaining 26 mice were implanted with telemetric transmitters prior to the experiments to allow measurement of heart rate, core body temperature, and locomotor activity. The mice were free of all viral, bacterial, and parasitic pathogens listed in the FELASA recommendations.33 Health status was monitored by a sentinel program throughout the experiments.
Mice generally were housed in pairs; each transmitter-implanted mouse was housed with a nonimplanted companion of the same strain, sex, and age. Mice were kept in Eurostandard type III open-top plastic cages (425 mm × 266 mm × 155 mm, floor area 820 cm2, Tecniplast, Indulab, Gams, Switzerland) with autoclaved aspen bedding (80 to 90 g per cage; LTE E-001 Abedd, Indulab). Autoclaved hay (8 to 12 g per cage) and 2 cotton nesting pads (each 5 × 5 cm; Nestlets, Indulab) were provided as nesting materials. A standard cardboard house (Ketchum Manufacturing, Brockville, Canada) served as a shelter. Mice were fed a pelleted mouse diet (3431, Provimi Kliba, Kaiseraugst, Switzerland) ad libitum and had unrestricted access to sterilized drinking water provided in a water bottle. The 12:12-h light:dark cycle in the room was established with artificial light (approximately 40 lx in the cage; lights on, 0300 to 1500). The temperature was 21 ± 1 °C, with a relative humidity of 50% ± 5% and 15 complete changes of HEPA-filtered air hourly. To avoid interfering influences, all necessary husbandry and management procedures were completed in the room at least a d before the start of an experiment or data recording, and disturbances (for example, visitors or unrelated experimental procedures) were not allowed. The animal room was insulated to exclude electronic noise.
The study and all procedures and protocols were approved by the Cantonal Veterinary Office (Zurich, Switzerland) under license number 111/2007. Housing and experimental procedures were in accordance with Swiss animal protection law and conformed to the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (Council of Europe nr.123 Strasbourg 1985).10 Housing and experimental procedures also were in accordance with the Guide for the Care and Use of Laboratory Animals22 and conformed to the AALAS position statement on the humane care and use of laboratory animals.
Transmitter implantation.
Prior to the experiments, at age 10 wk, 26 mice were instrumented with telemetric transmitters (TA10ETA-F20, Data Sciences International, St Paul, MN) to measure heart rate, core body temperature, and locomotor activity in freely moving mice.2,44 Briefly, mice were anesthetized with sevoflurane (Sevorane, Abbott, Baar, Switzerland), and the transmitter body was implanted in the abdomen under aseptic conditions. One wire-loop electrode was fixed with silk sutures (6-0 Perma-Handseide, Ethicon, Norderstedt, Germany) between the muscles located to the right of the trachea, and the other loop lead was sutured to the xiphoid process. Muscle layers and skin were closed with resorbable sutures (6-0 Vicryl, Ethicon). Postoperative pain was treated with flunixine (5 mg/kg SC twice daily; Biokema Flunixine, Biokema SA, Crissier-Lausanne, Switzerland) for 4 d.38 After transmitter implantation, mice were allowed to recover for 6 wk before the first experiment.
Experimental setting.
All experiments were conducted when the mice were 16 to 36 wk of age, with body weights ranging from 25 to 30 g. To avoid any influence of circadian rhythm, all experiments and weighing procedures were done between 1500 and 1800. The study was designed for the experiments and anesthesia to be performed at the beginning of the dark phase for these mice. Anesthesia was performed in a separate operating area within the animal room to avoid transportation of the mice and to ensure stable conditions of humidity, air pressure, and room temperature and sufficient removal of gases and smells through the ventilation system.
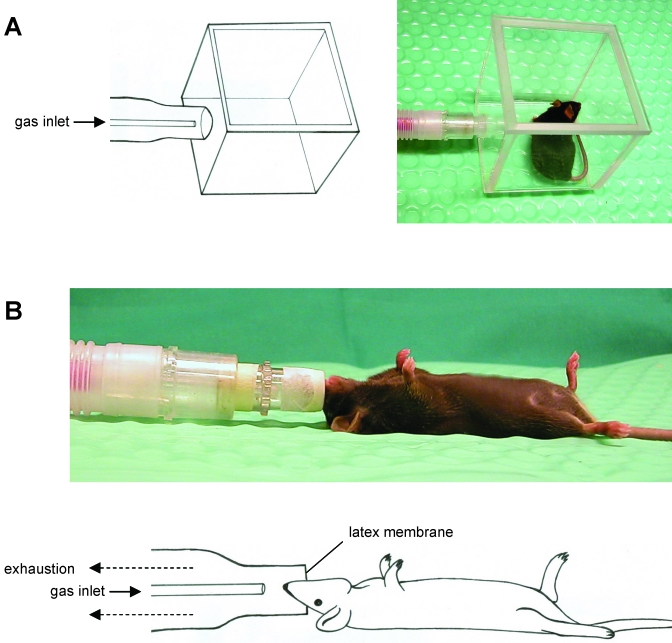
The method of delivering inhalation anesthesia was modified slightly from that described elsewhere.6 Briefly, sevoflurane was provided by using a commercially available rodent inhalation anesthesia apparatus (Provet, Lyssach, Switzerland), which was equipped with a sevoflurane vaporizer (Ohmeda Sevotec 5, Abbott, Baar, Switzerland) and a pump-driven filter system to eliminate waste anesthetic gas. As carrier gas, pressurized air was used at a flow rate of 600 mL/min. The anesthetic gas was introduced into the induction chamber or nose mask (Figure 1).
Figure 1.
(A) Chamber for induction of sevoflurane inhalation anesthesia. (B) Nose mask for maintaining sevoflurane anesthesia. The mask was equipped with a latex membrane, which had a hole in the center that fit around the nose of each mouse, with the dual purpose of preventing both withdrawal of environmental air into the nose mask and leakage of anesthetic gas from it.
Premedications.
As injectable drugs, we used fentanyl (0.04 mg/kg SC; Kantonsapotheke Zurich, Zurich, Switzerland) mixed with midazolam (4 mg/kg SC; Dormicum, Roche Pharma Schweiz AG, Reinach, Switzerland) in one protocol and S-ketamine (30 mg/kg SC; Keta-S, Graeub AG, Bern, Switzerland) in another. All drugs were dissolved in PBS immediately before injection in such a manner that dosing could be achieved by application of an injection volume of 2 μL/kg body weight. Injections were provided as premedication, that is, 5 to 7 min before sevoflurane anesthesia was induced.
Determination of minimal alveolar concentration.
Sevoflurane inhalation anesthesia was standardized by establishing minimum alveolar concentrations during sevoflurane monoanesthesia and after the premedications described earlier. To this end, we anesthetized 72 nontransmitter-implanted mice 2 to 4 times each; care was taken that mice had a break of at least 2 wk between tests.
Minimal alveolar concentration was determined according to commonly accepted procedures used in mice.6,7,14,23,42,43 For each protocol, 4 consecutive sevoflurane concentrations differing by 0.25% were tested; 25 mice were tested per concentration. The bracketed study design41 was adapted to our anesthesia protocols to measure minimal alveolar concentration at a defined time point of anesthesia, that is, at 12 min after inducing inhalant anesthesia (equivalent to 17 to 19 min after subcutaneous injection of premedication). Thus, after inducing sevoflurane anesthesia in the induction chamber (Figure 1 A) for 1.5 min at a maximal concentration of 8% sevoflurane, the mouse was taken out of the chamber and placed in dorsal recumbency on a warmed mat. Anesthetic gas then was applied at the test concentration by using a nose mask, with the mouse breathing spontaneously (Figure 1 B). After an equilibration time of 10 min, 3 noxious stimuli were applied sequentially: pinching of the tail (tail pinch reflex), interdigital webbing (pedal withdrawal reflex), and abdominal skin (abdominal skin pinch reflex). All stimuli were induced by the same investigator by using blunt forceps with a spacer between its arms to allow uniform application of pressure. Any motor response (for example, movement of the tail or an extremity, head jerking) to one or more of the 3 noxious stimuli was judged as purposeful movement, indicating that sevoflurane at the concentration applied did not induce anesthesia in the mouse evaluated. After testing the response to the 3 noxious stimuli (that is, after 12 to 13 min of inhalant anesthesia), administration of the anesthetic gas ceased, and the mouse was allowed to recover. By using the responses to the noxious stimuli, the mouse's minimal alveolar concentration was calculated as the average of the 2 partial pressures bracketing the positive response (that is, purposeful movement) or lack of response in the animal.
Anesthesia experiments.
Mice were allocated randomly to 1 of 3 anesthesia protocols. The 3 protocols consisted of fentanyl (0.04 mg/kg) and midazolam (4 mg/kg) as premedication and 3.3% sevoflurane (FMS); S-ketamine (30 mg/kg) as premedication and 5% sevoflurane (KS); and 5% sevoflurane as monoanesthesia (S). After premedication in the FMS and KS protocols, the mice were examined for 5 to 7 min in their home cage for behavioral aberrations. Inhalant anesthesia then was induced by placing each mouse in a clear induction chamber (8 × 8 × 8 cm; volume, 512 mL) into which 8% sevoflurane (Sevorane, Abbott, Baar, Switzerland) was introduced. The mouse's behavior in the induction chamber and the time point at which it became immobile were observed and noted. After 1.5 min, the mouse was transferred rapidly to the nose mask, through which anesthesia was maintained with sevoflurane. Mice breathed spontaneously while lying in dorsal recumbency on a water-filled warming mat (Gaymar, TP500, Orchard Park, NY) set at 39 °C ± 1 °C.
Tail pinch, pedal withdrawal, and abdominal skin pinch reflexes each were tested at 5-min intervals. All reflex tests were induced by the same investigator by using blunt forceps with a spacer between its arms to allow uniform application of pressure. The reflex tests were registered as positive or negative (that is, whether any motor response was present or not).
Respiratory rate was counted from the movement of the thorax wall and recorded at 5-min intervals. During anesthesia, mice were observed for any abnormality in their respiratory rhythm. In addition, heart rhythm alterations were monitored by using real-time telemetric electrocardiograms.
Anesthesia was stopped after 50 min by removing the nose from the mask and letting the mouse breathe room air. Mice were left on the warming mat and allowed to recover from anesthesia for 10 min before being placed back in their home cages.
Telemetric data acquisition and analysis.
Telemetric data were recorded from 8 mice per anesthetic protocol by using the Dataquest LabPRO program (Data Sciences International). Data collection was initiated by switching on the transmitter by using a magnet. Data acquisition started 3 d before anesthesia and continued for 3 d after anesthesia.
To estimate the acute effects of anesthesia (that is, after premedication and during anesthesia), heart rate and core body temperature were recorded for 4 s every 15 s (4 data points of 4 s per minute). From these data, mean values of heart rate and core body temperature were calculated for each minute for each mouse. Baseline values represent means from 1500 to 1800 (that is, the same time frame during which anesthesia occurred) during the 3 d prior to the experiment.
To establish baseline values (3 d before anesthesia) and to investigate postanesthetic effects (3 d after anesthesia), heart rate was measured for 30 s every 5 min, and core body temperature was measured for 10 s every 5 min. Locomotor activity was recorded continuously and stored at 5-min intervals.
For analysis of long-term postanesthetic effects, we took into account that values vary greatly during a 24-h cycle because mice are active mainly at night. Therefore, the means of the telemetric values for each mouse were calculated separately for the 12-h dark (night) and 12-h light (day) phases. A mouse's baseline values were established by calculating means from the 3 d prior to anesthesia. For each day after anesthesia, a mouse's baseline value was subtracted individually from its daytime and nighttime means; the differences are reported as delta (Δ) values.
Changes in body weight.
Body weight in transmitter-implanted mice was monitored for 3 d before and 3 d after anesthesia. Weights were obtained by using a precision balance (PR 2003 Delta Range, Mettler-Toledo AG, Greifensee, Switzerland) that specifically was adjusted for use with moving animals. Body weights were corrected to account for the weight of the transmitter (3.6 g). Mean baseline weight (from 3 consecutive daily measurements prior to the experiment) was calculated for each mouse and compared with that recorded on each of the 3 d after the experiment.
Acid–base balance and blood gas concentration.
At 3 to 4 wk after determination of minimal alveolar concentration determination, arterial blood was collected from the same 72 nonimplanted mice to assess acute effects of anesthesia on respiration and acid–base balance. Arterial blood was obtained after 10, 30, and 50 min of anesthesia from 8 mice per anesthetic protocol and time point.
Blood sampling and analyses were carried out as described previously.1,6 Briefly, the anterior neck was incised, the right common carotid artery was dissected out, a small hole in the artery was created by using fine-blade scissors, and arterial blood was collected in a heparinized syringe. Acid–base balance (pH), pCO2 (mm Hg), and pO2 (mm Hg) were determined immediately by using a blood-gas analyzer (Compact 3, AVL List, Graz, Austria). These mice died immediately due to the subsequent rapid loss of blood under anesthesia. Reference values of pH, pCO2, and pO2 for comparison had been established by using arterial blood from 20 HanIbm:NMRI mice that were similar in age to those in the current study.1
Statistical analysis.
All data are presented as mean ± 1 SD. Statistical analysis (version 17.0, SPSS for Windows, SPSS, Chicago, IL) was done to validate the results. All data were tested for normal distribution and homogeneity of variance and met the necessary assumptions for parametric analyses. One-way ANOVA was performed to compare group means of minimal alveolar concentrations and time until immobilization as well as heart rate, core body temperature, and locomotor activity at each of the first 3 d after anesthesia. Post hoc analysis with Bonferroni tests was done to identify significant differences between groups. For comparison of baseline values with corresponding experimental group means of heart rate, core body temperature, and locomotor activity during and at each of the 3 d after anesthesia, a dependent t test for paired samples was used. P values less than or equal to 0.05 were considered significant.
Results
Minimal alveolar concentration.
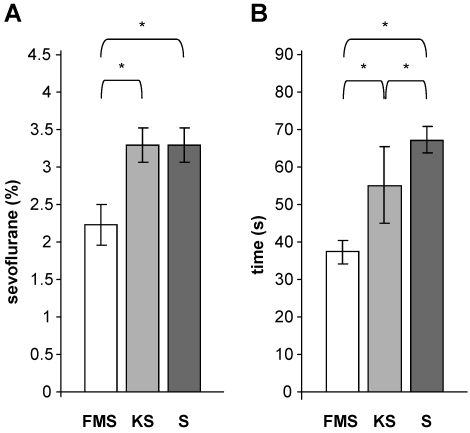
The minimal alveolar concentration (mean ± 1 SD) for sevoflurane monoanesthesia and with premedication using S-ketamine in adult female C57BL/6J mice was 3.3% ± 0.18% (Figure 2 A). Premedication with fentanyl–midazolam significantly (P = 0.0005) decreased the mean minimal alveolar concentration for sevoflurane to 2.2% ± 0.27% compared with that for the other 2 protocols. This decrease represents a gas savings of 33%.
Figure 2.
(A) Mean (n = 50 mice; bar, 1 SD) minimum alveolar concentrations for sevoflurane in adult C57BL/6J female mice. The gas-saving effect is evident from the decrease in minimum alveolar concentration seen after fentanyl–midazolam premedication with sevoflurane (FMS) compared with S-ketamine premedication with sevoflurane (KS) and sevoflurane alone (S). *, P ≤ 0.05 between values. (B) The mean time (n = 8 mice; bar, 1 SD) required until immobilization after mice were placed in the sevoflurane-filled induction chamber differed between all protocols. *, P ≤ 0.05 between values.
We considered that providing sevoflurane at 1.5 times the minimal alveolar concentration would prevent mice from responding to noxious stimulation (that is, surgical tolerance is achieved). Therefore all subsequent anesthesia experiments were conducted by using 3.3% sevoflurane after fentanyl–midazolam premedication but by using 5% sevoflurane after S-ketamine premedication and during sevoflurane monoanesthesia.
Induction of anesthesia.
Approximately 2 min (107.5 ±18.3 s) after injection with fentanyl–midazolam, all mice showed signs of sedation (for example, absence of locomotion and stationary activity, sleep-like posture). Approximately 5 min (306 ± 55.8 s) after injection with S-ketamine, all mice exhibited symptoms of tremor, ataxia, and dizziness.
When placed in the induction chamber, most nonpremedicated mice (that is, the sevoflurane monoanesthesia group) showed behaviors including defecation, urinating, shaking the head or limbs, jumping, and locomotion (Table 1). These behaviors were less frequent after S-ketamine premedication and were nearly totally absent after fentanyl–midazolam premedication. One transmitter-implanted mouse died after S-ketamine premedication when the animal was exposed to sevoflurane in the induction chamber; this animal was replaced.
Table 1.
Behaviors of mice (n = 8 per protocol) during induction of anesthesia with sevoflurane.
| % of animals showing |
||||||
| Anesthesia | Locomotion with or without ataxia | Jumping | Shaking head or limbs or both | Urination | Defecation | Apnea or death |
| FMS | 12.5 | 0 | 0 | 0 | 0 | 0 |
| KS | 100 | 0 | 0 | 37.5 | 0 | 12.5 |
| S | 100 | 50 | 100 | 100 | 62.5 | 0 |
The time until immobilization differed among all 3 protocols. The shortest time was associated with the FMS protocol and the longest with sevoflurane monoanesthesia (FMS compared with S, P = 0.0005; FMS compared with KS, P = 0.0005; KS compared with S, P = 0.004; Figure 2 B).
Effects during anesthesia.
During anesthesia, none of the mice showed any motor response to testing of the pedal withdrawal reflex, tail pinch, or abdominal skin pinch.
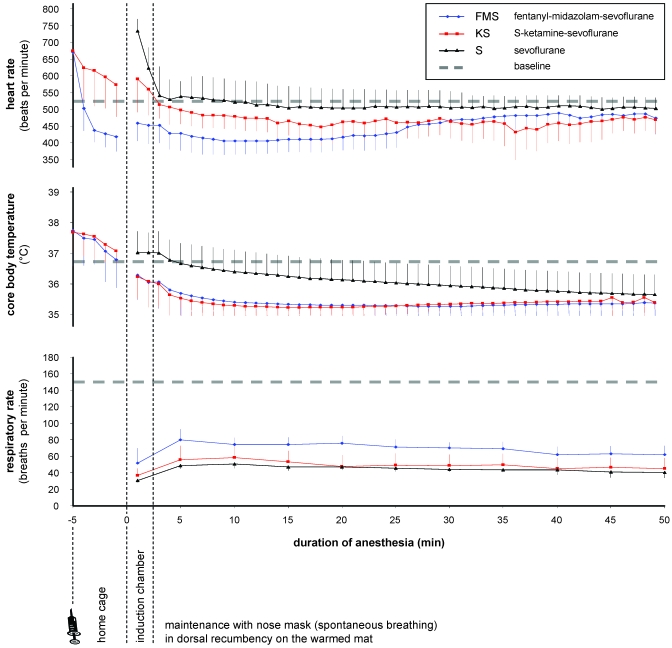
During the 50-min anesthesia period in all 3 protocols, heart rate and core body temperature remained within the general physiologic boundaries for this species (350 to 800 bpm, 35 to 38 °C; Figure 3). Heart rate was 446 ± 51 bpm during FMS anesthesia, 470 ± 59 bpm during KS anesthesia, and 519 ± 60 bpm during sevoflurane monoanesthesia. Compared with the mean baseline heart rate at the corresponding time of day (525 ± 80 bpm), the decreases in heart rate during FMS (P = 0.001) and KS (P = 0.030) were significant.
Figure 3.
Mean (n = 8 mice; bar, 1 SD) heart rate, core body temperature, and respiratory rate after premedication in the home cage, in the induction chamber, and during 50-min sevoflurane anesthesia while mice breathed spontaneously and lay in dorsal recumbency on the warming mat. Dashed lines indicate mean baseline values (measured before anesthesia) at the same time of day in conscious mice. The baseline respiratory rate was established by counting the movement of the thorax wall in resting mice before anesthesia.
Compared with the baseline core body temperature at the same time of day (36.8 ± 0.7 °C), core body temperature was decreased significantly during FMS (35.4 ± 0.6 °C; P = 0.0005) and KS (35.4 ± 0.4 °C; P = 0.0005) anesthesia. Core body temperature showed a tread toward a decrease during sevoflurane monoanesthesia (36.1 ± 0.7 °C; P = 0.058).
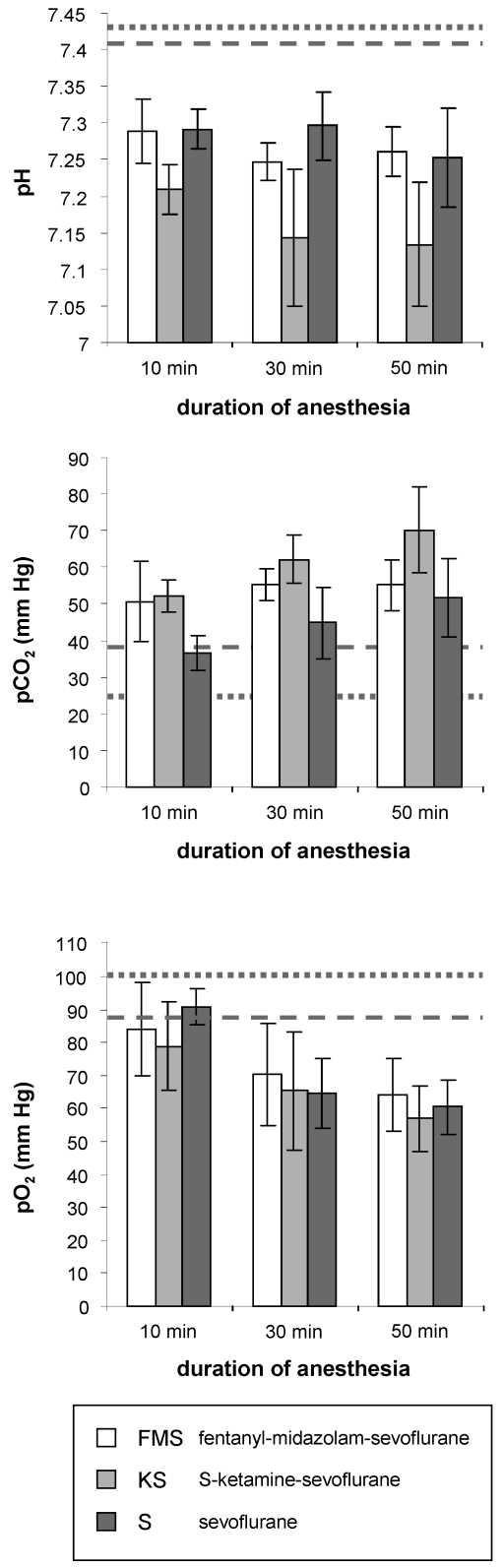
During all 3 protocols, the respiratory rate declined immediately after the onset of sevoflurane anesthesia and remained markedly depressed during the 50-min anesthesia session compared with baseline respiration in resting mice at the same time of day (150 ± 10 breaths per minute; Figure 3). The respiratory rate during anesthesia was 68.5 ± 7.7 breaths per minute for FMS, 48.8 ± 5.4 breaths per minute for KS, and 44 ± 5.1 breaths per minute for sevoflurane monoanesthesia. After 10, 30 and 50 min of anesthesia in all 3 protocols, blood gas and pH measurements of arterial blood showed prominent acidosis, hypercapnia, and hypoxia, with values markedly exceeding the physiologic range (Figure 4).
Figure 4.
Mean (n = 8 mice; bar, 1 SD) acid–base balance (pH), pCO2, and pO2 in arterial blood after 10, 30, and 50 min of sevoflurane anesthesia. Dotted lines indicate baseline levels established from HanIbm:NMRI mice in a previous study.1 Dashed lines indicate published values from conscious C57BL/6J mice.24
During anesthesia with KS, all mice displayed cardiac arrhythmia and episodes of apnea followed by tachypnea. None of these events occurred during either of the other 2 protocols. One transmitter-implanted mouse died at 15 min into KS anesthesia and was replaced.
Mice began showing increasing respiratory rate and muscle rigor within 1 min after sevoflurane was discontinued. In all 3 protocols, the mice had turned to ventral recumbency and were able to move within approximately 2 min after sevoflurane withdrawal.
Effects during the first 3 d after anesthesia.
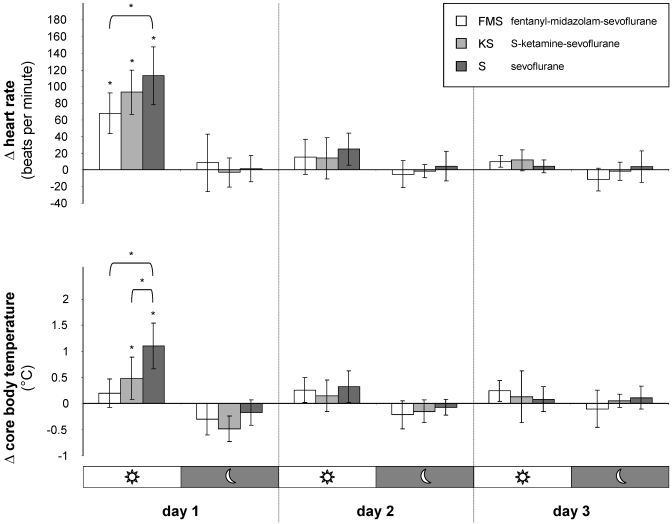
Compared with baseline values, telemetric measurements revealed a significant (P = 0.0005) increase in heart rate during the first 12 h after anesthesia in all 3 protocols (Figure 5). Comparing between protocols, the increase in heart rate after sevoflurane monoanesthesia was significantly (P = 0.0005) higher than that after FMS anesthesia, whereas heart rate after KS anesthesia did not differ significantly from that in the other 2 protocols.
Figure 5.
Mean (n = 8 mice; bar, 1 SD) postanesthetic measurements of the effects of 3 anesthesia protocols on heart rate and core body temperature. Delta (Δ) values represent deviations from baseline values (established prior to anesthesia) during the corresponding 12-h day and night periods. *, P ≤ 0.05 compared with baseline values and between protocols.
Compared with baseline values, core body temperature increased significantly during the first 12 h after anesthesia with KS anesthesia (P = 0.005) and sevoflurane monoanesthesia (P = 0.0005) but not after FMS anesthesia (Figure 5). Core body temperature was significantly higher after sevoflurane monoanesthesia compared with KS and FMS (S compared with FMS, P = 0.0005; S compared with KS, P = 0.006).
Locomotor activity and body weight were unchanged in all groups after anesthesia relative to baseline values before anesthesia.
Discussion
All 3 protocols tested provided a reliable 50-min period of anesthesia in laboratory mice, with short induction and recovery phases and lack of motor response to noxious stimuli. Subcutaneous injection of fentanyl–midazolam prior to sevoflurane inhalant anesthesia induced a gas-saving effect and had the advantage of inducing immediate sedation and preventing aversive reactions as well as extensive movements at the time of induction with sevoflurane. Injection of S-ketamine, the S(+)-enantiomer of ketamine, initially induced behavioral aberrations suggestive of excitation but attenuated aversive behaviors when mice were exposed to sevoflurane. In contrast, when sevoflurane anesthesia was induced without premedication, mice responded with defecation, urination, and locomotion including jumping and abnormal stationary movements. Compared with sevoflurane monoanesthesia, both premedication regimens shortened the time required to reach immobilization after exposure to sevoflurane; this effect was most pronounced with fentanyl–midazolam.
During anesthesia, while mice were warmed by a water-filled mat, core body temperature and heart rate were depressed compared with baseline values obtained at the time of day but before anesthesia. Both premedications intensified these effects, but all values during all 3 protocols remained within the ranges considered to be normal for mice. The most important adverse side effect that occurred during anesthesia was marked respiratory depression, as indicated by respiratory rates that were far below those of normal resting mice. This respiratory depression was accompanied by pronounced hypoxia, hypercapnia, and acidosis, all of which increased with time during anesthesia. Such changes in acid–base balance and blood gasses are well-known side effects of inhalant as well as injectable anesthesia.3,6,46 The degree of respiratory depression was nearly equal among all protocols, but apnea, tachypnea, and cardiac arrhythmia occurred with KS anesthesia, and 2 mice in this group died.
During the first 12 h after anesthesia, heart rate increased in all protocols; this increase was most pronounced during sevoflurane monoanesthesia and least apparent during the FMS protocol. Core body temperature was increased at 12 h after sevoflurane monoanesthesia and to a lesser extent after KS anesthesia. Because locomotor activity was unchanged after anesthesia regardless of protocol, physical activity is unlikely to be the reason for these effects. Postanesthetic measurements, including monitoring of body weight, indicated that all 3 protocols had only a short-term effect on the physiology and general condition of the mice.
The minimal alveolar concentration of sevoflurane was determined according to standard principles,41 including generally accepted adaptations for the particular species-specific conditions of mice.23,42,43 These modifications mainly concern the fact that constant-rate infusions and mechanical ventilation are not performed during determination of minimal alveolar concentration in mice. Furthermore, measurements of minimal alveolar concentration in mice were based on the inspired concentration of the inhalant, instead of on the end-tidal value, as is typical for larger animal species. In addition to the common single noxious stimulus induced by pinching the tail of the mouse,7 we applied 2 other noxious stimuli. The hindlimb withdrawal reflex has been shown to be useful for estimating depth of anesthesia in mice.24 Because applying a clamp between the toes was described as useful during the determination of minimal alveolar concentration of isoflurane in mice,14 we incorporated this stimulus in the form of pinching the interdigital webbing of the paw (pedal withdrawal reflex) in a reproducible manner. As a third noxious stimulus, the abdominal skin pinch reflex was applied as described earlier.1 For determination of the minimal alveolar concentration of sevoflurane, we applied these 3 noxious stimuli only once at a predefined time point of inhalant anesthesia to standardize the experimental conditions in regard to sevoflurane concentration and the single injection of fentanyl–midazolam or S-ketamine, with a view to determining the pharmacokinetics of the injected agents. Therefore, minimal alveolar concentration was determined at 12 min of sevoflurane anesthesia, which is congruent with 17 to 19 min after subcutaneous injection of the premedication.
The minimal alveolar concentration determined for sevoflurane monoanesthesia (3.3%) for the female C57BL/6J mice we tested here was similar to values in from the literature.6,29 Analgesic substances are known to reduce the minimal alveolar concentration during inhalant anesthesia in many animal species.12,51 In humans, both fentanyl and midazolam induce a gas-saving effect when combined with volatile anesthetics.21,31,39 In the current study, applying 0.04 mg/kg fentanyl in combination with 4 mg/kg midazolam as a subcutaneous bolus injection prior to anesthesia reduced the requirement for sevoflurane gas by one third. A similar gas-saving effect with isoflurane has been described for ketamine in dogs,40 but combination of S-ketamine with sevoflurane did not have this effect in our mice. The most probable explanation for this lack is that we could not administer S-ketamine as a target-controlled intravenous infusion (as is possible in large animals and humans) but rather as a single subcutaneous bolus injection. Therefore, from a pharmacokinetic viewpoint, the effects of S-ketamine might already have been decreasing when we determined the minimal alveolar concentration (that is, at 17 to 19 min after subcutaneous injection of 30 mg/kg S-ketamine).36
After standardization of the dosages by establishing minimal alveolar concentrations, we then compared the 3 protocols at dosages of sevoflurane representing 1.5 times the minimal alveolar concentrations. At this dosage, it is generally postulated that 99.9% of animals will not react to noxious stimuli,11,13 that is, that the animals have reached surgical tolerance. However, because we did not confirm analgesia by, for example, measuring heart rate, blood pressure, or respiration in response to a substantially noxious stimulus (for example, skin incision), we cannot claim definitively that surgical tolerance was achieved in the current study. However, motor reflex responses to noxious stimuli were suppressed in all mice for the entire duration of anesthesia (that is, 50 min).
Shortly (within approximately 2 min) after injection with fentanyl–midazolam, all mice exhibited reduced physical activity and a sleep-like posture, likely due to the sedative effect of these agents. In contrast, injection of S-ketamine gave rise to muscle tremors and ataxia. The spike (up to 800 bpm) in heart rate that we noted in the early phase of induction during sevoflurane monoanesthesia may be a normal reaction to removal of the mouse from its cage and placing it in a foreign environment (that is, induction chamber). The markedly lower heart rate during the induction phase of the FMS protocol suggests bradycardia due to fentanyl but also indicates the potential benefits of sedation, through stress reduction, during the initial phase of anesthesia.
During the 50-min anesthesia, mice anesthetized with FMS and KS displayed lower heart rate and core body temperature than did those anesthetized with S alone. This result can be explained by the known influences of fentanyl and ketamine on thermoregulation.4,50 In addition, the typical cardiovascular effects of the opioid might have potentiated the well-known cardiopulmonary depression caused by the volatile anesthetic sevoflurane. However, all heart rate and core body temperature measurements remained within the normal physiologic ranges of mice in all 3 protocols tested. Given that many other anesthetic regimens can decrease in core body temperature by more than 4 °C in just a few minutes, we consider the changes in the current study to be acceptable.1,52 Nevertheless, these findings underline the necessity for thermal support (as supplied in our experiments) during anesthetic procedures in small laboratory rodents.
In all protocols, the respiratory rate declined far below baseline values in resting mice. Blood gasses and pH in the arterial blood were impaired by all protocols to a similar extent and to values clearly different from published reference values from conscious HanIbm:NMRI and C57BL/6J mice.1,27 Therefore, respiratory depression—as evidenced by the marked decrease of respiratory rate in the presence of acidosis, hypoxia, and hypercapnia—was the most prominent side effect observed and it was present in all of the anesthetic protocols we tested. These symptoms may mainly reflect the effect of sevoflurane on cardiopulmonary function but also may be potentiated by fentanyl and—to a lesser extent—through S-ketamine. Although surgical stimulation can restore ventilation toward a less deleterious level,25,45 surgery was not performed in our experiments. Therefore, lack of constant surgical stimulation may have exacerbated the respiratory depression associated with duration of anesthesia. The oxygen content of the air used as a carrier gas likely is insufficient to prevent hypoxia. Therefore, increasing the inspiratory oxygen fraction (FiO2) above 0.3 by mixing oxygen into the carrier gas would be useful to minimize hypoxia due to anesthetic-induced respiratory depression.
The time required for recovery (that is, resposture and motion) after anesthesia was similar for all groups (1 to 2 min). This fact should be highlighted as a distinct advantage over most injectable anesthetic regimens in mice.1 The avoidance of postoperative complications associated with a prolonged recovery period and the resulting inability to correct physiologic impairment (for example, hypoxia, hypothermia, hypoglycemia, dehydration) is a key challenge in developing novel anesthesia methods for small laboratory rodents.
Heart rate and core body temperature increased in all 3 protocols during the first 12 h after the 50-min anesthesia, but these changes were attenuated or absent in mice that received FMS anesthesia. Therefore, the influence of our tested anesthesia protocols on physiology seemed to be of only short-term duration. It should also be noted that, during the 3 d after anesthesia, none of the anesthetic protocols showed an adverse effect on body weight, thus suggesting their negligible effects on the animals’ general condition.
In the current study, we tried to apply the most useful and efficient substances and dosages of injectable drugs and volatile anesthetic available for the adult female C57BL/6J mice we used. Because dosages vary greatly depending on the specific characteristics of the animals used (for example, strain, age, sex) as well as the ambient conditions of the laboratory, our protocols should carefully be adapted for use in other circumstances. Depending on the animals’ anesthetic needs and the severity of the intervention that they will undergo, higher or lower dosages—particularly of the volatile anesthetic sevoflurane—may be required. In addition, we suggest that the use of S-ketamine as a premedication might be improved by administering it at a lower dose or in combination with a minor tranquilizer. Such optimization could decrease the frequency, duration, and severity of side effects such as apnea, arrhythmia (including fatal abnormalities), and excessive excitation.
In conclusion, premedication with subcutaneous injection of fentanyl in combination with midazolam improved standard sevoflurane monoanesthesia of mice in our laboratory setting. Advantages included a short and quiet induction phase and decreased negative postanesthetic side effects on heart rate and core body temperature. A gas-saving effect was evident in the FMS treatment, corroborating the analgesic potential of the opioid component (fentanyl) in this modular anesthesia protocol.
Although all 3 protocols used here may be useful for anesthesia in mice, the combination of injection anesthesia with inhalation anesthesia could be superior to the widely used standard inhalation monoanesthesia, provided that appropriate drugs are combined and dosages are adapted to the requirements of the specific animals and laboratory. However, the choice of a specific anesthetic regimen should always be based on careful deliberation, considering arguments of animal welfare, feasibility, and any potential interference with the research project for which the anesthesia is required.
Acknowledgments
This work was sponsored by the ECLAM and ESLAV Foundation. We thank Robin Schneider and the staff of the Central Biological Laboratory for support in housing mice. We thank Professor Kurt Burki for generously providing research facilities and resources.
References
- 1.Arras M, Autenried P, Rettich A, Spaeni D, Rulicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456 [PubMed] [Google Scholar]
- 2.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. 2007. Assessment of postlaparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45 [DOI] [PubMed] [Google Scholar]
- 4.Bhargava HN. 1981. Antagonism of ketamine-induced anesthesia and hypothermia by thyrotropin releasing hormone and cyclo(His–Pro). Neuropharmacology 20:699–702 [DOI] [PubMed] [Google Scholar]
- 5.Boehm CA, Carney EL, Tallarida RJ, Wilson RP. 2010. Midazolam enhances the analgesic properties of dexmedetomidine in the rat. Vet Anaesth Analg 37:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, Arras M. 2010. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim 44:329–336 [DOI] [PubMed] [Google Scholar]
- 7.Chae YJ, Zhang J, Au P, Sabbadini M, Xie GX, Yost CS. 2010. Discrete change in volatile anesthetic sensitivity in mice with inactivated tandem-pore potassium ion channel TRESK. Anesthesiology 113:1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiba S, Nishiyama T, Yoshikawa M, Yamada Y. 2009. The antinociceptive effects of midazolam on 3 different types of nociception in mice. J Pharmacol Sci 109:71–77 [DOI] [PubMed] [Google Scholar]
- 9.Cornett PM, Matta JA, Ahern GP. 2008. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol Pharmacol 74:1261–1268 [DOI] [PubMed] [Google Scholar]
- 10.Council of the European Communities. Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off J Eur Communities L358:1-28. [Google Scholar]
- 11.de Jong RH, Eger EI., 2nd 1975. MAC expanded: AD50 and AD95 values of common inhalation anesthetics in man. Anesthesiology 42:384–389 [PubMed] [Google Scholar]
- 12.Doherty TJ, Frazier DL. 1998. Effect of intravenous lidocaine on halothane minimum alveolar concentration in ponies. Equine Vet J 30:300–303 [DOI] [PubMed] [Google Scholar]
- 13.Doherty TJ, Geiser DR, Frazier DL. 1997. Comparison of halothane minimum alveolar concentration and minimum effective concentration in ponies. J Vet Pharmacol Ther 20:408–410 [DOI] [PubMed] [Google Scholar]
- 14.Eckel B, Richtsfeld M, Starker L, Blobner M. 2010. Transgenic Alzheimer mice have a larger minimum alveolar anesthetic concentration of isoflurane than their nontransgenic littermates. Anesth Analg 110:438–441 [DOI] [PubMed] [Google Scholar]
- 15.Eilers H. 2008. Anesthetic activation of nociceptors: adding insult to injury? Mol Interv 8:226–229 [DOI] [PubMed] [Google Scholar]
- 16.Enderle AK, Levionnois OL, Kuhn M, Schatzmann U. 2008. Clinical evaluation of ketamine and lidocaine intravenous infusions to reduce isoflurane requirements in horses under general anaesthesia. Vet Anaesth Analg 35:297–305 [DOI] [PubMed] [Google Scholar]
- 17.Filibeck U, Castellano C. 1980. Strain-dependent effects of ketamine on locomotor activity and antinociception in mice. Pharmacol Biochem Behav 13:443–447 [DOI] [PubMed] [Google Scholar]
- 18.Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. 2003. Heritable differences in respiratory drive and breathing pattern in mice during anaesthesia and emergence. Br J Anaesth 91:541–545 [DOI] [PubMed] [Google Scholar]
- 19.Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. 2004. Influence of volatile anaesthetics on hypercapnoeic ventilatory responses in mice with blunted respiratory drive. Br J Anaesth 92:697–703 [DOI] [PubMed] [Google Scholar]
- 20.Hendrickx JF, Eger EI, 2nd, Sonner JM, Shafer SL. 2008. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg 107:494–506 [DOI] [PubMed] [Google Scholar]
- 21.Inagaki Y, Sumikawa K, Yoshiya I. 1993. Anesthetic interaction between midazolam and halothane in humans. Anesth Analg 76:613–617 [DOI] [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research. 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 23.Joo DT, Gong D, Sonner JM, Jia Z, MacDonald JF, Eger EI, 2nd, Orser BA. 2001. Blockade of AMPA receptors and volatile anesthetics: reduced anesthetic requirements in GluR2-null mutant mice for loss of the righting reflex and antinociception but not minimum alveolar concentration. Anesthesiology 94:478–488 [DOI] [PubMed] [Google Scholar]
- 24.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. 2003. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J 17:250–252 [DOI] [PubMed] [Google Scholar]
- 25.Lam AM, Clement JL, Knill RL. 1980. Surgical stimulation does not enhance ventilatory chemoreflexes during enflurane anaesthesia in man. Can Anaesth Soc J 27:22–28 [DOI] [PubMed] [Google Scholar]
- .Lamont LA, Mathews KA. 2007. Opioids, nonsteroidal anti-inflammatories, and analgesic adjuvants, p 241–271. In: Tranquilli WJ, Thurmon JC, Grimm KA. Lumb & Jones’ veterinary anesthesia and analgesia. Oxford (UK): Blackwell Publishing. [Google Scholar]
- 27.Lee EJ, Woodske ME, Zou B, O'Donnell CP. 2009. Dynamic arterial blood gas analysis in conscious, unrestrained C57BL/6J mice during exposure to intermittent hypoxia. J Appl Physiol 107:290–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemke KA. 2007. Anticholinergics and sedatives, p 203–239. In: Tranquilli WJ, Thurmon JC, Grimm KA. Lumb & Jones’ veterinary anesthesia and analgesia. Oxford (UK): Blackwell Publishing. [Google Scholar]
- 29.Liao M, Laster MJ, Eger EI, 2nd, Tang M, Sonner JM. 2006. Naloxone does not increase the minimum alveolar anesthetic concentration of sevoflurane in mice. Anesth Analg 102:1452–1455 [DOI] [PubMed] [Google Scholar]
- 30.Lin HC. 2007. Dissociative anesthetics, p 301–353. In: Tranquilli WJ, Thurmon JC, Grimm KA. Lumb & Jones’ veterinary anesthesia and analgesia. Oxford (UK): Blackwell Publishing. [Google Scholar]
- 31.Melvin MA, Johnson BH, Quasha AL, Eger EI., 3rd 1982. Induction of anesthesia with midazolam decreases halothane MAC in humans. Anesthesiology 57:238–241 [DOI] [PubMed] [Google Scholar]
- 32.Muir WW, 3rd, Wiese AJ, March PA. 2003. Effects of morphine, lidocaine, ketamine, and morphine–lidocaine–ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res 64:1155–1160 [DOI] [PubMed] [Google Scholar]
- 33.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B. 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42 [DOI] [PubMed] [Google Scholar]
- 34.Nishizawa N, Nakao S, Nagata A, Hirose T, Masuzawa M, Shingu K. 2000. The effect of ketamine isomers on both mice behavioral responses and c-Fos expression in the posterior cingulate and retrosplenial cortices. Brain Res 857:188–192 [DOI] [PubMed] [Google Scholar]
- 35.Richardson CA, Flecknell PA. 2005. Anaesthesia and postoperative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 33:119–127 [DOI] [PubMed] [Google Scholar]
- 36.Ryder S, Way WL, Trevor AJ. 1978. Comparative pharmacology of the optical isomers of ketamine in mice. Eur J Pharmacol 49:15–23 [DOI] [PubMed] [Google Scholar]
- 37.Sarton E, Teppema LJ, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL, Dahan A. 2001. The involvement of the µ-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg 93:1495–1500 [DOI] [PubMed] [Google Scholar]
- 38.Schuler B, Rettich A, Vogel J, Gassmann M, Arras M. 2009. Optimized surgical techniques and postoperative care improve survival rates and permit accurate telemetric recording in exercising mice. BMC Vet Res 5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sebel PS, Glass PS, Fletcher JE, Murphy MR, Gallagher C, Quill T. 1992. Reduction of the MAC of desflurane with fentanyl. Anesthesiology 76:52–59 [DOI] [PubMed] [Google Scholar]
- 40.Solano AM, Pypendop BH, Boscan PL, Ilkiw JE. 2006. Effect of intravenous administration of ketamine on the minimum alveolar concentration of isoflurane in anesthetized dogs. Am J Vet Res 67:21–25 [DOI] [PubMed] [Google Scholar]
- 41.Sonner JM. 2002. Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg 95:609–614 [DOI] [PubMed] [Google Scholar]
- 42.Sonner JM, Gong D, Eger EI., 2nd 2000. Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg 91:720–726 [DOI] [PubMed] [Google Scholar]
- 43.Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. 1999. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg 89:1030–1034 [DOI] [PubMed] [Google Scholar]
- 44.Spani D, Arras M, Konig B, Rulicke T. 2003. Higher heart rate of laboratory mice housed individually versus in pairs. Lab Anim 37:54–62 [DOI] [PubMed] [Google Scholar]
- 45.Sutherland RW, Drummond GB. 1996. Effects of surgical skin incision on respiration in patients anaesthetized with enflurane. Br J Anaesth 76:777–779 [DOI] [PubMed] [Google Scholar]
- 46.Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K. 2004. Long-term anaesthesia using inhalatory isoflurane in different strains of mice—the haemodynamic effects. Lab Anim 38:64–69 [DOI] [PubMed] [Google Scholar]
- 47.Thal SC, Plesnila N. 2007. Noninvasive intraoperative monitoring of blood pressure and arterial pCO2 during surgical anesthesia in mice. J Neurosci Methods 159:261–267 [DOI] [PubMed] [Google Scholar]
- 48.Theil DR, Stanley TE, 3rd, White WD, Goodman DK, Glass PS, Bai SA, Jacobs JR, Reves JG. 1993. Midazolam and fentanyl continuous infusion anesthesia for cardiac surgery: a comparison of computer-assisted versus manual infusion systems. J Cardiothorac Vasc Anesth 7:300–306 [DOI] [PubMed] [Google Scholar]
- 49.Tonner PH. 2005. Balanced anaesthesia today. Best Pract Res Clin Anaesthesiol 19:475–484 [DOI] [PubMed] [Google Scholar]
- 50.Ulugol A, Dost T, Dokmeci D, Akpolat M, Karadag CH, Dokmeci I. 2000. Involvement of NMDA receptors and nitric oxide in the thermoregulatory effect of morphine in mice. J Neural Transm 107:515–521 [DOI] [PubMed] [Google Scholar]
- 51.Valverde A, Doherty TJ, Hernandez J, Davies W. 2004. Effect of lidocaine on the minimum alveolar concentration of isoflurane in dogs. Vet Anaesth Analg 31:264–271 [DOI] [PubMed] [Google Scholar]
- 52.Zeller A, Arras M, Jurd R, Rudolph U. 2007. Mapping the contribution of β3-containing GABAA receptors to volatile and intravenous general anesthetic actions. BMC Pharmacol 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]